Most patients with follicular lymphoma (FL) have somatically mutated V genes with intraclonal variation, consistent with location in the germinal center site. Using our own and published sequences, we have investigated the frequency of potential N-glycosylation sites introduced into functional VH genes as a consequence of somatic mutation. FL cells were compared with normal memory B cells or plasma cells matched for similar levels of mutation. Strikingly, novel sites were detected in 55 of 70 (79%) patients with FL, compared to 7 of 75 (9%) in the normal B-cell population (P < .001). Diffuse large B-cell lymphoma (DLCL) showed an intermediate frequency (13 of 32 [41%] patients). Myeloma and the mutated subset of chronic lymphocytic leukemia showed frequencies similar to those of normal cells in 5 of 64 (8%) patients and 5 of 40 (13%) patients, respectively. In 3 of 3 random patients with FL, immunoglobulin was expressed as recombinant single-chain Fv inPichia pastoris, and glycosylation was demonstrated. These findings indicate that N-glycosylation of the variable region may be common in FL and in a subset of DLCL. Most novel sites are located in the complementarity-determining regions. VH sequences of nonfunctional VH genes contained few sites, arguing for positive selection in FL. One possibility is that the added carbohydrate in the variable region contributes to interaction with elements in the germinal center environment. This common feature of FL may be critical for tumor behavior.
Introduction
B-cell malignancies express many features of their normal B-cell counterparts, and these can reveal the stage of B-cell differentiation of the cell of origin. Follicular lymphoma (FL), which represents approximately 40% of all non-Hodgkin lymphomas, has a characteristic nodular or follicular architecture resembling that seen in the normal germinal center (GC) of a reactive lymph node. Most cases are surface immunoglobulin positive and express markers consistent with those of GC B cells. It has been assumed, therefore, that neoplastic transformation has led to an arrest of differentiation, with the accumulation of tumor cells in this site.
Further understanding of the nature of the cell of origin in FL has been provided by analysis of the immunoglobulin variable-region gene sequences of the tumor cells. During differentiation, normal B lymphocytes undergo a series of recombinatorial and mutational changes in their immunoglobulin variable-region genes. The V(D)J rearrangements of VH and VL genes occur mainly in the bone marrow, and, after encounter with antigen, the somatic mutation mechanism is activated in centroblasts in the GC.1-3 In this site, certain mutated sequences are selected by antigens held on follicular dendritic cells, leading to affinity maturation of the antibody response. Survival, maturation, and subsequent fate of selected B cells are directed by several additional elements in the GC, including CD40L+ T cells and cytokine milieu.1-3
For FL, it is clear that the cell of origin has undergone somatic mutation and that in many patients this process has continued after transformation, leading to intraclonal variation of V gene sequences.4-6 This behavior is consistent with location in the GC. Because normal B cells rely on engagement of the B-cell receptor for activation of the mutation mechanism, the finding of continuing mutational activity has led to debate about the role of antigens in stimulating FL.7 However, it appears that ongoing mutational activity may be limited in FL,8 but it is not apparent in cases that have transformed to diffuse lymphoma after chemotherapy.5,9 Uncertainty about the role of antigen in FL also cannot easily be resolved by analysis of mutational patterns in V genes because it is now evident that there is a natural tendency of the complementarity-determining region (CDR) sequences to accumulate replacement mutations.10 It remains unclear, therefore, whether antigen has a role in influencing the behavior of tumor cells in FL.
However, the importance of immunoglobulin expression in FL is highlighted by the fact that in most patients with FL, expression is retained, in spite of the fact that one allele of chromosome 14 is commonly disrupted at 14q32 by the t(14;18) translocation.11 12 Consequent overexpression of bcl-2 protein by the nonfunctional immunoglobulin allele is one mechanism that contributes to tumor cell survival. The almost universal conservation of immunoglobulin expression by the remaining functional allele might indicate a selective advantage for tumor cells in FL. The question arises as to whether this is dependent on stimulation by antigen.
Immunoglobulin carries N-glycosylated oligosaccharides located mainly in the heavy-chain constant regions. These act as spacers for the immunoglobulin molecule that are important for maintaining effector functions.13 Because of locations of the sites in the interstitial region between the CH2 domains, the oligosaccharide chains are incompletely galactosylated and sialylated.14 Human antibodies do not generally contain O-linked oligosaccharides, with the exception of IgA, which can be O-glycosylated in the hinge region.15 Glycosylation of the variable region is less common, but it has been found in some antibody molecules. Approximately 18% of the VH sequences in the Kabat database16 have been reported to contain a potential N-glycosylation site,17 but this includes tumor and normal sequences. The motif for N-glycosylation is Asn-X-Ser/Thr, where X is any amino acid except Pro, Asp or Glu. A few germline VHgenes have a naturally occurring N-glycosylation site, including V1-08, V4-34, and VH5a. Studies of monoclonal immunoglobulins with antibody activity have shown that binding to antigen can be increased15,18 or decreased19 by the presence of carbohydrate in the V region. There is also evidence that V-region glycosylation can alter physical properties20 and other characteristics normally attributed to the Fc region.17
We have been engaged in expressing recombinant variable region sequences from patients with B-cell malignancies, and we were struck by the frequency of apparent glycosylation in patients with FL. This led us to investigate our own and the large number of available published sequences for potential sites, which revealed that novel sites may be a feature of tumors of the germinal center.
Materials and methods
Patient material
Diagnostic lymph node biopsy specimens were received fresh, on the day of surgery, from 14 of 17 patients with FL and were processed at that time. In one patient, a frozen diagnostic biopsy was used as the source of material. In 2 of 17 patients (F12 and F72: Table1), repeat biopsy specimens, taken at the time of first and third recurrence, respectively, were received fresh. In all patients, the diagnosis of FL was made using clinical, histologic, and immunophenotypic data. Cell suspensions were made by dispersion through the wire mesh of a fine sieve into sterile RPMI medium (Gibco, Oxford, United Kingdom). The cells were collected, centrifuged, and washed once in RPMI. After resuspension, viability was assessed with 0.2% trypan blue stain, and aliquots of 107cells/mL were frozen in liquid N2 in 10% dimethyl sulfoxide, 50% decomplemented human AB serum, and 40% RPMI solution. An aliquot was used to determine the surface immunoglobulin isotype by fluorescence-activated cell sorter analysis. In a few patients, RNA was extracted from archived frozen tissue. Where possible, in those patients the immunophenotype was determined using immunohistochemical staining of paraffin sections.
V gene profiles and incidence of novel N-glycosylation sites resulting from somatic mutation in patients with FL
| VH . | VL . | Total . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient . | Germline donor . | Homology (%) . | JH . | No. sites . | Germline donor . | Homology (%) . | JL . | No. sites . | |
| F1 | V3-48 | 89 | JH3a | 1 | VκIII/A27 | 95 | Jκ1 | 0 | 1 |
| F2 | V3-48 | 92 | JH3b | 1 | VκIV/B3 | 96 | Jκ4 | 1 | 2 |
| F7 | V3-48 | 93 | JH4a | 2 | VκI/012 | 86 | Jκ3 | 1 | 3 |
| F8 | V3-48 | 80 | JH4 | 2 | VκIII/L2 | 96 | Jκ4 | 1 | 3 |
| F10 | V3-48 | 89 | JH4b | 1 | VκI/02 | 92 | Jκ2 | 0 | 1 |
| F11 | V3-48 | 91 | JH6b | 2 | VκI/A30 | 97 | Jκ1 | 0 | 2 |
| F15 | V3-48 | 96 | JH5a | 1 | VκIII/L6 | 97 | Jκ4 | 1 | 2 |
| F12 | V3-48 | 89 | JH6b | 1 | VκII/A17 | 99 | Jκ3 | 1 | 2 |
| F4* | V3-11 | 91 | JH6c | 2 | VκIII/L6 | 96 | Jκ4 | 1 | 3 |
| F72 | V3-15 | 92 | JH4b | 1 | VκI/A30 | 95 | Jκ2 | 0 | 1 |
| F17 | V3-21 | 91 | JH4 | 1 | Vλ1c | 94 | Jλ2 | 0 | 1 |
| F6 | V3-30 | 87 | JH4b | 0 | VκI/012 | 97 | Jκ2 | 1 | 1 |
| F3 | V3-49 | 87 | JH6b | 2 | VκI/012 | 88 | Jκ1 | 1 | 3 |
| F14 | P1† | 87 | JH4b | 1 | VκIV/B3 | 94 | Jκ1 | 0 | 1 |
| F5* | V4-34 | 83 | JH2 | 1 | VκIII/A27 | 89 | Jκ2 | 1 | 2 |
| F9 | V4-39 | 86 | JH5b | 1 | VκIV/A26 | 98 | Jκ2 | 0 | 1 |
| F16* | V4-59 | 86 | JH4b | 1 | Vλ1 | 96 | Jλ2 | 1 | 2 |
| VH . | VL . | Total . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient . | Germline donor . | Homology (%) . | JH . | No. sites . | Germline donor . | Homology (%) . | JL . | No. sites . | |
| F1 | V3-48 | 89 | JH3a | 1 | VκIII/A27 | 95 | Jκ1 | 0 | 1 |
| F2 | V3-48 | 92 | JH3b | 1 | VκIV/B3 | 96 | Jκ4 | 1 | 2 |
| F7 | V3-48 | 93 | JH4a | 2 | VκI/012 | 86 | Jκ3 | 1 | 3 |
| F8 | V3-48 | 80 | JH4 | 2 | VκIII/L2 | 96 | Jκ4 | 1 | 3 |
| F10 | V3-48 | 89 | JH4b | 1 | VκI/02 | 92 | Jκ2 | 0 | 1 |
| F11 | V3-48 | 91 | JH6b | 2 | VκI/A30 | 97 | Jκ1 | 0 | 2 |
| F15 | V3-48 | 96 | JH5a | 1 | VκIII/L6 | 97 | Jκ4 | 1 | 2 |
| F12 | V3-48 | 89 | JH6b | 1 | VκII/A17 | 99 | Jκ3 | 1 | 2 |
| F4* | V3-11 | 91 | JH6c | 2 | VκIII/L6 | 96 | Jκ4 | 1 | 3 |
| F72 | V3-15 | 92 | JH4b | 1 | VκI/A30 | 95 | Jκ2 | 0 | 1 |
| F17 | V3-21 | 91 | JH4 | 1 | Vλ1c | 94 | Jλ2 | 0 | 1 |
| F6 | V3-30 | 87 | JH4b | 0 | VκI/012 | 97 | Jκ2 | 1 | 1 |
| F3 | V3-49 | 87 | JH6b | 2 | VκI/012 | 88 | Jκ1 | 1 | 3 |
| F14 | P1† | 87 | JH4b | 1 | VκIV/B3 | 94 | Jκ1 | 0 | 1 |
| F5* | V4-34 | 83 | JH2 | 1 | VκIII/A27 | 89 | Jκ2 | 1 | 2 |
| F9 | V4-39 | 86 | JH5b | 1 | VκIV/A26 | 98 | Jκ2 | 0 | 1 |
| F16* | V4-59 | 86 | JH4b | 1 | Vλ1 | 96 | Jλ2 | 1 | 2 |
Expressed as recombinant scFv in P pastorisand in which functional glycosylation was demonstrated.
P1 is a member of VH 3 family, but the locus has not been identified.
Identification of tumor-derived V(D)J gene sequences
Total RNA was extracted from the cell suspensions or from 5-μm cut sections of frozen tissue using TRI Reagent following the supplier's instructions (Sigma, St Louis, MO). This is the preferred approach to identify functional transcripts, because it reduces the likelihood of amplifying the aberrantly rearranged allele. An aliquot of total RNA was then reverse transcribed using an oligo-d(T) primer and a first-strand cDNA synthesis kit (Amersham Pharmacia Biotech UK, Little Chalfont, United Kingdom). For identification of the tumor VH genes, 1 to 3 μL cDNA was amplified by polymerase chain reaction using 5′ VH leader primers (VH1 to VH6) either as a mix or as individual VHprimers, together with a 3′ constant region primer (Cμ, Cγ, or Cα) as previously described.21,22 If the isotype was unknown, a consensus JH primer was generally used as the 3′ primer. For VL genes, mixes of 5′ framework (FR) 1 primers in combination with the appropriate 3′ J region primers were used as previously described.23 At least 2 independent PCR amplifications were performed for each sample. Amplified products were separated by agarose gel electrophoresis, purified using the GeneClean kit (Bio101 Inc, Vista, CA), and cloned into the pGEM-T vector (Promega, Madison, WI). Plasmids were isolated from randomly selected bacterial clones and were sequenced using the M13-20 and reverse primers on an ABI 377 automatic sequencer (Foster City, CA). Tumor-related V genes were identified as predominant repeated or similar sequences with clonally related CDR3.23 Sequence alignment analysis used MacVector software (Oxford Molecular, Oxford, United Kingdom) and was aligned to Entrez and V-BASE databases with the DNA plot program available on the Internet (www.mrc-cpe.cam.ac.uk/imt-doc/INTRO.html).
VH gene sequence cohorts
FL VH gene sequences included in this study were obtained from our own laboratory (GenBank accession numbersAF398949-AF398965) and from the study by Noppe et al6(accession numbers AJ234156-AJ234298). Sequences from normal memory B cells and plasma cells included human monoclonal antibodies specific for foreign protein antigens (accession numbers S55287, 89-90, 92,M20003, 31, L26531-40, L37310-1, S67981-8, L01410-3, M97802-5,L03677-84, M87789-924-31); IgM+IgD+ CD27+ peripheral blood B cells (accession numbers AJ231545-AJ23168532); normal memory B cells (accession numbers Z80363-Z8077033); and IgA- and IgM-secreting intestinal plasma cells (accession numbersAJ002639-AJ002674, AJ009516-AJ00954534). Sequences chosen in this cohort all had a mutation frequency of more than 5%, similar to that of the FL cohort. Sequences from DLCL, multiple myeloma (MM), and the mutated subset of chronic lymphocytic leukemia (CLL) were obtained using our own data and those from the Entrez database. Accession numbers are as follows: DLCL, Z93849-Z93863,AF283779-AF283782, 84-87, 89, 91, 93, AF283800-02, 05-0635,36; MM, Z70256-257, Z75556-5557, X98899-99003,AJ238036-AJ23804037,38) and 48 VH sequences presented by Vescio et al39; mutated CLL subset,AJ239330-AJ239391, Z80836-Z80855.40 41
Sequences in the nonfunctional VH gene cohort were from normal memory B cells and plasma cells,32,33 42 all of which had a mutation frequency of more than 5% and had either an in-frame stop-codon or a nucleotide deletion that resulted in a frameshift. Gaps were introduced when sequences with deletion were translated to maintain the correct reading frame. Accession numbers for these sequences are AJ231557, 91, 99, AJ231601, 05, 30, 32 39, X87019, 75, 82, Z80464, 668, 708-9, Y13167-8, 70, and Z93132, 53-54, 58-59, 198, 214.
Expression of recombinant single-chain Fv in Pichia pastoris
For single-chain Fv (scFv) expression in the yeast P pastoris, tumor-derived VH and VLsequences were assembled as scFv as previously described.23 They were then cloned into the expression vector pPICZα, which contains the α-factor secretion signal derived from Saccharomyces cerevisiae, the c-myc epitope tag and a 6 × His tag at the carboxy-terminus (Invitrogen, Carlsbad, CA). Plasmids were introduced into yeast cells by electroporation, and transformants were selected on YPD plates containing 50 μg/mL zeocine. Single colonies were inoculated into 5 mL BMGY medium and grown in a shaking incubator at 30°C to an OD600 of 4. Cells were harvested by centrifugation, and expression was induced by resuspending the cells in 20 mL BMMY medium and shaking them at 30°C for 24 hours. Culture supernatants were recovered by centrifugation and were put through 0.22-μm filters. Supernatants were analyzed for scFv expression immediately or stored at −80°C.
Analysis of glycosylation status of scFv
For Western blot analysis, scFv supernatants were run through a NuPAGE Bis-Tris gradient polyacrylamide (4%-12%) gel (Invitrogen). Separated protein bands were electrotransferred onto Nylon filter, probed with biotinylated anti-myc monoclonal antibody 9E10, and were visualized by chemiluminescence using streptavidin-labeled horseradish peroxidase and the enhanced chemiluminescence plus reagents (Amersham Pharmacia Biotech UK). To remove N-linked carbohydrates, scFv supernatants were treated with Peptide:N-glycosidase (PNGase) (New England Biolabs, Beverly, MA).
Results
Analysis of N-glycosylation sites in single chain Fv from patients with FL
For 17 patients with FL, we identified VH and VL sequences for assembly as scFv during the preparation of DNA vaccines for a clinical trial. Analysis of the sequences (Table 1) showed that, apart from higher usage of the V3-48 gene in this cohort, the features of the VH sequences were typical of FL with a mean mutation level of 11% (range, 7%-20%). The VL genes were mainly Vκ (15 of 17) and had a lower mean mutation level of 6% (range, 1%-14%). The VHsequences were examined for potential N-glycosylation sites with the motif Asn-X-Ser/Thr (N-X-S/T), where X could be any amino acid apart from Pro, Asp, or Glu. Because, with one exception (F5), such sites were not present in the germline sequences, they must have been introduced by somatic mutation. Almost all (16 of 17) had at least one novel site, and 5 of 17 had 2 sites (Table 1). The incidence of novel sites in VL sequences was less, but 10/17 had one, and the patient (F6) with no site in VH did have a site in VL. The V4-34 gene was used by 1 of 17 patients (F5), and this site was a natural glycosylation site. However, in F5, mutational events caused the loss of this natural site and the gain of a new site. Therefore, in this cohort of FL, all patients had at least one new potential N-glycosylation site in the V-region sequence. Although all VH sequences showed evidence of intraclonal heterogeneity, as expected in FL, this process did not involve the N-glycosylation sites (data not shown).
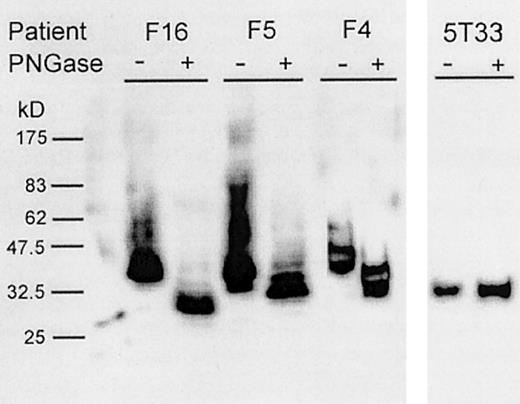
Glycosylation of novel sites in recombinant scFv proteins.
Using a P pastoris expression system, we made scFv proteins from 3 randomly chosen patients with FL (F4, F5, and F16) (Table 1). On sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), all the expressed proteins migrated more slowly than expected from the molecular weight of the encoded protein (approximately 30 kd) and showed heterogeneity consistent with the variable addition of carbohydrate (Figure1). On treatment of the scFv proteins with N-glycosidase, migration was increased to the expected position, and the bands became sharper. Remaining heterogeneity likely resulted from the incomplete removal of oligosaccharide. In contrast, migration of a scFv protein from a mouse myeloma (5T33),43containing no potential sites for N-glycosylation, was similar to the enzyme-treated human scFv proteins and was unaffected by treatment with N-glycosidase (Figure 1). These findings confirm that oligosaccharide side chains had been added to the N-glycosylation sites in the human scFv proteins during expression in yeast and that they are, therefore, functional.
Glycosylation of scFv proteins expressed in
P pastoris. Culture supernatants containing scFv proteins derived from patients (F4, F5, and F16) and from the 5T33 mouse myeloma were separated by SDS-PAGE and visualized by Western blotting using 9E10 monoclonal antibody. The scFv proteins were either untreated (−) or treated (+) with PNGase.
Glycosylation of scFv proteins expressed in
P pastoris. Culture supernatants containing scFv proteins derived from patients (F4, F5, and F16) and from the 5T33 mouse myeloma were separated by SDS-PAGE and visualized by Western blotting using 9E10 monoclonal antibody. The scFv proteins were either untreated (−) or treated (+) with PNGase.
Comparative incidence of novel N-glycosylation sites in normal B cells and in other B-cell tumors
To extend the investigation of FL and to assess the incidence of potential N-glycosylation sites in normal B cells in a range of other B-cell tumors, we analyzed somatically mutated V gene sequences from the databases. The analysis was confined to VH because of the higher levels of mutation in these genes and to the low numbers of available VL sequences. All cases analyzed were matched for similar levels of somatic mutation, and the VH gene usage was not significantly different among the various cohorts. Results on a further 53 patients with FL confirmed our small initial study, with 79% of all patients showing novel sites (Table2). However, normal B cells had only a low frequency (9%) of these sites. Normal cells included antibody-secreting cells,9 memory cells,49and plasma cells,17 and glycosylation sites were uncommon in all cell types. Because B cells are in rapid transit through the germinal center, it is difficult to know how to assess glycosylation patterns in so-called normal germinal center B cells. In one published study in which VH gene sequences from single B cells picked from germinal centers were available, glycosylation sites were evident in 3 of 7 patients.44 Clearly, these numbers are too small to interpret at present, and more data are required. Plasma cells of multiple myeloma had a similar incidence (8%) to normal B cells. The incidence of novel sites in a mutated subset of CLL (13%) was close to the normal level.
Frequency of novel N-glycosylation sites in disease cohorts compared to normal memory B cells and plasma cells
| Cohort . | Homology to GL (%) . | No. patients analyzed . | No. patients with new sites (%) . | Statistical significance* . | |
|---|---|---|---|---|---|
| Median . | Range . | ||||
| FL | 88 | 76-96 | 70 | 55 (79) | P < .001 |
| DLCL | 88 | 78-99 | 32 | 13 (41) | P = .014 |
| Mutated CLL | 92 | 86-94 | 40 | 5 (13) | NS |
| MM | 92 | 84-97 | 64 | 5 (8) | NS |
| Normal | 91 | 83-95 | 75 | 7 (9) | — |
| Cohort . | Homology to GL (%) . | No. patients analyzed . | No. patients with new sites (%) . | Statistical significance* . | |
|---|---|---|---|---|---|
| Median . | Range . | ||||
| FL | 88 | 76-96 | 70 | 55 (79) | P < .001 |
| DLCL | 88 | 78-99 | 32 | 13 (41) | P = .014 |
| Mutated CLL | 92 | 86-94 | 40 | 5 (13) | NS |
| MM | 92 | 84-97 | 64 | 5 (8) | NS |
| Normal | 91 | 83-95 | 75 | 7 (9) | — |
Compared to normal cell cohort (χ2test).
NS indicates not significant.
Interestingly, DLCL appeared to be heterogeneous; 41% of patients had novel sites (Table 2). All DLCLs were primary tumors, with 24 of 32 obtained from lymph node biopsy specimens and 8 of 32 from extranodal sites. Novel sites appeared more commonly in those obtained from lymph node (11 of 24, 46%) than in those obtained from extranodal sites (2 of 8, 25%), though the numbers were too small to reach statistical significance. Only a few sequences of patients in the 2 subsets of DLCL, as defined by microarray analysis,45 were available, but N-glycosylation sites were observed in the activated B-cell subset (3 of 7 patients) and the germinal center subset (2 of 7 patients).
Location of novel N-glycosylation sites in the VHregion of patients with FL
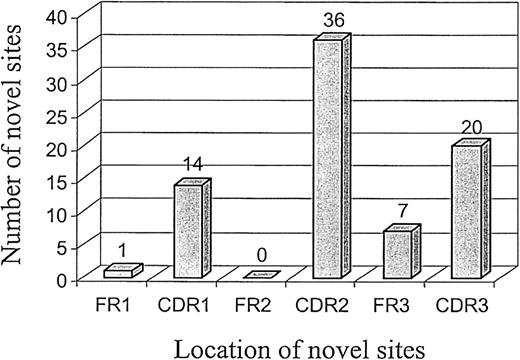
The distribution of novel glycosylation sites across the VH region sequences of 55 cases of FL is shown in Figure2. The vast majority (90%) of sites were located in the CDRs, with CDR2 having the largest number (Figure 2). As expected from its internal position in the variable region,46 FR2 had no sites.
Distribution of novel glycosylation site motifs in VH gene sequences of FL.
Locations of novel glycosylation sites within the VH sequences (FR1 to CDR3) of 55 patients with FL were analyzed. The number of novel glycosylation sites in each region is indicated.
Distribution of novel glycosylation site motifs in VH gene sequences of FL.
Locations of novel glycosylation sites within the VH sequences (FR1 to CDR3) of 55 patients with FL were analyzed. The number of novel glycosylation sites in each region is indicated.
A more detailed analysis (Figure 3) of 50 sequences of patients with no natural glycosylation sites showed that the novel sites are limited to a few positions in the CDRs. In the 11 patients with a new site in CDR1, the location was at codons 33 to 35. In 20 sequences with acquisition of a new site in CDR2, codon 50, a known hotspot for somatic mutation,33 was mutated to Asn at the N-terminus of CDR2. In contrast, in normal B cells or in other tumors, replacement amino acids in this hot spot position rarely included Asn. In certain germline genes (eg, V3-11, V3-23, and V3-48), a mutation to Asn at codon 50 generates an Asn-X-Ser motif (Figure 3). In FL, there was a tendency to retain the involved Ser or to replace it with Thr. To compare mutational events in a single V gene between B cells of different origin, we focused on the commonly used V3-23 gene. In FL, 5 of 5 patients had accumulated glycosylation sites (Figure 3), with 4 of 5 in CDR1, CDR2, or FR 3. In normal B cells, CLL, and MM, there were 2 of 17, 1 of 7, and 0 of 4 sites, respectively. This indicates that the minor asymmetries of VH gene usage among the B-cell sources do not account for the major differences in the frequency of glycosylation sites. With regard to distribution of sites in the totality of FL sequences, it is clear that replacement Asn residues were also commonly acquired at or near the N-terminus of the CDR3 sequence (Figure 3) through codons that may be derived from N-addition or from D-segment genes.
Location of novel glycosylation sites within the deduced amino acid sequences of the VH regions of FL.
Sequences of FL are aligned to the closest GL counterparts, with amino acid numbering according to Kabat.16 Dots represent identity with the representative GL sequences. Novel glycosylation site motifs are highlighted. Sequences of FL determined from this study are indicated by patient numbers.
Location of novel glycosylation sites within the deduced amino acid sequences of the VH regions of FL.
Sequences of FL are aligned to the closest GL counterparts, with amino acid numbering according to Kabat.16 Dots represent identity with the representative GL sequences. Novel glycosylation site motifs are highlighted. Sequences of FL determined from this study are indicated by patient numbers.
Effect of somatic mutation on VH sequences containing a natural glycosylation site
The V1-08, V4-34, and VH5a germline gene sequences all have naturally occurring N-glycosylation sites. In all tumors, there was a tendency to lose this site with increasing levels of somatic mutation, as has been reported for V4-34 in normal B cells.47 Among FL (8 patients), CLL (11 patients), and MM (4 patients), the 9 of 23 patients without a natural site had a mean mutational level of 11% (range, 7%-24%) compared to 6% (range, 1%-12%) in the remaining 14 sequences. V1-08 was used in one patient with FL and in one patient with MM. Both kept the natural site in FR3, and neither gained additional sites. Three patients with MM used VH5a; 2 of 3 retained the natural site in CDR2, and 1 of 3 lost the site as a result of somatic mutation.
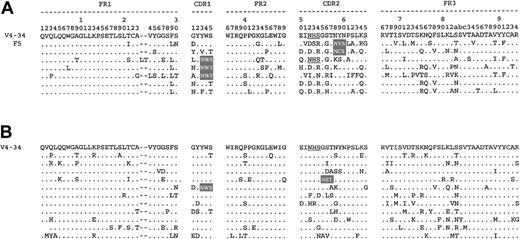
The V4-34 gene has a natural site in CDR2 (codons 52-54). The effects of somatic mutation on this site and the creation of new sites in our cohorts of FL and CLL are shown in Figure4 (A and B, respectively). Interestingly, 6 of 7 patients with FL lost the natural site, but 4 of 6 of these generated novel sites in either CDR1 or CDR2. One patient with FL retained the natural site and acquired another site in CDR1. This small analysis suggests that control over the natural site is independent of the accumulation of new sites in FL. In the mutated CLL group, 5 of 11 patients lost the natural site in V4-34 without gaining new sites (Figure 4B). However, 2 of 11 patients who retained the natural site acquired an additional new site.
Amino acid sequences of VH of FL and CLL derived from V4-34.
VH sequences of FL and CLL are aligned to the V4-34 GL sequence, with amino acid numbering according to Kabat et al.16 Dots represent identity with the representative GL sequences. The natural glycosylation site in the GL sequence is underlined, and novel glycosylation sites are highlighted. Sequences of FL determined from this study are indicated by patient number.
Amino acid sequences of VH of FL and CLL derived from V4-34.
VH sequences of FL and CLL are aligned to the V4-34 GL sequence, with amino acid numbering according to Kabat et al.16 Dots represent identity with the representative GL sequences. The natural glycosylation site in the GL sequence is underlined, and novel glycosylation sites are highlighted. Sequences of FL determined from this study are indicated by patient number.
Incidence of glycosylation motifs in somatically mutated nonfunctional VH genes
To assess whether acquired novel glycosylation sites were positively selected in FL, nonfunctional mutated VHsequences available from normal B cells were scanned for the Asn-X-Ser/Thr (N-X-S/T) motif in the sequences aligned to the VH of origin. In the 29 available patients with mutational levels greater than 5%, there were novel sites in 4 (14%), an incidence comparable to that in normal B cells. To ensure that the nonfunctional sequences arose at an early stage of B-cell maturation, before antigen encounter, VDJ sequences containing pseudogenes (1 sequence) or stop codons in CDR3 (19 sequences) were analyzed separately. The incidence of novel sites was 3 of 20 (15%), similar to the overall incidence in the nonfunctional cohort in the databases. It appears, therefore, that the presence of novel sites in FL does not arise from the accumulation of unselected mutations but represents a positively selected feature.
Discussion
Oligosaccharide chains are commonly displayed by the glycolipids or glycoproteins of cell surfaces, where they play a major role in interaction with the environment.48 The terminal sugars of the chains confer specificity on interactions with receptors, and they can influence multiple functions, including adhesion, migration, and binding to growth factors.49 In epithelial tumors, malignant transformation is often associated with changes in glycosylation patterns that can increase metastatic behavior.50
The finding that apparently functional N-glycosylation sites, created by somatic mutation, are a feature of FL is unexpected. The site of acquisition of the required Asn-X-Ser/Thr motif in the VHsequences is mainly in the CDRs, with common involvement of a known hot spot at position 50. In normal cells, mutations of the TAC (Tyr) codon at this position in functional and nonfunctional V3-11, V3-48, and DP58 genes occur with similar frequency at all 3 nucleotides.33 This generates a variety of replacement amino acids. However, in FL, the codon commonly changes from TAC to AAC (Asn), presumably reflecting a selective process. Sites located in CDR3, especially those in the N-terminal amino acids, could have arisen during recombinatorial or somatic mutational events. If the former, the lack of such sites in normal cells would imply a selective process for tumor development at an early stage. This seems less likely than the interpretation that accumulation of sites in CDR3 occurs by somatic mutation, in parallel with those in CDR1 and CDR2.
The finding that motifs are not common in normal somatically mutated B cells or in nonfunctional VH sequences strongly suggests that the sites are positively selected. The inference must be that the added carbohydrate confers an advantage on the tumor cells. It argues against the alternative view that normal B cells must protect the antigen-binding site against this modification, a process that would lead to negative selection against glycosylation sites. If that were generally the case, sites would still accumulate in the nonfunctional sequences. However, the level of 15% of these sites in the nonfunctional sequences likely to have arisen during VHDJH recombination indicates that there is no natural tendency to concentrate these sites by unselected mutational activity. It is equally unlikely that the process of antigen selection in the cell of FL origin could have been affected by the presence of the oligosaccharide chains in the binding site. It would be surprising if the myriad antigen-binding specificities represented in FL were influenced in any consistent direction by this addition. In contrast, the small proportion of normal B cells that does have novel sites could produce the subset of antibodies in which affinity for antigen is increased by the presence of carbohydrate.15
One clue to the function of the oligosaccharide may be provided by the pattern in DLCL, in which a subset shares this feature. The preliminary indication is that glycosylation may be more apparent in nodal tumors, perhaps indicating a role for cells retained in the GC. A similar increase in novel sites is also evident in the few sequences so far analyzed in cells of Burkitt lymphoma (data not shown). The relative lack of motifs in normal memory B cells, normal plasma cells, MM, and CLL indicates that cells that have exited from the germinal center do not require N-glycosylation.
Oligosaccharides of cell surface glycoproteins are multifunctional, with properties dictated by the oligosaccharide composition and sequence. Terminal sugars are critical for the recognition of ligands, with sialic acid particularly important. For example, the immunoglobulin superfamily receptors of hematopoietic cells CD22, CD33, and sialoadhesin all belong to the family of I-type lectins that interact with sialic acid through variable regionlike domains.51 In addition, glycosylated cell surface molecules such as CD44 and CD77 are up-regulated on B cells by CD40 ligation, which occurs in the GC.52 53 Clearly there are many molecules on B cells that can express oligosaccharides, and these have critical effects on cell–cell interactions.
The multiplicity of glycosylated molecules expressed by B cells in the GC might argue against a simple adhesive role for the added oligosaccharides in the V-region of FL. A more intriguing possibility is that the presence of the carbohydrate in the antigen-binding site allows an interaction with lectins in the GC that can then signal through the surface immunoglobulin. Binding to elements in the site might be related to the polyreactive behavior observed for immunoglobulins expressed by FL cells,54 which, for antibody molecules, apparently can be influenced by glycosylation.55,56 However, polyreactivity cannot be the only explanation because some myeloma proteins also exhibit this characteristic.57 The location of the oligosaccharide in the V-region appears critical, possibly because, in contrast to the oligosaccharides in the constant region,14 the chains exposed in the V-region may be more available to the glycosyl transferases and therefore may be fully glycosylated. Even location within the V-region could be important because the natural site in V4-34 does not appear to be conserved in FL. There is a similar tendency to lose this site in highly mutated mucosal plasma cells.47 The pattern of terminal sugars at the new sites in tumor cells could determine interaction with lectins in the vicinity. Low-level signaling through the BCR appears to influence the survival of mature B cells.58 For FL, signaling by way of oligosaccharide interactions may free FL cells from dependence on antigen and may contribute to tumor cell persistence or growth. If these speculations are confirmed by experiments in progress, it could open the possibility of inhibiting this interaction,59leading to new therapeutic approaches for FL.
We thank Drs B. Mead, T. Illidge, H. Myint, and D. G. Oscier for supplying clinical material from patients with FL and CLL.
Supported by the Leukaemia Research Fund, the Cancer Research Campaign, and Tenovus UK.
D.Z. and H.M. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Freda K. Stevenson, Molecular Immunology Group, Tenovus Laboratory, Southampton University Hospitals Trust, Southampton SO16 6YD, United Kingdom; e-mail: fs@soton.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal