Cytosolic Ca++ induces the shedding of microvesicles and nanovesicles from erythrocytes. Atomic force microscopy was used to determine the sizes of these vesicles and to resolve the patchy, fine structure of the microvesicle membrane. The vesicles are highly enriched in glycosyl phosphatidylinositol–linked proteins, free of cytoskeletal components, and depleted of the major transmembrane proteins. Both types of vesicles contain 2 as-yet-unrecognized red cell proteins, synexin and sorcin, which translocate from the cytosol to the membrane upon Ca++ binding. In nanovesicles, synexin and sorcin are the most abundant proteins after hemoglobin. In contrast, the microvesicles are highly enriched in stomatin. The membranes of both microvesicles and nanovesicles contain lipid rafts. Stomatin is the major protein of the microvesicular lipid rafts, whereas synexin and sorcin represent the major proteins of the nanovesicular rafts in the presence of Ca++. Interestingly, the raft proteins flotillin-1 and flotillin-2 are not found in the vesicles but remain in the red cell membrane. These data indicate the presence of different types of lipid rafts in the erythrocyte membrane with distinct fates after Ca++ entry. Synexin, which is known to be vital to the process of membrane fusion, is suggested to be a key component in the process of vesicle release from erythrocytes.
Introduction
Erythroctes are known to respond to physiological stimuli via a diversity of receptors and effectors.1 In particular, prostaglandin E2 and lysophosphatidic acid (LPA) induce Ca++-dependent processes.2,3 The rise of cytosolic Ca++ in erythrocytes triggers a sequence of biochemical and morphologic changes that finally result in the release of hemoglobin-containing exovesicles. Two sorts of vesicles, differing in size, have been described; these have been named microvesicles (150 nm diameter) and nanovesicles (60 nm diameter).4 Elevated cytosolic Ca++ levels cause alterations in the membrane protein pattern, notably the aggregation of proteins catalyzed by transaminases and cytoskeletal rearrangements owing to the proteolysis of protein 4.1.5 Another Ca++-mediated effect is the breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate with a concomitant increase in 1,2-diacylglycerol and phosphatidic acid.6Moreover, the phospholipid-scramblase is activated and the aminophospholipid translocase is inhibited,7 thereby leading to a randomization of the phospholipid asymmetry over the 2 membrane leaflets. The Ca++-activated potassium efflux via the Gardos channel causes the loss of cell water and concomitant shrinkage of the erythrocyte.8 The latter factors are essential for the release of the vesicles from the echinocytic erythrocytes.
The physiological importance of the vesiculation process can be seen in a protection strategy of the red cell against the destruction by complement.9 An erythrocyte being attacked by the complement components C5b through C9 faces a local Ca++ influx and responds by a rapid release of vesicles containing the membrane attack complex. A similar defense mechanism has been described for platelets,10neutrophils,11 and oligodendrocytes.12 The clinical importance of the vesicles themselves has been highlighted by the finding that, owing to the loss of phospholipid asymmetry, they provide substrates for the secretory phospholipase A2 to generate the proinflammatory lipid mediator LPA.13 LPA is an important second messenger and has been shown to open Ca++ channels in human erythrocytes,3 thereby stimulating further vesiculation.
The vesicles released from human erythrocytes after treatment with Ca++ and ionophore A23187 are free of cytoskeletal components and are specifically enriched in glycosyl phosphatidylinositol (GPI)–anchored proteins, such as acetylcholinesterase (AChE) and decay accelerating factor (CD55).14 They are also enriched in the transmembrane protein complement receptor 1 (CD35).15 Particularly, in nanovesicles, AChE is highly concentrated in relation to total protein and phospholipid content.4 Similarly, in mechanically induced vesiculation of erythrocytes, the specific enrichment of GPI-linked CD59 in the vesicles has been shown.16Interestingly, red cells of patients with paroxysmal nocturnal hemoglobinuria, which lack GPI-linked proteins, have an impaired ability to vesiculate.17 Extensive studies on different cell types unequivocally show that GPI-linked proteins are specifically located in cholesterol- and sphingolipid-rich domains of the plasma membrane, also termed lipid rafts.18 In the erythrocyte membrane, GPI-anchored proteins are similarly organized within lipid rafts,19,20 with stomatin, flotillin-1, and flotillin-2 being present in great abundance at the cytoplasmic face.20 This finding led us to investigate whether the Ca++-induced vesiculation is a lipid raft–based process and whether stomatin21-27 and the flotillin proteins28 29 are enriched within the released vesicles.
In this study, we show that stomatin, in contrast to the flotillin proteins, is enriched within the microvesicles. Moreover, synexin and sorcin are present in microvesicles and are highly enriched in nanovesicles. These vesicles contain lipid rafts, with synexin and sorcin being Ca++-dependent raft-associated proteins. Our data shed new light on the organization and dynamics of red cell lipid rafts and the generation of exovesicles.
Materials and methods
Cells
Whole blood was obtained from healthy donors by venipuncture at the general hospital of Vienna, collected into heparinized tubes and either used immediately or stored overnight at 4°C. Erythrocytes were pelleted (at 200g for 10 minutes) and subsequently washed 5 times with 10 mM Tris-Cl, 150 mM NaCl, pH 7.5 (Tris-buffered saline [TBS]).
Preparation of microvesicles and nanovesicles
Method A.
Vesicles were prepared according to Allan et al.4 Briefly, erythrocytes were resuspended in 3 volumes TBS containing 1 mM CaCl2 and 5 μM ionophore A23187 (Sigma, St Louis, MO) and incubated at 37°C for 30 minutes. After addition of EDTA to a final concentration of 5 mM, the erythrocytes were pelleted (at 15 000g for 30 seconds), and the supernatant was centrifuged (at 15 000g, 4°C, for 20 minutes). The resulting pellet of microvesicles was resuspended in an appropriate volume of TBS and cleared of contaminating erythrocytes by a short centrifugation as above, whereas the nanovesicles in the supernatant were pelleted by an ultracentrifugation step (Beckman SW50.1 rotor [Beckman Instruments, Palo Alto, CA], at 100 000g, 4°C, for 60 minutes) and resuspended in an appropriate volume of TBS. The vesicles were assayed for AChE activity and either used immediately for further experiments or stored overnight at 4°C.
Method B.
Erythrocytes were resuspended in 9 vol TBS containing 1 mM CaCl2 and 5 μM ionophore A23187 and incubated at 37°C for 30 minutes. After the addition of EDTA to a final concentration of 5 mM, the erythrocytes were pelleted (at 15 000g for 30 seconds), and the supernatant was subjected to 4 subsequent centrifugation steps. The first 3 steps were performed in 1.5-mL plastic tubes at 15 000g, 4°C, for 10, 20, and 30 minutes, respectively, and the subsequent ultracentrifugation step was performed as above (Beckman SW50.1 rotor, at 100 000g,4°C, for 60 minutes). Each vesicle pellet was resuspended in an appropriate volume of TBS, assayed for AChE activity, and used immediately for further experiments.
Identification of proteins
Protein samples were analyzed by gel electrophoresis and silver staining, as described below, or by immunoblotting with monoclonal antibodies against band 3 (Sigma); flotillin-1, flotillin-2, and synexin (Transduction Laboratories, San Diego, CA); sorcin (Zymed Laboratories, San Francisco, CA); and stomatin22; subsequent detection was by horseradish peroxidase–goat-antimouse immunoglobulin G (Promega, Madison, WI) and the SuperSignal chemiluminescence kit (Pierce, Rockford, IL). For mixed peptide sequencing, proteins were separated on 12% polyacrylamide gels, electroblotted onto Immobilon-P (Millipore, Bedford, MA), and stained by Amido Black (1 mg/mL in 5% acetic acid, 10% methanol). Selected stained bands were cut out, and cyanogen bromide (CNBr) cleavage was performed as described,30 with minor modifications. Briefly, the membrane pieces were washed in 5% acetic acid, 10% methanol, and twice in water, and then immersed for a short time in a CNBr solution (500 mg CNBr/mL 70% formic acid), fixed above the CNBr solution in a sealed plastic vial, and incubated at 34°C for 2 hours. The membrane pieces were vacuum dried (SpeedVac, Savant, Fullerton, CA) and directly subjected to automated Edman degradation (ABI model 476A, Applied Biosystems, Foster City, CA). The mixed peptide sequence data were matched to the public databases by means of the FASTF or TFASTF algorithms.
Silver staining
Electrophoresis gels were fixed in 50% methanol, 12% acetic acid, and 0.5 mL/L 37% formaldehyde for 1 hour and then washed with 50% ethanol for 1 hour (2 changes). The gels were pretreated with 0.2 g/L Na2S2O3 for 1 minute, rinsed 3 times with water, and incubated with 2 g/L AgNO3 and 0.75 mL/L 37% formaldehyde for 20 minutes, in the dark. Subsequently, the gels were rinsed twice in water; developed in a solution containing 60 g Na2CO3, 4 mg Na2S2O3, 0.5 mL 37% formaldehyde per liter; stopped with 50% methanol and 12% acetic acid; and equilibrated with 30% methanol and 2% glycerol before drying.
Measurement of AChE activity
AChE activity was determined according to Ellman‘s method, as described by Steck and Kant31 with minor modifications. Briefly, an aliquot of the sample, not exceeding 50 μL, was mixed with an equal volume 0.5% Triton X-100 in TBS and incubated at 37°C for 5 minutes to assure solubilization of lipid rafts. Then, 100 mM sodium phosphate, pH 7.5, was added, up to 700 μL; next, 50 μL 10 mM 5,5′-dithiobis-(2-nitrobenzoic acid) in 100 mM sodium phosphate, pH 7.0, and 50 μL 12.5 mM acetylthiocholine chloride (Sigma) were added and mixed, and the reaction was followed spectrophotometrically (U-2000 Hitachi, Tokyo, Japan) at 412 nm at room temperature. The initial increase in absorbance was measured for 60 seconds.
Identification of ganglioside GM1
First, 10-μL aliquots of the sucrose gradient fractions were dotted onto nitrocellulose membrane and vacuum dried for 2 hours. The membrane was blocked by incubation with 5% milk powder and 0.1% Tween-20 in TBS for 1 hour at room temperature and subsequently incubated with cholera toxin B subunit–peroxidase conjugate (Sigma) diluted 1/1000 in 0.1% Tween-20 in TBS at 4°C overnight. The membrane was washed 3 times for 15 minutes in 0.1% Tween-20 in TBS, and GM1 was detected by means of the SuperSignal chemiluminescence kit (Pierce).
Determination of the membrane association of vesicle proteins
Equivalent amounts of microvesicles and nanovesicles (according to their AChE activity) were mixed, adjusted to 50 μL with TBS, and lysed by incubation with 100 μL 0.5% saponin in TBS containing 1.5 mM CaCl2 or 7.5 mM EDTA, for 20 minutes, on ice. The vesicle membranes were pelleted by centrifugation in a fixed-angle rotor (Beckman, TLA-100.1, at 200 000g, 4°C, for 30 minutes). The pellets were resuspended in lysis buffer to give an equal volume to the supernatants, and aliquots of the supernatants and pellet suspensions were analyzed by gel electrophoresis/silver staining and immunoblotting. Bands of interest were scanned and quantified by densitometry by means of the ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Phase separation of vesicle proteins in Triton X-114 solution
The phase separation of membrane proteins in Triton X-114 according to the difference in hydrophobicity32 was applied with slight modifications. To purify Triton X-114, the precondensation of the detergent was performed 3 times, as recommended.32 Equivalent amounts of microvesicles and nanovesicles, according to their AChE activity, were mixed and adjusted to 50 μL with TBS. The vesicles were lysed by addition of 50 μL cold 1% Triton X-114 in TBS, containing 2 mM CaCl2 or 10 mM EDTA, and incubated on ice for 20 minutes. This lysate was placed on top of a 200-μL sucrose cushion (6% sucrose, 0.06% Triton X-114 in TBS, containing 2 mM CaCl2 or 10 mM EDTA) in an Eppendorf vial and incubated at 30°C for 5 minutes for condensation. The tubes were placed in a swinging bucket rotor (Heraeus Variofuge 3.0, Hanau, Germany), and the detergent phase was pelleted at room temperature (at 300g for 5 minutes). The upper, aqueous phase was removed and cooled on ice, and the separation was repeated by addition of fresh Triton X-114 (0.5% final concentration). After incubation on ice for 20 minutes, the aqueous phase was placed on top of the previously used sucrose cushion, incubated at 30°C, and centrifuged as before. The aqueous phase and the detergent phase (oily droplet) were collected, and TBS or Triton X-114 was added to obtain equal volumes and composition. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis sample buffer was added, and aliquots of the samples were analyzed by gel electrophoresis/silver staining and immunoblotting.
Flotation assay
Microvesicles or nanovesicles (50 μL) were lysed by the addition of 50 μL 1% Triton X-100 in TBS, containing either 2 mM CaCl2 or 10 mM EDTA, incubated on ice for 20 minutes, and mixed with 100 μL 80% sucrose, 0.5% Triton X-100 in TBS containing 2 mM CaCl2 or 10 mM EDTA. The resulting suspensions were placed in centrifuge tubes (Beckman, 13 × 51 mm), and each was overlaid with 1250 μL 30% sucrose in TBS containing 1 mM CaCl2 or 5 mM EDTA, and 333 μL 10% sucrose in TBS containing 2 mM CaCl2 or 10 mM EDTA, and centrifuged in a precooled SW50.1 rotor (Beckman) at 230 000g, 4°C, for 17 hours. Thirteen fractions, 140 μL each, were collected from the top, and aliquots were analyzed by gel electrophoresis/silver staining, immunoblotting, and GM1 dot blotting and were also analyzed for AChE activity and cholesterol content (Unisys kit, Roche Diagnostics, Mannheim, Germany).
Atomic force microscopy
A Macmode PicoSPM magnetically driven dynamic-force microscope (Molecular Imaging, Phoenix, AZ) was used. The topography images were recorded in the Magnetic AC (Mac) mode33,34with MacLevers (Molecular Imaging) of about 1 N/m nominal spring constant in aqueous buffer solutions at room temperature. Measurements were performed with 5 nm free-tip oscillation amplitude at 40 kHz driving frequency, and the feedback-loop was driven at 20% amplitude reduction. Amplitude images were recorded simultaneously. Image size was 1 μm × 1 μm to 30 μm × 30 μm at 1 Hz lateral scan rate. Microvesicles and nanovesicles were specifically bound to wheat germ agglutinin (WGA)–coated mica surfaces. For this, mica sheets (Gröpl, Tulln, Austria) were first derivatized with ethanolamine as previously described,35 36 then incubated for 1.5 hours with a solution of 1 mg/mL ethylene glycol-bis(succinimidylsuccinate) (Pierce) in chloroform containing 0.5% triethylamine, washed in chloroform, and dried with N2. Subsequently, a solution of 0.5 mg/mL WGA (Sigma) in 10 mM sodium phosphate and 150 mM NaCl, pH 7.5 (phosphate-buffered saline), was applied for 2 hours, and the excess of WGA was removed. Finally, the vesicles were bound to the mica surface in Hepes-buffered saline (5 mM Hepes, 150 mM NaCl, pH 7.3) for 15 minutes at appropriate concentrations and imaged in the same buffer by means of atomic force microscopy (AFM).
Results
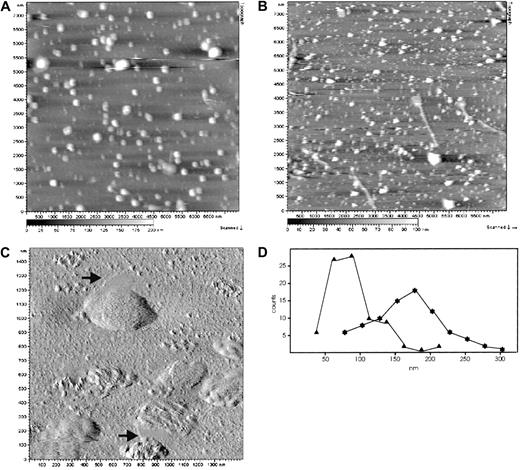
Microvesicles and nanovesicles released from erythrocytes after Ca++/A23187 treatment were prepared according to method A and studied by AFM to assess their sizes und structure under physiological conditions. The vesicles were immobilized in solution by WGA, covalently attached to the mica support, and imaged by means of dynamic-force microscopy.33,34 In this procedure, a magnetically oscillated cantilever scans the surface of the sample, touching it upon every downstroke, thus reducing the oscillation amplitude. The amplitude is held constant by a feedback loop so that the cantilever follows the surface topography, yielding the height image shown in Figure 1. Owing to the small oscillation amplitudes of only a few nanometers, this mode provides a most gentle tip-surface interaction, minimizing the compression of the soft vesicular structures on the surface. Single vesicles with almost no deterioration or disassembling of the structures during imaging were clearly resolved (Figure 1). Apparently, the vesicles were tightly bound to the surface; this was most likely accomplished by numerous, strong bonds between the WGA molecules on the mica surface and carbohydrate moieties on the membrane surface. Microvesicles (Figure 1A) appear considerably larger in both diameter and height than nanovesicles (Figure 1B). Higher magnification amplitude images (Figure 1C) revealed a patchy, texturelike, fine structure of the microvesicular membrane. As the microvesicles are essentially free of cytoskeletal components, this fine structure reflects an inherent organization of the vesicular membrane itself. Some of the microvesicles (Figure 1C) appear associated with flat membrane sheets, possibly reflecting a segregation of vesicular membrane domains. In Figure 1D, the size distribution of nanovesicles and microvesicles is shown. For nanovesicles, the maximum of distribution is centered around 81 nm (n = 85), with 62 nm peak width at half maximum; for microvesicles, the maximum is found at 179 nm (n = 82), with 92 nm peak width at half maximum. These data are in good agreement with earlier results obtained by electron microscopy.4 Additionally, the size distribution indicates the cross-contamination of nanovesicles with small microvesicles (about 16%) and of microvesicles with large nanovesicles owing to the limited separation efficiency of method A.
AFM images of erythrocyte microvesicles and nanovesicles.
Microvesicles (panels A, C) and nanovesicles (panel B) were adsorbed to WGA-coated mica surfaces. (A) (B) Topography images were obtained with the use of the Mac mode in buffered saline. Singly distributed vesicular structures are clearly resolved. Heights are indicated by a gray scale bar, ranging from 0 nm (black) to 200 nm (white) (panel A) and from 0 nm (black) to 100 nm (white) (panel B). Image size was 7.5 μm. (C) The high-resolution AFM amplitude image reveals distinct, texturelike structures on the microvesicular membranes and flat membrane patches that are occasionally associated with the microvesicles (arrows). Image size was 1.5 μm. (D) Size distribution of microvesicles (n = 82, asterisks) and nanovesicles (n = 85, triangles). Maxima are found at 179 nm and 81 nm, respectively.
AFM images of erythrocyte microvesicles and nanovesicles.
Microvesicles (panels A, C) and nanovesicles (panel B) were adsorbed to WGA-coated mica surfaces. (A) (B) Topography images were obtained with the use of the Mac mode in buffered saline. Singly distributed vesicular structures are clearly resolved. Heights are indicated by a gray scale bar, ranging from 0 nm (black) to 200 nm (white) (panel A) and from 0 nm (black) to 100 nm (white) (panel B). Image size was 7.5 μm. (C) The high-resolution AFM amplitude image reveals distinct, texturelike structures on the microvesicular membranes and flat membrane patches that are occasionally associated with the microvesicles (arrows). Image size was 1.5 μm. (D) Size distribution of microvesicles (n = 82, asterisks) and nanovesicles (n = 85, triangles). Maxima are found at 179 nm and 81 nm, respectively.
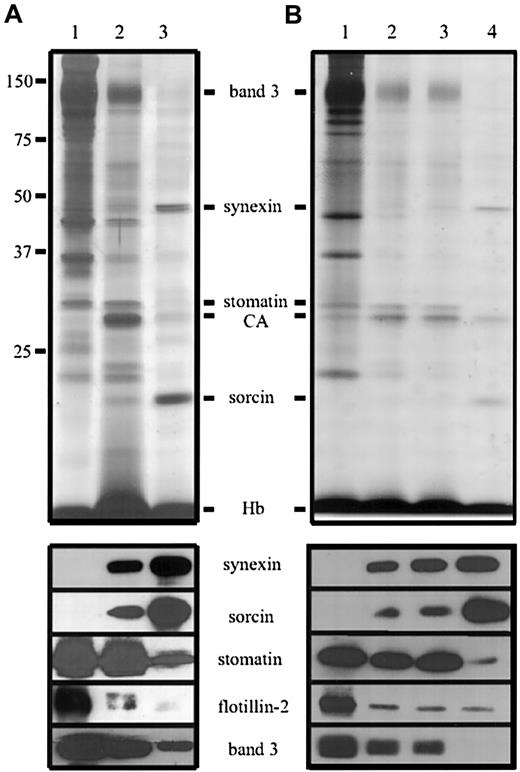
As the GPI-linked protein AChE is abundant in the microvesicles and nanovesicles,4 we were specifically interested whether other lipid raft proteins, like stomatin and the flotillins, are similarly enriched within these vesicles. Figure2A shows the protein pattern of washed erythrocyte membranes (lane 1) compared with that of whole microvesicles and nanovesicles (lanes 2 and 3), normalized to AChE activity. Both types of vesicles contain the major cytosolic protein components, hemoglobin (16-kd monomers) and carbonic anhydrase (30 kd). The AChE activity relative to the hemoglobin content is highly enriched in both types of vesicles, with roughly 80 times (in microvesicles) and 250 times (in nanovesicles) enrichment when compared with erythrocytes (data not shown), as previously reported.4 The vesicles are essentially free of cytoskeletal proteins, and the relative amounts of the major integral membrane proteins band 3 and glycophorins are diminished. Stomatin (band 7.2) is the only major erythrocyte membrane protein that parallels the specific enrichment of the GPI-anchored protein AChE in microvesicles (Figure 2A). Interestingly, only trace amounts of the flotillins are found in the vesicles.
Identification of proteins in erythrocyte microvesicles and nanovesicles.
(A) Erythrocytes were treated with Ca++/A23187, and microvesicles and nanovesicles were prepared according to method A. Aliquots of the erythrocyte membranes (lane 1), microvesicles (lane 3), and nanovesicles (lane 3), normalized to AChE activity, were analyzed by 12% polyacrylamide gel electrophoresis/silver staining (upper panel) and immunoblotting (lower panel) as indicated. (B) Vesicles were prepared according to method B. Aliquots, normalized to AChE activity, of the erythrocyte membranes (lane 1) and vesicles obtained after low-speed centrifugation for 10 minutes (lane 2) and 20 minutes (lane 3) and ultracentrifugation (lane 4) were analyzed as in panel A. CA indicates carbonic anhydrase; Hb, hemoglobin.
Identification of proteins in erythrocyte microvesicles and nanovesicles.
(A) Erythrocytes were treated with Ca++/A23187, and microvesicles and nanovesicles were prepared according to method A. Aliquots of the erythrocyte membranes (lane 1), microvesicles (lane 3), and nanovesicles (lane 3), normalized to AChE activity, were analyzed by 12% polyacrylamide gel electrophoresis/silver staining (upper panel) and immunoblotting (lower panel) as indicated. (B) Vesicles were prepared according to method B. Aliquots, normalized to AChE activity, of the erythrocyte membranes (lane 1) and vesicles obtained after low-speed centrifugation for 10 minutes (lane 2) and 20 minutes (lane 3) and ultracentrifugation (lane 4) were analyzed as in panel A. CA indicates carbonic anhydrase; Hb, hemoglobin.
In nanovesicles, 2 major proteins can be identified by gel electrophoresis/silver staining as prominent bands at 47 kd and 22 kd, respectively; they represent the most abundant proteins after hemoglobin. Similar bands were already described as band 4.5 and band 8 by Allan et al4; so far, they have remained uncharacterized. Mixed peptide sequencing identified the 47-kd component as synexin (annexin VII) and the 22-kd component as sorcin. These results were confirmed by immunoblotting (Figure 2). Synexin is a member of the large annexin protein family,37 which has important functions in membrane organization and intracellular traffic. Upon Ca++ binding, synexin translocates to the membrane and acts as a membrane-fusion protein, for instance in the exocytotic secretion of chromaffin granules.38 Sorcin, too, translocates to the membrane in a Ca++-dependent manner39,40 and has been shown to bind to specific membrane proteins and synexin.41
Synexin and sorcin are also found in the microvesicle preparation (Figure 2A, lane 2), and stomatin is present in the nanovesicle preparation (Figure 2A, lane 3); however, this could be due, at least partly, to cross-contamination of the respective vesicle pools (Figure1D). To address this question, we modified the preparation method and fractionated the vesicles by extended differential centrifugation (method B). The 4 centrifugation steps recovered 68% (10 minutes), 15% (20 minutes), 5% (30 minutes), and 12% (ultracentrifugation) of the total AChE activity in the pellet. After normalization to the AChE activity, the protein patterns of the vesicle preparations were determined (Figure 2B). Apparently, the 10- and 20-minute vesicles are very similar and probably represent different size populations of microvesicles, in accordance with the size distribution analysis (Figure 1D). The nanovesicles obtained by method B (Figure 2B, lane 4) completely lack band 3, indicating that there is no cross-contamination with microvesicles. Synexin is present in both microvesicles and nanovesicles, yet is enriched in the nanovesicles. Sorcin is highly enriched in nanovesicles compared with microvesicles; in contrast, only trace amounts of stomatin can be found in nanovesicles. Thus, stomatin and sorcin can be used as marker proteins for microvesicles and nanovesicles, respectively.
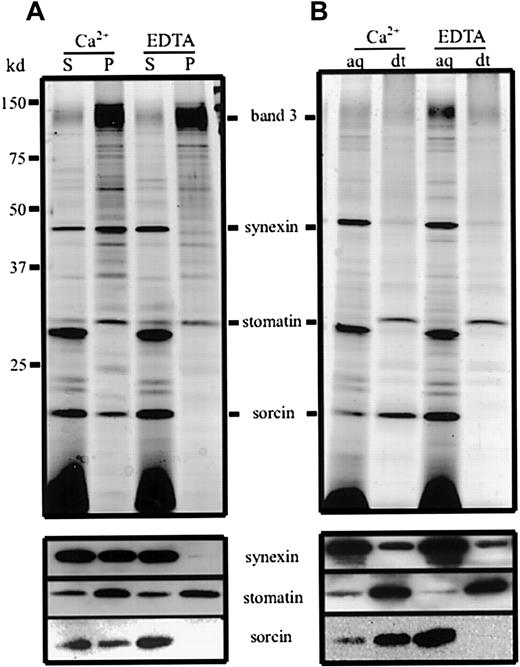
To address the question of whether synexin and sorcin reside in the lumen or at the membrane of the vesicles, we permeabilized the vesicles with saponin in the presence of Ca++ or EDTA and isolated the vesicular membranes (Figure 3A). In the presence of Ca++, synexin and sorcin remained partly associated with the vesicular membranes, with more than 50% and 25%, respectively, being present in the pellet. Probably the actual percentage of the membrane-bound fraction of synexin and sorcin is higher, because the integral proteins stomatin and band 3 were also partly solubilized under the given conditions. The unequal distribution of synexin and sorcin between supernatant and pellet may suggest that these proteins are partly organized in different protein complexes. When Ca++ was replaced by EDTA, the membrane association of both proteins was completely abolished.
Ca++ dependence of the synexin and sorcin membrane association and change in sorcin hydrophobicity.
(A) Mixtures of microvesicles and nanovesicles were lysed by 0.5% saponin in the presence of Ca++ or EDTA. The vesicle membranes were pelleted, and aliquots of the supernatants (S) and pellets (P) were analyzed by 12% polyacrylamide gel electrophoresis/silver staining (upper panel) and immunoblotting (lower panel). (B) Mixtures of microvesicles and nanovesicles were dissolved in Triton X-114 in the presence of Ca++ or EDTA, and phase separation was performed. Aliquots of the aqueous (aq) and detergent (dt) phases were analyzed by 12% polyacrylamide gel electrophoresis/silver staining (upper panel) and immunoblotting (lower panel).
Ca++ dependence of the synexin and sorcin membrane association and change in sorcin hydrophobicity.
(A) Mixtures of microvesicles and nanovesicles were lysed by 0.5% saponin in the presence of Ca++ or EDTA. The vesicle membranes were pelleted, and aliquots of the supernatants (S) and pellets (P) were analyzed by 12% polyacrylamide gel electrophoresis/silver staining (upper panel) and immunoblotting (lower panel). (B) Mixtures of microvesicles and nanovesicles were dissolved in Triton X-114 in the presence of Ca++ or EDTA, and phase separation was performed. Aliquots of the aqueous (aq) and detergent (dt) phases were analyzed by 12% polyacrylamide gel electrophoresis/silver staining (upper panel) and immunoblotting (lower panel).
To investigate whether this membrane association was due to Ca++-dependent binding of membrane components or to a Ca++-induced change in hydrophobicity of the respective proteins, we analyzed the distribution of the vesicle proteins after Triton X-114 phase separation (Figure 3B). In the presence of Ca++, sorcin predominantly partitioned into the detergent phase, whereas in the absence of Ca++ it was exclusively present in the aqueous phase. This gain in hydrophobicity upon Ca++ binding has already been described for recombinant sorcin.39 In contrast, synexin did not change its partitioning characteristic in the presence of Ca++, reflecting an unaltered hydrophilic behavior. This finding is in accordance with the idea that Ca++ ions mediate the binding of synexin to the membrane by association with acidic phospholipids rather than inducing a conformational change.
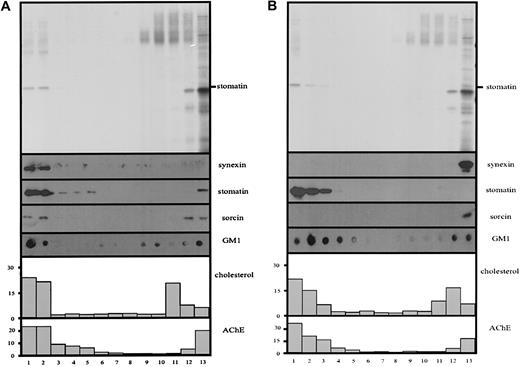
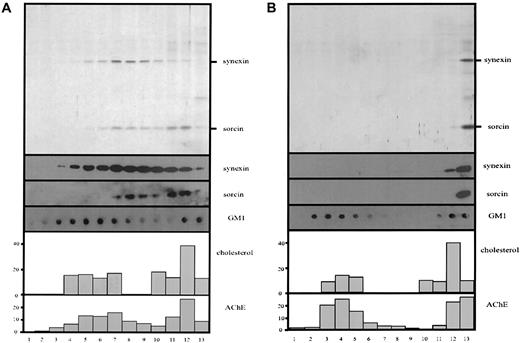
The patchy, fine structure of the microvesicular membrane (Figure 1C) and the specific enrichment of the lipid raft components stomatin and AChE in microvesicles suggested the presence of lipid rafts within the vesicular membrane. Therefore, we prepared microvesicles by the 10-minute centrifugation according to method B, lysed them in cold Triton X-100, and analyzed the solution by density gradient equilibrium centrifugation for the presence of floating lipid rafts. Each experiment was done in parallel, with Ca++ or EDTA being present during lysis and in the sucrose gradient. The flotation assays in Figure 4 reveal that most of stomatin and AChE is present in the low-density region of the discontinuous density gradients. Moreover, the lipid raft markers cholesterol and ganglioside GM1 show a parallel distribution in the low-density region of the gradient. These results clearly indicate the existence of lipid rafts within the microvesicular membrane. Interestingly, in the presence of Ca++, most of synexin and part of sorcin comigrated with the lipid raft markers to low densities (Figure 4A). Replacement of Ca++ by EDTA completely abolished this association of synexin and sorcin with the lipid rafts (Figure 4B). Here we want to mention that some variation concerning the relative amount of the lipid raft markers in the low-density region can be observed among different blood samples. However, this is not specific for vesicular rafts but is true for erythrocyte rafts in general and is probably related to variations in the serum lipid content of the donors.
Ca++-dependent association of synexin and sorcin with microvesicular lipid rafts.
Microvesicles prepared according to method B (10-minute low-speed centrifugation) were lysed with cold 1% Triton X-100 in TBS, in the presence of Ca++ or EDTA, and subjected to discontinuous density gradient centrifugation. Thirteen fractions were collected from the top, and aliquots were analyzed by 12% polyacrylamide gel electrophoresis/silver staining (panel A) and immunoblotting (panel B), as indicated, and for AChE activity and cholesterol content (percentage of total).
Ca++-dependent association of synexin and sorcin with microvesicular lipid rafts.
Microvesicles prepared according to method B (10-minute low-speed centrifugation) were lysed with cold 1% Triton X-100 in TBS, in the presence of Ca++ or EDTA, and subjected to discontinuous density gradient centrifugation. Thirteen fractions were collected from the top, and aliquots were analyzed by 12% polyacrylamide gel electrophoresis/silver staining (panel A) and immunoblotting (panel B), as indicated, and for AChE activity and cholesterol content (percentage of total).
In parallel, we investigated whether lipid rafts are also present in nanovesicles prepared by method B, which virtually lack the raft protein stomatin. Again, the lipid raft markers AChE, cholesterol, and GM1 are partly present at low densities in the discontinuous density gradient, indicating the existence of lipid rafts in the nanovesicular membrane (Figure 5). In the presence of Ca++, most of synexin and a part of sorcin are found in the lower-density fractions of the sucrose gradient. However, the distribution of synexin and, particularly, of sorcin is not strictly correlated with that of the lipid raft markers but is shifted toward higher densities (Figure 5A). When Ca++ is replaced by EDTA, neither synexin nor sorcin shows a floating behavior, but both are found in the bottom fraction of the gradient (Figure 5B).
Ca++-dependent association of synexin and sorcin with nanovesicular lipid rafts.
Nanovesicles prepared according to method B (ultracentrifugation) were lysed with cold 1% Triton X-100 in TBS, in the presence of Ca++ or EDTA, and subjected to discontinuous density gradient centrifugation. Thirteen fractions were collected from the top, and aliquots were analyzed by 12% polyacrylamide gel electrophoresis/silver staining (panel A) and immunoblotting (panel B), as indicated, and for AChE activity and cholesterol content (percentage of total).
Ca++-dependent association of synexin and sorcin with nanovesicular lipid rafts.
Nanovesicles prepared according to method B (ultracentrifugation) were lysed with cold 1% Triton X-100 in TBS, in the presence of Ca++ or EDTA, and subjected to discontinuous density gradient centrifugation. Thirteen fractions were collected from the top, and aliquots were analyzed by 12% polyacrylamide gel electrophoresis/silver staining (panel A) and immunoblotting (panel B), as indicated, and for AChE activity and cholesterol content (percentage of total).
Discussion
The data presented here show the involvement of stomatin-specific lipid rafts and the as-yet-unrecognized red cell components synexin and sorcin in the Ca++-induced exovesiculation process of erythrocytes. AFM determination of the sizes of quasi-native microvesicles and nanovesicles (Figure 1A,B) is in good agreement with the data obtained by electron microscopy.4 The patchy, fine structure of the microvesicular membrane revealed at higher magnification (Figure 1C) may reflect various lipid domains and/or lipid-protein complexes.Whereas the size distribution of nanovesicles is rather narrow, the microvesicles reveal a higher size variation (Figure 1D).
The specific enrichment of stomatin in microvesicles and of synexin and sorcin in microvesicles and nanovesicles (Figure 2) indicates a vital function for these proteins in the vesiculation process. Stomatin is an oligomeric integral membrane protein,20,26 which is palmitoylated,27 is associated with lipid rafts,20,42 and has been suggested to play a role as a scaffolding component20,42 similar to the raft protein caveolin. Synexin (annexin VII) was first isolated from adrenal medullary chromaffin cells and shown to cause Ca++-dependent aggregation of isolated chromaffin granules.38 The 13 members of the annexin protein family share a common core domain that is composed of 4 highly conserved repeats that are thought to mediate binding of Ca++ions.37 However, their N-terminal domains are highly variable and function as docking sites for specific interaction partners. Synexin, which contains an extraordinarily long N-terminus, binds to sorcin in a Ca++-dependent manner.41Sorcin belongs to the penta- EF-hand family and plays a role in the Ca++-mediated signal transduction by association with specific membrane proteins at specific subcellular locations.41,43-45 Interestingly, the N-terminal domains of sorcin and synexin contain similar structural motifs and mediate the interaction of these proteins.41,46 This interaction, which has been studied in the adrenal medullary tissue41and in differentiating myoblasts,47 interferes with the membrane-aggregation activity of synexin, indicating a regulatory role for sorcin in the exocytotic process.
Our finding that sorcin and synexin translocate to the erythrocyte membrane upon Ca++ entry (Figure 3A) is in good agreement with the reported characteristics of these proteins in other tissues. Sorcin is the only red cell vesicular protein that increases its hydrophobicity in a Ca++-dependent manner (Figure 3B). This gain in hydrophobicity is due to a conformational change accompanying Ca++-binding, a property common to all EF-hand proteins, and suggests that exposed hydrophobic parts of sorcin directly insert into the membrane. The Ca++-dependent membrane association of synexin is based on a different mechanism: synexin-bound Ca++ ions are thought to act like an electrostatic glue that mediates binding to negatively charged phospholipids.48
We have recently shown that stomatin and the related flotillins are the major integral proteins of erythrocyte lipid rafts.20Interestingly, these proteins behave differently in the vesiculation process. Whereas stomatin is specifically concentrated in the microvesicles, flotillins are virtually absent from microvesicles and remain in the red cell membrane (Figure 2). This result suggests that there are at least 2 distinct sets of lipid rafts in the erythrocyte membrane, with stomatin and the flotillins being respective marker proteins. In accordance with this concept is the finding that stomatin and the flotillins are organized in independent oligomeric complexes.20 Generally, the coexistence of functionally different types of lipid rafts at the plasma membrane provides an exciting new perspective in the hypothesis of membrane microdomains.49-51
In microvesicles, the lipid raft components stomatin and AChE are highly enriched relative to other membrane proteins. We show that lipid rafts exist within the microvesicular membrane (Figure 4), with most of stomatin and AChE being raft-associated. We suggest that the patchy, fine structure of the microvesicular membrane seen by AFM reflects the inherent lipid organization in raft and nonraft domains (Figure 1C). Interestingly, synexin and, to a lesser extent, sorcin are also associated with the microvesicular rafts in a Ca++-dependent manner. This is the first report to show the lipid raft association of synexin. Several other members of the annexin protein family have already been described as associating with lipid rafts in various cellular contexts. Annexin II and the interacting protein p11 are the major lipid raft components in Madin-Darby canine kidney cells.52 In baby hamster kidney cells, the membrane association of annexin II is partially Ca++ independent but sensitive to cholesterol sequestration,53 thereby proving a tight interaction of annexin II with lipid rafts. Annexin XIIIb, which is myristoylated at the N-terminus, associates with lipid rafts and plays a role in the apical delivery of vesicular carriers.54 In smooth muscle cells, the concerted action of annexins II, V, and VI is thought to influence Ca++-dependent lipid raft dynamics, with important consequences for signaling events and Ca++flux.55
In our study of nanovesicles, the flotation experiments in Figure 5A,B show a distinct pool of GM1, cholesterol, and AChE comigrating in the low-density region of the sucrose gradient, indicating the existence of lipid rafts. As the erythrocyte raft marker proteins, stomatin and the flotillins, are virtually absent from nanovesicles, it is conceivable that the nanovesicular rafts represent another type of microdomain in the erythrocyte membrane. In the presence of Ca++, synexin and sorcin show a floating behavior; however, their distribution lacks correlation with the lipid raft markers. Our interpretation of this phenomenon is that there is a certain reversibility in the calcium-mediated binding of synexin and sorcin to the nanovesicular lipid rafts, with an equilibrium between raft-bound and unbound protein. During the centrifugation, these partitioning states of the respective proteins encounter opposing forces, with the raft-bound form floating to lower densities and the unbound form sedimenting to higher densities. This would result in the segregation and observed difference in the distribution of the lipid raft markers and synexin and sorcin. In this light, the difference in the distribution of synexin and sorcin suggests a lower affinity of sorcin to the nanovesicular rafts as compared with synexin. Sorcin has not yet been described as a lipid raft protein, and it remains to be determined whether sorcin interacts directly with raft lipids or whether it is associated via the N-terminus of synexin. Moreover, comparison of Figures 4A and 5A suggests that the calcium-dependent association of synexin and sorcin is stronger for microvesicular than for nanovesicular rafts. However, further lipid and proteomic analyses are necessary to define the difference between microvesicular and nanovesicular rafts and to understand their roles in the vesiculation process of erythrocytes.
There is increasing evidence that lipid rafts are involved in membrane budding and vesiculation during diverse biological processes.56 It is well documented that lipid rafts play a key role in the vesicle-based exocytic and endocytic transport (for review, see Ikonen57). Lipid rafts were shown to serve as platforms for the inclusion of sorting receptors and cargo molecules to the forming vesicle.58 Moreover, it has been shown that viral assembly takes place within lipid rafts of the plasma membrane and that the viral envelope is enriched in lipid raft components.59-61 Our data suggest that the calcium-induced microvesiculation and nanovesiculation of erythrocytes are also lipid raft–based processes, with stomatin and synexin being the major raft proteins, respectively. It remains to be determined whether the membrane-aggregating38 and fusogenic62,63activities of synexin play a role in these processes, with sorcin being a modulator.41 Our finding that lipid rafts specific for stomatin, flotillin, synexin, and sorcin are segregated during the vesiculation process introduces a novel aspect of the dynamics and organization of the erythrocyte membrane.
We thank Diethelm Gauster for peptide sequencing, Elisabeth Legenstein for quantitative cholesterol analyses, and Tim Skern for critically reading the manuscript.
Supported by grants P12907 (R.P.) and P12802 (P.H.) from the Austrian Science Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Rainer Prohaska, Institute of Medical Biochemistry, University of Vienna, Vienna Biocenter, Dr Bohr-Gasse 9/3, A-1030 Vienna, Austria; e-mail: prohaska@bch.univie.ac.at.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal