Diffuse large B-cell lymphoma (DLBCL), a histologically well-defined subset of non-Hodgkin lymphoma, is clinically and genetically heterogenous. By G-banding, most cases showed complex hyperdiploid karyotypes and diverse cytogenetic abnormalities that included recurring and nonrecurring translocations, deletions, duplications, and marker chromosomes. While G-banding provided valuable leads to identification of specific rearrangements that enabled gene discovery and clinical correlations, many aberrations remained uncharacterized because of their complexity. The molecular cytogenetic technique spectral karyotyping (SKY), on the other hand, enables complete characterization of all aberrations in a tumor cell karyotype and, hence, precise quantitation of chromosome instability. We report here, for the first time, SKY analysis of a panel of 46 DLBCL cases previously analyzed by G-banding, ascertained at the Memorial Sloan-Kettering Cancer Center. This analysis provided a cytogenetic profile of DLBCL that was characterized by a higher level of instability, qualitatively as well as quantitatively, compared with G-banding. Thus, 551 breakpoints were detected by SKY, in contrast to the 295 by G-banding. Several new recurring breakpoints, translocations, and regions of gain and loss were identified, which included 13 breakpoints not previously identified by G-banding, 10 breakpoints that were underrepresented by G-banding, and 4 previously unrecognized translocations: der(14)t(3;14)(q21;q32), t(1;13)(p32;q14), t(1;7)(q21;q22), and der(6)t(6;8)(q11;q11). We identified new clinical associations involving recurring breakpoints detected by SKY. These studies emphasize the value of SKY analysis for redefinition of chromosomal instability in DLBCL to enhance gene discovery as well as clinical correlation analysis.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common form of non-Hodgkin lymphoma (NHL), representing 40% of all adult cases, and comprises several entities characterized by genetic, morphologic, and clinical features. This heterogeneity has been suggested to derive from the developmental stage of the B cell at which it is transformed—ie, germinal center or post–germinal center B cell—as well as transformation to DLBCL of a lower histologic grade.1-3 Clinically, less than 45% of patients with DLBCL achieve complete remission and become cured, while the remaining succumb to disease. However, very few biologic or genetic markers that define the heterogeneity or precisely predict clinical behavior are available.
The cytogenetic features of DLBCL have been partly defined by G-banding analysis.4 Approximately 50% of cases exhibit chromosomal translocations involving one of the IG gene sites, which lead to deregulated expression of a variety of genes. The remaining cases display diverse types of chromosomal rearrangements that include translocations, deletions, and undefined aberrations such as additions and marker chromosomes.4 The recent introduction of spectral karyotyping (SKY) has made possible the identification of each of the human chromosomes by a different color, facilitating precise identification of all rearrangements in a tumor karyotype.5 A limited number of hematopoietic tumors such as multiple myeloma6,7 and acute leukemia8-11have been studied by SKY. These studies showed that SKY detected a several-fold increase in random as well as nonrandom chromosomal aberrations compared with G-banding. So far, there have been no attempts to examine clinical correlations based on SKY data in hematopoietic or other neoplasms. There have also been no SKY-based studies of NHL. Here we report SKY analysis of a panel of 46 DLBCLs ascertained at the Memorial Sloan-Kettering Cancer Center. We identified 13 new recurring breakpoints; 4 new recurring translocations; a number of cryptic, balanced, and unbalanced translocations; and regions of gain and loss. We identified new clinical associations involving recurring breakpoints detected by SKY.
Materials and methods
Tumor ascertainment
The ongoing ascertainment of consecutive NHL cases for cytogenetic analysis initiated at the Memorial Sloan-Kettering Cancer Center in 1984 has been described.12,13 A subset of 46 cases with DLBCL for which archived methanol-acetic acid–fixed metaphase preparations were available were selected from this ascertainment for SKY analysis. All patients were histologically reclassified according to the World Health Organization classification14 and staged by the Ann Arbor staging system. None of the cases had histologic evidence or were suspected to have preexisting low-grade lymphoma. Of the 46 cases, 29 were ascertained pretreatment and 17 posttreatment.
Cytogenetic and SKY analysis
Cytogenetic analysis was performed on metaphase spreads obtained from lymph node biopsy (43), bone marrow (2), or pleural effusion (1) as previously described.12 Clonal chromosomal abnormalities were described according to the International System of Human Cytogenetic Nomenclature.15 SKY analysis was performed as previously described.5,6 SKY images were acquired with an SD300 Spectracube (Applied Spectral Imaging, Migdal Ha-Emck, Israel) mounted on a Nikon Eclipse E800 microscope using a custom-designed optical filter (SKY-1) (Chroma Technology, Brattleboro, VT). For each case, 5 to 30 metaphases were analyzed. Breakpoints on G-banded chromosomes were scored according to previously established criteria.4 Breakpoints on the SKY-painted chromosomes were determined by comparison with corresponding DAPI and G-banded karyotype of the same tumor as previously described.6
Fluorescence in situ hybridization analysis
Fluorescence in situ hybridization (FISH) with whole chromosome painting probes for chromosomes 1, 3, and 14 (Vysis, Downers Grove, IL) was performed according to manufacturer-supplied protocols. To confirm 14q32 rearrangements, the PAC clone 120H10, derived from the region immediately telomeric to IGHV, was used in FISH analysis.16 For each case, 5 to 13 metaphases were analyzed using the Quips Pathvision (Applied Imaging, Santa Clara, CA).
Statistical analysis of data
Differences in percentage of variables were tested for significance using the 2-tailed Fischer exact test. Survival times were estimated by Kaplan-Meier analysis and compared by log-rank test. Cytogenetic variables included 32 recurring break sites. Clinical variables included age, sex, performance status, stage, B symptoms, bulky disease, lactate dehydrogenase, extranodal disease, number of extranodal sites, international prognostic index,17 and response. Time to treatment failure was measured from time of initiation of treatment until disease progression. Overall survival was measured from initiation of treatment (45 cases) or the date of diagnosis, if the patient was observed (1 case), until the date last seen.
Results
Clinical and histologic features of patients
The clinical and histologic features of the 46 cases with reconfirmed DLBCL at diagnosis are summarized in Table1. Morphologic variants included 28 centroblastic, 5 plasmablastic, 4 immunoblastic, 3 pleomorphic, and 5 T-cell–rich/histiocyte-rich lymphomas. The 1 case with primary effusion lymphoma (2612) could not be subtyped. Of the 46 cases, 3 (2293, 2473, 1800) were primary mediastinal large B-cell lymphomas. The male:female ratio was 1:1.19 (21 males, 25 females). The age range was 21 to 85 years (median, 57.5). Eight patients had stage I disease, 11 had stage II disease, 10 had stage III disease, and 16 had stage IV disease. Of the 46 patients, 28 were at low or low-intermediate risk, whereas 14 were at high or high-intermediate risk. Clinical data were not available for 1 patient (980), and 3 patients (980, 2358, 2433) were lost to follow-up. All patients, with the exception of cases 2202 and 2627, received anthracycline-containing combination chemotherapy as part of their initial treatment.
Clinical and histologic features of the 46 DLBCLs
| Subject no. . | Case . | Age/Sex . | Rx . | Study . | Specimen . | Morphology . | Stage . | IPI . | Response . | TTP, mo . | OS, mo . | Status . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 980 | 32/M | UT | ID | Cervical node | CB | — | — | — | — | — | LF |
| 2 | 1942 | 29/M | UT | ID | Colonic mass | CB | II XEA | LR | CR | 47.0 | 47.0 | NED |
| 3 | 1965 | 58/F | UT | ID | Jugular node | CB | II A | LR | CR | 40.0 | 40.0 | NED |
| 4 | 1997 | 44/M | UT | ID | Axillary node | CB | III B | HR | CR | 9.6 | 16.3 | DOD |
| 5 | 2045 | 68/F | UT | ID | Tonsil | CB | II EA | LR | CR | 39.4 | 39.4 | NED |
| 6 | 2180 | 33/M | UT | ID | Stomach | CB | I EA | LR | CR | 15.4 | 15.4 | NED |
| 7 | 2202 | 82/M | UT | ID | Retroperitoneal mass | CB | III A | LR | NR* | 4.8 | 4.8 | DOD |
| 8 | 2270 | 59/M | UT | PRD | Inguinal node | CB | I A | LR | NR | 2.9 | 28.8 | NED |
| 9 | 2326 | 50/M | UT | ID | Neck node | CB | III A | LR | CR | 12.9 | 12.9 | NED |
| 10 | 2358 | 74/F | UT | ID | Neck mass | TCR/HR | IV A | HR | — | — | — | LF |
| 11 | 2417 | 21/F | UT | ID | Cervical node | PL | IV A | LR | CR | 6.8 | 8.1 | AWD |
| 12 | 2438 | 57/F | UT | ID | Inguinal node | CB | IV B | HR | CR | 11.7 | 11.7 | NED |
| 13 | 2447 | 65/F | UT | ID | Right thigh | PB | II EX | HR | CR | 9.4 | 9.4 | NED |
| 14 | 2451 | 71/M | UT | ID | Inguinal node | CB | IV A | HR | CR | 10.3 | 10.3 | NED |
| 15 | 2453 | 64/F | UT | ID | Axillary node | TCR/HR | IV A | HR | POD | 2.7 | 5.6 | DOD |
| 16 | 2511 | 55/M | UT | ID | Axillary node | TCR/HR | III A | LR | CR | 6.8 | 6.8 | NED |
| 17 | 2526 | 77/F | UT | ID | Cervical node | PB | I A | LR | CR | 5.1 | 5.1 | NED |
| 18 | 2536 | 72/F | UT | ID | Axillary node | CB | IV A | HR | CR | 4.8 | 4.8 | AWD |
| 19 | 2545 | 83/M | UT | ID | Inguinal node | TCR/HR | III A | LR | No Rx | 5.3 | 5.3 | AWD |
| 20 | 2625 | 61/F | UT | ID | Neck mass | CB | I A | LR | CR | 5.1 | 5.1 | NED |
| 21 | 2627 | 82/F | UT | ID | Spleen | CB | I A | HR | CR* | 4.3 | 4.3 | NED |
| 22 | 2629 | 59/F | UT | ID | Hepatic artery node | CB | II A | LR | CR | 5.6 | 5.6 | AWD |
| 23 | 2609 | 53/M | UT | ID | Bone marrow | IM | IV B | LR | POD | 4.6 | 5.9 | DOD |
| 24† | 2612 | 52/F | UT | ID | Ascitic fluid | — | IV A | LR | CR | 18.7 | 18.7 | NED |
| 25 | 2433 | 73/F | UT | ID | Jugular node | TCR/HR | III A | HR | — | — | — | LF |
| 26 | 2411 | 60/M | UT | ID | Neck mass | IM | IV A | LR | CR | 8.5 | 8.5 | NED |
| 27 | 2055 | 71/F | UT | ID | Epiglottis | PB | I EA | LR | CR | 25.7 | 25.7 | DOD |
| 28 | 2308 | 77/F | UT | ID | Pelvic node | CB | III BE | HR | CR | 17.8 | 17.8 | NED |
| 29 | 2549 | 85/F | UT | ID | Tonsil | CB | IV A | LR | CR | 5.8 | 5.8 | NED |
| 30 | 2216 | 35/F | T | 2nd RP | Omentum | PL | II XA | LR | NR | 4.4 | 10.2 | DOD |
| 31‡ | 2293 | 37/F | T | 2nd RP | Level II neck node | CB | II XA | LR | CR | 20.6 | 58.8 | AWD |
| 32 | 2616 | 43/M | T | PRD | Bone marrow | CB | IV A | LR | POD | 4.6 | 10.1 | DOD |
| 33‡ | 2473 | 37/M | T | PRD | Supraclavicular node | CB | II XEEB | HR | POD | 6.7 | 14.7 | NED |
| 34 | 2631 | 57/M | T | 2nd RP | Antecubital fossa | CB | IV A | HR | CR | 11 | 24.2 | AWD |
| 35 | 2369 | 39/M | T | PRD | Pancreas | IM | IV XB | HR | NR | 4.7 | 10.5 | DOD |
| 36 | 2552 | 46/M | T | 2nd RP | Cervical node | CB | III A | LR | CR | 11.7 | 22.8 | AWD |
| 37 | 2498 | 57/F | T | 1st RP | Axillary node | IM | I A | LR | CR | 6 | 12.3 | NED |
| 38 | 2005 | 42/F | T | 1st RP | Axillary node | PL | III ARE | LR | CR | 8.7 | 37.8 | DOD |
| 39 | 1778 | 37/F | T | 2nd RP | Jugular node | CB | IV A | HR | CR | 15.7 | 30.1 | DOD |
| 40 | 2020 | 63/M | T | 1st RP | Spleen | CB | IV A | HR | CR | 16.5 | 36.8 | DOD |
| 41 | 1983 | 66/F | T | 1st RP | Scalene node | CB | I A | LR | CR | 8.6 | 33.0 | DOD |
| 42‡ | 1800 | 28/M | T | PRD | Supraclavicular node | CB | II A | LR | PR | 5.6 | 45.7 | NED |
| 43 | 2006 | 23/M | T | 1st RP | Axillary node | PB | III A | LR | CR | 7.2 | 12.1 | DOD |
| 44 | 2287 | 48/M | T | 2nd RP | Scalene node | CB | IV A | HR | PR | 9.1 | 16.7 | DOD |
| 45 | 1686 | 61/F | T | 1st RP | Cervical node | PB | II XA | LR | CR | 243.4 | 319.2 | NED |
| 46 | 2635 | 82/F | T | 2nd RP | Axillary node | CB | II A | HR | CR | 155.9 | 226.2 | DOD |
| Subject no. . | Case . | Age/Sex . | Rx . | Study . | Specimen . | Morphology . | Stage . | IPI . | Response . | TTP, mo . | OS, mo . | Status . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 980 | 32/M | UT | ID | Cervical node | CB | — | — | — | — | — | LF |
| 2 | 1942 | 29/M | UT | ID | Colonic mass | CB | II XEA | LR | CR | 47.0 | 47.0 | NED |
| 3 | 1965 | 58/F | UT | ID | Jugular node | CB | II A | LR | CR | 40.0 | 40.0 | NED |
| 4 | 1997 | 44/M | UT | ID | Axillary node | CB | III B | HR | CR | 9.6 | 16.3 | DOD |
| 5 | 2045 | 68/F | UT | ID | Tonsil | CB | II EA | LR | CR | 39.4 | 39.4 | NED |
| 6 | 2180 | 33/M | UT | ID | Stomach | CB | I EA | LR | CR | 15.4 | 15.4 | NED |
| 7 | 2202 | 82/M | UT | ID | Retroperitoneal mass | CB | III A | LR | NR* | 4.8 | 4.8 | DOD |
| 8 | 2270 | 59/M | UT | PRD | Inguinal node | CB | I A | LR | NR | 2.9 | 28.8 | NED |
| 9 | 2326 | 50/M | UT | ID | Neck node | CB | III A | LR | CR | 12.9 | 12.9 | NED |
| 10 | 2358 | 74/F | UT | ID | Neck mass | TCR/HR | IV A | HR | — | — | — | LF |
| 11 | 2417 | 21/F | UT | ID | Cervical node | PL | IV A | LR | CR | 6.8 | 8.1 | AWD |
| 12 | 2438 | 57/F | UT | ID | Inguinal node | CB | IV B | HR | CR | 11.7 | 11.7 | NED |
| 13 | 2447 | 65/F | UT | ID | Right thigh | PB | II EX | HR | CR | 9.4 | 9.4 | NED |
| 14 | 2451 | 71/M | UT | ID | Inguinal node | CB | IV A | HR | CR | 10.3 | 10.3 | NED |
| 15 | 2453 | 64/F | UT | ID | Axillary node | TCR/HR | IV A | HR | POD | 2.7 | 5.6 | DOD |
| 16 | 2511 | 55/M | UT | ID | Axillary node | TCR/HR | III A | LR | CR | 6.8 | 6.8 | NED |
| 17 | 2526 | 77/F | UT | ID | Cervical node | PB | I A | LR | CR | 5.1 | 5.1 | NED |
| 18 | 2536 | 72/F | UT | ID | Axillary node | CB | IV A | HR | CR | 4.8 | 4.8 | AWD |
| 19 | 2545 | 83/M | UT | ID | Inguinal node | TCR/HR | III A | LR | No Rx | 5.3 | 5.3 | AWD |
| 20 | 2625 | 61/F | UT | ID | Neck mass | CB | I A | LR | CR | 5.1 | 5.1 | NED |
| 21 | 2627 | 82/F | UT | ID | Spleen | CB | I A | HR | CR* | 4.3 | 4.3 | NED |
| 22 | 2629 | 59/F | UT | ID | Hepatic artery node | CB | II A | LR | CR | 5.6 | 5.6 | AWD |
| 23 | 2609 | 53/M | UT | ID | Bone marrow | IM | IV B | LR | POD | 4.6 | 5.9 | DOD |
| 24† | 2612 | 52/F | UT | ID | Ascitic fluid | — | IV A | LR | CR | 18.7 | 18.7 | NED |
| 25 | 2433 | 73/F | UT | ID | Jugular node | TCR/HR | III A | HR | — | — | — | LF |
| 26 | 2411 | 60/M | UT | ID | Neck mass | IM | IV A | LR | CR | 8.5 | 8.5 | NED |
| 27 | 2055 | 71/F | UT | ID | Epiglottis | PB | I EA | LR | CR | 25.7 | 25.7 | DOD |
| 28 | 2308 | 77/F | UT | ID | Pelvic node | CB | III BE | HR | CR | 17.8 | 17.8 | NED |
| 29 | 2549 | 85/F | UT | ID | Tonsil | CB | IV A | LR | CR | 5.8 | 5.8 | NED |
| 30 | 2216 | 35/F | T | 2nd RP | Omentum | PL | II XA | LR | NR | 4.4 | 10.2 | DOD |
| 31‡ | 2293 | 37/F | T | 2nd RP | Level II neck node | CB | II XA | LR | CR | 20.6 | 58.8 | AWD |
| 32 | 2616 | 43/M | T | PRD | Bone marrow | CB | IV A | LR | POD | 4.6 | 10.1 | DOD |
| 33‡ | 2473 | 37/M | T | PRD | Supraclavicular node | CB | II XEEB | HR | POD | 6.7 | 14.7 | NED |
| 34 | 2631 | 57/M | T | 2nd RP | Antecubital fossa | CB | IV A | HR | CR | 11 | 24.2 | AWD |
| 35 | 2369 | 39/M | T | PRD | Pancreas | IM | IV XB | HR | NR | 4.7 | 10.5 | DOD |
| 36 | 2552 | 46/M | T | 2nd RP | Cervical node | CB | III A | LR | CR | 11.7 | 22.8 | AWD |
| 37 | 2498 | 57/F | T | 1st RP | Axillary node | IM | I A | LR | CR | 6 | 12.3 | NED |
| 38 | 2005 | 42/F | T | 1st RP | Axillary node | PL | III ARE | LR | CR | 8.7 | 37.8 | DOD |
| 39 | 1778 | 37/F | T | 2nd RP | Jugular node | CB | IV A | HR | CR | 15.7 | 30.1 | DOD |
| 40 | 2020 | 63/M | T | 1st RP | Spleen | CB | IV A | HR | CR | 16.5 | 36.8 | DOD |
| 41 | 1983 | 66/F | T | 1st RP | Scalene node | CB | I A | LR | CR | 8.6 | 33.0 | DOD |
| 42‡ | 1800 | 28/M | T | PRD | Supraclavicular node | CB | II A | LR | PR | 5.6 | 45.7 | NED |
| 43 | 2006 | 23/M | T | 1st RP | Axillary node | PB | III A | LR | CR | 7.2 | 12.1 | DOD |
| 44 | 2287 | 48/M | T | 2nd RP | Scalene node | CB | IV A | HR | PR | 9.1 | 16.7 | DOD |
| 45 | 1686 | 61/F | T | 1st RP | Cervical node | PB | II XA | LR | CR | 243.4 | 319.2 | NED |
| 46 | 2635 | 82/F | T | 2nd RP | Axillary node | CB | II A | HR | CR | 155.9 | 226.2 | DOD |
Rx indicates treatment; IPI, international prognostic index; TTP, time to progression; OS, overall survival; UT, untreated; T, treated; ID, initial diagnosis; PRD, primary refractory disease; RP, relapse; CB, centroblastic; TCR/HR, T-cell-rich/histiocyte-rich; PL, pleomorphic; PB, plasmablastic; IM, immunoblastic; HR, high and high-intermediate risk; LR, low and low-intermediate risk; CR, complete remission; NR, no response; POD, progressive disease; PR, partial remission; DOD, died of disease; NED, no evidence of disease; LF, lost to follow-up; AWD, alive with disease.
Did not receive anthracycline-containing combination chemotherapy as part of their initial treatment.
Primary effusion lymphoma.
Mediastinal large B-cell lymphoma.
Chromosome involvement and breakpoints identified by SKY analysis versus G-banding
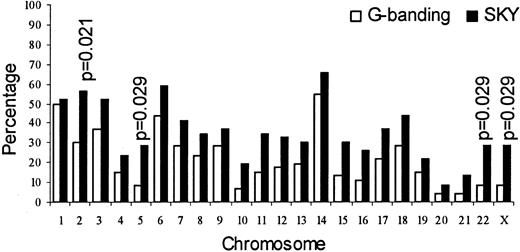
Figure 1 shows the percentage involvement of each chromosome. With the exception of chromosome 1, all chromosomes were affected more frequently in the SKY analysis than in the G-banding analysis; however, the difference in involvement only of chromosomes 2, 5, 22, and X was significant (P ≤ .05).
Involvement of chromosomes by SKY versus G-banding.
The difference was significant for chromosomes 2, 5, 22, and X.
Involvement of chromosomes by SKY versus G-banding.
The difference was significant for chromosomes 2, 5, 22, and X.
Figure 2 shows the distribution of breakpoints, and a representative SKY karyotype with multiple and cryptic rearrangements is shown in Figure3A. A total of 551 breakpoints were identified by SKY, compared with 295 by G-banding. SKY analysis confirmed 234 (80%) breakpoints identified by G-banding and revised 53 (20%). Most of the revisions comprised breakpoints that were classified by G-banding as terminal, which were identified by SKY to be interstitial. Breakpoints on chromosomes 1, 2, 3, 6, and 8 were misidentified most frequently. In addition, SKY analysis identified 264 new breakpoints, including 60 that could not be precisely assigned to a band. The 551 breakpoints identified by SKY analysis were located at 171 bands and, of these, 125 (73%) were recurring (≥ 2 cases). In the case of G-banding, the 295 breakpoints were located at 144 bands and, of these, 63 (44%) were recurring. While the number of recurring sites noted in more than 10% of the cases was 32 in SKY analysis, it was only 10 in G-banding. With the exception of 1q32, 6q23, 8q24, 14q32, and 19q13, all the recurring sites (27) were noted to be involved more frequently in SKY analysis than in G-banding. Breaks at 2 sites, 16q11-13 and 17q11-21, were significantly more frequent in SKY analysis (P ≥ .05). Sites most frequently identified to be involved in rearrangement in SKY analysis were 14q32 (57%), 6q11-15 (30%), 18q21 (30%), 1q11-21 (28%), 3q27 (28%), and 9p13 (22%); sites most frequently identified in G-banding analysis were 14q32 (57%), 1q11-21 (24%), 3q27 (20%), 6q11-15 (17%), and 18q21 (15%).
Distribution of breakpoints.
Idiogram shows distribution of breakpoints identified by G-banding (left) and SKY (right). The number of breakpoints in each chromosome that were identified by SKY, but could not be precisely assigned to a band, are noted on top of the chromosomes. Each dot represents a single breakpoint. ★ represents 10 breakpoints; ✖ represents a single breakpoint misidentified by G-banding; ✜ represents a single breakpoint misidentified by G-banding but within the same chromosome.
Distribution of breakpoints.
Idiogram shows distribution of breakpoints identified by G-banding (left) and SKY (right). The number of breakpoints in each chromosome that were identified by SKY, but could not be precisely assigned to a band, are noted on top of the chromosomes. Each dot represents a single breakpoint. ★ represents 10 breakpoints; ✖ represents a single breakpoint misidentified by G-banding; ✜ represents a single breakpoint misidentified by G-banding but within the same chromosome.
Representative karyotypes illustrating the various chromosomal rearrangements identified in SKY analysis.
(A) SKY karyotype of case 2612 with primary effusion lymphoma showing several multiple complex and cryptic translocations, notably, t(3;14)(q27;q32) and der(14)t(1;14)(?;q32). The display colors are shown on the left and the classification colors on the right. (B) Confirmation of t(3;14)(q27;q32) by FISH using PAC probe 120H10 (14q32). The lack of green signal on chromosome 1 confirms the nonreciprocal nature of der(14)t(1;14). (C) The same metaphase was washed and rehybridized with whole chromosome painting probe for chromosomes 1 (green) and 14 (red). Presence of green signal on chromosome 14 confirms der(14)t(1;14)(?;q32). (D) Partial karyotypes (from left to right) showing the 3 new and 2 potentially new recurring translocations. Inverted DAPI band image of the involved chromosome is shown on the left and the classification color on the right.
Representative karyotypes illustrating the various chromosomal rearrangements identified in SKY analysis.
(A) SKY karyotype of case 2612 with primary effusion lymphoma showing several multiple complex and cryptic translocations, notably, t(3;14)(q27;q32) and der(14)t(1;14)(?;q32). The display colors are shown on the left and the classification colors on the right. (B) Confirmation of t(3;14)(q27;q32) by FISH using PAC probe 120H10 (14q32). The lack of green signal on chromosome 1 confirms the nonreciprocal nature of der(14)t(1;14). (C) The same metaphase was washed and rehybridized with whole chromosome painting probe for chromosomes 1 (green) and 14 (red). Presence of green signal on chromosome 14 confirms der(14)t(1;14)(?;q32). (D) Partial karyotypes (from left to right) showing the 3 new and 2 potentially new recurring translocations. Inverted DAPI band image of the involved chromosome is shown on the left and the classification color on the right.
To identify new recurring breakpoints, all breakpoints identified in SKY and G-banding analyses were compared. Thirteen new recurring breakpoints were identified by SKY, with 16q11-13 (13%), 12p11, and 11p11 (9% each) as the most frequent (Table2). Because the number of cases in the present cohort was small, the 551 breakpoints identified by SKY also were compared with the 1021 breakpoints identified by G-banding in our previously reported cohort of 363 consecutively ascertained DLBCLs.4 All the new recurring sites identified in the present study by SKY were either nonrecurring or were undetected by G-banding in this larger cohort.
New recurring breakpoints and their corresponding incidence by G-banding in this cohort and the previous cohort (Cigudosa et al, 1999)
| Subject no. . | Site . | SKY . | G-banding . | |
|---|---|---|---|---|
| This cohort . | Cigudosa et al . | |||
| 1 | 16q11-13 | 6 (13.0) | — | 1 (0.46) |
| 2 | 12p11 | 4 (8.7) | — | 1 (0.46) |
| 3 | 11p11 | 4 (8.7) | 1 (2.2) | — |
| 4 | 18p11 | 3 (6.5) | 1 (2.2) | — |
| 5 | 20q11-13 | 3 (6.5) | 1 (2.2) | — |
| 6 | 17q11 | 3 (6.5) | — | — |
| 7 | 4p11 | 2 (4.3) | — | — |
| 8 | 4p14 | 2 (4.3) | 1 (2.2) | — |
| 9 | 5q11 | 2 (4.3) | — | 1 (0.46) |
| 10 | 16p11 | 2 (4.3) | — | — |
| 11 | 17q23 | 2 (4.3) | — | — |
| 12 | 18q11 | 2 (4.3) | — | — |
| 13 | Xp11 | 2 (4.3) | — | — |
| Subject no. . | Site . | SKY . | G-banding . | |
|---|---|---|---|---|
| This cohort . | Cigudosa et al . | |||
| 1 | 16q11-13 | 6 (13.0) | — | 1 (0.46) |
| 2 | 12p11 | 4 (8.7) | — | 1 (0.46) |
| 3 | 11p11 | 4 (8.7) | 1 (2.2) | — |
| 4 | 18p11 | 3 (6.5) | 1 (2.2) | — |
| 5 | 20q11-13 | 3 (6.5) | 1 (2.2) | — |
| 6 | 17q11 | 3 (6.5) | — | — |
| 7 | 4p11 | 2 (4.3) | — | — |
| 8 | 4p14 | 2 (4.3) | 1 (2.2) | — |
| 9 | 5q11 | 2 (4.3) | — | 1 (0.46) |
| 10 | 16p11 | 2 (4.3) | — | — |
| 11 | 17q23 | 2 (4.3) | — | — |
| 12 | 18q11 | 2 (4.3) | — | — |
| 13 | Xp11 | 2 (4.3) | — | — |
To determine the effect of therapy on the incidence of breakpoints, data from treated and untreated cases were compared. The mean number of breaks per case was higher in treated cases (16) than untreated cases (9). Most (24 of 32) of the recurring breakpoints were noted to be affected more frequently in the treated cases; however, the difference was significant only for breaks at 17q11-21 (P ≤ .05).
Chromosome structural aberrations identified by SKY analysis versus G-banding
Multiple complex structural aberrations (translocations, deletions, duplications, isochromosomes, inversions, insertions, additions, and markers) were present in 85% of the cases in this study. Of the 46 cases with clonal chromosome abnormalities, the G-banded karyotype of only 4 cases remained unchanged after SKY analysis. In the remaining 42 cases, SKY provided additional cytogenetic information. G-banding analysis detected a total of 240 aberrations, of which 98 were undefined (73 additions, 24 markers, and 1 ring chromosome). All the undefined aberrations were resolved by SKY. Of the 240 aberrations, SKY confirmed 74 and revised 166. Of the marker and addition chromosomes detected by G-banding, 75% represented translocations. In addition, SKY identified 79 new aberrations. A total of 211 translocations were identified by SKY. Of these, 64 were new, including 30 that were cryptic. Several of the latter were confirmed by FISH using whole chromosome painting or locus-specific probes (Figure 3B,C).
While rearrangements affecting one of the IG gene sites were the most common aberrations detected by both the techniques, they were detected at a slightly higher rate by SKY (66%) than by G-banding (61%) (Table 3). Of the 25 cases with translocations affecting 14q32 detected by SKY, 6 had t(3;14)(q27;q32), 5 had t(14;18)(q32;21), 4 had t(9;14)(p13;q32), and 1 each had t(8;14)(q24;q32) and t(8;14;18)(q24;q32;q21), while 10 had other 14q32-associated translocations (Table4). The incidence of translocations affecting 14q32 detected by G-banding analysis was the same as that detected by SKY (25 cases); however, 3 of the 14q32 breakpoints were misidentified by G-banding. Translocations affecting 22q11 were noted in 7 cases by SKY, compared with 2 by G-banding. Of the former 7 cases, 3 cases had t(3;22)(q27;q11) and 4 had other 22q11 translocations (Table 4). Translocations involving 2p11 were less frequent and were detected in 2 cases by SKY alone (Table 4).
Frequency of IG gene site and 3q27 involvement detected by SKY and by G-banding
| Translocation . | Number of cases (%) . | ||
|---|---|---|---|
| SKY . | G-banding . | Misidentified by G-banding . | |
| 14q32 | |||
| t(3;14)(q27;q32) | 6 (13.04) | 5 (10.87) | |
| t(14;18)(q32;q21) | 5 (10.87) | 5 | 1 (2.17) |
| t(8;14;18)(q24;q32;q21) | 1 (2.17) | — | |
| t(8;14)(q24;q32) | 1 (2.17) | 1 (2.17) | |
| t(9;14)(p13;q32) | 4 (8.70) | 6 (13.04) | 2 (4.35) |
| Other 14q323-150 | 10 (21.74) | 8 (17.39) | 1 (2.17) |
| Overall 14q32 | 26 (56.52)3-151 | 26 (56.52)3-151 | 4 (8.70) |
| 22q11 | |||
| t(3;22)(q27;q11) | 3 (6.52) | 2 (4.35) | |
| Other 22q11 | 4 (8.70) | — | |
| Overall 22q11 | 7 (15.23) | 2 (4.35) | |
| 2p11 | 2 (4.35) | — | |
| Overall IG gene site | 31 (67.40)3-152 | 28 (60.87) | 3 (6.52) |
| 3q27 | |||
| Other 3q27 | 5 (10.87) | 2 (4.35) | |
| Overall 3q27 | 13 (28.26)3-153 | 9 (19.55) | |
| Translocation . | Number of cases (%) . | ||
|---|---|---|---|
| SKY . | G-banding . | Misidentified by G-banding . | |
| 14q32 | |||
| t(3;14)(q27;q32) | 6 (13.04) | 5 (10.87) | |
| t(14;18)(q32;q21) | 5 (10.87) | 5 | 1 (2.17) |
| t(8;14;18)(q24;q32;q21) | 1 (2.17) | — | |
| t(8;14)(q24;q32) | 1 (2.17) | 1 (2.17) | |
| t(9;14)(p13;q32) | 4 (8.70) | 6 (13.04) | 2 (4.35) |
| Other 14q323-150 | 10 (21.74) | 8 (17.39) | 1 (2.17) |
| Overall 14q32 | 26 (56.52)3-151 | 26 (56.52)3-151 | 4 (8.70) |
| 22q11 | |||
| t(3;22)(q27;q11) | 3 (6.52) | 2 (4.35) | |
| Other 22q11 | 4 (8.70) | — | |
| Overall 22q11 | 7 (15.23) | 2 (4.35) | |
| 2p11 | 2 (4.35) | — | |
| Overall IG gene site | 31 (67.40)3-152 | 28 (60.87) | 3 (6.52) |
| 3q27 | |||
| Other 3q27 | 5 (10.87) | 2 (4.35) | |
| Overall 3q27 | 13 (28.26)3-153 | 9 (19.55) | |
One case had dup(14)(q11q32).
Both the homologs of chromosome 14 were involved in 1 case.
Two IG gene sites were affected in 4 cases (14q32 and 22q11 in 3 cases and 14q32 and 2p11 in 1 case).
One case had both t(3;14)(q27;q32) and t(3;22)(q27;q11).
Translocations affecting IG gene sites other than those involving 3q27, 8q24, and 18q21
| IG gene sites . | Case . | Translocation/derivative . |
|---|---|---|
| 14q32 | 2545 | der(14)t(3;14)(q21;q32) |
| 2433 | der(1)ins(1;12)(p34-36;q13q24)t(1;3;14) (q32;q21;q32) | |
| 2552 | der(3)t(3;21)(p13;q11)t(3;14)(q21;q11) t(14;17)(q32;?)t(4;17)(?;?) | |
| 2612 | der(14)t(1;14)(p?;q32) | |
| 2629 | t(1;14)(q42;q32) | |
| 2438 | der(11;14)(p11;q32) | |
| 2453 | der(14)t(19;14)(q12;q32) | |
| 1800 | der(14)t(14;15)(q32;q?) | |
| 980 | der(14)t(2;14)(p11;q32) | |
| 22q11 | 2473 | t(16;22)(p11;q11) |
| 2552 | t(19;22)(q13.3;q11) | |
| 2020 | der(12)t(12;22)(p11;q11) | |
| 1778 | der(22)t(6;22)(p11;q11) | |
| 2p11 | 980 | der(14)t(2;14)(p11;q32) |
| 2616 | der(17)t(2;17)(p11;q25) |
| IG gene sites . | Case . | Translocation/derivative . |
|---|---|---|
| 14q32 | 2545 | der(14)t(3;14)(q21;q32) |
| 2433 | der(1)ins(1;12)(p34-36;q13q24)t(1;3;14) (q32;q21;q32) | |
| 2552 | der(3)t(3;21)(p13;q11)t(3;14)(q21;q11) t(14;17)(q32;?)t(4;17)(?;?) | |
| 2612 | der(14)t(1;14)(p?;q32) | |
| 2629 | t(1;14)(q42;q32) | |
| 2438 | der(11;14)(p11;q32) | |
| 2453 | der(14)t(19;14)(q12;q32) | |
| 1800 | der(14)t(14;15)(q32;q?) | |
| 980 | der(14)t(2;14)(p11;q32) | |
| 22q11 | 2473 | t(16;22)(p11;q11) |
| 2552 | t(19;22)(q13.3;q11) | |
| 2020 | der(12)t(12;22)(p11;q11) | |
| 1778 | der(22)t(6;22)(p11;q11) | |
| 2p11 | 980 | der(14)t(2;14)(p11;q32) |
| 2616 | der(17)t(2;17)(p11;q25) |
Translocations involving 3q27 were the next most frequent after those affecting the IG gene sites. Eight cases exhibited t(3;14)(q27;q32) or t(3;22)(q27;q11), and 5 involved other sites (Table5). Only 9 of these translocations were detected by G-banding. Translocations affecting 1q11-21 (15%), 1p32-36 (11%), and 1q42-44 (9%) were also common and noted equally in SKY and G-banding analyses. Of note were 10 other non-IGH sites identified by SKY that were promiscuously involved in balanced and unbalanced translocations: 2q31, 12q11-13 (15%), 1p11-13, 2p13, 7q11, 16q11-13, 17q11-21 (13% each), 3p21, 7q22, and 15q13-15 (11% each). These sites were either undetected or underrepresented in G-banding analysis.
Translocations affecting band 3q27 other than those involving 14q32 and 22q11
| Case . | Translocation/derivative . |
|---|---|
| 1778 | t(3;5)(q27;p13) |
| 2451 | t(3;12)(q27;q22) |
| 2552 | der(3)t(3;6)(q27;p21) |
| 2545 | der(3)t(3;7)(p21;p13)t(X:3)(q27;q27) |
| 2308 | der(3)t(2;3)(?;q27)del(3)(p21) |
| Case . | Translocation/derivative . |
|---|---|
| 1778 | t(3;5)(q27;p13) |
| 2451 | t(3;12)(q27;q22) |
| 2552 | der(3)t(3;6)(q27;p21) |
| 2545 | der(3)t(3;7)(p21;p13)t(X:3)(q27;q27) |
| 2308 | der(3)t(2;3)(?;q27)del(3)(p21) |
SKY is known for its ability to unambiguously characterize complex chromosomal aberrations and thus facilitate identification of new or hidden recurring translocations. Indeed, 4 new recurring translocations were identified in 2 cases each (Table 6) (Figure 3D). The translocations t(3;14)(q21;q32) and t(1;7)(q21;q22) were also identified by G-banding; the former was nonrecurring. Interestingly, 3 translocations, t(1;13)(p32;q14), t(1;7)(q21;q22), and der(6)t(6;8)(q11;q11), were observed only in cases without t(3q27) or t(14q32). In addition, 2 potentially new recurring translocations were identified in 2 cases each: t(5;16)(?;q11-12) and t(19;22)(q13;q11-13). A search in our database revealed another t(14;18)(q32;q21)-negative follicular large cell lymphoma with a t(19;22)(q13;q13). The ability to precisely delineate chromosomal abnormalities also facilitated the correct identification of known specific recurring translocations. Thus, in 2 cases, SKY revealed the presence of t(3;14)(q27;q32) and t(3;22)(q27;q11), respectively, that were overlooked by G-banding due to poor morphology. In 3 other cases, t(9;14)(p13;q32) (2 cases) and t(14;18)(q32;q21) (1 case), identified by G-banding, were revised by SKY and shown to be otherwise.
New recurring translocations in DLBCL
| Case . | SKY . | G-banding . | t(14q32) . | t(3q27) . |
|---|---|---|---|---|
| New | ||||
| 2545 | der(14)t(3;14)(q21;q32) | der(14)t(4;14)(q21;q32) | + | + |
| 2433 | der(1)ins(1;12)(p34-36; q13q24)t(1;3;14)(q32; q21;q32) | der(1)add(1)(p36)t(1;3; 14)(q32;q21;q32) | + | − |
| 2631 | der(1)t(1;13)(p32;q14)t (X;1)(?;q32) | der(1)add(1)(p32)add(1) (q32) | − | − |
| 2369 | t(1;13)(p32;q14) | add(1)(p34) | − | − |
| 1983 | der(1)t(1;7)(q21;q22) | der(1)t(1;7)(q21;q22) | − | − |
| 2616 | t(1;7)(q21;q22) | t(1;7)(q21;q22) | − | − |
| 2005 | der(6)t(6;8)(q11;q11) | add(6)(q23) | − | − |
| 2498 | der(6)t(6;8)(q11;q11) | del(6)(q21q23) | − | − |
| Potentially new | ||||
| 2438 | der(16)t(5;16)(?;q11) | mar | − | + |
| 1983 | der(16)t(5;16)(?;q11-12) | inv(16)(q22q24) | − | − |
| 2552 | t(19;22)(q13;q11-13) | add(19)(q13) | − | + |
| 2616 | der(22)t(19;22)(q?;q13) | 22 | − | − |
| 9816-150 | − | t(19;22)(q13;q13) | − | − |
| Case . | SKY . | G-banding . | t(14q32) . | t(3q27) . |
|---|---|---|---|---|
| New | ||||
| 2545 | der(14)t(3;14)(q21;q32) | der(14)t(4;14)(q21;q32) | + | + |
| 2433 | der(1)ins(1;12)(p34-36; q13q24)t(1;3;14)(q32; q21;q32) | der(1)add(1)(p36)t(1;3; 14)(q32;q21;q32) | + | − |
| 2631 | der(1)t(1;13)(p32;q14)t (X;1)(?;q32) | der(1)add(1)(p32)add(1) (q32) | − | − |
| 2369 | t(1;13)(p32;q14) | add(1)(p34) | − | − |
| 1983 | der(1)t(1;7)(q21;q22) | der(1)t(1;7)(q21;q22) | − | − |
| 2616 | t(1;7)(q21;q22) | t(1;7)(q21;q22) | − | − |
| 2005 | der(6)t(6;8)(q11;q11) | add(6)(q23) | − | − |
| 2498 | der(6)t(6;8)(q11;q11) | del(6)(q21q23) | − | − |
| Potentially new | ||||
| 2438 | der(16)t(5;16)(?;q11) | mar | − | + |
| 1983 | der(16)t(5;16)(?;q11-12) | inv(16)(q22q24) | − | − |
| 2552 | t(19;22)(q13;q11-13) | add(19)(q13) | − | + |
| 2616 | der(22)t(19;22)(q?;q13) | 22 | − | − |
| 9816-150 | − | t(19;22)(q13;q13) | − | − |
Identified from our database. The case was histologically diagnosed as follicular large cell lymphoma and was t(14;18)-negative.
Gains and losses of chromosomes
Resolution of all derived, addition, and marker chromosomes by SKY resulted not only in the detection of a 2-fold increase in the gains and losses but also in their correct identification. Fifty percent of deletions and 11% of translocations identified by G-banding were found to represent translocations and duplications, respectively, by SKY. Figure 4 shows the regions of partial- or whole-arm gains and losses identified by SKY. Gains (89%) were noted more frequently than losses (80%). While gains were noted equally in both treated and untreated groups (90% vs 88%), losses were more frequent in the treated group than the untreated (94% vs 72%). All the chromosomes were affected by gains. Chromosomes frequently involved in gains were 7 (39%), 1 (37%), 3 (35%), 12 (33%), 2 (30%), and 18 (28%). Region of common cytogenetic gain in these chromosomes comprised 7q11, 7q22, 1q11-23, 3q21-29, 12q13-15, 12q22-24, 2q21, 2p13-21, and 18q21-23. Most of the chromosomes also were affected by deletions, with del(6q) (35%) being the most frequent. Five regions of common cytogenetic deletions, in decreasing order of incidence, were observed: 6q23, 6q21, 6q25-27, 6q15, and 6q11-13. Other chromosomes involved in deletions were 2 (22%), 13q (17%), 1 (15%), and 17p (13%). The region commonly deleted on chromosome 13 was 13q22-32. Deletions affecting chromosomes 1 and 2 were highly heterogenous and involved both arms of each chromosome.
Idiogram showing distribution of gain and loss of genetic material.
The bars on the right indicate gains, and the bars on the left indicate loss.
Idiogram showing distribution of gain and loss of genetic material.
The bars on the right indicate gains, and the bars on the left indicate loss.
Correlation of the recurring breakpoints with clinical features
A correlation analysis of recurring breakpoints identified by SKY with clinical features was performed. Of 32 such sites, 5 showed a significant association with one of the clinical features (P ≤ .05): 7q11 with female, 3p21 with male, 3q27 with stage III-IV disease, 3q27 and 2q31 with more than 2 high or high-intermediate risk, and 2q31 and 7q22 with poor response to treatment (Table 7). Correlation with time to treatment failure and overall survival were not performed because the median follow-up was short.
Summary of significant correlations between the clinical features and recurring breakpoints
| Clinical feature . | Recurring site . | Present (%) . | Absent (%) . | P . |
|---|---|---|---|---|
| Female | 7q11 | 06/06 (100) | 19/40 (47) | .025 |
| Male | 3p21 | 05/05 (100) | 14/41 (34) | .008 |
| Stage III-IV | 3q27 | 11/13 (85) | 15/32 (47) | .024 |
| High or high-intermediate risk | 2q31 | 06/06 (100) | 23/36 (63) | .016 |
| 3q27 | 13/13 (100) | 17/30 (57) | .004 | |
| Poor response to treatment | 2q31 | 04/07 (57) | 06/36 (18) | .040 |
| 7q22 | 03/05 (60) | 07/38 (18) | .033 |
| Clinical feature . | Recurring site . | Present (%) . | Absent (%) . | P . |
|---|---|---|---|---|
| Female | 7q11 | 06/06 (100) | 19/40 (47) | .025 |
| Male | 3p21 | 05/05 (100) | 14/41 (34) | .008 |
| Stage III-IV | 3q27 | 11/13 (85) | 15/32 (47) | .024 |
| High or high-intermediate risk | 2q31 | 06/06 (100) | 23/36 (63) | .016 |
| 3q27 | 13/13 (100) | 17/30 (57) | .004 | |
| Poor response to treatment | 2q31 | 04/07 (57) | 06/36 (18) | .040 |
| 7q22 | 03/05 (60) | 07/38 (18) | .033 |
Discussion
DLBCL is a histologically well-defined entity that is clinically and genetically heterogeneous. Previous G-banding studies by us and others have shown that most cases are characterized by complex hyperdiploid karyotypes. The types of chromosomal rearrangements are diverse and include translocations, deletions, duplications, and several undefined aberrations such as additions and marker chromosomes.4,12 18-21 This study is the first reported to use SKY to fully characterize the chromosomal rearrangements in a panel of histologically reconfirmed DLBCL. In more than 90% of the cases, SKY provided additional cytogenetic information. Resolution of all the derived, addition, and marker chromosomes by SKY revealed several new recurring breakpoints, translocations, and regions of gains and losses.
By SKY, 13 new recurring translocation breakpoints (Xp11, 4p11, 4p14, 5q11, 11p11, 12p11, 16p11, 16q11-13, 17q11, 17q23, 18p11, 18q11, 20q11-13) were detected that were noted as unique occurrences by G-banding (this study and Cigudosa et al4). The frequency of 10 other recurring translocation breakpoints (1p11-13, 2p13, 2q31, 3p21, 7q11, 7q22, 12q11-13, 15q13-15, 17q11-21, 22q11) was higher in SKY analysis compared with G-banding (11%-17% vs 1%-5%).4,12,18-20 Based on G-banding analysis, we have previously shown that translocations involving 14q32 and 3q27 were the most frequent and together affected 50% of cases. Among these, the partner chromosomes involved remained unidentified in 5% to 15% of the cases due to poor morphology or karyotype complexity. SKY unambiguously identified all the partner chromosomes involved in these translocations, and this in turn enabled the detection of a new recurring translocation, t(3;14)(q21;q32). We have previously observed t(3;12)(q27;q22) (case 862) as nonrecurring in our larger G-banded series.4 The identification of a t(3;12)(q27;q22), designated by G-banding as t(3;12;14)(q29;q22;q32) in the present cohort (2541), makes this a recurring translocation.
The incidence of the known recurring translocations, t(3;14)(q32;q27), t(14;18)(q32;21), t(3;22)(q27;q11), and t(8;14)(q24;q32), was in keeping with the literature. The incidence of t(9;14)(p13;q32) was comparatively higher in the present cohort and may be due to case selection. Apart from detecting some of these recurring translocations with a higher frequency, SKY also enabled their correct identification. Rearrangements affecting bands 3q27 and/or 14q32 were absent in 39% of the cases. We have previously shown, by G-banding analysis, that this subset is characterized by deletions and numerous unidentified additions.4 SKY facilitated the complete cytogenetic characterization of this subset and identified 3 new recurring translocations, t(1;13)(p32;q14), t(1;7)(q21;q22), and der(6)t(6;8)(q11;q11). We also identified 2 potentially new recurring translocations in this group: t(5;16)(?;q11-12) and t(19;22)(q13;q11-13). A search of our database revealed another t(14;18)(q32; q21)-negative follicular large cell lymphoma with a t(19;22)(q13;q13).
Recurring chromosomal changes that lead to gain and/or loss of genetic material constitute important events in both disease transformation and progression.1,22 In this study, SKY identified gains and losses in more than 80% of the cases, a higher frequency of detection than by G-banding. Delineation of all the unbalanced translocations, additions, and marker chromosomes by SKY allowed us to narrow the regions of gain and loss in several chromosomes, namely, gain of 2p13-22, 2q21, 3q27-29, 9p11-24, 13q22-34, 18q21, and 19q11-13 and loss of 6q11-13 and 18p11-13. Although these regions have previously not been identified by G-banding, they have been delineated more precisely by comparative genomic hybridization.22-25 Interestingly, all the cases without translocations of 14q32 or 3q27 showed gains. Gain of 3 (53%), 7q (65%), and 18q21 (41%) and loss of 6q11-13 (59%) were significantly more frequent, suggesting that gain and/or loss of genetic material may play a more proximal role in the development of this subset.
Several studies have attempted to correlate cytogenetic findings with clinical features and/or patient outcome, and the results have been generally contradictory. Most studies failed to show an association between the recurring translocations t(3q27)/BCL6, t(14;18)/BCL2, and t(8;14)/MYC and clinical outcome.26-31 In some studies, changes such as trisomies of chromosomes 2/2p, 3/3p, 5, 6, or 18, monosomy 7, del(6q), abnormalities of 17 [−17, del(17p), i(17q)], and breaks or duplications of 1q have been shown to correlate with advanced stage disease and poor prognosis.13,19 32-36 In the present study, SKY analysis identified 3 sites (2q31, 3q27, 7q22) that significantly associated with advanced-stage disease or poor treatment response. Two of these, 2q31 and 7q22, have previously not been described by G-banding studies. However, we recognize that as interesting as these results are, they need to be confirmed on larger series of consecutively ascertained and uniformly treated DLBCLs. As shown by SKY in this study, most patients, even prior to treatment, harbored multiple genetic changes, making consistent correlations difficult.
The SKY studies reported here and the previously reported comparative genomic hybridization studies by us and others22,23 25present a picture of impressive genetic instability of DLBCL manifested at the cytogenetic level. Much of it remained undetected by conventional G-banding studies that most likely contributed to the conflicting reports in the literature, especially in the clinical correlation analyses. Therefore, the studies reported here emphasize the need for redefinition of chromosomal instability in DLBCL and other NHL subsets applying modern molecular cytogenetic techniques, together with conventional G-banding, for gene discovery as well as meaningful clinical correlations.
We thank Jane Houldsworth for careful reading of the manuscript and constructive criticism.
Supported by the National Cancer Institute grants CA34775, CA66999, and CA80814 (R.S.K.C.).
G.N. and P.H.R. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
R. S. K. Chaganti, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10021; e-mail: chagantr@mskcc.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal