Serotonin (5-HT), a well-known neurotransmitter of the central nervous system, has been implicated in diverse aspects of immune regulation. Here we show that 5-HT can efficiently drive programmed cell death in established Burkitt lymphoma (BL) lines that remain faithful to the original biopsy phenotype (group 1). Group 1 BL cells cultured in the presence of 5-HT exhibited marked suppression of DNA synthesis that was accompanied by extensive apoptosis—serotonin-driven apoptosis was complete within 24 hours, was preceded by early caspase activation, and was accompanied by a decline in mitochondrial membrane potential. BL cells that had drifted to a lymphoblastic group 3 phenotype were relatively resistant to these actions of serotonin, and the forced ectopic expression of either bcl-2 orbcl-xL provided substantial protection from 5-HT–induced apoptosis. 5-HT receptor antagonists (SDZ205-557, granisetron, methysergide) failed to inhibit serotonin-induced apoptosis, whereas the selective serotonin reuptake inhibitors (SSRI)—fluoxetine (Prozac), paroxetine (Paxil), and citalopram (Celexa)—substantially blocked the monoamine actions. Western blot analysis showed that BL cells expressed protein for the 5-HT transporter, and transport assays confirmed active uptake of serotonin by the cells. Unlike what was suggested for neuronal cells, there was no evidence that intracellular oxidative metabolites were responsible for the 5-HT–induced programmed death of BL cells. These data indicate that serotonin drives apoptosis in biopsylike BL cells after its entry through an active transport mechanism, and they suggest a novel therapeutic modality for Burkitt lymphoma.
Introduction
Outside the central nervous system, serotonin (5-HT) is produced mainly by enterochromaffin cells of the gut and is taken up by an active transport mechanism to a number of cell types, with platelets providing the richest reservoir of 5-HT in the periphery.1 Release of platelet-stored 5-HT can be rapid and triggered, for example, by platelet-activating factor, thrombin, C3a, C5a, and immunoglobulin (Ig)E-containing immune complexes. Thus, at sites of inflammation and platelet activation, local concentrations of 5-HT could be expected to greatly exceed the relatively low amounts normally found in serum.2-4 Moreover, primary and secondary lymphoid organs are innervated with noradrenergic nerve fibers that have the potential to accumulate serotonin and to release it on stimulation. Because the nerve terminals are in close contact with, for example, lymphocytes at these sites, this indicates that immune cells can be exposed directly to 5-HT flow.5
There are numerous reports of 5-HT modulating natural killer cell, macrophage, and T-cell function [reviewed in 5]. Of the 14 known receptor subtypes for 5-HT, mRNA for 8 of these was recently demonstrated in rat immune tissues.6,7 Particularly for the promotion of T-cell proliferation, a major target for serotonin action appears to be the 5-HT1A receptor, a property conserved between mammals and fish.8,9 B lymphocytes also carry this receptor subtype (among others) and, as with T cells, display NF-κB–dependent up-regulation of mRNA and protein for the 5-HT1A receptor.10 At least for rodent splenic B cells, this receptor subtype seems to be involved in the 5-HT–promoted augmentation of mitogenic responses to lipopolysaccharide and dextran sulfate.11 B cells additionally express the serotonin transporter (SERT) with Epstein-Barr virus (EBV)–transformed B-cell lines providing an important resource for genotyping polymorphisms in the promoter region of the transporter.1 12 However, no function has as yet been ascribed to B-cell SERT.
Monoamines, including 5-HT, have been reported to induce apoptosis in cultured neuronal cells, and cerebellar granule neurons are particularly sensitive.13 Indeed, serotonin-induced neuronal cell death has been implicated as a possible cause of neurodegenerative and neuropsychiatric disorders.14 We wanted to ask whether the neoplastic B cells of Burkitt lymphoma (BL), either through a receptor-mediated or an active transport mechanism, might be similarly encouraged to undergo apoptosis in response to serotonin. A long-recognized health problem in the malarial belts of equatorial Africa, northeastern Brazil, and Papua, New Guinea, the incidence of Burkitt lymphoma is increasing alarmingly outside these endemic regions because of its association with human immunodeficiency virus (HIV) infection. Among North Americans there is a 1000-fold increased incidence in patients with acquired immunodeficiency syndrome (AIDS), a trend likely to rise as these patients live longer through better management of their immune deficiency.15-17
Burkitt lymphoma, though a highly aggressive tumor, is nonetheless characterized by a predisposition to apoptosis, as evidenced by its classical starry sky histology.18 This almost defining feature of the malignancy echoes its germinal center (GC) origin, where a propensity for spontaneous apoptosis among the constituent cells within these regions of secondary lymphoid tissues reflects a need to select for high-affinity mutations that target the immunoglobulin V region genes in proliferating centroblasts.19-21 By necessity, GC B cells fail to express the prosurvival protein Bcl-2, a feature retained by BL cells in situ.22 Importantly, BL cell lines in early passage stay faithful to the biopsy phenotype. In addition to retaining GC B-cell markers, they remain Bcl-2 negative and can be prompted into accelerated apoptosis by a variety of signals, including those induced by antigen-receptor cross-linking.23 These so-called group 1 BL cell lines provide excellent models for investigating treatments that might shift the inherent imbalance between proliferation and apoptosis in this disease in favor of the latter. Group 2 and, eventually, group 3 lines develop on long-term passage of EBV-positive BL (that constitutes virtually all endemic but only a minority of nonendemic cases) because of pressure from de novo–expressed viral latent genes. Group 3 lines develop resistance to apoptotic signals caused partly by an induction of bcl-2 but also of other survival-promoting genes.23-25
We herein document serotonin as a rapid promoter of programmed cell death in BL lines that remain faithful to the original biopsy phenotype. This action of 5-HT is mediated through an active serotonin uptake mechanism and is the first report of SERT regulating function in a B-lineage cell. The serotonin transporter would appear to represent a novel therapeutic target in Burkitt lymphoma.
Materials and methods
Cell lines
Group 1 Burkitt lymphoma cell lines L3055 (EBVneg), BL2 (EBVneg), Elijah (EBVpos), and MUTU I (EBVpos) were maintained in early passage as described previously.26 The late-passage group 3 BL line, MUTU III (EBVpos), was also used with all cells cultured in RPMI 1640 medium supplemented with 10% Serum Supreme (BioWhittaker, Wokingham, United Kingdom), 2 mM glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin. Stable bcl-2 andbcl-xL transfectants of L3055 cells, together with those carrying the mammalian expression vector pEF-MC1 neopA alone as controls, were generated and characterized as detailed elsewhere.27 A stable line of HEK 293 cells carrying full-length human SERT has been described previously.28
Reagents
5-HT, fluoxetine (Prozac), pargyline, clorgyline, and deprenyl were purchased from Sigma (Dorset, United Kingdom). The 5-HT receptor antagonists SDZ205-557 and methysergide were from Sandoz (Basel, Switzerland), and granisetron was from Smith-Kline Beecham (Harlow, Essex, United Kingdom). 5-HT binoxalate, [1,2-3H(N)-] was obtained from NEN (Zaventem, Belgium). Paroxetine (Paxil) and citalopram (Celexa) are from Smith-Kline Beecham and Lundbeck (Copenhagen, Denmark), respectively. Mouse monoclonal anti-human SERT antibody was purchased from Mab Technologies (Stone Mountain, GA). Affinity-purified goat F(ab′)2 antibody fragment to human IgM was purchased from ICN Biomedicals (Cleveland, OH). JC-1 (5,5′,6,6′-tetrachlorol, 1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide) cationic dye was purchased from Molecular Probes (Leiden, Holland). Supersignal chemiluminescence reagent and horseradish peroxidase–conjugated rabbit anti-mouse antibody were from Pierce (Chester, United Kingdom). Syto 16 was from Molecular Probes Europe (Leiden, The Netherlands). All other chemicals were obtained from Sigma (Poole, United Kingdom) and were of the best grade available.
Assessment of DNA synthesis
DNA synthesis was determined by measuring3H-thymidine ([3H]Tdr; Amersham, Little Chalfont, United Kingdom) incorporation into cellular DNA.29 Cells were cultured in 200 μL supplemented medium at densities and for times indicated in the text in flat-bottomed, 96-well tissue culture plates (Becton Dickinson, Oxford, United Kingdom) and then were pulsed for the final 4 hours with 50 μL of 10 μCi (0.37 MBq)/mL [3H]Tdr before harvesting on a Skatron cell harvester (Helis Bio, Newmarket, United Kingdom).
Viability assays
Changes in the viability of cells cultured under conditions indicated in the text were quantified by assessment of forward and 90° (side) light scatter of cells, as described,30 using an EPICS XL-MCL flow cytometer (Beckman Coulter, Miami, FL). Before analysis, cells were harvested into polythene fluorescence cell sorter analysis (FACS) tubes and were fixed at 4°C in 400 μL FACS buffer (phosphate-buffered saline [PBS] with 5% NGS and 2% formaldehyde) [BDH/Merck]). Cells were gated in 2 populations (viable and dead) according to their forward and side light scatter.30Viability was expressed as a percentage of the total number of cells residing in the viable and nonviable gates. Cells were also analyzed according to Schuurhuis et al31 to assess the percentages within treated populations of those that were viable, apoptotic, or necrotic. Syto 16 was added at a concentration of 250 nM in PBS to 500 000 cells and was incubated at room temperature for 30 minutes, at which time 5 μg/mL propidium iodide (PI) was added. Samples were then analyzed immediately by FACS, and a 2-dimensional plot was generated of syto 16 fluorescence versus PI fluorescence. Syto 16 is taken up only by viable cells, whereas PI exclusively enters necrotic cells. Syto 16−ve/PI−ve cells are deemed apoptotic.31
Cell cycle analysis
Cells cultured under conditions indicated in the text were harvested into 100 μL 1× PBS followed by 400 μL cell cycle buffer containing Triton X-100 and PI and were left to incubate at 4°C for 1 hour to allow staining of DNA. The degree of fluorescence emitted by bound PI was directly proportional to the amount of DNA in each cell and was measured by flow cytometry as previously described.29 Results are expressed as percentage of viable cells residing in each phase of the cycle.
Measures of apoptosis
Apoptosis was assessed by staining cells with acridine orange and visualizing nuclear morphology exactly as described previously.27 Viable cells displayed a homogeneous chromatin-staining pattern, whereas apoptotic cells characteristically showed condensed and fragmented chromatin. Mitochondrial depolarization was assessed using the JC-1 cationic dye. Cells were incubated with 10 μM JC-1 for 30 minutes before they were visualized on an LSM 510 confocal microscope (Zeiss, Oberkochen, Germany). JC-1 exhibited potential-dependent accumulation in mitochondria accompanied by a shift in fluorescence emission from 525 nm (green) to 590 nm (red). Mitochondrial depolarization was indicated by a decrease in the red–green fluorescence ratio.
Detection of caspase activity
Caspase activity was detected using the CaspaTag Caspace Activity Kit (Intergen, Oxford, United Kingdom). Cells cultured under the conditions indicated in the text were harvested in 300 μL culture medium at a cell density of 106cells/mL, followed by the addition of 5 μL of a 30× working solution of FAM-VAD-FMK. Cells were then incubated for 1 hour at 37°C under 5% CO2, protecting the tubes from light. At the end of the incubation, they were washed twice in 2 mL 1× washing buffer, followed by the addition of 2 μL PI to distinguish dead cells from live cells, and then analyzed on an EPICS XL-MCL flow cytometer or by confocal microscopy. For the latter, treated cells were harvested in 300 μL culture medium containing 10 μL FAM-VAD-FMK before incubation for 1 hour under the conditions described above. Next, 1.5 μL Hoechst 33342 stain was added for 5 minutes, and cells were washed twice in 2 mL 1× washing buffer after the addition of 1 μL PI.
Detection of oxidative damage to DNA
Oxidative damage to DNA was quantified using the OxyDNA assay (Biotrin, Dublin, Ireland). After the culture of cells under conditions indicated in the text, they were washed first in 1× PBS, then in wash solution, and then by the addition of 100 μL blocking solution with a 1-hour incubation at 37°C. After 2 washes in working solution, cells were incubated with 100 μL FITC conjugate for 1 hour in the dark at room temperature before they were washed twice again in washing solution and once in PBS. Finally, cells were resuspended in FACS buffer and were analyzed on an EPICS XL-MCL flow cytometer.
Western blot analysis
Cell pellets were resuspended in 100 μL lysis buffer (25 mM Tris, pH 7.6, 150 mM NaCl, 1 μg/mL aprotonin, 10 μg/mL leupeptin, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 1 mM EDTA, and 1% Triton X-100). Samples were incubated for 1 hour at 4°C and were pelleted at high speed for 10 minutes at 4°C, and 100 μg protein per sample was subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis. SERT-transfected HEK cells were used as a positive control.28 After running, gels were equilibrated in transfer buffer (25 mM Tris, pH 8.3, 150 mM glycine, 20% (vol/vol) methanol), and proteins were transferred to polyvinylidene difluoride membranes. Blots were blocked in buffer (10 mM Tris, pH 7.5, 10 mM NaCl, 0.1% Tween 20) containing 5% (wt/vol) milk and were probed overnight at 4°C with a 1/5000 dilution of mouse monoclonal anti-SERT antibody. Blots were developed with a horseradish peroxidase–conjugated anti-mouse secondary antibody and were visualized using enhanced chemiluminescence.
5-HT uptake assay
Transport assays were performed by incubating 2 × 107 cells with 100 nM 5-HT plus 400 nM3H-5-HT for 0 to 60 minutes at 37°C in Hanks balanced salt solution containing 1% bovine serum albumin and 10 mM HEPES (pH 7.2). The assay was stopped by 5-fold dilution in ice-cold PBS. Cell pellets were resuspended in scintillation fluid, and the total counts per pellet were determined. Nonspecific uptake was assayed in parallel at 4°C under the same conditions. Data for nonspecific uptake were subtracted from the total uptake values to give the specific uptake.
Results
5-HT inhibits DNA synthesis in group 1 BL lines
The EBV-negative L3055 line maintained in early passage has been extensively characterized and widely used as an in vitro model for biopsylike BL cell behavior.26,27,29,30 32-36 In initial experiments, L3055 cells were treated with concentrations of 5-HT ranging from 1 to 150 μM, and their proliferative capacity was assessed by [3H]-thymidine incorporation at 24, 48, and 72 hours. Maximum effects were observed after 24 hours, and these data are shown in Figure 1A. It can be seen that 5-HT caused a reduction in DNA synthesis in a concentration-dependent manner, as evidenced by decreasing [3H]-thymidine incorporation with increasing concentrations of 5-HT.
5-HT promotes growth cessation in group 1 BL cells.
(A) L3055 BL cells plated at an initial density of 5 × 104/mL in flat-bottomed microwells, with concentrations of 5-HT ranging from 1 to 150 μM as indicated, cultured for 24 hours, and DNA synthesis (expressed as % control) assessed by [3H]Tdr incorporation over the last 4 hours. (B) As for panel A, but cells plated at different cell numbers indicated per 200 μL with 5-HT at 5 μM (■), 50 μM (○), 150 μM (♦). (C) Four group 1 BL lines (L3055, BL2, Elijah, and Mutu I) and one group 3 cell line (Mutu III) were treated with 125 μM 5-HT, as in panel A. (D) L3055 cells plated at 5 × 104/mL were cultured for 24 hours either alone (black histogram), with 5-HT at 150 μM (open histogram), or with anti-μ chain antibody at 10 μg/mL (shaded histogram), then were harvested for analysis of their cell cycle profile. All data are given as mean ± SEM from 8 separate experiments (A) and from 3 separate experiments (B-D).
5-HT promotes growth cessation in group 1 BL cells.
(A) L3055 BL cells plated at an initial density of 5 × 104/mL in flat-bottomed microwells, with concentrations of 5-HT ranging from 1 to 150 μM as indicated, cultured for 24 hours, and DNA synthesis (expressed as % control) assessed by [3H]Tdr incorporation over the last 4 hours. (B) As for panel A, but cells plated at different cell numbers indicated per 200 μL with 5-HT at 5 μM (■), 50 μM (○), 150 μM (♦). (C) Four group 1 BL lines (L3055, BL2, Elijah, and Mutu I) and one group 3 cell line (Mutu III) were treated with 125 μM 5-HT, as in panel A. (D) L3055 cells plated at 5 × 104/mL were cultured for 24 hours either alone (black histogram), with 5-HT at 150 μM (open histogram), or with anti-μ chain antibody at 10 μg/mL (shaded histogram), then were harvested for analysis of their cell cycle profile. All data are given as mean ± SEM from 8 separate experiments (A) and from 3 separate experiments (B-D).
Sensitivity to 5-HT was found to correlate with the number of cells plated. Thus, although more than 50 μM 5-HT was required to achieve 50% inhibition of DNA synthesis when cells were cultured at the relatively high plating density of 105/mL (2 × 104/well), when plated at 2.5 × 104/mL (5 × 103/well), 50% inhibition was achieved with approximately 5 μM 5-HT. Similarly, at a fixed 5-HT concentration of 50 μM 5-HT, approximately 35%, 70%, and greater than 90% inhibitions of DNA synthesis were achieved for cells plated at 2 × 104, 104, and 5 × 103/well, respectively (Figure 1B). Particularly in early passage, even 1 μM 5-HT could effect a significant reduction in the level of DNA synthesis otherwise occurring in L3055 cells at lower numbers (data not detailed).
Three other group 1 BL cell lines were investigated—Mutu I, Elijah, and BL2. When these were treated with a high concentrations (125 μM) of 5-HT for 24 hours of culture, a substantial decrease in DNA synthesis as measured by [3H]-thymidine incorporation was again noted. In contrast, Mutu III, a group 3 BL cell line, showed only marginally decreased DNA synthesis in response to 5-HT (Figure 1C). The unrelated T-cell leukemia line, Jurkat, was found to be insensitive to 5-HT actions (data not detailed).
It is well documented that the cessation of DNA synthesis can be achieved in group 1 BL cells through the engagement of BCR. Here, this is accompanied by growth arrest in the G0/G1compartment of cell cycle and subsequent entry into programmed death.23,27 36 We wanted to determine whether 5-HT might similarly encourage arrest during a distinct phase of the cell cycle. Figure 1D compares the cell cycle status of L3055 cells after 24-hour treatment with 5-HT or anti-μ chain antibody (to ligate the BCR). Each was used at its maximal concentration for inhibiting DNA synthesis and inducing cell death. Although BCR ligation exerted a greater influence on the cell cycle profile, each treatment resulted in an accumulation of arresting cells in the G0/G1compartment, with a corresponding disappearance from the S and the G2/M phases of cycle compared with control cultures.
5-HT–promoted inhibition of DNA synthesis in group 1 BL lines is accompanied by cell death
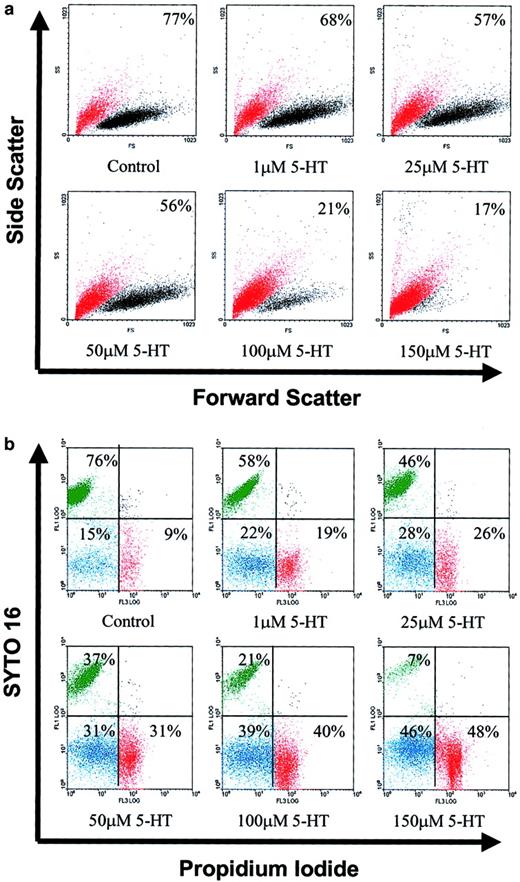
To ascertain whether serotonin-promoted cessation of DNA synthesis was accompanied by cell death, L3055 cells were again treated with 1 to 150 μM 5-HT and were cultured for 24 hours. At the end of incubation, we used FACS to analyze them for forward versus side scatter profiles. Cells were gated into 2 populations based on different light scatter properties, large live cells and shrunken dead cells. As seen from Figure 2A, 5-HT treatment resulted in a dramatic concentration-dependent increase in the number of L3055 cells acquiring light scatter characteristics of dead cells. The background presence of 20% to 25% dead cells is typical of early-passage BL lines maintaining biopsy traits. In an analogous set of experiments (on earlier passage cells), 5-HT–treated cells were double stained with syto 16 and PI. Syto 16 is taken up only by viable cells, whereas PI only enters cells whose membranes have become permeabilized. It can be seen from Figure 2B that the number of viable cells (syto 16+ve/PI−ve) decreased in a concentration-dependent manner in response to serotonin, with a trend similar to that assessed from changes in light scatter properties as described above. By the end of a 24-hour exposure to 5-HT, approximately equal numbers of cells had assumed characteristics of necrosis (syto 16−ve/PI+ve) versus apoptosis (syto 16−ve/PI−ve).
5-HT induces cell death in group 1 BL cells.
L3055 cells cultured at an initial density of 105/mL were treated with 1 to 150 μM of 5-HT for 24 hours, as indicated. At the end of the incubation, cells were analyzed as follows. (A) By forward (FS) versus side scatter (SS) analysis and gated into 2 different populations: viable (relatively high FS and low SS) cells (black dots) and dead (relatively low FS and high SS) cells (red dots). Percentage viable cells are indicated for each treatment. (B) By FACS analysis of styo 16–PI double-stained cells: syto 16+ve/PI−ve (viable) cells shown in upper left quadrant in green, syto 16−ve/PI−ve shown in lower left quadrant in blue, syto 16−ve/PI+veshown in lower right quadrant in red. Cells used in panel A were approximately at passage 40, whereas those for panel B were approximately at passage 35. All results shown are representative of 3 similar experiments.
5-HT induces cell death in group 1 BL cells.
L3055 cells cultured at an initial density of 105/mL were treated with 1 to 150 μM of 5-HT for 24 hours, as indicated. At the end of the incubation, cells were analyzed as follows. (A) By forward (FS) versus side scatter (SS) analysis and gated into 2 different populations: viable (relatively high FS and low SS) cells (black dots) and dead (relatively low FS and high SS) cells (red dots). Percentage viable cells are indicated for each treatment. (B) By FACS analysis of styo 16–PI double-stained cells: syto 16+ve/PI−ve (viable) cells shown in upper left quadrant in green, syto 16−ve/PI−ve shown in lower left quadrant in blue, syto 16−ve/PI+veshown in lower right quadrant in red. Cells used in panel A were approximately at passage 40, whereas those for panel B were approximately at passage 35. All results shown are representative of 3 similar experiments.
Characteristics of 5-HT–promoted apoptosis in group 1 BL lines
One of the changes that arose from the turn-on of viral latent genes as EBV-positive group 1 BL cell lines drifted to a group 3 phenotype on long-term culture was the induction of Bcl-2 expression.25 The finding that Mutu III cells were resistant to the actions of 5-HT when compared to their group 1 counterparts indicated that the expression of anti-apoptotic genes may, at least in part, be responsible for the difference observed. We directly investigated the role of survival genes on the 5-HT–dependent changes in BL cells by examining the influence of the monoamine on L3055 cells that had been stably transfected with eitherbcl-2 or bcl-xL and compared this with the effects on cells that were carrying the transfection vector alone.
It is evident from results presented in Figure3A that even with the highest concentration of 5-HT used in these studies, the forced expression of either Bcl-2 or Bcl-xL afforded substantial protection to L3055 cells from the antiproliferative actions of the monoamine when compared to those carrying vector alone. Staining of treated cells with the nuclear dye acridine orange revealed characteristic apoptotic morphology for 5-HT–treated L3055 cells, exemplified by chromatin condensation and nuclear fragmentation together with overt cell shrinkage. These changes failed to develop in Bcl-2–overexpressing cells exposed to 5-HT (Figure 3B).
Characteristics of 5-HT–induced apoptosis in group 1 BL cells.
(left) (A) L3055 cells transfected with either bcl-2,bcl-xL, or control vector were treated with 125 μM 5-HT and DNA synthesis (expressed as mean % control ± SEM of 3 experiments) was assessed exactly as for Figure 1A. (B) After 24 hours of culture, with control medium [(i), (iii)] or 125 μM 5-HT [(ii), (iv)], L3055 bcl-2-transfectants [(i), (ii)], and L3055 vector control cells [(iii), (iv)] were stained with the fluorescent nuclear dye acridine orange and were visualized by microscopy. Results are representative of 3 experiments. (right) L3055 cells were treated with control medium or with 150 μM 5-HT as indicated. (C) Cultured for 24 hours, stained with the cationic dye JC-1, and visualized by confocal microscopy. (D, E) Cultured for 6 hours, then stained with a fluorescent probe for active caspases (FAM-VAD-FMK). (D) Results obtained by flow cytometry (% cells active caspase positive indicated in top right corner). (E) Confocal microscopy analysis of cells counterstained with the Hoechst nuclear dye (blue), with PI to detect dead and membrane-compromised cells (red); activated caspases are stained green. Results are representative of 4 experiments.
Characteristics of 5-HT–induced apoptosis in group 1 BL cells.
(left) (A) L3055 cells transfected with either bcl-2,bcl-xL, or control vector were treated with 125 μM 5-HT and DNA synthesis (expressed as mean % control ± SEM of 3 experiments) was assessed exactly as for Figure 1A. (B) After 24 hours of culture, with control medium [(i), (iii)] or 125 μM 5-HT [(ii), (iv)], L3055 bcl-2-transfectants [(i), (ii)], and L3055 vector control cells [(iii), (iv)] were stained with the fluorescent nuclear dye acridine orange and were visualized by microscopy. Results are representative of 3 experiments. (right) L3055 cells were treated with control medium or with 150 μM 5-HT as indicated. (C) Cultured for 24 hours, stained with the cationic dye JC-1, and visualized by confocal microscopy. (D, E) Cultured for 6 hours, then stained with a fluorescent probe for active caspases (FAM-VAD-FMK). (D) Results obtained by flow cytometry (% cells active caspase positive indicated in top right corner). (E) Confocal microscopy analysis of cells counterstained with the Hoechst nuclear dye (blue), with PI to detect dead and membrane-compromised cells (red); activated caspases are stained green. Results are representative of 4 experiments.
Staining of monoamine-treated L3055 cells with carbocyanine liquid crystal forming probe JC-1 highlighted a loss of mitochondrial membrane potential accompanying the 5-HT–induced apoptosis of group 1 BL cells. Thus, after exposure to serotonin, there was a complete disappearance of the intense red emission from JC-1 aggregates, which, in control cells, form within mitochondria maintaining their membrane potential integrity. Instead, monoamine-treated cells developed a diffuse green emission that resulted from the disseminated distribution of relatively unconcentrated JC-1 monomers (Figure 3C).
Another hallmark of programmed cell death is caspase activation, though for some B lymphocyte subsets, caspase-independent routes to apoptosis have been described.37 38 To assess whether the caspase cascade was triggered by 5-HT in L3055 cells, we used the carboxyfluorescence probe FAM-VAD-FMK, which binds irreversibly to caspases in active configuration. Results illustrated in Figure3 demonstrated increased activation of caspases over background levels in L3055 group 1 BL cells by 6 hours of treatment with 5-HT. This can be seen both by FACS-based analysis (Figure 3D) and by laser-scanning confocal microscopy, whereby treated cells, counterstained by the Hoechst nuclear dye (blue) and with PI to reveal dead cells (red), exhibited green cytoplasmic staining for active caspases (Figure 3E).
Selective serotonin reuptake inhibitors inhibit actions of 5-HT on group 1 BL cells
The actions of 5-HT on neuronal cells and lymphocytes can be mediated through the many different receptor subtypes to be found on the surfaces of the target cells or through an active uptake mechanism that relies on the serotonin transporter.1,5,6 To explore which of these pathways was responsible for the observed effects of 5-HT on group 1 BL lines, L3055 cells were pretreated with a range of 5-HT receptor antagonists and selective serotonin reuptake inhibitors. Three receptor antagonists were tested: SDZ205-557, a 5-HT4receptor antagonist; granisetron, a highly selective 5-HT3receptor antagonist; and methysergide, which antagonizes 5-HT1A, 5-HT1B, 5-HT1D, 5-ht1E, 5-ht1F, 5-HT2A, 5-HT2B, 5-HT2C, 5-ht5A, 5-ht5B, 5-HT6, and 5-HT7receptors.6 All 3 receptor antagonists, at concentrations considerably higher than their affinities for the respective 5-HT receptor subtypes,6 and even when combined, failed to influence the ability of 5-HT to inhibit DNA synthesis in L3055 cells (Figure 4A). By contrast, 3 structurally distinct selective SSRIs—fluoxetine, citalopram, and paroxetine—each substantially reversed, in a concentration-dependent manner, the 5-HT–dependent cessation of DNA synthesis that was otherwise provoked (Figure 4B). Rank order and relative potencies for each of the SSRIs inhibiting 5-HT–promoted cessation of DNA synthesis in L3055 cells correlated well with their known IC50 values for blocking 5-HT uptake by neuronal SERT.39 The apparent failure of the SSRI to impart complete protection from 5-HT actions seemed to reflect (at least in part) a tendency to inhibit [3H]-thymidine incorporation in L3055 cells when used at increasingly higher concentrations (unpublished observations, Serafeim and Gordon, 2001).
Influence of receptor antagonists and selective serotonin reuptake inhibitors on 5-HT actions against group 1 BL cells.
(A) L3055 cells were pretreated for 30 minutes with the 5-HT receptor antagonists (SDZ205-557 [10 μM], granisetron [1 μM], methysergide [1 μM]), alone or in combination, before the addition of 125 μM 5-HT (+ 5-HT) or control medium (control), and DNA synthesis was assessed as in Figure 1A. (B) As for panel A, but cells were cultured with 150 μM 5-HT alone (0; striped bar) or with 5-HT after 30-minute pretreatment with the SSRI, fluoxetine (black bars), citalopram (white bars), or paroxetine (gray bars) at concentrations indicated. Results are expressed as % control (no addition) values. Data are represented as the mean ± SEM of 3 (A) or 4 (B) separate experiments.
Influence of receptor antagonists and selective serotonin reuptake inhibitors on 5-HT actions against group 1 BL cells.
(A) L3055 cells were pretreated for 30 minutes with the 5-HT receptor antagonists (SDZ205-557 [10 μM], granisetron [1 μM], methysergide [1 μM]), alone or in combination, before the addition of 125 μM 5-HT (+ 5-HT) or control medium (control), and DNA synthesis was assessed as in Figure 1A. (B) As for panel A, but cells were cultured with 150 μM 5-HT alone (0; striped bar) or with 5-HT after 30-minute pretreatment with the SSRI, fluoxetine (black bars), citalopram (white bars), or paroxetine (gray bars) at concentrations indicated. Results are expressed as % control (no addition) values. Data are represented as the mean ± SEM of 3 (A) or 4 (B) separate experiments.
The actions of the SSRI on 5-HT–dependent inhibition of DNA synthesis were reflected in their ability to reverse 5-HT–dependent cell death and caspase activation in L3055 cells. As seen from Table 1, optimal concentrations of each of the 3 SSRIs were able to block substantially the loss of viability arising from a 24-hour exposure to 5-HT, as judged by changes in forward versus side scatter properties of the cells. Similarly, 5-HT–induced caspase activation at 6 hours was abolished almost completely by pretreatment of cells with each SSRI studied (Table 1).
Reversal of 5-HT actions by SSRI
| Treatments* . | % Viable cells† (24 h) . | % Caspase activation‡ (6 h) . |
|---|---|---|
| Control | 69.3 ± 1.20 | 27.3 ± 1.20 |
| 5-HT | 21.3 ± 8.65 | 58.3 ± 2.03 |
| Fluoxetine + 5-HT | 54.7 ± 0.88 | 30.3 ± 2.91 |
| Citalopram + 5-HT | 55.3 ± 4.98 | 29.3 ± 1.33 |
| Paroxetine + 5-HT | 58.0 ± 3.06 | 29.3 ± 4.98 |
| Treatments* . | % Viable cells† (24 h) . | % Caspase activation‡ (6 h) . |
|---|---|---|
| Control | 69.3 ± 1.20 | 27.3 ± 1.20 |
| 5-HT | 21.3 ± 8.65 | 58.3 ± 2.03 |
| Fluoxetine + 5-HT | 54.7 ± 0.88 | 30.3 ± 2.91 |
| Citalopram + 5-HT | 55.3 ± 4.98 | 29.3 ± 1.33 |
| Paroxetine + 5-HT | 58.0 ± 3.06 | 29.3 ± 4.98 |
L3055 cells were cultured with or without 5-HT (150 μM), as indicated. SSRI: fluoxetine (5 μM), citalopram (10 μM), paroxetine (2 μM).
At the end of a 24-hour culture period with treatments shown, cells were analyzed by FACS for forward versus side light scatter properties, as in Figure 2. Percentage viable cells remaining are given as means ± SEM of 3 experiments.
After 6-hour treatment, L3055 cells were stained with FAM-VAD-FMK and analyzed for active caspases as for Figure 3. Results are given as percentage cells active caspase positive and are represented as means ± SEM of 3 experiments.
Evidence that 5-HT–induced apoptosis is not caused by oxidative stress
When 5-HT is transported into cells, it can be stored in vesicles until released or converted to 5-hydroxyindoleacetic acid (5-HIAA) by the mitochondrial enzyme monoamine oxidase (MAO).1 This enzyme exists in 2 forms, MAO-A and MAO-B.40 Because 5-HT catabolites are highly oxidative, we wanted to assess whether, as described for some neuronal cells, 5-HT signaled group 1 BL cells to undergo apoptosis by inducing oxidative stress.13 First, we explored the effect of pretreating cells with various concentrations of the relatively broad-acting MAO inhibitor, pargyline, before adding 5-HT. Even at the highest concentration of 100 μM, pargyline, which had no deleterious effect by itself, failed to influence 5-HT–induced cessation of DNA (Figure 5A). The same outcome was observed when using clorgyline and deprenyl, which display high-selectivity inhibition for the MAO-A and MAO-B isoforms, respectively40 (Figure 5B).
Evidence that 5-HT–induced apoptosis in BL cells is not due to oxidative stress.
(A) L3055 cells at 105/mL were treated with 10 to 100 μM of the MAO inhibitor pargyline in the presence of control medium or 125 μM 5-HT, as indicated. Cells were cultured for 24 hours in flat-bottomed microwells, and DNA synthesis was assessed by [3H]Tdr incorporation as for Figure 1A. (B) As for panel A, but cells were treated with concentrations indicated for the selective MAO-A and MAO-B inhibitors clorgyline and deprenyl, respectively. Data are represented as the mean ± SEM of 3 separate experiments. Note that cells used in panel A were of later passage (passages 40-45) than those in panel B (passages 60-65). (C) L3055 cells were cultured at 105/mL for 6 hours in control medium (histogram in bold), with 150 μM 5-HT (i) or 30 μM H2O2 (ii). OxyDNA-FITC conjugate was used to stain 8-oxodGuo residues on oxidatively damaged DNA. Data shown are representative of 3 identical experiments.
Evidence that 5-HT–induced apoptosis in BL cells is not due to oxidative stress.
(A) L3055 cells at 105/mL were treated with 10 to 100 μM of the MAO inhibitor pargyline in the presence of control medium or 125 μM 5-HT, as indicated. Cells were cultured for 24 hours in flat-bottomed microwells, and DNA synthesis was assessed by [3H]Tdr incorporation as for Figure 1A. (B) As for panel A, but cells were treated with concentrations indicated for the selective MAO-A and MAO-B inhibitors clorgyline and deprenyl, respectively. Data are represented as the mean ± SEM of 3 separate experiments. Note that cells used in panel A were of later passage (passages 40-45) than those in panel B (passages 60-65). (C) L3055 cells were cultured at 105/mL for 6 hours in control medium (histogram in bold), with 150 μM 5-HT (i) or 30 μM H2O2 (ii). OxyDNA-FITC conjugate was used to stain 8-oxodGuo residues on oxidatively damaged DNA. Data shown are representative of 3 identical experiments.
Although the above offers evidence that oxidative stress was not the cause of 5-HT–induced apoptosis in BL cells, we decided to examine more directly the generation of potential reactive oxygen species as a consequence of 5-HT uptake. For this we used a relatively new assay, which quantifies 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo) residues generated as a result of oxidative damage to DNA. In Figure 5C it can be seen that there was no increase in the level of oxidatively damaged DNA on treating L3055 cells with concentrations of 5-HT that otherwise effectively promoted apoptosis. By contrast, treating cells with 30 μM H2O2 (which also efficiently promoted apoptosis; data not detailed) resulted in a large increase in the level of 8-oxoguanosine residues generated.
Burkitt lymphoma cells express a functional serotonin transporter
Reversing 5-HT actions on group 1 BL cells by SSRI indicated that the monoamine exerts its effects through an active transport mechanism. Although the presence of SERT has been described for EBV-transformed B lymphoblasts and normal peripheral blood lymphocytes,1,12 41 there have been no previous reports of its existence or characteristics on lymphoma cells of any description.
Western blot analysis of cell lysates with an antibody raised to a unique sequence within SERT revealed the presence of protein migrating as a single band with an approximate apparent molecular weight of 70 kd in all BL lines used in this study (Figure6A). This ran faster than immunoblotted protein from HEK293 cells that had been transfected with human neuronal SERT; wild-type HEK293 cells were negative for immunoreactive protein. Alternative posttranslational modification of SERT in different cell types has been suggested to account for the varying size range reported for the transporter, including that of a prominent 60-kd species in some tissues.42,43 The Mutu III line, which was relatively resistant to the inhibitory actions of 5-HT (Figure 1C), nevertheless carried equivalent levels of SERT protein to its group 1 counterpart; fully resistant L3055 bcl-2 transfectants also retained high levels of SERT. Although the group 1 Elijah BL cells consistently displayed a lower level of SERT protein than the other BL lines, a demonstrable immunoreactive band was always present (Figure 6A). When assessed for first-order kinetics of 5-HT uptake, L3055 cells were shown to carry a transporter with properties similar to those previously described by one of us for full-length neuronal SERT44 (Figure 6B).
BL cells express the serotonin transporter.
(A) Western blotting analysis for the expression of human SERT protein in HEK 293 cells transfected with human SERT, HEK 293 wild-type cells, and BL lines. Upper band indicates ectopically expressed human neuronal SERT (apparent molecular weight of 90 kd). Lower arrow indicates the approximate 70-kd hSERT immunoreactive protein in BL cells. The blot shown is representative of 3 different experiments. (B) 5-HT uptake was assayed for 0 to 60 minutes at 37°C, at a substrate concentration of 500 nM, in 2 × 105 L3055 cells. Data are the mean ± standard error of 4 experiments.
BL cells express the serotonin transporter.
(A) Western blotting analysis for the expression of human SERT protein in HEK 293 cells transfected with human SERT, HEK 293 wild-type cells, and BL lines. Upper band indicates ectopically expressed human neuronal SERT (apparent molecular weight of 90 kd). Lower arrow indicates the approximate 70-kd hSERT immunoreactive protein in BL cells. The blot shown is representative of 3 different experiments. (B) 5-HT uptake was assayed for 0 to 60 minutes at 37°C, at a substrate concentration of 500 nM, in 2 × 105 L3055 cells. Data are the mean ± standard error of 4 experiments.
Discussion
Burkitt lymphoma is treated by aggressive combination chemotherapy, usually over a 6-month period and requiring frequent hospital stays.45 Although there is a 3-year relapse-free survival rate of approximately 80% for patients with local disease, patients with disseminated tumor respond less well to chemotherapy and have poor survival rates. Limited medical resources in regions in which BL is endemic also limit survival. In patients with AIDS, non-Hodgkin lymphomas tend to be aggressive and advanced at diagnosis. The median survival of HIV-positive patients with non-Hodgkin lymphomas is 6 months. Burkitt lymphoma comprises approximately 20% of such lymphomas and develops in some 2% of AIDS patients.46-48 Intensive chemotherapy is clearly not the most desirable course of treatment for immunosuppressed patients.
Whatever the epidemiology, BL is invariably characterized by a chromosomal translocation of the c-myc locus at the 8q24 region and one or other immunoglobulin gene loci. This most commonly (80% of patients) gives rise to the t8:14(q24;q32) translocation involving the immunoglobulin heavy chain gene.49 As a result of the translocation, c-mycgene expression is deregulated and, through its paradoxical behavior, likely contributes to the dichotomous biology of BL—aggressive, uncontrolled proliferation coupled with high-rate apoptosis.50 51 Although current therapies predominantly target the former, simpler interventions that encourage a shift toward the latter may be particularly beneficial to patients who are immunocompromised or for whom expensive drug regimens, with the attendant intensive hospitalization required, prove problematic. Our current findings indicate a novel attack on BL whereby a simple monoamine, serotonin, effectively redirects the balance in the neoplastic clone away from proliferation and toward apoptosis.
The study was prompted by a combination of numerous reports on 5-HT modifying immune cell behavior and others implicating serotonin in promoting programmed death of neuronal cell subsets.1,5,8-11,13 Moreover, B-lineage cells have been described as carrying a variety of 5-HT receptor subtypes and the 5-HT transporter, though with no function having been ascribed to the latter.1,5,12 Not only were we able to demonstrate high-rate serotonin-dependent apoptosis of biopsylike BL cells, we also showed that the action of the monoamine was most likely delivered through an active 5-HT transport mechanism. BL cells carried immunoreactive protein for SERT and displayed first-order uptake kinetics, similar to those previously described for the transporter.44 Importantly, the induction of BL cell apoptosis by 5-HT was substantially blocked in the presence of selective serotonin reuptake inhibitors, but not by 5-HT receptor antagonists, strongly implicating SERT as the monoamine target for promoting programmed death. Whether other relevant amines, such as histamine, norepinephrine, or dopamine, might also modify BL cell behavior, albeit by mechanisms independent of SERT, now clearly warrants attention.
For neuronal cells, oxidative metabolites of monoamines have been implicated in driving the apoptotic program.13 Although we do not want to fully rule out this mechanism for the 5-HT–induced apoptosis of BL cells, we failed to observe any reversal of the effect in the presence of monoamine oxidase inhibitors, nor could we detect consequences of reactive oxygen species as monitored by an assay designed to quantify 8-oxoguanosine residues generated de novo on damaged DNA. If the generation of reactive oxygen species was not responsible for serotonin-dependent apoptosis of BL cells, then clearly, alternatives must be considered. One possibility we are pursuing is that on monoamine binding, SERT itself stimulates signal transduction pathways that lead to the downstream activation of the apoptosis program. Precedents for the activation of SERT-dependent signaling pathways by 5-HT have been established.52,53 The involvement of the mitogen-activated protein kinase pathway is one focus for study in this regard.53 Another signaling component warranting attention is that of protein phosphatase 2A (PP2A), by which the catalytic subunit (PP2Ac) has been shown to associate with biogenic amine transporters in regulated heteromeric assemblies.54 Interestingly, functional SERT activity has been reported as a requirement for the induction of apoptosis in human placental choriocarcinoma cells by the appetite depressant, d-fenfluramine. As in our study on serotonin, the actions of this amphetamine analog could be blocked by fluoxetine.55
The ectopic expression of bcl-2 fully protected group 1 BL cells from 5-HT–induced apoptosis. This is of interest with regard to the above in that Lee and Shacter reported a failure of Bcl-2 to guard BL cells against oxidant-induced cell death.56 More important, all recirculating normal B lymphocytes carry high steady-state levels of Bcl-2.22 Indeed, we have found that even at the highest concentrations of 5-HT used in the current study, no deleterious effects resulted on B-cell viability or on the ability to respond to polyclonal activation (unpublished observations, Holder, Serafeim, and Gordon, 2001). However, it remains unclear whether the behavior of the physiological equivalents of group 1 BL, GC B cells, might be modified by 5-HT uptake. Secondary lymphoid tissues such as spleen have been shown to be rich in 5-HT, and their innervation by serotonergic neurons could provide high microenvironmental levels of the monoamine.1 5 We are investigating possible functions of SERT on normal GC B cells. The finding that Bcl-2 and Bcl-xL offered protection to BL cells from the effects of serotonin indicates that the range of tumors potentially susceptible to mononamine anti-proliferative and pro-apoptotic actions are limited, a finding borne out by the complete insensitivity of the Jurkat T-cell leukemia line in this regard, despite the fact that Jurkat cells carry readily detectable immunoreactive SERT (unpublished observations, Serafeim, 2001). The observation of SERT immunoreactivity even in nonresponsive cells means we can state unequivocally that any apoptosis observed must lie downstream of 5-HT uptake and arise from a cell-specific expression (or, rather, lack) of survival genes.
Although our studies indicate the 5-HT transporter as a novel therapeutic target in Burkitt lymphoma, it is tempting to speculate on a role for endogenous 5-HT in the development and progress of the disease. Although high levels of 5-HT were used for most of our studies so that effects could be assessed on sufficient numbers of cells, it was evident, even with concentrations as low as 1 μM, that a significant increase in apoptosis could be achieved, especially when using fewer cells in early passage. Basal levels of 5-HT in blood are lower than 100 nM but can systemically reach more than 1 μM during inflammation or ischemia.1 The upper limit possible in the local environment of a tumor is unknown, but, if innervated by serotonin-containing nerve fibers, it might be sufficient to influence the dynamics of the lymphoma. Admittedly fanciful, it is nonetheless intriguing to consider whether the intimate bidirectional flow that undoubtedly exists between the nervous system and the immune system57 58 could, by way of classical neurotransmitters such as 5-HT, alter the natural history of a lymphoid malignancy such as Burkitt lymphoma.
Finally, our demonstration of SERT as a potential target to treat Burkitt lymphoma affords an important challenge: the identification of a suitable pharmaceutical agent by which to deliver the therapeutic strategy. Clearly, 5-HT itself is not appropriate, but the known pharmacology of compounds such as d-fenfluramine59provides optimism that a feasible exogenous compound can be developed. Key to the identification of a candidate drug will be the elucidation of the precise molecular mechanism of 5-HT–induced apoptosis of biopsylike Burkitt lymphoma cells.
We thank Michelle Holder and Anita Challa for their help maintaining the cell lines, and Anne Milner and Rachel Chapman for invaluable assistance with the syto 16 assay.
Supported by a program grant from the Medical Research Council and by a grant from the National Institutes of Health (NIH DA07390). J.G. is an MRC Non-Clinical Research Professor.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John Gordon, MRC Centre for Immune Regulation, The University of Birmingham, Vincent Dr, Birmingham B15 2TT, United Kingdom; e-mail: j.gordon@bham.ac.uk.

![Fig. 1. 5-HT promotes growth cessation in group 1 BL cells. / (A) L3055 BL cells plated at an initial density of 5 × 104/mL in flat-bottomed microwells, with concentrations of 5-HT ranging from 1 to 150 μM as indicated, cultured for 24 hours, and DNA synthesis (expressed as % control) assessed by [3H]Tdr incorporation over the last 4 hours. (B) As for panel A, but cells plated at different cell numbers indicated per 200 μL with 5-HT at 5 μM (■), 50 μM (○), 150 μM (♦). (C) Four group 1 BL lines (L3055, BL2, Elijah, and Mutu I) and one group 3 cell line (Mutu III) were treated with 125 μM 5-HT, as in panel A. (D) L3055 cells plated at 5 × 104/mL were cultured for 24 hours either alone (black histogram), with 5-HT at 150 μM (open histogram), or with anti-μ chain antibody at 10 μg/mL (shaded histogram), then were harvested for analysis of their cell cycle profile. All data are given as mean ± SEM from 8 separate experiments (A) and from 3 separate experiments (B-D).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/7/10.1182_blood.v99.7.2545/6/m_h80722355001.jpeg?Expires=1767709481&Signature=rcBd4VrVMtS2Vke7SQYbGV49xa2Yu9TbOL9C2b0UAowKfThguC3vH73SViQ2kx~GTZrUXlH0DM9ZImcO2Fz~P~Al4RE9nMz4dFa2nLcA~P1NtJ2embbmNp5p8Fc2fidKxF-d6~ILOP9N3pCjjN3H7A1nWeWxp~UU-PrA41tK3~2oZUFtjR5giHkOQMYhFE~4VfRyJB4wWL~g1-T3-HaCB3HW2ed6n66fj7MXWDTVgC-i2u5AK6CF0d26WbzIsKqDKwwWMotRTHr~jfOjyKAg~ZU5yLtgho9Dg8fyPnrF2Y4FcAldN8HbYa7rU4fnA8YPM1VvNSBGvRjDdl04KqFnFA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Characteristics of 5-HT–induced apoptosis in group 1 BL cells. / (left) (A) L3055 cells transfected with either bcl-2,bcl-xL, or control vector were treated with 125 μM 5-HT and DNA synthesis (expressed as mean % control ± SEM of 3 experiments) was assessed exactly as for Figure 1A. (B) After 24 hours of culture, with control medium [(i), (iii)] or 125 μM 5-HT [(ii), (iv)], L3055 bcl-2-transfectants [(i), (ii)], and L3055 vector control cells [(iii), (iv)] were stained with the fluorescent nuclear dye acridine orange and were visualized by microscopy. Results are representative of 3 experiments. (right) L3055 cells were treated with control medium or with 150 μM 5-HT as indicated. (C) Cultured for 24 hours, stained with the cationic dye JC-1, and visualized by confocal microscopy. (D, E) Cultured for 6 hours, then stained with a fluorescent probe for active caspases (FAM-VAD-FMK). (D) Results obtained by flow cytometry (% cells active caspase positive indicated in top right corner). (E) Confocal microscopy analysis of cells counterstained with the Hoechst nuclear dye (blue), with PI to detect dead and membrane-compromised cells (red); activated caspases are stained green. Results are representative of 4 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/7/10.1182_blood.v99.7.2545/6/m_h80722355003.jpeg?Expires=1767709481&Signature=0g0X-esKc3nglXHSsrjeTDHRYggJraUZwcKAWwdZ2lUUZaQ5T7IZDE2az7QvyIrBAF2oSi3x4DhrOiyPqUWEs34Fe7CVZNkvaFsLsfWPuEeoQkFgtnfoo8bv7~jm-WvQVtaIAyjIYj2s-sRE3L6fdGf6sJRDw-lmGAmYepaiUqD6FwW~kzvEn-BDzFpND8k8gUw0PTarPafNcecC6Un53TgsikxFWdVSI6~VVqbMbvMoTiKhmlGCIPUlawuA5dhkOTd1XkPq8ZHAjI1lIuszWFpDjkpX8CYdCHYNmVC1loVJg1hg-25rEjkx1C10-LN63Tasi4NGTnnY1P1erhJT7A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Influence of receptor antagonists and selective serotonin reuptake inhibitors on 5-HT actions against group 1 BL cells. / (A) L3055 cells were pretreated for 30 minutes with the 5-HT receptor antagonists (SDZ205-557 [10 μM], granisetron [1 μM], methysergide [1 μM]), alone or in combination, before the addition of 125 μM 5-HT (+ 5-HT) or control medium (control), and DNA synthesis was assessed as in Figure 1A. (B) As for panel A, but cells were cultured with 150 μM 5-HT alone (0; striped bar) or with 5-HT after 30-minute pretreatment with the SSRI, fluoxetine (black bars), citalopram (white bars), or paroxetine (gray bars) at concentrations indicated. Results are expressed as % control (no addition) values. Data are represented as the mean ± SEM of 3 (A) or 4 (B) separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/7/10.1182_blood.v99.7.2545/6/m_h80722355004.jpeg?Expires=1767709481&Signature=dpEdGT5pWnvrBrihzJxe2WUd03gj5W7bijat9kbToUTKiiIqYKOGYZLd2EtGa4OlOfqd68LncrqapCeHOQlhk5mE9jyJxgwb9Xr4Rm9nevCW3yv3PDsZBWmVBlrVE6E21fM78w5KqL1lKSA4SFF4YXXy7WhajU6v7Ow-W0ALc4MukfUtG5pOmq9Q40XUJ94kWK0QUc-s5u9X0m8Bhz0CDGSLoonMLz8LRG64vFLaMl3i0gXec-OMwUr7TOza3Kycc9L-qbj5Doq03F4CR0M-Iq0ywG5TXYMOwvFoK-abHkO6quYzIUtKwwD2sxaPtAM9Z9RRAdI1WYo0NPmjM85RrA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Evidence that 5-HT–induced apoptosis in BL cells is not due to oxidative stress. / (A) L3055 cells at 105/mL were treated with 10 to 100 μM of the MAO inhibitor pargyline in the presence of control medium or 125 μM 5-HT, as indicated. Cells were cultured for 24 hours in flat-bottomed microwells, and DNA synthesis was assessed by [3H]Tdr incorporation as for Figure 1A. (B) As for panel A, but cells were treated with concentrations indicated for the selective MAO-A and MAO-B inhibitors clorgyline and deprenyl, respectively. Data are represented as the mean ± SEM of 3 separate experiments. Note that cells used in panel A were of later passage (passages 40-45) than those in panel B (passages 60-65). (C) L3055 cells were cultured at 105/mL for 6 hours in control medium (histogram in bold), with 150 μM 5-HT (i) or 30 μM H2O2 (ii). OxyDNA-FITC conjugate was used to stain 8-oxodGuo residues on oxidatively damaged DNA. Data shown are representative of 3 identical experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/7/10.1182_blood.v99.7.2545/6/m_h80722355005.jpeg?Expires=1767709481&Signature=X7by7swC59QyC3uRlXMSIyqis2omrvEO9~2dRDEneWBf8g4Lww3Mu9AUDbKFEjrpVx0BzFTzB8lhlWU8lENlrMEHTPKc~oSZL3GhQBe3niDRCzgqVz98zzetPFE0tRZJe7nTZ0gzbizHdLrfkyYDs32uXWL42hO9bt4WUwkdj-i2GsHcyBdavuROV73D0SQrEzNrkDdLUhApiGiFpnVCIlfuYZ7wTkWDhsGkZeqNNQx3f-IHIdE0jMtYLQrENH0HgBMj45Wy6WLuIV6LkahKkV1uINtx8kYzANjiHYnkxDMNtNu0JxXJzoIqjC4Ajg-61RpvdwNMT6Qv-pr2lmUdqw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal