Abstract
We studied the effects of administration of several cytokines, including progenipoietin-1 (ProGP-1), Flt-3 ligand (FL), granulocyte colony-stimulating factor (G-CSF), and granulocyte-macrophage colony-stimulating factor in a pegylated form (pGM-CSF), on dendritic cell (DC) populations in mouse spleen. ProGP-1 produced the most striking increase in overall DC numbers, apparently more than its constituent FL and G-CSF components. However, the expansion in DC numbers was strongly subpopulation selective, with ProGP-1 and FL producing selective expansion of CD8+ DCs, whereas pGM-CSF produced selective expansion of CD8− DCs. Surprising differences were observed between the effects of murine and human recombinant FL preparations on murine DCs. Many of the biologic functions of the DC subpopulations expanded by cytokines remained intact, including the capacity of the ProGP-1– and FL-expanded CD8+ DCs to produce the T-helper-1–biasing cytokine interleukin 12 (IL-12). However, the expanded DCs from all but G-CSF–treated mice were deficient in the ability to make interferon γ, and the CD8+ DCs produced with pGM-CSF treatment had an abrogated capacity to form bioactive IL-12. Such selective expansion of DC populations and alterations in their cytokine-secretion capacity have implications for clinical use of the studied cytokines in immune modulation.
Introduction
Dendritic cells (DCs) are potent antigen-presenting cells with a unique ability to activate naive T cells.1,2They are distributed throughout lymphoid and nonlymphoid tissues but are relatively rare, constituting less than 1% of spleen cells. DCs show heterogeneity in surface phenotype, origin, and some functional characteristics.3-8 We previously identified 3 distinct DC populations, delineated by the expression of CD4 and CD8 (CD4+CD8−, CD4−CD8−, and CD4−CD8+), in spleens of normal mice.4 These 3 DC populations appear to derive from separate developmental streams, as indicated by their different kinetics of bromodeoxyuridine uptake and lack of evidence of a precursor-product relationship among them.9 Functional variations among the splenic DCs include differences in cytokine production: CD8+ DCs are the principal producers of interleukin-12 (IL-12)10,11 and interferon-α (IFN-α),11 whereas CD4−CD8−DCs are the principal producers of IFN-γ.11
DCs are candidate targets for immune system modulation, particularly for immunotherapy of tumors. One strategy used to target DCs specifically is ex vivo manipulation of these cells, such as loading DCs with tumor antigens before administration to the patient.12,13 Another strategy is in vivo administration of cytokines that selectively increase DC numbers. Cytokines that have been found to increase DC numbers dramatically in mice include Flt-3 ligand (FL), used alone14,15 or with CD40L,16and granulocyte-macrophage colony-stimulating factor (GM-CSF) in a polyethylene glycol–conjugated or pegylated form (pGM-CSF) that extends its life span in the circulation.17,18 In humans, DC numbers were increased by administering FL19 or granulocyte colony-stimulating factor (G-CSF).20 In addition, progenipoietin 1 (ProGP-1), an agonist of the Flt-3 and G-CSF receptors, markedly enhanced DC numbers in mice.21However, the various DC populations are not absolutely dependent on GM-CSF or FL for development. In the absence of GM-CSF receptor or GM-CSF itself, little change occurs in the number or phenotype of splenic DCs.18,22 Although DC numbers are reduced in FL knockout mice, DCs of both CD8− and CD8+subsets are still present.23
If expansion of DC populations by in vivo cytokine administration is to be exploited for therapy, it is essential to understand the changes in DC subpopulation balance and the characteristics of expanded DCs relative to those of normal DCs. Accordingly, we compared the phenotype and function of DCs expanded by ProGP-1, FL of mouse (muFL) and human (huFL) origin, G-CSF, FL together with G-CSF, and pGM-CSF. We found that certain cytokines induced marked differential expansion of functionally distinct DC subsets and that some cytokines caused changes in the cytokine-secretion capacity of these DCs.
Methods
Mice and in vivo administration of cytokines
All mice were bred under specific-pathogen–free conditions at the Walter and Eliza Hall Institute (WEHI) animal-breeding facility. For studies in which cytokines were administered, male C57BL/6J WEHI mice 6 to 9 weeks of age were used. Mice were injected (subcutaneously at the nape of the neck) once daily with cytokines in phosphate-buffered saline (PBS) containing mouse serum albumin (MSA; 1 μg/mL). PBS containing MSA alone was used in control injections (untreated mice). Male CBA/J mice 7 to 10 weeks of age were the sources of responder T cells.
Cytokines
For in vivo administration, ProGP-121 and recombinant (r) huFL were provided by Pharmacia (St Louis, MO), pGM-CSF by Immunex (Seattle, WA), and human rG-CSF by Dr A. Roberts (WEHI) and Kirin Brewery (Takasaki, Japan). The rmuFL was produced from a Chinese hamster ovary cell line provided by Professor N. Nicola (WEHI) and purified in our laboratory. The murine rGM-CSF and murine rIL-4 used for in vitro DC stimulation were gifts from Immunex. Rat rIFN-γ (bioactive in mice) and murine rIL-18 were purchased from PeproTech (Rocky Hill, NJ). Murine rIL-12 p70 was purchased from R&D Systems (Minneapolis, MN).
DC stimulants
Fixed and heat-killed Staphylococcus aureus (SAC; Pansorbin) was purchased from Calbiochem-Novabiochem (Alexandria, Australia). Oligonucleotides containing a fully phosphorothioated (ph) CpG motif were synthesized by GeneWorks (Adelaide, Australia) according to a published sequence (CpG166824).
Isolation of DCs
DCs were isolated as described previously.4Briefly, spleen fragments were digested for 20 minutes at room temperature with collagenase and deoxyribonuclease and then treated for 5 minutes with EDTA to disrupt T-cell–DC complexes. Light-density cells were selected by centrifugation over Nycodenz medium (1.077 g/cm3, murine osmolarity). When DCs were purified from mice treated with FL or ProGP-1, the procedure was modified and 10 mL Nycodenz was used for each spleen equivalent to avoid overloading the separation medium. Cells not of DC lineage were depleted by first incubating the cells with titrated levels of anti-CD3 (KT3), anti–Thy-1 (T24/31.7), anti-B220 (RA3-6B2), anti–Gr-1 (RB6-8C5), and antierythrocyte (Ter-119) monoclonal antibodies (mAbs). The antibody-coated cells were then removed by incubation with sheep anti–rat IgG–coupled magnetic beads (Dynabeads M-450; Dynal, Oslo, Norway) or goat anti–rat IgG–coupled magnetic beads (Paesel and Lorei, Duisburg, Germany). The DCs prepared in this way were at least 85% pure at this stage. Immunofluorescent labeling and then sorting or gating during flow cytometry were used to complete purification of the DCs.
Immunofluorescent labeling of DCs
The mAbs, fluorescent conjugates, and multicolor labeling procedures were all described previously.4 To identify and sort all DCs, the pan-DC markers used were high levels of class II major histocompatibility complex (MHC), CD11c, or both, together with high forward light scatter. Anti-CD11c (N418) was used as a Cy5 or fluorescein isothiocyanate (FITC) conjugate. Anti–class II MHC (N22 or M5/114) was used as a FITC or Alexa 594 conjugate; conjugation levels were deliberately kept at less than maximal to ensure that the strong staining for class II MHC at saturation did not cause inaccurate color-compensation problems in other channels. The markers used to separate the splenic DC subpopulations were CD8α and CD4. Anti-CD8α (YTS169.4) was used as a phycoerythrin (PE) or Cy5 conjugate and anti-CD4 (GK1.5) as a PE or Alexa 594 conjugate. The staining for costimulator molecules employed anti-CD80 (16-10A1), anti-CD86 (GL1), and anti-CD40 (FGK45.5), used as FITC conjugates. Anti–DEC-205 (NLDC-145) and anti-CD11b (M-170) were used as FITC conjugates. Propidium iodide (PI; 1 μg/mL) was included in the final wash after immunofluorescent staining to label dead cells.
Flow cytometric analysis and sorting of DCs
Most analyses were done on a fluorescence-activated cell-sorter scanner (FACStar Plus; Becton Dickinson, San Jose, CA), as described previously,4 by using up to 4 fluorescent channels for the immunofluorescent staining (FL1 for FITC, FL2 for PE, FL3 for Cy5, and FL4 for Alexa 594) with the FL5 channel set to exclude PI-positive dead cells and autofluorescent cells. During gating, care was taken to ensure that any cells brightly fluorescent in FL3 and spilling over into FL5 were not gated out as dead cells. A MoFlo instrument (Cytomation, Fort Collins, CO) was used to sort DC populations. To sort or gate for DCs, the class II MHC and CD11c markers, together with the forward and side scatter gates, were set to select for cells with DC characteristics. The expression of CD4 and CD8 was used to segregate DC subpopulations (CD8+CD4−, CD8 intermediate [int] CD4−, CD8−CD4−, and CD4+CD8−). Reanalysis of sorted DC populations was done, and only DCs with a purity of greater than 95% were used for further functional analyses.
Stimulation of DCs for cytokine production
Sorted splenic mouse DCs (0.5-2 × 106/mL) were cultured in 96-well round-bottomed plates in modified RPMI-1640 medium containing 10% fetal-calf serum (FCS) at 37°C in an atmosphere of 10% carbon dioxide (CO2) in air, as described previously.25 The culture supernatant was collected, separated from the cells by centrifugation, and stored at −20°C until analysis. Conditions previously established for optimum production of each cytokine were used.11 For production of IL-12, the stimulation mixture was GM-CSF (200 U/mL), IFN-γ (20 ng/mL), CpG-ph (0.5 μM), or SAC (10 μg Pansorbin/mL) in the presence or absence of IL-4 (100 U/mL). Incubation time was 16 to 24 hours. For production of IFN-γ, IL-12 and IL-18 (20 ng/mL each) were used for stimulation and the incubation time was 3 or 4 days.
Analysis of IL-12 and IFN-γ by enzyme-linked immunosorbent assay
Aliquots of DC culture supernatants were assessed by a 2-site enzyme-linked immunosorbent assay (ELISA) as previously described.11 29 Briefly, 96-well polyvinyl chloride microtiter plates (Dynatech Laboratories, Chantilly, VA) were coated with an appropriate purified capture mAb, namely R2-9A5 (anti–IL-12 p70; hybridoma obtained from the American Type Culture Collection [ATCC], Manassas, VA), C15.6 (anti–IL-12 p40; PharMingen, San Diego, CA), or R4-6A4 (anti–IFN-γ; ATCC). Cytokine binding was then detected with an appropriate biotinylated detection mAb, namely R1-5D9 (anti–IL-12 p40; ATCC), C17.8 (anti–IL-12 p40; hybridoma provided by L. Schofield, WEHI), or XMG1.2 (anti–IFN-γ; ATCC).
Allostimulatory mixed leukocyte reaction
CD4+ and CD8+ T cells were purified from pooled mesenteric, axillary, brachial, and inguinal lymph nodes of CBA/J mice. Briefly, cell suspensions from lymph nodes were obtained by passing the organs through a fine mesh sieve. Cells were washed with PBS containing 2% FCS. The cells were then depleted of cells other than T cells by incubation with a mixture of the following antibodies: Ter119, RA3-6B2, M1-70, RB6-6CS, and either anti-CD8 (53.6.7) or anti-CD4 (GK1.5). Antibody-coated cells were removed by incubation with sheep anti–rat IgG magnetic beads (Dynal). The CD4+ or CD8+ T cells (98% and 96% pure, respectively) were washed, suspended in culture medium (modified RPMI-164025containing 10% FCS), and counted. Replicate culture trays were incubated at 37°C in 10% CO2 in air for 2.5 to 5.5 days. On days 2.5, 3.5, 4.5, and 5.5, a culture tray was pulsed with tritium-thymidine (1 μCi [0.0185 MBq]/well) for 6 hours and then frozen. The trays were thawed, the cells were harvested on glass fiber filters, and the amount of thymidine incorporated was measured by using liquid scintillation. All cultures (all time points) were done in triplicate or greater, and background controls with T cells only were included for each time point.
Results
Dose response of ProGP-1
One aim of this study was to compare the effect on DC expansion of ProGP-1, an agonist of the Flt-3 and G-CSF receptors, with that of the individual cytokines FL, G-CSF, and pGM-CSF. The optimal dose of injected FL (10 μg/day for 10 days) and pGM-CSF (2 μg/day for 5 days)14,17 18 for normal enhancement of DCs in mouse spleens was previously determined by others, and these regimens also worked well in our laboratory. To establish the optimal dose and duration of ProGP-1 administration, mice were injected with 10, 20, 50, or 100 μg ProGP-1/day for either 7 or 10 days, and splenic DCs were isolated and counted. The optimal regimen was found to be 20 μg/day for 10 days, which increased DC numbers to about 5 × 107extracted DCs/spleen. Higher doses of ProGP-1 provided similar or occasionally higher DC yields but were associated with complications caused by excessive hematopoiesis. For example, some mice had such marked expansion of vertebral bone marrow that hematopoietic cells encroached on regional peripheral nerves, nerve roots, and the spinal cord. In some of these mice, paresis or paralysis developed. All mice given ProGP-1 had enlarged spleens (weight increase ∼5-fold) and lymph nodes. Serum samples from these mice were examined for anti–ProGP-1 antibodies by ELISA (data not shown). Mice that received 1 course of treatment (10 days) or 2 courses of treatment (10 days each with a 2-week break between them) had anti–ProGp-1 serum titers that were 2-fold higher than those of untreated controls (mean titer, 1:512 versus 1:256; 5 mice/group). Moreover, mice that received 2 courses of ProGP-1 treatment had a normal response to the cytokine treatment. Thus, there was little evidence suggesting that the ProGP-1 chimeric cytokine was itself immunogenic in vivo.
Effect of ProGP-1 treatment on mouse spleen DCs
To investigate the effect of ProGP-1 on murine DCs, groups of mice were injected with 20 μg ProGP-1/day for 10 days and splenic DCs were isolated and analyzed for various surface markers by flow cytometry. The results were compared with those in untreated control mice and in mice given both FL and G-CSF (10 μg of each/day); muFL was used initially to provide the best control for effects on DCs in murine spleen. The total numbers of splenic DCs of the main subtypes after these treatments are shown in Table 1. Results of the flow cytometric analysis of the surface phenotypes of the DCs after treatment with ProGP-1 are provided in Figure1.
Effect of treatment with cytokines on number of dendritic cells (DCs) in mouse spleens
| DC subset . | Cytokine used . | ||||||
|---|---|---|---|---|---|---|---|
| None . | ProGP-1 (20 μg/d) . | G-CSF (10 μg/d) . | muFL (10 μg/d) . | huFL (10 μg/d) . | G-CSF + muFL (10 μg/d) . | pGM-CSF (2 μg/d) . | |
| Total DCs | 1.60 | 53.4 | 3.6 | 15.0 | 38.7 | 18.8 | 19.0 |
| CD4+CD8− | 0.75 | 0.9 | 1.5 | 1.8 | 4.3 | 1.4 | 9.9 |
| CD4−CD8+ | 0.35 | 45.4 | 1.0 | 9.5 | 17.0 | 13.5 | 2.0 |
| CD4−CD8− | 0.46 | 4.3 | 0.8 | 2.1 | 16.6 | 2.6 | 6.7 |
| DC subset . | Cytokine used . | ||||||
|---|---|---|---|---|---|---|---|
| None . | ProGP-1 (20 μg/d) . | G-CSF (10 μg/d) . | muFL (10 μg/d) . | huFL (10 μg/d) . | G-CSF + muFL (10 μg/d) . | pGM-CSF (2 μg/d) . | |
| Total DCs | 1.60 | 53.4 | 3.6 | 15.0 | 38.7 | 18.8 | 19.0 |
| CD4+CD8− | 0.75 | 0.9 | 1.5 | 1.8 | 4.3 | 1.4 | 9.9 |
| CD4−CD8+ | 0.35 | 45.4 | 1.0 | 9.5 | 17.0 | 13.5 | 2.0 |
| CD4−CD8− | 0.46 | 4.3 | 0.8 | 2.1 | 16.6 | 2.6 | 6.7 |
Values are the number of DCs/per spleen × 106. Treatment was done for 10 days (d), except for that with pegylated granulocyte-macrophage colony-stimulating factor (pGM-CSF), which was done for 5 days. Values are from 1 experiment representative of at least 3 experiments using a minimum of 3 mice for each cytokine treatment and for untreated controls.
ProGP-1 indicates progenipoietin-1; G-CSF, granulocyte colony-stimulating factor; muFL, murine Flt-3 ligand; and huFL, human Flt-3 ligand.
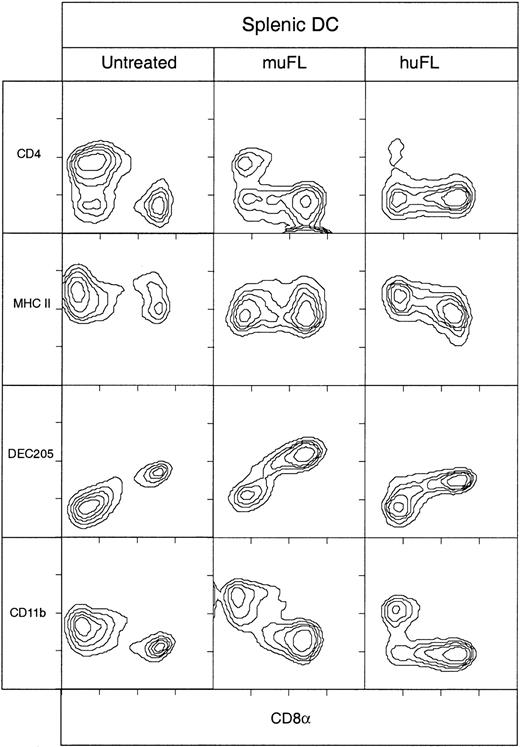
Surface phenotype of splenic DCs from mice treated with ProGP-1.
DCs isolated from spleens of C57BL/6 mice that were either untreated or treated for 10 days with ProGP-1 (20 μg/day) were immunofluorescence stained with antibodies directed to typical murine DC markers and costimulation molecules. The DCs were analyzed by gating on high forward light scatter and high levels of expression of CD11c, MHC class II, or both. Data are representative of more than 10 experiments, all of which examined pooled splenic DCs from at least 3 mice.
Surface phenotype of splenic DCs from mice treated with ProGP-1.
DCs isolated from spleens of C57BL/6 mice that were either untreated or treated for 10 days with ProGP-1 (20 μg/day) were immunofluorescence stained with antibodies directed to typical murine DC markers and costimulation molecules. The DCs were analyzed by gating on high forward light scatter and high levels of expression of CD11c, MHC class II, or both. Data are representative of more than 10 experiments, all of which examined pooled splenic DCs from at least 3 mice.
ProGP-1 treatment increased the total DCs per spleen about 30 fold. This increase was heavily biased toward CD8+ DCs, which increased 130 fold. The CD8+ DC population was composed of CD8-high (hi) cells, as well as many CD8intcells. The CD8int DCs could be separated as a distinct population not only by their level of CD8 expression but also by their forward-scatter profile, which was intermediate between that of CD4−CD8− DCs and that of CD4−CD8+ DCs (data not shown). Although the absolute number of DCs increased and the subset balance changed, the surface phenotype of these subsets with respect to basic DC markers and activation markers was unchanged. Thus, all CD8+ DCs were DEC-205+ CD11b−CD4−, whereas the CD4+CD8− DCs and the CD4−CD8− DCs were all DEC-205−CD11b+ (Figure 1). The mixture of FL and G-CSF also increased total DC numbers, with a similar bias toward CD8+DCs, but the effects were much less pronounced than with ProGP-1.
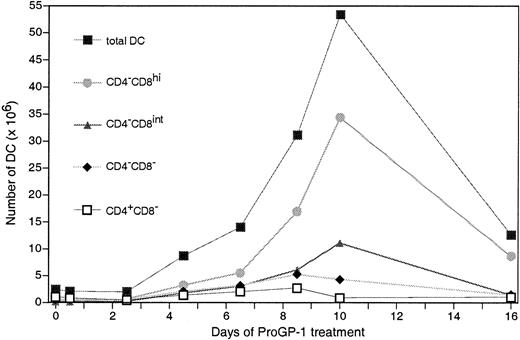
Kinetics of DC response to ProGP-1
We previously showed that the 3 main splenic DC subsets (CD4+CD8−, CD4−CD8−, and CD4−CD8+) have different turnover rates and independent developmental kinetics.9 To determine whether the selective enhancement of CD8+ DCs after ProGP-1 treatment occurred throughout the response, DCs from mouse spleens were isolated, enumerated, and analyzed throughout the 16 days of ProGP-1 treatment (Figure 2). We found that the DCs in each subset had increased in size, as assessed by forward light scatter, as early as 12 hours after ProGP-1 injection (data not shown). Moreover, as early as 2.5 days after ProGP-1 treatment began, the CD8+ DCs had become the principal subset in the spleen. By day 4.5 of treatment, the number of CD8hi DCs had increased 7 fold and the number of CD8int DCs had increased 11 fold. CD8+ DCs then remained the dominant subtype, showing an increase of about 130 fold at the day-10 peak. In contrast, CD4−CD8− DCs and CD4+CD8− DCs had maximal increases of 8 fold and 2.5 fold, respectively, on day 8.5. After 16 days of ProGP-1 treatment, the numbers of CD8− DCs were close to those in untreated mice. The numbers of CD8+ DCs were still elevated (by about 16 fold), although the maximal effects of ProGP-1 on CD8+ DC numbers were observed on about day 10 of treatment. The surface markers shown in Figure 1 remained unchanged throughout the treatment (data not shown).
Increase in splenic DCs during ProGP-1 treatment.
CD8+ DCs constituted the principal DC population after 2.5 days of treatment. DCs were isolated from spleens of mice that were either untreated or treated with ProGP-1 for 0.5 to 16 days. The DCs were gated on high forward scatter and high expression of CD11c and MHC class II and analyzed for CD4 and CD8 expression; then the number of DCs in each subset was recorded. Data are from 1 experiment but findings at each time point were analyzed on at least 3 occasions, with similar results.
Increase in splenic DCs during ProGP-1 treatment.
CD8+ DCs constituted the principal DC population after 2.5 days of treatment. DCs were isolated from spleens of mice that were either untreated or treated with ProGP-1 for 0.5 to 16 days. The DCs were gated on high forward scatter and high expression of CD11c and MHC class II and analyzed for CD4 and CD8 expression; then the number of DCs in each subset was recorded. Data are from 1 experiment but findings at each time point were analyzed on at least 3 occasions, with similar results.
Effect of rmuFL and rhuFL on mouse spleen DCs
To provide a direct comparison with ProGP-1, the effect of FL alone on DCs in mouse spleens was determined. Initially, an rmuFL preparation derived from a mammalian cell line was used because we wanted to compare the effect of ProGP-1 with that of the normal ligand of the murine Flt-3 receptor. As shown in Table 1 and Figure3, muFL treatment increased DC numbers about 11 fold, and this increase was heavily biased toward CD8+ DCs, which increased 32 fold. Thus, the effect of FL was similar in type, though not in magnitude, to that of ProGP-1.
Surface phenotype of splenic DCs from mice treated with FL.
DCs isolated from spleens of C57BL/6 mice that were either untreated or treated for 10 days with muFL or huFL (10 μg/day) were immunofluorescence stained with antibodies directed to typical murine DC markers. The DCs were analyzed by gating on high forward light scatter and high levels of expression of CD11c. The data are representative of more than 5 experiments that all examined pooled splenic DCs from at least 3 mice.
Surface phenotype of splenic DCs from mice treated with FL.
DCs isolated from spleens of C57BL/6 mice that were either untreated or treated for 10 days with muFL or huFL (10 μg/day) were immunofluorescence stained with antibodies directed to typical murine DC markers. The DCs were analyzed by gating on high forward light scatter and high levels of expression of CD11c. The data are representative of more than 5 experiments that all examined pooled splenic DCs from at least 3 mice.
This result, however, differed from findings in previous studies in which a more extensive and more balanced enhancement of both the CD8− and CD8+ subsets was obtained by using an rhuFL.14 17 Therefore, in the current study, we also administered rhuFL to mice for 10 days. The huFL provided a much greater increase in total DC numbers than the muFL (Table 1) and produced about an equal enhancement of CD4−CD8− and CD4−CD8+ DCs (Figure 3), results more similar to data in previous studies. The CD4+CD8− DCs were increased about 6 fold by huFL treatment, a larger increase than observed with either muFL or ProGP-1. CD8int DCs were present but to a lesser extent than after muFL or ProGP-1 treatment. The CD4−CD8− DC population after huFL treatment was unusual in that nearly half of these cells expressed DEC-205 rather than their all being DEC-205− as in the control mice or the mice given muFL or ProGP-1 (Figure 3). These DEC-205+CD4−CD8− DCs did not express CD11b.
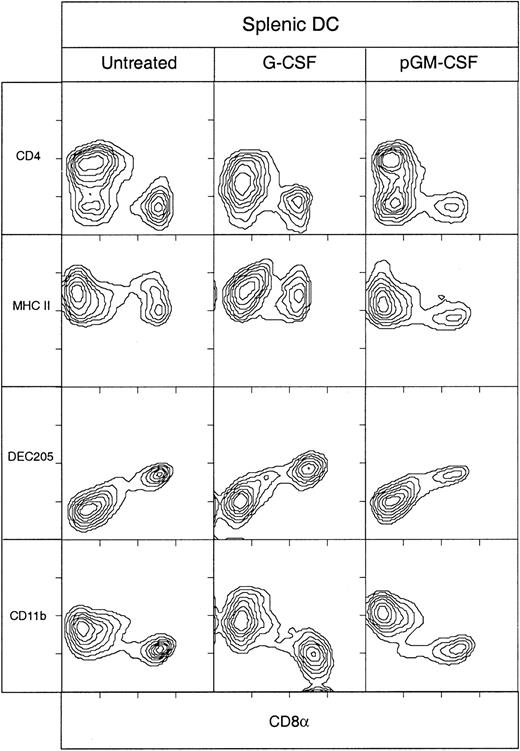
Effect of G-CSF and pGM-CSF on mouse spleen DCs
The effects on splenic DCs of 2 myeloid cytokines, G-CSF and pGM-CSF, were compared with the effects of FL and ProGP-1. Treatment with G-CSF yielded a small increase (2.3 fold) in total DC numbers, with a small bias (3-fold increase) toward CD8+ DCs (Table1 and Figure 4). Apart from typical CD11chi MHC class IIhi DCs, G-CSF treatment resulted in a large population of cells that were CD11cintCD8int and positive for MHC class II; because it was not clear that these were bona fide DCs, however, they are not included in Table 1 or Figure 4.
Phenotype of DCs isolated from mice treated with G-CSF or pGM-CSF.
DCs isolated from spleens of C57BL/6 mice that were either untreated or treated for 10 days with G-CSF (10 μg/day) or for 5 days with pGM-CSF (2 μg/d), were immunofluorescent stained with antibodies directed to typical murine DC markers. The DCs were analyzed by gating on high forward light scatter and high levels of expression of CD11c. Data for each cytokine treatment are representative of 3 experiments that examined pooled splenic DCs from at least 3 mice.
Phenotype of DCs isolated from mice treated with G-CSF or pGM-CSF.
DCs isolated from spleens of C57BL/6 mice that were either untreated or treated for 10 days with G-CSF (10 μg/day) or for 5 days with pGM-CSF (2 μg/d), were immunofluorescent stained with antibodies directed to typical murine DC markers. The DCs were analyzed by gating on high forward light scatter and high levels of expression of CD11c. Data for each cytokine treatment are representative of 3 experiments that examined pooled splenic DCs from at least 3 mice.
Treatment with pGM-CSF provided the greatest contrast with the effects of all the other cytokines tested, and the results were similar to those of a previous study.18 Previously, it was not known whether pGM-CSF differentially affected the CD4+CD8− and CD4−CD8− DC populations. We found that the total DC level increased about 12 fold and that this increase was due largely to similar increases in the CD4−CD8−and CD4+CD8− DC populations (Table 1 and Figure 4). The surface phenotype of these enhanced populations was CD11b+DEC-205−, as in untreated mice or mice treated with ProGP-1 or muFL. In addition, there was a small increase (about 5 fold) in the CD8+DEC-205+ DC population, although many of these CD8+ DCs expressed intermediate, rather than high, levels of CD8.
Maturation of cytokine-treated DCs in culture
To determine whether the DCs from mice given cytokines could be matured further in culture and whether one subset could be transformed into another, sorted DCs from mice treated with ProGP-1, muFL, both muFL and G-CSF, and pGM-CSF were stained and analyzed after overnight culture in medium alone or with various cytokines and stimuli. DCs from cytokine-treated mice behaved in a manner similar to that of the corresponding DC population from normal mice.9Specifically, all subpopulations could be matured further by culture in medium alone (as indicated by increases in class II MHC expression and forward light scatter), and such maturation could be enhanced further by adding GM-CSF and factors such as tumor necrosis factor α (TNF-α) or CpG-ph. With all cytokine and stimulus combinations tested (including GM-CSF together with IFN-γ, TNF-α, IL-1, IL-3, FL, ProGP-1, CpG-ph, and various combinations of these agents), the CD4 and CD8 phenotype of the DCs from treated or control mice did not change except for a decrease in the level of CD4 expression. This confirmed earlier results with DCs from untreated mice9 indicating that the DC populations did not transform into other populations under conditions of maturation or activation. Moreover, the new group of CD8int DCs identified in the treated mice remained CD8int after overnight culture, after 60 hours of culture, and under all conditions tested, despite the further maturation of these DCs indicated by increases in their size and MHC class II expression.
IL-12 production by DCs from cytokine-treated mice
CD8+ splenic DCs in normal mice are by far the greatest producers of all isoforms of IL-12, including the bioactive p70 form, the p40 monomer, and the receptor antagonist (p40)2 form.11,17,26-29 Optimal production of the p70 form of IL-12 requires a stimulus of bacterial, microbial, or T-cell–dependent origin and an appropriate mixture of cytokines, including IL-4.30 31 Under the same conditions but in the absence of IL-4, production of p40—most notably the (p40)2antagonist form—is favored. Because CD8+ DCs were increased 130 fold with ProGP-1 and more than 30 fold with muFL in our experiments, we conducted studies to establish whether these cytokine-enhanced CD8+ DCs were indeed capable of high levels of IL-12 production. We also examined whether the ratio of total p40 to p70 was similarly regulated by IL-4 in the treated DCs. Therefore, all the sorted DC subpopulations from cytokine-treated mice were stimulated in culture with CpG-ph in the presence of GM-CSF and IFN-γ and with or without IL-4 (data not shown), and the production of IL-12 p70 and p40 in the culture supernatants was determined by ELISA (Figure 5). In addition, the production of p40, p70, and (p40)2 was assessed by Western blotting (data not shown).
IL-12–producing capacity of DC populations from mice treated with cytokines.
Sorted subpopulations of splenic DCs (0.5 × 106/mL) from cytokine-treated mice were stimulated in culture with CpG-ph (0.5 μM), IL-4 (100 U/mL), IFN-γ, (20 ng/mL), and GM-CSF (200 U/mL). The FL data are from treatment with muFL, but treatment with huFL yielded similar results. Supernatants were harvested after 16 to 20 hours and then analyzed by ELISA for production of IL-12 p40 and p70. Data are from 1 experiment; error bars represent the range of results with duplicate samples. Similar results were obtained in more than 5 experiments using DCs from mice treated with ProGP-1 and FL and in 3 experiments using DCs from mice treated with G-CSF and pGM-CSF.
IL-12–producing capacity of DC populations from mice treated with cytokines.
Sorted subpopulations of splenic DCs (0.5 × 106/mL) from cytokine-treated mice were stimulated in culture with CpG-ph (0.5 μM), IL-4 (100 U/mL), IFN-γ, (20 ng/mL), and GM-CSF (200 U/mL). The FL data are from treatment with muFL, but treatment with huFL yielded similar results. Supernatants were harvested after 16 to 20 hours and then analyzed by ELISA for production of IL-12 p40 and p70. Data are from 1 experiment; error bars represent the range of results with duplicate samples. Similar results were obtained in more than 5 experiments using DCs from mice treated with ProGP-1 and FL and in 3 experiments using DCs from mice treated with G-CSF and pGM-CSF.
The highly abundant CD8+ DCs from mice treated with ProGP-1, muFL, and both muFL and G-CSF all produced high per-cell levels of p70 (maximized in the presence of IL-4) and p40, similar to results with CD8+ DCs from control mice. The CD8int DCs from these mice produced levels of IL-12 resembling those of the CD8hi DCs (Figure 5). IL-12 production of CD8+ DCs from G-CSF–treated mice was about 70% of control values. However, the minority population of CD8+ DCs and CD8int DCs from mice treated with pGM-CSF produced only small amounts of IL-12 p70 but near normal levels of p40 and, in line with this finding, Western blot analysis showed a predominance of the antagonistic (p40)2 form, even in the presence of IL-4 (data not shown).
The CD4+CD8− and CD4−CD8− DCs from all cytokine-treated mice remained like those from normal mice in that they were low producers of IL-12 p70 and relatively low producers of IL-12 p40. However, cytokine treatment changed the levels of IL-12 p40 production somewhat, with the CD4+CD8− and CD4−CD8− DCs from mice treated with ProGP-1, muFL, and both muFL and G-CSF showing enhanced production, whereas those from mice treated with G-CSF and pGM-CSF showed reduced production.
Splenic DCs from mice treated with ProGP-1, muFL, and both muFL and G-CSF all followed the same pattern of IL-12 regulation observed in normal mice (Figure 5), since when IL-4 was omitted, p70 production dropped, p40 production increased, and levels of the antagonistic (p40)2 form increased (data not shown). However, this tight control was abrogated in splenic DCs from pGM-CSF–treated mice, since even in the presence of IL-4, there was a negligible amount of p70 and a predominance of p40 and (p40)2. This regulation and production of IL-12 by each of the DC populations from cytokine-treated mice followed the same pattern when other stimuli, such as SAC or CD40L, were used (data not shown). Most notably, as was the case with DCs from untreated mice, none of the DC populations from treated mice produced appreciable levels of bioactive IL-12 in the absence of a microbial or T-cell–derived stimulus.
IFN-γ production by DCs from cytokine-treated mice
In contrast to the situation with IL-12, CD4−CD8− DCs of normal mice have the greatest potential to produce IFN-γ, as revealed by ELISA assay of culture supernatants after stimulation with a combination of IL-12 and IL-18.11 The capacity of the CD4−CD8− DCs from the cytokine-treated mice to produce IFN-γ is illustrated in Figure6. Only the CD4−CD8− DCs from mice treated with G-CSF produced levels of IFN-γ comparable to control values; the CD4−CD8− DCs from mice treated with ProGP-1, muFL, both muFL and GM-CSF, and pGM-CSF all showed severe defects in the production of IFN-γ. The CD4+CD8− and CD4−CD8+ DC populations all produced small amounts of IFN-γ—less than half of that produced by the equivalent DC subsets of normal mice (data not shown). The defect in IFN-γ production in ProGP-1–treated mice was manifest as early as 2 days after treatment began and was extensive at 5 days; at 10 days, production of IFN-γ was barely detectable (Figure 6).
IFN-γ–producing capacity of CD4−CD8− DCs from cytokine-treated mice.
Sorted subpopulations of CD4−CD8− splenic DCs (0.5 × 106/mL) from cytokine-treated mice were stimulated in culture with IL-12 and IL-18 (20 ng/mL each). Data for 2 and 5 days (d) of ProGP-1 treatment are shown. The FL data are from treatment with muFL, but treatment with huFL yielded similar results. Supernatants were harvested after 3 days and then analyzed by ELISA for IFN-γ production. Data are from 1 experiment; error bars represent the range of results with duplicate samples. Similar results were obtained in more than 5 experiments using DCs from mice treated with ProGP-1 and FL and in 3 experiments using DCs from mice treated with G-CSF and pGM-CSF.
IFN-γ–producing capacity of CD4−CD8− DCs from cytokine-treated mice.
Sorted subpopulations of CD4−CD8− splenic DCs (0.5 × 106/mL) from cytokine-treated mice were stimulated in culture with IL-12 and IL-18 (20 ng/mL each). Data for 2 and 5 days (d) of ProGP-1 treatment are shown. The FL data are from treatment with muFL, but treatment with huFL yielded similar results. Supernatants were harvested after 3 days and then analyzed by ELISA for IFN-γ production. Data are from 1 experiment; error bars represent the range of results with duplicate samples. Similar results were obtained in more than 5 experiments using DCs from mice treated with ProGP-1 and FL and in 3 experiments using DCs from mice treated with G-CSF and pGM-CSF.
Allostimulatory capacity of DCs from cytokine-treated mice
Because an exceptional capacity to stimulate T cells in an allogenic mixed leukocyte reaction (MLR) is a hallmark of mature DCs, we tested whether the DC subpopulations from cytokine-treated mice maintained this capacity. All DC subpopulations in normal mouse spleens have a strong capacity to activate naive allogenic CD4 or CD8 T cells to cycle, and we found this also to be true of DC populations from treated mice. The main DC populations (CD4+CD8−, CD4−CD8−, and CD4−CD8+ DCs) from mice treated with ProGP-1, muFL, and G-CSF for 10 days were compared with those from normal mice (Figure 7). There was little difference between the DCs from treated mice and those from normal mice in their ability to stimulate naive CD4 T cells; thus, this aspect of antigen presentation was intact in the cytokine-treated mice. The allostimulatory capacity of DCs from pGM-CSF–treated mice was previously studied by others17 and also found to be intact.
Stimulation capacity of DCs from cytokine-treated mice.
Purified DC populations (103 cells/well) from mice treated for 10 days with G-CSF, ProGP-1, or FL were tested for their ability to stimulate naive CD4+ T cells (20 × 103cells/well) in an allostimulatory MLR and compared with the same DC populations from untreated mice. For clarity, only the maximum value for thymidine uptake (day 3.5) is shown for untreated mice and only the muFL data are shown, although DCs from mice given huFL stimulated T cells to a similar extent. The average count per minute from triplicate values is shown for each time point; error bars represent the range of results with triplicate samples. Data are from 1 experiment that compared all DC populations from treated and untreated mice. Similar results were obtained in a second experiment that compared DCs from G-CSF–treated mice with DCs from untreated mice and in 3 additional experiments that compared DCs from ProGP-1–treated and FL-treated mice with controls.
Stimulation capacity of DCs from cytokine-treated mice.
Purified DC populations (103 cells/well) from mice treated for 10 days with G-CSF, ProGP-1, or FL were tested for their ability to stimulate naive CD4+ T cells (20 × 103cells/well) in an allostimulatory MLR and compared with the same DC populations from untreated mice. For clarity, only the maximum value for thymidine uptake (day 3.5) is shown for untreated mice and only the muFL data are shown, although DCs from mice given huFL stimulated T cells to a similar extent. The average count per minute from triplicate values is shown for each time point; error bars represent the range of results with triplicate samples. Data are from 1 experiment that compared all DC populations from treated and untreated mice. Similar results were obtained in a second experiment that compared DCs from G-CSF–treated mice with DCs from untreated mice and in 3 additional experiments that compared DCs from ProGP-1–treated and FL-treated mice with controls.
We also assessed the CD8int DC population that was a minor component of normal spleens but was enhanced in spleens from cytokine-treated mice, since these DCs might have represented immature forms of CD8hi DC. With care taken to eliminate autofluorescent macrophages,4 we obtained a small sample of these CD8int DCs from normal spleens and found their stimulatory capacity to be similar to that of CD4−CD8hi DCs. The CD8int DCs from ProGP-1–treated mice had a similar stimulatory capacity, and those from muFL-treated mice had an enhanced capacity. However, CD8int DCs from G-CSF–treated mice were unusual in that they expressed only intermediate levels of CD11c, although they expressed class II MHC. These cells failed to show allostimulatory capacity at the usual 3.5-day peak of thymidine incorporation, although some proliferation was observed at 4.5 to 5.5 days of culture. Thus, they could not be classified as typical mature DCs, although they may have represented an immature DC form.
Discussion
Of all the cytokines tested in this study, ProGP-1, an agonist of the Flt-3 and G-CSF receptors, was the most effective in increasing the number of spleen DCs. It was substantially more effective than FL or G-CSF used alone or together. However, its effects were strongly selective, increasing the number of CD4−CD8+DCs 130 fold so that they dominated the final DC population. Selective enhancement of CD8+ DCs was also observed after treatment with muFL, which increased the number of CD8+ DCs about 30 fold and, to some extent, with G-CSF, which produced a 3-fold increase. Conversely, pGM-CSF, although it increased CD8+ DC numbers about 5 fold, produced a selective 14-fold increase in CD4−CD8− DCs, a finding similar to results reported previously.17,18 We found that pGM-CSF increased the newly described CD4+CD8− DC population4 and the CD4−CD8− DC population equally. The strong selective effects we observed represented different expansions of particular DC subsets rather than elimination of others, since all DC subsets in all treated mice were present in numbers at least equal to those in normal mice. Except in mice given huFL, the surface phenotypes of the expanded DC subsets resembled those of DC subsets from normal mice with respect to other markers, including markers of activation, although the cells were larger, as indicated by forward scatter profiles. The presence of allostimulatory ability in the expanded DC subsets after full treatment also suggested that the cells were similar in maturity to those from normal mice.
One surprising finding was the difference between results from administration to mice of rmuFL prepared in mammalian cells and administration of rhuFL. This difference partly accounts for why a strong selective effect of FL was not observed in an earlier study using huFL.14 The huFL produced equally large increases in CD8− and CD8+ DCs, thereby providing evidence against a CD8+-selective effect, although there was a selective increase in CD4− DCs. However, because many of the CD8− DCs expressed DEC-205, like normal splenic CD8+ DCs do, it may be that selective expansion of the same subset had occurred without acquisition of CD8α. However, these cells did not produce substantial amounts of IL-12 and thus functionally did not resemble CD8+ DCs from normal mice or DEC-205+ splenic DCs from CD8 knockout mice.11Because CD8−DEC-205+ DCs are found in lymph nodes in normal mice32 and these cells also do not make appreciable amounts of IL-12,11 another explanation might be aberrant seeding of the cells into the spleen in the huFL-treated mice.
The exact reason for the different effects of the muFL and huFL preparations remains unclear. It may be simply that different recombinant expression systems produce differentially glycosylated or folded FL proteins with large differences in blood life span or other bioactivities. However, it does appear that there is a qualitative as well as a quantitative difference between the preparations that may reflect a species-specific difference between them. FL is structurally related to other members of the tyrosine kinase receptor family,33 and possibly huFL is “promiscuous” in the murine system and able to bind to other, related members of this family. Alternatively, differences in affinity of binding to the Flt-3 receptor may lead to differences in signaling and consequential downstream biologic activities. Several signaling pathways and molecules found to be important in Flt-3 signaling34-37 may be differentially activated by muFL and huFL. Only studies of the kinetics and affinity of binding to Flt-3 receptors and downstream intracellular signaling events would clarify this issue.
One “new” splenic DC subtype emphasized by this study consists of DCs bearing intermediate levels of CD8α. These DCs are present in small numbers in normal spleen but are not normally segregated as a distinct subtype. However, CD8int DCs are a more prominent feature of normal lymph node DCs and, we found here, of spleens from mice given ProGP-1 or muFL. In spleens from normal mice and most mice given cytokines, these cells expressed high levels of CD11c and class II MHC and stimulated naive allogenic T cells; therefore, they were by definition relatively mature DCs. They did not appear to represent a transient stage but seemed functionally similar to CD8hi DCs. An exception to this was the CD8int cells isolated from spleens of G-CSF–treated mice, which although being class II MHChi, expressed only low levels of CD11c and were poor stimulators of allogenic T cells. These may have been immature DCs or cells not even of the DC lineage. It is pertinent that in humans, in vivo treatment with G-CSF leads to an expansion of DC-2–type immature DC cells or plasmacytoid cells, which are also poor stimulators of naive T cells.20 In future experiments, we will attempt to determine whether CD8intDCs from G-CSF–treated mice are analogous to human plasmacytoid cells.
A consequence of the marked enhancement of CD8+ DCs with ProGP-1 or FL treatment is potentially high levels of IL-12–producing DCs with a capacity to bias T-cell immune responses to the T-helper-1 (Th1) type. It was therefore important to determine whether the expanded CD8+ DCs had the same capacity to produce IL-12 and if the process was under normal regulation. We found the capacity to produce high levels of IL-12 p70 and p40 was indeed maintained and restricted to CD8+ DCs from mice treated with ProGP-1, FL, and G-CSF. The IL-12 regulatory systems also seemed intact. This result has important implications for clinical use of these cytokines in immune modulation, since treatment with these agents could lead—for better or for worse—to enhanced inflammatory responses. However, the mice treated with pGM-CSF had a severe reduction in their capacity to make bioactive IL-12 p70, and this possible “anti-Th1” effect was magnified by the predominance of (p40)2, the antagonist of the IL-12 receptor. It is not clear whether the regulatory system of the CD8+ DCs from these mice was disrupted or whether a separate CD8+ DC population was induced by this myeloid hormone.
Despite the functional and phenotypic normality of most of the expanded DC populations, a marked deficit was found in their ability to make IFN-γ. This occurred with all cytokine treatments except G-CSF, including pGM-CSF treatment, which markedly expanded the IFN-γ–producing CD4−CD8− DCs. Thus, the spleens of pGM-CSF–treated mice were devoid of DCs producing either of the Th1-inducing cytokines, IL-12 or IFN-γ. As a result, these mice represent an interesting experimental model.
Overall, this study found that ProGP-1, along with FL, G-CSF, and pGM-CSF, are useful tools for expanding DC populations. However, the expansion is often strongly selective for particular DC subpopulations and this could have important biologic and clinical implications. Many of the biologic functions of the expanded DCs remain intact, but some—particularly the capacity to produce certain cytokines—appear to change. We do not know whether the cytokine-regulatory systems are disrupted in such cells or whether there is more DC heterogeneity than would be expected from surface phenotype alone and perhaps populations of non–cytokine-producing DCs have been selectively expanded. Because of the potential clinical uses of these cytokines, it is important to determine whether the same effects occur in human DC subpopulations.
We thank V. Lapatis, D. Kaminaris, J. Chan, C. Tarlinton, G. Paukovics, and F. Battye for excellent FACS assistance.
Supported in part by research funding from Pharmacia, St Louis, MO (K.S.). H.H. was supported by a Deutsche Krebshilfe fellowship.
Two of the authors (R.E. and S.W.) are employed by Pharmacia Corporation, St Louis, MO.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Meredith O'Keeffe, Walter and Eliza Hall Institute of Medical Research, Royal Melbourne Hospital, Victoria, 3050, Australia.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal