Abstract
Conflicting results obtained from animal studies suggest that B cells play a role in maintaining long-term T-cell memory and in skewing T-cell response toward a T-helper 2 (TH2) phenotype. X-linked agammaglobulinemia (XLA) is a genetic human disease characterized by the lack of circulating B cells due to the mutation of Bruton tyrosine kinase. This disease thus represents a unique model for studying the role of B lymphocytes in regulating T-cell functions in humans. To this aim, we analyzed hepatitis B envelope antigen (HBenvAg)–specific T-cell memory in a series of XLA patients vaccinated against hepatitis B virus (HBV). We found HBenvAg-specific T lymphocytes producing interferon–γ, interleukin-4, or both in the peripheral blood of XLA patients up to at least 24 months after completing the standard anti-HBV immunization protocol. The HBenvAg-specific T-cell frequencies and the percentage of patients with these responses were not significantly different from healthy vaccinated controls. By combining cell purification and enzyme-linked immunospot assay, we found that effector CD27− T cells, which promptly produced cytokines in response to antigen (Ag), and memory-resting CD27+ T cells, which required Ag restimulation to perform their functions, were maintained in both XLA patients and controls for up to 24 months after the last vaccination boost. These data strongly suggest that B cells are not an absolute requirement for the generation of effective T-cell memory in humans, nor do they seem to influence TH1/TH2 balance.
Introduction
X-linked agammaglobulinemia (XLA) is an X-chromosome–linked recessive genetic disease characterized by a lack of mature B cells, which results in primary immunodeficiency.1,2 Affected subjects show a defective humoral immune response that renders them susceptible to recurrent bacterial infections.3 Early intravenous immunoglobulin replacement therapy is effective in preventing severe acute bacterial infections.4 The T-cell counts and function are generally normal in these patients,5 and this explains why these patients cope normally with viral infections during infancy. However, in some patients severe echovirus-dependent encephalitis and meningitis may develop.6
The molecular basis of this disorder is mutations in Bruton tyrosine kinase (btk) gene, whose malfunction leads to an arrest of B-cell differentiation.7-10 This tyrosine kinase is expressed in myeloid cells as well as in B-lineage cells, but the effects of mutations in Btk appear to have no clinically significant effects on myeloid cell function.11
It has been suggested that B lymphocytes not only are involved in humoral immune response, but may also have a role in influencing different facets of T-cell immunity. First, B cells have been shown to function as efficient antigen-presenting cells (APCs), owing to their peculiar capacity to uptake soluble antigens (Ags) at very low concentration through their surface immunoglobulins.12,13Second, it has been hypothesized that B lymphocytes are involved in maintaining long-term T-cell memory.14-16 Third, the role played by B cells in influencing the cytokine pattern of T-helper (TH) cells has been analyzed in a variety of studies. As is well known, T lymphocytes recognize Ags by engaging the T-cell receptor (TCR) with peptide–major histocompatibility complexes (MHCs) displayed on the surface of APCs.17 Following TCR triggering, CD4+ T lymphocytes polarize toward TH1 or TH2 cells that produce different sets of cytokines, such as interferon-γ (IFN–γ) or interleukin-4 (IL-4), IL-5, and IL-13, respectively.18-20 Conversely, TH0 cells are nonpolarized effector cells producing all types of cytokines. The Ag, the cytokine/chemokine microenvironment, or the type of APC involved are all factors that critically influence the skewing toward a preferential TH1 or TH2 response.21 In this regard, studies in mice have shown that Ag-specific B cells might either preferentially favor selective TH2 cell expansion upon direct CD40/CD40-ligand interaction with CD4+ T cells,22,23 or drive dendritic cells (DCs) to suppress polarized TH1-cell responses.24 Moreover, both B-cell–deficient andbtk-mutant mice have been shown to be unable to efficiently clear extracellular pathogens owing to a predominant TH1-cell polarization.25 26
However, the majority of data on the B-cell role in influencing T-cell responses derives from animal models because of the evident difficulties in addressing these complex issues directly in humans. Therefore, XLA may represent a unique human model of B-cell deficiency for studying the role of B lymphocytes in influencing Ag-specific responses of memory-resting and memory-effector T cells. To this aim, we evaluated here the hepatitis B envelope Ag (HBenvAg)–specific T-cell response in a series of XLA patients vaccinated against hepatitis B virus (HBV) up to at least 24 months after completion of the immunization protocol, comparing them with healthy age-matched immunized volunteers.
Vaccination against HBV is highly effective in preventing both acute and chronic HBV infection. Protection is believed to be mediated by the presence of both protective HBenvAg-specific antibodies (Abs) and by the induction and expansion of HBenvAg-specific memory CD4+T lymphocytes.27 In particular, we decided to study XLA patients immunized against HBV, as this vaccine has been proved to reduce serum HBV DNA in chronically infected patients in the absence of neutralizing Abs,28 indicating that specific CD4+ T-cell–mediated response plays a pivotal role in directly suppressing HBV replication.29 30
Patients, materials, and methods
Patients
The present study included 9 male patients with very low immunoglobulin serum levels and no circulating B cells (1% or fewer) referred to the Pediatric Department of University of Brescia (mean age, 16 years; range, 6-30 years). In 7 patients, the diagnosis was confirmed by btk gene sequence analysis, revealing mutations as indicated in Table 1. By contrast, in the remaining 2 patients, no mutations were identified, although the patients exhibited markedly reduced levels of the btktranscripts (not shown). Also included were 6 healthy age-matched controls (mean age, 20.6 years; range, 7-32 years). All subjects from both experimental groups were immunized with Engerix-B (SmithKline Beecham, Rixensart, Belgium) anti–hepatitis B recombinant vaccine according to the standard vaccination procedure. Peripheral blood mononuclear cells (PBMCs) were obtained from XLA patients or control subjects after informed consent either before or 1, 12, or 24 months after the third (last) boost of anti-HBV vaccine; PBMCs were cryopreserved in liquid nitrogen and thawed immediately prior to functional or phenotypical studies. Clinical data of the XLA patients studied are summarized in Table 1. Approval was obtained from the Institutional Review Board for these studies. Informed consent was provided according to the declaration of Helsinki.
Characteristics of patients with X-linked agammaglobulinemia
| Patient no. . | Age, y . | IgG, g/L . | IgA, g/L . | IgM, g/L . | B cells/ mm3 (%) . | T cells/ mm3 (%) . | btk mutation . |
|---|---|---|---|---|---|---|---|
| 1 | 30 | 1.9 | < .06 | .19 | < 10 (0.8) | 1712 (75) | 1114C > T |
| 2 | 16 | 1.61 | < .06 | .10 | < 10 (0.6) | 2021 (94) | 188A > T |
| 3* | 9 | 2.62 | .34 | .24 | 14 (0.7) | 2425 (90) | 994C > T |
| 4* | 11 | 3.55 | .29 | .20 | 17 (0.8) | 2440 (94) | 994C > T |
| 5 | 20 | 1.41 | < .06 | .06 | 18 (0.8) | 1838 (74) | del C534 |
| 6 | 16 | 1.00 | < .06 | .03 | < 10 (0.1) | 1650 (90) | Unknown |
| 7 | 6 | 2.08 | .08 | .35 | 15 (0.8) | 3455 (92) | Unknown |
| 8 | 22 | 1.63 | .13 | .14 | 13 (1.0) | 1751 (89) | IVS15 + 1G > T |
| 9 | 14 | 1.08 | < .06 | .38 | < 10 (0.5) | 1835 (94) | 656-657ins-T |
| Patient no. . | Age, y . | IgG, g/L . | IgA, g/L . | IgM, g/L . | B cells/ mm3 (%) . | T cells/ mm3 (%) . | btk mutation . |
|---|---|---|---|---|---|---|---|
| 1 | 30 | 1.9 | < .06 | .19 | < 10 (0.8) | 1712 (75) | 1114C > T |
| 2 | 16 | 1.61 | < .06 | .10 | < 10 (0.6) | 2021 (94) | 188A > T |
| 3* | 9 | 2.62 | .34 | .24 | 14 (0.7) | 2425 (90) | 994C > T |
| 4* | 11 | 3.55 | .29 | .20 | 17 (0.8) | 2440 (94) | 994C > T |
| 5 | 20 | 1.41 | < .06 | .06 | 18 (0.8) | 1838 (74) | del C534 |
| 6 | 16 | 1.00 | < .06 | .03 | < 10 (0.1) | 1650 (90) | Unknown |
| 7 | 6 | 2.08 | .08 | .35 | 15 (0.8) | 3455 (92) | Unknown |
| 8 | 22 | 1.63 | .13 | .14 | 13 (1.0) | 1751 (89) | IVS15 + 1G > T |
| 9 | 14 | 1.08 | < .06 | .38 | < 10 (0.5) | 1835 (94) | 656-657ins-T |
Ig indicates immunoglobulin.
Patients 3 and 4 are brothers.
Purification of PBMC subpopulations
PBMCs were isolated by Lymphoprep (Nycomed Pharma, Oslo, Norway) gradient centrifugation. Cells were cryopreserved in liquid nitrogen and then thawed for use in the different assays. To obtain CD27-enriched or CD27-depleted T-cell populations, CD2+ T cells were first positively isolated by means of anti-CD2 monoclonal Ab (mAb)–coated immunomagnetic beads (Dynabeads, Dynal, Oslo, Norway) according to the manufacturer's instructions. Purified CD2+ T cells were then incubated with anti-CD27 mAb (PharMingen, San Diego, CA) for 30 minutes on ice, washed 4 times, and incubated with Dynabeads conjugated with goat antimouse IgG mAb (Dynal).31 Cells were then detached from the magnetic beads by Detachabeads (Dynal) for 45 minutes at room temperature (RT). Positively selected CD27+ cells were 99% pure, whereas the CD27-depleted population consisted of 85% CD27− cells. Both populations coexpressed CD3 (more than 95%).
Proliferative response
All proliferation assays were set up by seeding 2 × 105 PBMCs per well in 96-well round-bottomed plates, as previously described.32 Cultures were incubated for 5 days at 37°C in 5% CO2, in the presence of different concentrations of recombinant (r) HBenvAg (a d subtype) (10, 5, 1, and 0 μg/mL) containing the 15-52 sequence of pre-S1 domain, the 133-145 sequence of pre-S2 domain, and the entire S domain (SmithKline Beecham). Cells were then pulsed with 1 μCi (.037 MBq) tritiated thymidine (Amersham, Buckinghamshire, United Kingdom) per well for 18 hours. Plates were harvested, and tritiated thymidine incorporation was assessed by means of a liquid scintillation counter (MicroBeta Plus) (Wallac, Turku Finland). All assays were performed in triplicate.
Enzyme-linked immunospot assay
HBenvAg-specific IFN-γ– and IL-4–secreting T cells were detected by enzyme-linked immunospot (ELISPOT) assay, as previously described.31 33 This assay allows the frequencies of cytokine-secreting cells to be precisely estimated by directly counting the percentage of cytokine-specific spots formed upon Ag stimulation. Briefly, 96-well nitrocellulose-backed plates (MAHA S4510) (Millipore, Bedford, MA) were coated with 5 μg/mL mouse antihuman IFN-γ or IL-4 mAb (PharMingen). Plates were washed with phosphate-buffered saline (PBS) supplemented with 0.25% Tween 20 (Sigma, St Louis, MO) (PBS/0.25% Tween 20) and blocked with PBS/10% human serum. PBMCs were deposited in the wells in duplicate at 1 × 105 cells per well in the presence of different concentrations of HBenvAg (10, 5, 1, and 0 μg/mL). After 48 hours' incubation at 37°C, plates were washed with PBS/0.25% Tween 20, and then 2 μg/mL biotinylated mouse antihuman IFN-γ or IL-4 mAb (PharMingen) was added to the well. After 2 hours' incubation at RT, plates were washed 5 times; 50 μL streptavidin–horseradish peroxidase conjugate (PharMingen) (dilution 1:500) was added to the wells; and plates were incubated for a further 90 minutes at RT. Colorimetric reaction was obtained by means of P-nitroblue tetrazolium/ 5-bromo-4-chloro-3-indolylphosphatase as a substrate. Spots were quantified by means of an AID ELISPOT Reader (AID, Strassberg, Germany), a computer-based system allowing the semiautomatic interpretation of ELISPOT microtiter plates. Once background or artifacts had been eliminated, the counting software quantitated the number of spots in the well, and the data were exported for analysis. Potential cell ability to produce IFN-γ or IL-4 was verified by stimulating cells with mitogen phytohemoagglutinin (PHA) (Wellcome, Beckenham, United Kingdom).
Intracellular cytokine staining by flow cytometry
PBMCs from 6 XLA patients and 2 healthy controls were either cultured in the presence or absence of HBenvAg 10 μg/mL for 18 hours at 37°C or treated with phorbolmyristate acetate/ionomycin (Sigma-Aldrich, Milan, Italy) (20 ng/mL/1 μg/mL) for 6 hours. At 2 hours after starting cultures, 10 μg/mL brefeldin-A (Sigma-Aldrich) was added. Triple staining was then performed to detect IFN-γ– and IL-4–producing CD4+ T cells as follows: cells were incubated with human γ-globulins for 15 minutes at RT and then stained with CyChrome-conjugated anti-CD4 mAb (PharMingen) at 4°C for 30 minutes. Cells were washed twice with fluorescence-activated cell sorter (FACS) buffer (PBS 1 ×, 2% fetal calf serum, 0.01% sodium azide), fixed and permeabilized with Cytofix/Cytoperm solution (Cytofix/Cytoperm Kit) (PharMingen) at 4°C for 20 minutes, followed by 2 washes with Perm Wash Buffer (PharMingen). Fluorescein isothiocyanate–conjugated antihuman IFN-γ– and phycoerythrin-conjugated antihuman IL-4 mAbs (PharMingen) were added, and cells were incubated for 30 minutes at 4°C. Cells were then washed twice with FACS buffer, acquired with a FACScan flow cytometer (Becton Dickinson, San Jose, CA), and anlyzed by means of CellsQuest software (BD, Palo Alto, CA). Negative controls were obtained by staining cells with an irrelevant isotype-matched mAb.
Statistical analysis
Differences between groups were analyzed by a 2-tailed, 2-sample Student t test or by contingency tables (χ2) with 2 degrees of confidence. A value of P < .05 was considered to be statistically significant.
Results
PBMC proliferative response to HBenvAg
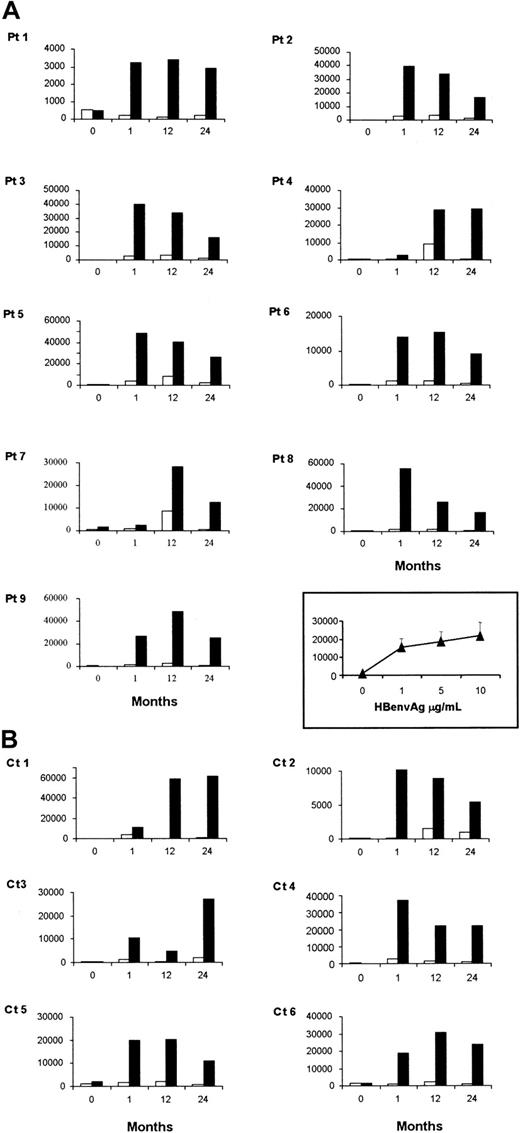
PBMCs isolated from patients or healthy controls either before or after vaccination against HBV were stimulated in vitro with HBenvAg. The results presented here were obtained with the use of HBenvAg at 10 μg/mL, a concentration that proved to be optimal in our experiments. At basal time, no patient or control subject had a significant proliferative response to HBenvAg, corresponding to a stimulation index (SI) below 3. SI was defined as the ratio of cpm from HBenvAg-stimulated PBMCs to unstimulated PBMCs. At 1 month after the end of the vaccination cycle, all individuals from both study populations had a significantly positive SI. Mean HBenvAg-specific SIs ± SEM for PBMCs obtained from XLA patients were as follows: 1.2 ± 0.29; 12 ± 2.73; 11.50 ± 3.05; 21.64 ± 3.14, assessed before and 1, 12, and 24 months after immunization, respectively. These results were not significantly different from controls (1.09 ± 0.15; 18.68 ± 7.98; 16.05 ± 5.50; 24.62 ± 9.32). Individual results for both XLA and control groups are shown in Figure 1.
HBenvAg-specific proliferation.
PBMCs from XLA patients (A) or control subjects (B) were assessed by3H-thymidine incorporation 5 days after incubation with (solid bars) or without (open bars) 10 μg/mL HBenvAg. Responses by XLA patients' PBMCs at various antigen concentrations 1 month after vaccination appear in the inset and are expressed as mean ± SEM. Similar concentration-dependent responses were also obtained in controls (not shown). The assays were performed at different times before and after anti-HBV immunization as indicated. Each value was calculated as the mean cpm of triplicate determinations. Pt indicates patient; Ct, control subject.
HBenvAg-specific proliferation.
PBMCs from XLA patients (A) or control subjects (B) were assessed by3H-thymidine incorporation 5 days after incubation with (solid bars) or without (open bars) 10 μg/mL HBenvAg. Responses by XLA patients' PBMCs at various antigen concentrations 1 month after vaccination appear in the inset and are expressed as mean ± SEM. Similar concentration-dependent responses were also obtained in controls (not shown). The assays were performed at different times before and after anti-HBV immunization as indicated. Each value was calculated as the mean cpm of triplicate determinations. Pt indicates patient; Ct, control subject.
Frequencies of HBenvAg-specific IFN-γ– and IL-4–producing T cells, as detected by ELISPOT assay
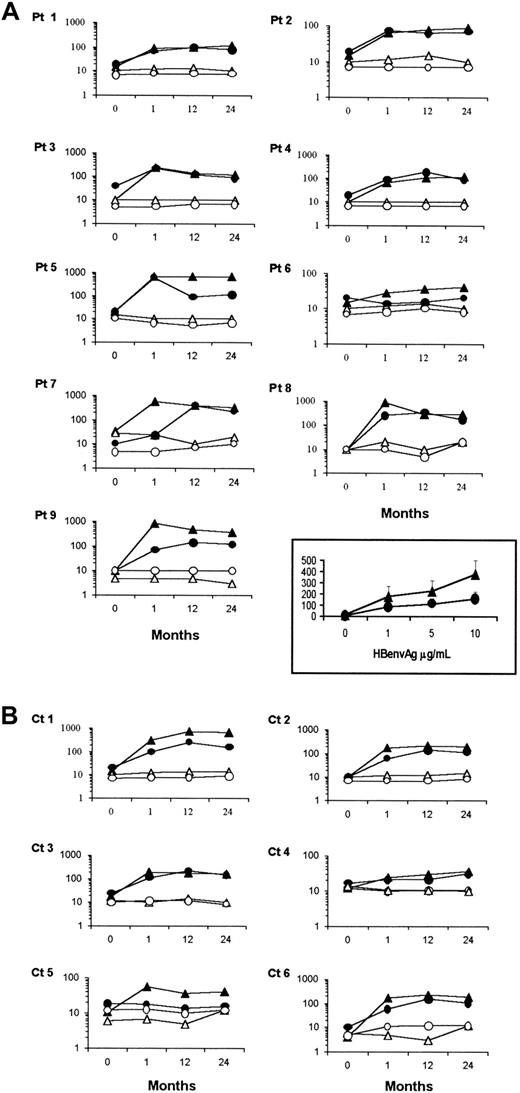
Calculations of either IFN-γ– or IL-4–producing HBenvAg-specific T-cell frequencies in the peripheral blood of either XLA patients or healthy subjects were carried out by ELISPOT assay. Spots were analyzed after 48 hours of culture in the presence or absence of HBenvAg. Potential cell capability to produce IFN-γ or IL-4 was verified by stimulating cells with mitogen PHA. Cells from all the subjects tested produced similar amounts of cytokines after this nonspecific stimulus (not shown). Before vaccination, no XLA patient or control subject showed any significant specific response, arbitrarily defined as the presence of at least 5 spots in the stimulated wells after subtraction of spots in the nonstimulated ones. Conversely, in response to HBenvAg, all the XLA patients, as well as all the healthy subjects, produced significant numbers of both IFN-γ and IL-4 spots (Figure 2). Frequencies of cytokine-producing cells were expressed as number of positive T cells per 1 × 106 total PBMCs and varied individually. Mean HBenvAg-specific cytokine-producing cells per 1 × 106PBMCs in XLA patients ± SEM, calculated after subtraction of spots in unstimulated wells from spots in HBenvAg-stimulated ones were as follows: for IFN-γ, 3.66 ± 0.66; 356.44 ± 123.07; 231 ± 82.37; 233 ± 64.12; and for IL-4, 3.77 ± 0.72; 138 ± 65.47; 139 ± 46.27; 100.66 ± 19.16, as assessed before and 1, 12, and 24 months after anti-HBV immunization, respectively. None of these data differed significantly from those obtained in control subjects (for IFN-γ, 2.5 ± 1.11; 143 ± 40.16; 229.5 ± 107.19; 200.83 ± 91.82; for IL-4, 7 ± 2; 51.83 ± 15.92; 124 ± 40.51; 83.16 ± 23.47).
Analysis of IFN-γ– or IL-4–producing HBenvAg-specific CD4+ T-cell frequencies.
PBMCs isolated from XLA patients (A) or control subjects (B) either before or after 1, 12, and 24 months from the immunization against HBV, were assessed by ELISPOT assay for their capacity to form IFN-γ or IL-4 spots in response to a 48-hour stimulation with 10 μg/mL HBenvAg. Responses by XLA patients' PBMCs at various antigen concentrations 1 month after vaccination appear in the inset and are expressed as mean ± SEM. Similar results were obtained in controls (not shown). Data are expressed as number of cytokine-producing CD4+ T cells in 1 × 106total PBMCs. For each patient, solid triangles and circles represent IFN-γ– or IL-4–producing cells, respectively, in the presence of HBenvAg. Open triangles and circles represent the respective cytokine-producing cells in the absence of HBenvAg-stimulation. Pt indicates patient; Ct, control subject.
Analysis of IFN-γ– or IL-4–producing HBenvAg-specific CD4+ T-cell frequencies.
PBMCs isolated from XLA patients (A) or control subjects (B) either before or after 1, 12, and 24 months from the immunization against HBV, were assessed by ELISPOT assay for their capacity to form IFN-γ or IL-4 spots in response to a 48-hour stimulation with 10 μg/mL HBenvAg. Responses by XLA patients' PBMCs at various antigen concentrations 1 month after vaccination appear in the inset and are expressed as mean ± SEM. Similar results were obtained in controls (not shown). Data are expressed as number of cytokine-producing CD4+ T cells in 1 × 106total PBMCs. For each patient, solid triangles and circles represent IFN-γ– or IL-4–producing cells, respectively, in the presence of HBenvAg. Open triangles and circles represent the respective cytokine-producing cells in the absence of HBenvAg-stimulation. Pt indicates patient; Ct, control subject.
Intracellular cytokine staining
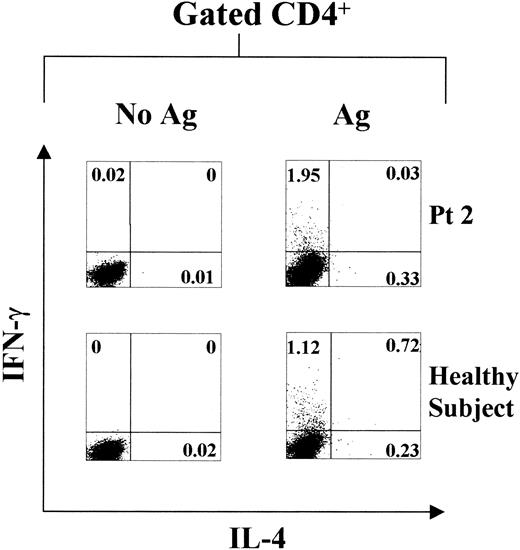
In order to substantiate that memory-effector (ME) T cells with TH1, TH2, or TH0 phenotype are long-lived, intracellular cytokine analysis by flow cytometry was determined in both XLA patients and healthy subjects at 24 months after the last vaccination boost. Specific intracytoplasmic IFN-γ and IL-4 productions were differently distributed in the peripheral T cells of the 2 study populations, upon 18 hours' incubation with HBenvAg in vitro (Figure 3; Table2). This supports the hypothesis that memory T cells with prompt TH1, TH2, or TH0 function are long-lived in humans and that their maintenance does not require the presence of B cells. Moreover, these data confirm those obtained with ELISPOT assay, showing the absence of a selective polarization toward the TH1 cell phenotype in XLA patients.
HBenvAg-specific intracellular cytokine production.
HBenvAg-stimulated or unstimulated PBMCs obtained from 1 representative XLA patient (patient 2) and 1 control subject and assayed by flow cytometry, are shown. Analysis was performed 24 months after the last anti-HBV vaccine boost. PBMCs were cultured with 10 μg/mL HBenvAg for 18 hours at 37°C, 5% CO2; at 2 hours after cultures were started, brefeldin-A was added. Fixed and permeabilized cells were triple stained with anti-CD4, anti–IFN-γ, and anti–IL-4 as described in “Patients, materials, and methods.” Cells were gated according to CD4 expression, and data are represented as IL-4–producing cells on the x-axis and IFN-γ–producing cells on the y-axis. The percentage of either single- or double-positive cells is indicated in the respective plot quadrants. Pt indicates patient.
HBenvAg-specific intracellular cytokine production.
HBenvAg-stimulated or unstimulated PBMCs obtained from 1 representative XLA patient (patient 2) and 1 control subject and assayed by flow cytometry, are shown. Analysis was performed 24 months after the last anti-HBV vaccine boost. PBMCs were cultured with 10 μg/mL HBenvAg for 18 hours at 37°C, 5% CO2; at 2 hours after cultures were started, brefeldin-A was added. Fixed and permeabilized cells were triple stained with anti-CD4, anti–IFN-γ, and anti–IL-4 as described in “Patients, materials, and methods.” Cells were gated according to CD4 expression, and data are represented as IL-4–producing cells on the x-axis and IFN-γ–producing cells on the y-axis. The percentage of either single- or double-positive cells is indicated in the respective plot quadrants. Pt indicates patient.
Flow cytometric analysis of intracellular cytokines in PBMCs stimulated or not stimulated with HBenvAg in vitro from XLA patients or healthy controls
| . | IFN-γ alone . | IL-4 alone . | IFN-γ and IL-4 . | |||
|---|---|---|---|---|---|---|
| No Ag . | Ag . | No Ag . | Ag . | No Ag . | Ag . | |
| Patient no. | ||||||
| 1 | 0 | 0.54 | 0 | 0.34 | 0 | 0.07 |
| 2 | 0.02 | 1.95 | 0.01 | 0.33 | 0 | 0.03 |
| 3 | 0 | 0.25 | 0.02 | 0.18 | 0 | 0 |
| 4 | 0 | 0.65 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0.83 | 0 | 0.48 | 0 | 0.10 |
| 6 | 0.01 | 1.18 | 0.01 | 0.65 | 0.02 | 0.8 |
| Control subject no. | ||||||
| 1 | 0 | 1.12 | 0.02 | 0.23 | 0 | 0.12 |
| 2 | 0 | 0.45 | 0 | 0.15 | 0 | 0.05 |
| . | IFN-γ alone . | IL-4 alone . | IFN-γ and IL-4 . | |||
|---|---|---|---|---|---|---|
| No Ag . | Ag . | No Ag . | Ag . | No Ag . | Ag . | |
| Patient no. | ||||||
| 1 | 0 | 0.54 | 0 | 0.34 | 0 | 0.07 |
| 2 | 0.02 | 1.95 | 0.01 | 0.33 | 0 | 0.03 |
| 3 | 0 | 0.25 | 0.02 | 0.18 | 0 | 0 |
| 4 | 0 | 0.65 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0.83 | 0 | 0.48 | 0 | 0.10 |
| 6 | 0.01 | 1.18 | 0.01 | 0.65 | 0.02 | 0.8 |
| Control subject no. | ||||||
| 1 | 0 | 1.12 | 0.02 | 0.23 | 0 | 0.12 |
| 2 | 0 | 0.45 | 0 | 0.15 | 0 | 0.05 |
Data are expressed as the percentage of positive cells expressing the indicated substance.
IFN indicates interferon; IL, interleukin; and Ag, antigen.
ELISPOT analysis in CD27+ and CD27−T-cell populations
To analyze memory-resting (MR) or ME T-cell responses, we performed some experiments comparing positively selected CD27+ T cells with CD27-depleted T cells or unfractionated populations in 2 XLA patients and 2 control subjects at 24 months after the anti-HBV vaccine protocol completion.34 We assessed the capacity of these fractionated or unfractionated cells to produce cytokines 4, 18, 48, and 96 hours after the HBenvAg stimulation, as detected by ELISPOT assay in vitro. HBenvAg was tested at different concentrations (10, 5, 1, and 0 μg/mL) and displayed a concentration-dependent response (not shown).
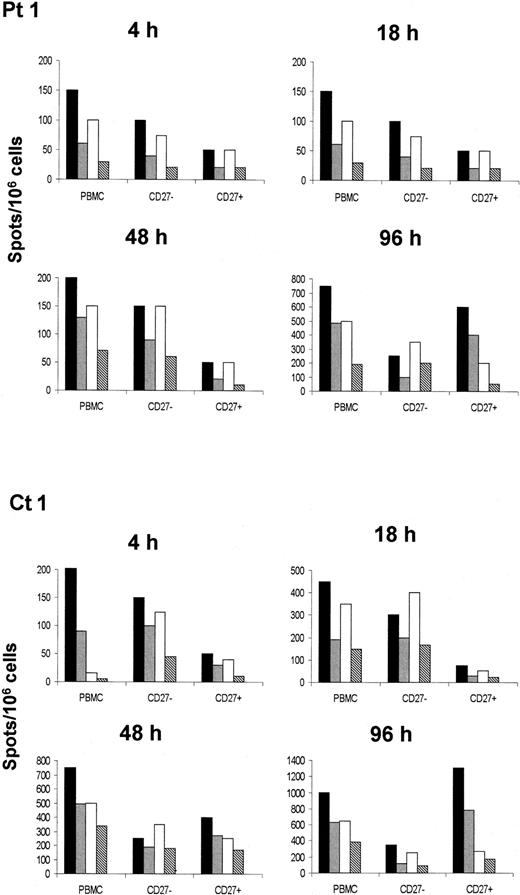
As indicated in Figure 4, both IFN-γ and IL-4 were produced in significantly higher amounts by the CD27− T cells than by their CD27+counterparts, as early as 4 hours after cultures were started in both the study populations. This is in agreement with the notion that CD27− cells correspond to effector cells requiring neither Ag restimulation nor IL-2 addition in vitro for differentiating in effectors.34 After 96 hours, the cytokine-producing cells were prevalently confined to the CD27+ population, which, in contrast to total PBMCs, showed an evident bias toward the TH1 phenotype35 (Figure 4). On the other hand, the number of both IFN-γ and IL-4 spots formed by CD27−T cells as detected 48 or 96 hours after cultures were started remained virtually unchanged, indicating that cytokine production by this T-cell subset occurred almost exclusively in the very early phase of culture. However, since ELISPOT assay of CD27+ and CD27− cells was performed 24 months after immunization in both XLA patients and healthy individuals, these data suggest that both MR and ME T cells seem to be long-lived.
Analysis of MR and ME T-cell functions.
MR T cells were distinguished from ME T cells on the basis of the presence or absence of CD27 on the cell membrane. Cell function was evaluated in terms of production of IFN-γ after stimulation with HBenvAg, 10 μg/mL (solid bars) or 1 μg/mL (gray bars), or in terms of production of IL-4 after stimulation with HBenvAg, 10 μg/mL (open bars) or 1 μg/mL (coarse bars); production was determined at the single-cell level by ELISPOT assay. Data are expressed as the number of cytokine-producing cells in 1 × 106 total PBMCs. At 24 months after the vaccination protocol was completed, PBMCs were isolated from XLA patients or control subjects and then tested in either unfractionated or purified form according to CD2 expression (of T cells) and subsequently enriched with, or depleted of, CD27+ cells as described in “Patients, materials, and methods.” The different cell populations were assayed 4, 18, 48, and 96 hours after culture in the presence of HBenvAg. Results obtained from 1 out of 2 XLA patients (Pt 1) and 1 out of 2 healthy subjects (Ct 1) are shown. Similar results were obtained in the other 2 individuals.
Analysis of MR and ME T-cell functions.
MR T cells were distinguished from ME T cells on the basis of the presence or absence of CD27 on the cell membrane. Cell function was evaluated in terms of production of IFN-γ after stimulation with HBenvAg, 10 μg/mL (solid bars) or 1 μg/mL (gray bars), or in terms of production of IL-4 after stimulation with HBenvAg, 10 μg/mL (open bars) or 1 μg/mL (coarse bars); production was determined at the single-cell level by ELISPOT assay. Data are expressed as the number of cytokine-producing cells in 1 × 106 total PBMCs. At 24 months after the vaccination protocol was completed, PBMCs were isolated from XLA patients or control subjects and then tested in either unfractionated or purified form according to CD2 expression (of T cells) and subsequently enriched with, or depleted of, CD27+ cells as described in “Patients, materials, and methods.” The different cell populations were assayed 4, 18, 48, and 96 hours after culture in the presence of HBenvAg. Results obtained from 1 out of 2 XLA patients (Pt 1) and 1 out of 2 healthy subjects (Ct 1) are shown. Similar results were obtained in the other 2 individuals.
Discussion
Owing to mutations of Btk genes, subjects affected by XLA lack circulating B cells and represent an excellent and unique model to study the role of these lymphocytes in modulating T-cell memory in humans. Indeed, although the current opinion is that B cells are unable to activate naive T lymphocytes or are even tolerogenic,36-38 several animal studies suggest that they might be required to maintain long-term T-cell memory.14-16
There is very little and contrasting data on T-cell memory in XLA patients. In one study, an impaired delayed cutaneous hypersensitivity reaction in XLA patients was reported.39 However, normal T-cell proliferation and cytokine production in response to either mitogens or tetanus toxoid (TT) in XLA patients up to 6 months after TT immunization boost have been subsequently shown.40
The conditions required to maintain T-cell memory are still the subject of intense debate and investigation. Various evidence suggests that neither B cells nor Ag persistence is required for maintaining either CD4+ or CD8+ T-cell memory, as these are both long-lived cell populations.14,41-43 On the other hand, different reports have proposed that memory CD4+ T cells require continual stimulation by APCs expressing MHC/Ag complexes to survive.14,44 According to this view, follicular DCs (FDCs) retain antigen on their surface in the form of immune complexes (ICs) for a very long time, functioning as an Ag reservoir for continual stimulation of memory CD4+ T cells.14,44 Hence, the presence of B cells producing specific Abs would be strictly necessary not only for producing the Abs required for IC formation, but also for providing an environment in which Ags may be trapped and retained.44
In order to define the role of B cells in influencing memory T-cell immunity in humans, we analyzed the HBenvAg-specific CD4+T-cell responses in terms of proliferation, frequency, and cytokine production. This study was performed on 9 XLA patients immunized against HBV who were compared with age-matched controls at different times after completion of the vaccination protocol. We found that HBenvAg-specific proliferative responses did not differ from those of controls, up to at least 24 months after vaccination. Proliferative assay quantifies the expansion of circulating-specific T cells, as assessed 6 days after incubation with HBenvAg and thus evaluates mainly the presence of memory T cells requiring Ag restimulation in order to perform their effector functions. An important factor is that memory T cells from XLA patients showed either a TH1 or a TH2 phenotype, as detected by both ELISPOT and intracytoplasmic cytokine analyses, with frequencies that did not differ from those of healthy controls up to at least 24 months after the last vaccination boost. All together, these data suggest that in contrast with most, but not all, animal models,22,23,26,36neither T-cell memory nor the balance between TH1- and TH2-cell responses are impaired by severe B-cell deficiency in humans. Furthermore, these findings might be only apparently in disagreement with a recent study that indicates a dominant TH1 polarization in TT-specific T-cell clones derived from the same type of patient.45 Indeed, our experimental procedure, monitoring antigen-specific T-cell responses ex vivo for a 24-month period after vaccination, may mainly detect early memory T cells with both TH1 and TH2 phenotype. Conversely, the Del Prete protocol, which studies the responses of long-term T-cell lines isolated more than 10 years after the first vaccination boost, may identify late memory T cells with strong TH1 polarization.45 This hypothesis may be reinforced by the possibility that the frequent bacterial infections that affect XLA patients during childhood may select a preferential TH1 polarization in the long run.46Nevertheless, our data clearly indicate that XLA patients are capable of mounting TH1- and/or TH2-cell responses, and this has important implications for setting up protective vaccination protocols against both intracellular and extracellular pathogens.
It could be argued that since XLA patients receive periodic administration of intravenous immunoglobulins, these might include specific Abs that form ICs with the immunizing HBenvAg and thus represent an important factor in maintaining the FDC-dependent memory.14 However, this possibility seems unlikely, since it has been shown that a lack of B cells cannot support the development of FDCs47 and that lymph node and splenic architecture are disrupted in both XLA patients and transgenic mice expressing nonfunctional mutated forms of Btk.48 49
We also studied the role of B cells in influencing both ME and MR T-cell immunity in humans, by performing a series of experiments using CD27-enriched and CD27-depleted T-cell populations.31,34,50 CD27 is a Traf-linked tumor necrosis factor receptor family member receptor,51 whose presence on the cell membrane has been strictly related to naive or MR T cells requiring Ag-driven proliferation for differentiating in effectors.21,31,34,50,52,53 MR T cells, also defined as “central memory,” are trapped in the secondary lymphoid organs by the lymph node homing receptors cc chemokine receptor-7 (CCR7) and L-selectin and require antigen-driven restimulation in order to differentiate into ME cells.21 In contrast, the CD27− (or the CCR7−) phenotype has been related to effector T cells, which are capable of migrating into inflamed tissues where they display prompt effector function without any need of further proliferation or cytokine intervention.21,31,34,50,52,53 The capability of MR T cells to provide effector T cells more promptly than naive T cells is essential to efficiently control those pathogens to which a given individual has been previously exposed. We confirm that the HBenvAg-specific CD27− T cells are bona fide ME cells since they performed their functions of cytokine synthesis within a few hours of contact with Ag in both vaccinated XLA patients and healthy controls. In contrast, MR T cells were confined to the CD27+ population and exerted full effector function only after several hours of Ag stimulation, suggesting that they require Ag-induced proliferation in vitro.34 The finding that the CD27+-enriched cell population produced only IFN-γ after 96 hours of culture, while total PBMCs produced both IFN-γ and IL-4, is in agreement with previous reports indicating that CD27 distinguishes a functionally distinct, but not the entire, memory T-cell population.35 Collectively, our data suggest that neither MR nor ME T cells require B cells or persisting Ags in humans because of the following considerations. First, both CD27+MR and CD27− ME T cells were retained in XLA patients lacking B cells 24 months after completion of vaccination, favoring the hypothesis that not only the MR, but also the ME, T-cell population is long-lived.41,54-56 This is further supported by the finding that the intracytoplasmic cytokines were detected in CD4+ T cells within a few hours of Ag stimulation, and again 24 months after vaccination. Second, it is very unlikely that a soluble antigen, such as the recombinant form of HBenvAg, can persist up to 2 years after the last vaccination boost in XLA patients lacking both B cells and FDCs, which are the most important candidates for the long-term storage of soluble Ag.14 Nonetheless, we cannot exclude the hypothetical intervention of cross-reactive Ags to stimulate T cells by Ag mimicry, although this hypothesis is contradicted by the observation that memory T cells are maintained for a very long time after their transfer in class I– or class II–deficient MHC molecule recipients.41 42
In conclusion, in this report we demonstrate that XLA patients immunized against HBV show a robust antigen-specific T-cell response in vitro, which is retained for at least 24 months after vaccination. Analysis of HBenvAg-specific cytokine production performed at the single-cell level by ELISPOT or by flow cytometry revealed that both MR and ME CD4+ T cells with TH1, TH2, or TH0 phenotype are long-lived in these patients, as well as in healthy controls. All these data lead us to conclude that severe B-cell deficiency in humans does not impair the generation of an effective Ag-specific T-cell memory. The detection of HBenvAg-specific MR and ME T cells with either TH1 or TH2 phenotype may provide important building blocks for setting up highly protective, T-cell–based vaccination protocols in XLA patients and for devising innovative strategies for the manipulation of antimicrobial responses in these subjects.
Supported by Ministero della Sanità-Istituto Superiore di Sanità (Progetti AIDS 1998-2000 and Epatite Virale 1997-1999) (V.B.); Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MURST), 40% 1997-1999 (V.B.); Progetto Associazione Italiana Sclerosi Multipla (AISM), 1997-1999 (V.B.); Progetto Finalizzato CNR Biotecnologie, European Community contract BMH4-CT98-3703 (V.B.); and MURST, 40% 1999 (A.P.).
M.P. and D.A. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Vincenzo Barnaba, Fondazione Andrea Cesalpino, Dipartimento di Medicina Interna, Policlinico Umberto I, V le del Policlinico 155, 00161, Rome, Italy; e-mail:vincenzo.barnaba@uniroma1.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal