Abstract
Interleukin 17 (IL-17) is a proinflammatory cytokine produced by activated CD4+ memory T cells. We previously showed that IL-17 increased the growth rate of human cervical tumors transplanted into athymic nude mice. To address the possible role of T cells in the biologic activity of IL-17 for tumor control, we grafted 2 murine hematopoietic immunogenic tumors (P815 and J558L) transfected with a complementary DNA encoding murine IL-17 into syngeneic immunocompetent mice. We found that growth of the 2 IL-17–producing tumors was significantly inhibited compared with that of mock-transfected tumors. In contrast to the antitumor activity of IL-17 observed in immunocompetent mice, we observed no difference in the in vivo growth of IL-17–transfected or mock-transfected P815 cells (P815–IL-17 and P815-Neo, respectively) transplanted into nude mice. We then showed that IL-17 increased generation of specific cytolytic T lymphocytes (CTLs) directed against the immunodominant antigens from P815 called A, B, C, D, and E, since all mice injected with P815–IL-17 developed a P815-specific CTL response, whereas only 6 of 16 mice immunized with P815-Neo had a specific CTL response against the antigens. The induction of CTLs was associated with establishment of a tumor-protective immunity. These experiments suggest that T lymphocytes are involved in the antitumor activity of IL-17. Therefore, IL-17, like other cytokines, appears to be a pleiotropic cytokine with possible protumor or antitumor effects on tumor development, which often depends on the immunogenicity of tumor models.
Introduction
Interleukin-17 (IL-17), originally identified by Rouvier et al1 as cytolytic T-lymphocyte (CTL)–associated antigen 8, is a T-cell–derived cytokine with homology toHerpesvirus saimiri.1,2 It is expressed mainly by activated CD4+CD45RO+ memory T cells.3,4 CD4 T cells can be classified into T-helper (Th) 1 cells, which secrete interferon (IFN) γ, IL-2, and tumor necrosis factor (TNF) β; Th2 cells, which produce IL-4, IL-5, IL-6, IL-10, and IL-13; and Th0 cells, a common precursor with the ability to release both IFNγ and IL-4. Thirty percent of Th0/Th1 clones have been shown to produce IL-17, whereas Th2 clones never express IL-17.5IL-2 and IL-15 were found to increase IL-17 production by human peripheral blood (PB) mononuclear cells.6
IL-17 is considered to be a proinflammatory cytokine because it increases IL-6 and IL-8 production by macrophages, fibroblasts, keratinocytes, and synovial cells2,3,7-9 and nitric oxide production by human osteoarthritic cartilage.10,11 IL-17 also induces secretion of IL-1β and TNF-α by human macrophages and endothelial cells.7,12 IL-17 activates the nuclear factor κB and activator protein 1 transcription factors, which may explain its proinflammatory properties.10,13 In addition, IL-17 induces production of granulocyte colony-stimulating factor and CXC chemokines that stimulate granulopoiesis and recruitment of neutrophils into tissues.14-16
The IL-17 receptor is a type I transmembrane protein that is expressed in virtually all cells and tissues, in contrast to the restricted expression of IL-17, which is confined to T cells.2,17This receptor has no sequence similarity with any other known cytokine receptor. It interacts with the adapter molecule TNF receptor–associated factor 6, which is required for IL-17 signaling.18
The exact role of IL-17 in disease is unknown. IL-17 has been found to promote cartilage destruction in various forms of arthritis.19,20 Overproduction of IL-17 has been observed in the synovium of patients with rheumatoid arthritis21 and in PB lymphocytes from patients with systemic sclerosis.22
In a previous study, we found that IL-17 promotes tumorigenicity of human cervical tumors in nude mice.23 Because this paradoxical tumor-promoting activity of IL-17 in the absence of T cells was unexpected, we conducted the current study to analyze the effect of IL-17 on the growth of syngeneic tumors in immunocompetent mice. We found that IL-17 inhibits the growth of 2 hematopoietic tumors, mastocytoma P815 and plasmocytoma J558L. Our findings strongly suggest that T cells are involved in this antitumor activity of IL-17.
Materials and methods
Mice and tumor cell lines
Female Balb/c, DBA/2, and athymic nude/nude mice 6 to 8 weeks of age (Iffa Credo L'Arbresle, France) were used in this study. The mouse plasmocytoma J558L and mastocytoma P815 cell lines were obtained from the American Type Culture Collection (Manassas, VA). The mouse squamous cell carcinoma KLN 205 was purchased from the European Collection of Cell Culture (Salisbury, Wiltshire, United Kingdom). The cell lines were cultured in RPMI supplemented with 10% fetal-calf serum (FCS), 2 mM L-glutamine (Sigma, St Louis, MO), 5 mM sodium pyruvate, 50 IU/mL penicillin, 50 μg/mL streptomycin, and 0.01% mercaptoethanol. P815 A-E−, an antigen-loss variant of P815 that had lost the 5 immunodominant antigens (A, B, C, D, and E) recognized by CTLs directed against P815, was obtained from the Ludwig Institute, Brussels, Belgium (Dr G. Warnier).
Transfection of P815 and J558L cells
A complementary DNA (cDNA) encoding murine IL-17 (mIL-17) was inserted into an expression vector under the control of the Sr-α promoter in pBR322, into which a neomycin resistance gene (NT-Neo) was introduced.24 NT-Neo containing or lacking the 1100-base-pair mIL-17 cDNA was linearized with ScaI restriction enzyme and stably transfected by electroporation into P815 and J558L. Electroporation was done with a Bio-Rad Gene Pulser (Hercules, CA) at a voltage of 260 V with a capacitance of 960 μF. Seventy-two hours after electroporation, transfectants were selected by culture in RPMI 1640 (Gibco-Life Technologies, Paisley, United Kingdom), supplemented with 1 mg/mL G418 (Geneticin; Gibco-Life Technologies). G418-resistant clones were expanded in selection medium and tested for expression of IL-17.
Enzyme-linked immunosorbent assay (ELISA) for mIL-17
An ELISA was used to measure mIL-17 in culture supernatants. Briefly, 96 breakaway microtiter plates with flat-bottomed wells (Nunc, Denmark) were coated with 100 μL anti–IL-17 antibody 8H4 (1 μg/mL) diluted in carbonate buffer (0.1 M sodium carbonate and sodium bicarbonate [pH 9.6]) overnight at 4°C. The plates were then saturated with 200 μL phosphate-buffered saline (PBS) and 1% bovine serum albumin (BSA) for 1 hour at room temperature. After washes with PBS and 0.05% Tween 20 (Merck, Schchardt, Germany), 100 μL recombinant mIL-17 or samples diluted in PBS and 1% BSA were added and incubation was allowed to proceed for 1.5 hours at 37°C. After washes, the plates were incubated with 100 μL nitrophenol (NIP)–labeled anti-IL-17 mAb 18H10 (0.5 μg/mL) diluted in PBS and 1% BSA for 1 hour at 37°C. After washes, 100 μL peroxidase-labeled anti-NIP (0.5 μg/mL) was added. One hour later, the reaction was revealed by addition of the peroxidase substrate (o-phenylenediamine dihydrochloride), and the optical density was read at a wavelength of 492 nm.
Reverse transcriptase–polymerase chain reaction amplification for expression of mIL-17 messenger RNA
RT-PCR was done as described previously.25 The following oligonucleotides were used: hypoxanthine phosphoribosyl transferase (HGPRT) sense, GTTGGATACAGGCCAGACTTTGTTG; HGPRT antisense, GATTCAACTTGCGCTCATCTTAGGC; mIL-17 sense, CACCCAGAGCACCAGCTGAT; and mIL-17 antisense, AATCAATAGCACGAACTG.
In vitro growth assay
P815 and J558L were plated on 96-well flat-bottomed plates at different concentrations. Cells were cultured for 3 days, and their proliferation was determined by using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described previously.23
Tumor growth in mice
P815 and J558L tumor cells (1.5 × 106) were injected subcutaneously into DBA/2 and Balb/c mice, respectively. In some experiments, tumor cells were injected into athymic nude/nude mice. Eight to 10 mice per group were used in each experiment. Tumor volume (in microliters) was estimated from the dimensions of the tumor by using the following formula: volume = ab2/2, in which a was the length and b the width of the tumor.
Immunization and detection of P815 ABCDE–specific CTLs
Live P815–IL-17 or P815-Neo cells were injected into mice subcutaneously at 2 sites (5 × 105 cells/site). Two weeks later, blood (100 μL) was collected. In vitro stimulation of blood lymphocytes was done by mixing 3 × 105 lymphocytes purified with the Ficoll method with 105 irradiated (100 Gy) P815 cells and 2 × 106 irradiated (30 Gy) normal syngeneic spleen cells in 48-well plates (total volume, 0.8 mL). Cells were cultured for 7 days in Dulbecco modified Eagle medium supplemented with 10% FCS, L-arginine hydrochloric acid (116 mg/mL), L-asparagine (36 mg/mL), L-glutamine (216 mg/mL), glucose (4.5 g/L final concentration), 10 mM HEPES, and 5 × 10−5M2-mercaptoethanol.
Effector cells were harvested and then tested against P815 or P815A-E− targets by using a conventional 4-hour chromium 51 (51Cr) release assay as described previously.26 Briefly, effector cells were diluted in duplicate in V-bottomed microtiter plates and mixed with 2000 51Cr-labeled target cells in a final volume of 150 μL. Six different effector-to-target ratios were tested. In all experiments, a 50-fold excess of cold P815A-E− cells were mixed with labeled targets to eliminate all cytotoxicity directed against the nonimmunodominant A, B, C, D, or E antigens derived from P815. Supernatants (100 μL) were collected after incubation for 4 hours at 37°C, and radioactivity was measured by using a 96-well plate counter (Wallac; Perkin Elmer, France). Data were expressed as P815 ABCDE–specific lytic units (LUs)/106 effector cells, obtained after subtraction of the LUs measured against the targets negative for P815A-E−.
LUs were defined as the number of effector cells that lysed 50% of 104 targets cells in 4 hours. This number was estimated by regression (1−e−kx) from the specific release obtained at 3 different effector-to-target ratios chosen in the linear range of the lysis curve. Results are expressed in number of LUs/106effector cells. An LU less than 0.1 was considered to be negative.
Tumor-protection experiments
DBA/2 mice were immunized with 5 × 104 live IL-17–transfected P815 cells. Eighteen days later, tumor-free mice were challenged with either wild-type P815 cells or KLN 205 carcinoma cells. In other experiments, DBA/2 mice were immunized with 5 × 104 IL-17–transfected P815 A-E− cells. Fifteen days later, tumor-free mice were challenged with wild-type P815 A-E− cells.
Statistical analysis
The Mann-Whitney test was used to analyze the in vivo differences in tumor size among the various groups of mice.
Results
Characterization of IL-17–transfected P815 and J558L cell lines
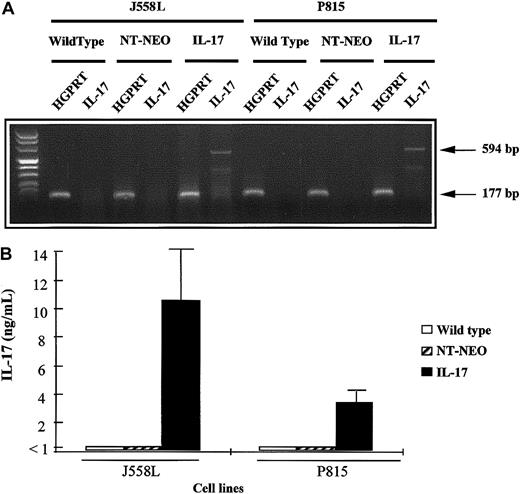
We transfected 2 hematopoietic cell lines, the mastocytoma P815 line and the plasmocytoma J558L line, with a cDNA encoding murine IL-17. Stable transfectants that expressed IL-17 mRNA after PCR amplification were obtained. In contrast, no mRNA expression for IL-17 was found in the parental cell lines or mock-transfected tumors (Figure1A). Because this IL-17 signal could have been derived from contaminating plasmids associated with the extracted mRNA, we also assessed the culture supernatant for the presence of the protein. P815–IL-17 and J558L–IL-17 cells cultured for 72 hours secreted considerable amounts of IL-17 in their supernatants (3.5 ng/mL to 10 ng/mL for 5 × 105 cells; Figure 1B).
Characterization of mIL-17–transfected P815 and J558L cell lines.
(A) The cDNA derived from mRNA extracted from wild-type, mock-transfected (NT-Neo), or mIL-17–transfected tumor cells (P815 or J558L) was amplified by PCR using primers specific for HGPRT or IL-17 mRNA. Amplified PCR products were then loaded on a 2% agarose gel and stained with ethidium bromide for UV visualization. (B) Wild-type, mock-transfected, or IL-17–transfected tumor cells (P815 or J558L) were plated on 24-well flat-bottomed plates at a density of 5 × 105 cells per well. The cells were cultured for 72 hours, and IL-17 concentrations in the supernatants were measured by ELISA.
Characterization of mIL-17–transfected P815 and J558L cell lines.
(A) The cDNA derived from mRNA extracted from wild-type, mock-transfected (NT-Neo), or mIL-17–transfected tumor cells (P815 or J558L) was amplified by PCR using primers specific for HGPRT or IL-17 mRNA. Amplified PCR products were then loaded on a 2% agarose gel and stained with ethidium bromide for UV visualization. (B) Wild-type, mock-transfected, or IL-17–transfected tumor cells (P815 or J558L) were plated on 24-well flat-bottomed plates at a density of 5 × 105 cells per well. The cells were cultured for 72 hours, and IL-17 concentrations in the supernatants were measured by ELISA.
We then investigated the growth rate of wild-type, mock-transfected, and IL-17–transfected cell lines. We found that IL-17–transfected and mock-transfected tumors and parent cells (P815 and J558L) had identical in vitro growth; Figure 2 shows a typical experiment. Similar results were obtained after the cells were counted on slides (data not shown).
In vitro proliferation of parental, mock-transfected, and IL-17–transfected P815 and J558L cells.
Wild-type, mock-transfected (NT-Neo), or IL-17–transfected P815 (A) or J558L (B) cells were plated on 96-well flat-bottomed plates at a density ranging from 102 to 2 × 103 cells per well. The cells were cultured for 3 days, and their proliferation was determined by MTT assay. For these experiments, all cells were cultured in the same medium without G418 or neomycin. For each cell line, a standard curve between the absorbance in the MTT test and the number of cells was determined. Values are mean results from triplicate cultures and are representative of 3 experiments.
In vitro proliferation of parental, mock-transfected, and IL-17–transfected P815 and J558L cells.
Wild-type, mock-transfected (NT-Neo), or IL-17–transfected P815 (A) or J558L (B) cells were plated on 96-well flat-bottomed plates at a density ranging from 102 to 2 × 103 cells per well. The cells were cultured for 3 days, and their proliferation was determined by MTT assay. For these experiments, all cells were cultured in the same medium without G418 or neomycin. For each cell line, a standard curve between the absorbance in the MTT test and the number of cells was determined. Values are mean results from triplicate cultures and are representative of 3 experiments.
Tumor growth of mock-transfected and IL-17–transfected P815 and J558L cells in immunocompetent mice
The cell lines P815 and J558L, transfected with mIL-17 cDNA and then transplanted into immunocompetent DBA/2 and Balb/c mice, respectively, had a slower growth rate than mock-transfected cells (Figure 3A and 3B). For example, on day 19 after transplantation, a mean tumor volume of 2540 μL was observed with transplanted mock-transfected P815 cells (P815-Neo), whereas the tumor volume of the IL-17–transfected P815 cells was 938 μL (P < .05). This inhibitory activity of IL-17 on tumor growth was often apparent 12 to 15 days after tumor transplantation (Figure 3A and 3B). We did not find any significant difference between the growth of parental and mock-transfected tumors (data not shown). When high concentrations of tumor cells (> 106 cells) were used, IL-17 did not affect the tumor incidence rate, but when low concentrations were employed, the uptake of IL-17–expressing tumors decreased (data not shown).
IL-17–transfected and mock-transfected tumors showed identical in vitro growth.
Mock-transfected or IL-17–transfected P815 (A) or J558L (B) cells were injected into DBA/2 and Balb/c mice, respectively, by subdermal inoculation of 1.5 × 106 cells. Eight to 10 mice per group were used in each experiment, and tumor size was assessed. Results are representative of 2 independent experiments. The asterisk indicates significant results (P < .05).
IL-17–transfected and mock-transfected tumors showed identical in vitro growth.
Mock-transfected or IL-17–transfected P815 (A) or J558L (B) cells were injected into DBA/2 and Balb/c mice, respectively, by subdermal inoculation of 1.5 × 106 cells. Eight to 10 mice per group were used in each experiment, and tumor size was assessed. Results are representative of 2 independent experiments. The asterisk indicates significant results (P < .05).
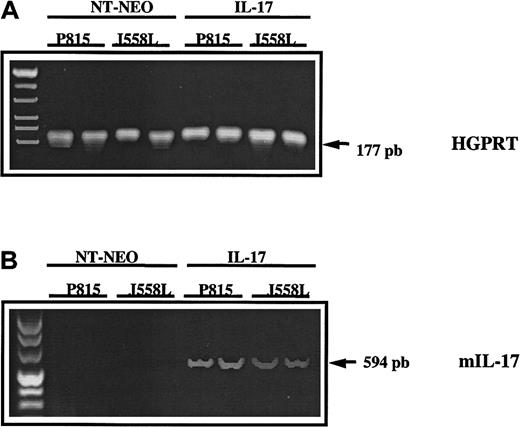
To determine the in vivo persistence of the IL-17 transgene in tumors transfected with mIL-17 cDNA and transplanted into mice, biopsies were done on day 20 after transplantation. Murine IL-17 cDNA was detected in all 4 biopsy specimens from mice given transplants of P815–IL-17 or J558L–IL-17 cells but was not observed in mice previously given injections of the control transfectants P815-Neo or J558L-Neo. These findings indicated that the transgene was not lost during tumor development (Figure 4).
In vivo persistence of the IL-17 transgene in tumor transfected with mIL-17 cDNA and transplanted into mice.
The cDNA derived from mRNA extracted from mock-transfected (NT-Neo) or IL-17–transfected P815 or J558L cells was amplified by PCR using primers specific for murine HGPRT (A) or mIL-17 (B). Amplified PCR products were loaded on a 2% agarose gel and stained with ethidium bromide for UV visualization.
In vivo persistence of the IL-17 transgene in tumor transfected with mIL-17 cDNA and transplanted into mice.
The cDNA derived from mRNA extracted from mock-transfected (NT-Neo) or IL-17–transfected P815 or J558L cells was amplified by PCR using primers specific for murine HGPRT (A) or mIL-17 (B). Amplified PCR products were loaded on a 2% agarose gel and stained with ethidium bromide for UV visualization.
Role of T lymphocytes in the antitumor activity of IL-17
Loss of IL-17–mediated antitumor activity in nude mice.
To investigate the possible role of T lymphocytes in the antitumor activity of IL-17, we transplanted mIL-17–transfected or mock-transfected P815 cells into nude mice. In contrast to our finding of inhibitory activity of IL-17 in immunocompetent mice, we observed no difference in the in vivo growth of P815–IL-17 or P815 cells transfected with the control plasmid P815-Neo (Figure5). In agreement with these results, IL-17 also did not inhibit growth of the J558L lymphoma in nude mice (data not shown).
IL-17 does not inhibit growth of the P815 mastocytoma in nude mice.
P815 cells transfected with cDNA encoding murine IL-17 (P815–IL-17) or with NT plasmid alone (Neo) were injected into nude mice by subdermal inoculation of 1.5 × 106 cells. Eight mice per group were used in each experiment, and tumor size was assessed. Results are representative of 2 independent experiments.
IL-17 does not inhibit growth of the P815 mastocytoma in nude mice.
P815 cells transfected with cDNA encoding murine IL-17 (P815–IL-17) or with NT plasmid alone (Neo) were injected into nude mice by subdermal inoculation of 1.5 × 106 cells. Eight mice per group were used in each experiment, and tumor size was assessed. Results are representative of 2 independent experiments.
IL-17 increases generation of P815-specific CTLs.
P815 cells express 5 immunodominant antigens (A, B, C, D, and E) recognized by most syngeneic CTLs.26 To measure specifically the induction of CTLs directed against these antigens, we used, in our cytotoxicity assay directed against P815, a 50-fold excess of an antigen-loss variant of P815 (P815A-E−) that no longer expressed these antigens. All mice given injections of P815–IL-17 developed a P815-specific CTL response, since the PB lymphocytes of 10 of the 16 immunized mice had high levels of cytotoxicity (LUs > 1/106 effector cells) directed against the immunodominant antigens of P815, whereas those of the remaining 6 mice showed moderate P815-specific CTL activity (Figure 6).
IL-17 increases the generation of P815-specific CTLs.
DBA/2 mice were given a subcutaneous injection of live P815–IL-17 or P815 Neo cells at 2 sites (5 × 105 cells/site). Fifteen days later, whole PB was collected and stimulated in vitro with irradiated P815. CTL activity was measured 7 days later by using a51Cr-release assay on targets P815 or P815 A-E−, an antigen-loss variant of P815 that had lost the 5 immunodominant antigens A, B, C, D, and E. Each point represents the lytic activity of the PB lymphocytes from an individual mouse. To measure induction of CTLs against the immunodominant antigens specifically, we used a 50-fold excess of cold P815 A-E− cells during the assay. Data are expressed as P815 ABCDE–specific LUs/106 effector cells, obtained after subtraction of the LUs measured against the targets negative for P815 A-E−.
IL-17 increases the generation of P815-specific CTLs.
DBA/2 mice were given a subcutaneous injection of live P815–IL-17 or P815 Neo cells at 2 sites (5 × 105 cells/site). Fifteen days later, whole PB was collected and stimulated in vitro with irradiated P815. CTL activity was measured 7 days later by using a51Cr-release assay on targets P815 or P815 A-E−, an antigen-loss variant of P815 that had lost the 5 immunodominant antigens A, B, C, D, and E. Each point represents the lytic activity of the PB lymphocytes from an individual mouse. To measure induction of CTLs against the immunodominant antigens specifically, we used a 50-fold excess of cold P815 A-E− cells during the assay. Data are expressed as P815 ABCDE–specific LUs/106 effector cells, obtained after subtraction of the LUs measured against the targets negative for P815 A-E−.
In contrast, only 6 of the 16 mice immunized with P815-Neo had a specific CTL response against the P815 immunodominant antigens (Figure6). This response was considered to be strong in only 2 of those mice (12%), whereas it was strong in 10 of the mice (62%) in which IL-17–producing tumor cells were used as immunogens (Figure6). Mice not given transplants (controls) had no P815-specific CTL response (data not shown). The increase in CTL generation induced by IL-17 was not restricted to immunodominant antigens, since enhancement of CTL activity specific for P815 A-E− was also observed in spleens of mice in which IL-17–transfected P815 A-E− cells had been transplanted (data not shown).
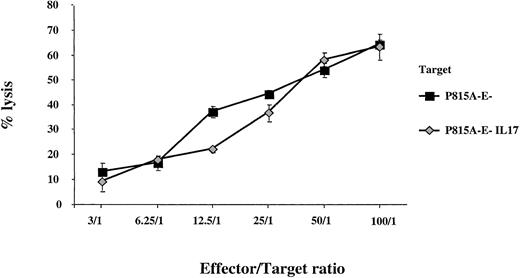
To eliminate a possible direct activity of IL-17 on CTL effector capacity, we measured the antitumor cytotoxicity of CTLs specific for P815 A-E− against P815 A-E− tumor cells either producing or not producing IL-17. We did not find any enhancement of the lysis of IL-17–transfected P815A-E− cells compared with that of P815A-E− cells (Figure 7). In addition, no increase in expression of CTL effector molecules, such as perforin or Fas-L, was observed when enriched CD8 T cells were stimulated with recombinant IL-17 (data not shown).
Analysis of the activity of IL-17 on CTL effector capacity.
We selected CTLs specific for P815 A-E− and compared their cytolytic activity with that of parent P815 A-E− cells or IL-17–producing P815 A-E− tumor cells. The target cells were labeled with chromium and incubated at a constant density with CTLs specific for anti-P815 A-E− at different effector-to-target ratios.
Analysis of the activity of IL-17 on CTL effector capacity.
We selected CTLs specific for P815 A-E− and compared their cytolytic activity with that of parent P815 A-E− cells or IL-17–producing P815 A-E− tumor cells. The target cells were labeled with chromium and incubated at a constant density with CTLs specific for anti-P815 A-E− at different effector-to-target ratios.
It is notable that the ability of IL-17 to increase the generation of CTLs against the immunodominant antigens of P815 was observed only when live tumor cells were used in the immunization procedure. In experiments in which irradiated P815-Neo or P815–IL-17 cells were injected into mice, IL-17 production did not confer any benefit on the in vivo frequency of CTL induction (data not shown).
Immunization with P815–IL-17 cells provides partial protection against a challenge with wild-type P815.
We tested whether induction of CTLs was associated with establishment of tumor-protective immunity. Because 40% to 60% of mice do not develop tumors when given subcutaneous injections with a small number of P815–IL-17 cells (5 × 104), we challenged tumor-free mice with parental P815. In the absence of immunization, all mice developed tumors when given transplants of P815 cells. In some experiments, a few spontaneous rejections subsequently occurred (Figure 8A).
Immunization with P815–IL-17 cells partly protects against challenge with wild-type P815 cells.
DBA/2 mice were immunized with live IL-17–transfected P815 cells (5 × 104). Eighteen days later, tumor-free mice were challenged with either wild-type P815 cells (5 × 105; A) or KLN 205 melanoma cells (106; B). Ten to 12 mice per group were used for each experiment. (C) DBA/2 mice were either not immunized or immunized with 5 × 104 IL-17–transfected P815 A-E− cells. Fifteen days later, tumor-free mice were challenged with wild-type P815 A-E− cells (5 × 105).
Immunization with P815–IL-17 cells partly protects against challenge with wild-type P815 cells.
DBA/2 mice were immunized with live IL-17–transfected P815 cells (5 × 104). Eighteen days later, tumor-free mice were challenged with either wild-type P815 cells (5 × 105; A) or KLN 205 melanoma cells (106; B). Ten to 12 mice per group were used for each experiment. (C) DBA/2 mice were either not immunized or immunized with 5 × 104 IL-17–transfected P815 A-E− cells. Fifteen days later, tumor-free mice were challenged with wild-type P815 A-E− cells (5 × 105).
In contrast, 80% of mice immunized with live P815–IL-17 cells were protected against challenge with wild-type P815 (Figure 8A). In control experiments to assess the specificity of this protection, we found that when irrelevant tumors, such as the KLN 205 melanoma, were transplanted into mice with or without immunization by P815–IL-17 cells, all mice developed tumors (Figure 8B). It was difficult to compare the protection induced by P815-IL–17 cells with that generated by P815 cells because, even at low concentrations, less than 15% of mice remained free of tumors after transplantation with P815 cells. Partial tumor protection against wild-type P815 A-E− cells was also observed in mice previously immunized with IL-17–transfected P815 A-E− cells (Figure 8C). In agreement with the results of the cytotoxicity assay, when mice were immunized with irradiated P815–IL-17 or P815 Neo and then challenged with wild-type P815, the tumor incidence ranged from 70% to 80%, with no differences related to the type of cells used in the vaccination protocol (data not shown).
Discussion
In this study, we found that IL-17 inhibited the growth rate of 2 hematopoietic tumors, mastocytoma P815 and lymphoma J558L, that were transplanted into immunocompetent mice. A direct effect of IL-17 on the in vivo proliferation of tumors seems unlikely to have occurred because wild-type, mock-transfected, and IL-17–transfected tumors showed the same in vitro proliferation kinetics (Figure 2). Several findings strongly suggest that a host-dependent mechanism involving T lymphocytes was involved in the antitumor activity of IL-17. First, when transplanted into nude mice, IL-17–transfected P815 cells and P815 cells transfected with the vector alone did not show any difference in growth in vivo (Figure 5). Second, IL-17 increased the generation of P815-specific CTLs, since all mice injected with P815–IL-17 developed a P815-specific CTL response, whereas CTL activity in the spleen was only found in 37% of mice given transplants of mock-transfected P815 (Figure 6). Third, when immunized with P815–IL-17, 80% of mice were protected against challenge with wild-type P815. Because of the unexpected tumor-inhibitory effects of control immunoglobulins, we could not demonstrate directly by means of depletion experiments that this protection was mediated by T lymphocytes. However, we showed that this tumor immunity was specific to the tumor cells used for immunization, since no protection was observed in mice challenged with irrelevant tumors. Furthermore, other researchers previously found that induction of protective immunity against P815 is always under the control of T lymphocytes.27 28
Different mechanisms may contribute to the IL-17 enhancement of tumor-specific T cells. We and others showed that IL-17 increases production of IL-6 by different cell lines.23,29 In addition, we here observed increased expression of IL-6 in biopsy specimens from IL-17–producing tumors compared with parent or mock-transfected tumors (data not shown). Because previous studies in immunogenic models showed that the antitumor activity of IL-6 was associated with induction of tumor-specific CTLs,30-32IL-6 may play a role in the activation of specific T cells by IL-17. Jovanovic et al7 reported that IL-17 stimulated the secretion of IL-12 by macrophages. IL-12 is known to be a cytokine that promotes Th1 immunity and leads to activation of CTLs.33It has also been used as an adjuvant to elicit CTLs against tumor antigens in mice.34
Increasing evidence suggests that tumor cells do not directly activate T lymphocytes and that dendritic cells (DCs) are responsible for the presentation of tumor antigens to T cells.35,36T-cell priming during tumor development may be inefficient because DCs are most often blocked in an immature state inside tumors.37 IL-17 promotes maturation of DC progenitors, as indicated by increases in cell-surface expression of costimulatory molecules, major histocompatibility complex class II antigens, and allostimulatory capacity when CD11+ DCs were treated with IL-17.38 This may lead to an improvement in T-cell priming by tumor cells producing IL-17. Furthermore, we have found that IL-17 recruits cells from the monocyte-macrophage lineage in the tumor microenvironment23 (data not shown) and that this process may be the result of induction of specific chemokines.39 In contrast, in neither the previous study23 nor the current study (data not shown) did we observe any recruitment of neutrophils at the tumor site.
A direct role of IL-17 on T lymphocytes could not be excluded because an IL-17 antagonist inhibited IL-2 production and T-cell proliferation induced by alloantigens or mitogens, suggesting a key role for endogenously produced IL-17 in T-cell growth.2 40 However, we did not observe a direct influence of IL-17 on the CTL effector capacity, since IL-17 did not enhance the activity of specific CTLs in a cytotoxicity assay. IL-17 also did not increase expression of CTL effector molecules such as perforin or Fas-L (data not shown).
It is notable that we found that irradiation of tumor cells eliminated the IL-17 effect on the enhancement of specific CTLs and the induction of tumor immunity. Other researchers have also reported that proliferating tumor cells deliver a stronger immunogenic signal than nonproliferating tumors.41 Furthermore, in some models, irradiation enhances the immunogenicity of tumors, and this could compete with and mask the adjuvant properties of other immunostimulatory molecules.42
Although our results indicate a role for T cells in the antitumor activity of IL-17, we cannot exclude the possibility that other effectors cooperate with T cells to inhibit tumor growth. Enhancement of natural killer activity appears to be associated with reduction of metastatic lung nodules in mice given transplants of tumors transfected with IL-17.43
In conclusion, IL-17 appears to be a pleiotropic cytokine, since human tumors transfected with IL-17 showed increased growth in nude mice,23 whereas immunogenic tumors were inhibited in immunocompetent mice by means of a T-cell–dependent mechanism. These opposite activities in the regulation of tumor growth are not restricted to IL-17 but may also be demonstrated by other cytokines.44,45 For example, IL-6 is considered to be an in vivo growth factor for lymphoma or plasmocytoma transplanted into immunocompetent mice, and it was found to increase the proliferation of human tumors transplanted into nude mice.46-48 However, IL-6 also inhibited the growth of immunogenic tumors by stimulating antitumor T-cell activity.30-32,49,50 Similarly, several studies showed that IL-4 has a potent antitumor activity that does not appear to be T-cell dependent.51,52 In contrast, a protumor activity of IL-4 mediated by Th2 lymphocytes has also been reported.53These antagonistic activities of cytokines in the regulation of tumor growth, which often depends on the immunogenicity of the tumor model, must be taken into consideration when these molecules are used in trials of immunotherapy.
We thank Anne Moineau and Isabelle Joyeux for expert technical assistance and John Abrams for providing the anti–IL-17 antibodies.
Note: During revision of the manuscript for this paper, Hirahara et al54 showed that IL-17 induced a T-cell–dependent, tumor-specific immunity in another tumor model.
Supported by Université Pierre et Marie Curie, the Institut National de la Santé et de la Recherche Médicale, the Ligue Nationale Contre le Cancer l'Association pour la Recherche contre le Cancer, and the Indo-French Center for the Promotion of Advanced Research. A.C. was a fellow of the Ligue Nationale contre le Cancer and the Fondation pour la Recherche Médicale.
F.F. has declared a financial interest in Schering Plough, whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Eric Tartour, Unité d'Immunologie Biologique, Hopital Européen Georges Pompidou, 20 Rue Leblanc, 75908 Paris Cedex 15; e-mail: eric.tartour@egp.ap-hop-paris.fr.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal