Abstract
Chronic myeloid leukemia is a clonal myeloproliferative expansion of transformed primitive hematopoietic progenitor cells characterized by high-level expression of BCR-ABL chimeric gene, which induces growth factor independence. However, the influence of BCR-ABL expression on cell-mediated cytotoxicity is poorly understood. In the present study, we asked whether BCR-ABL expression interferes with leukemic target sensitivity to natural killer (NK) cell cytolysis. Our approach was based on the use of 2 BCR-ABL transfectants of the pluripotent hematopoietic cell line UT-7 expressing low (UT-7/E8, UT-7/G6) and high (UT-7/9) levels of BCR-ABL. As effector cells, we used CD56bright, CD16−, CD2− NK cells differentiated in vitro from CD34 cord blood progenitors. We demonstrated that BCR-ABL transfectants UT-7/9 were lysed by NK cells with a higher efficiency than parental and low UT-7/E8.1 and UT-7/G6 transfectants. This enhanced susceptibility to lysis correlated with an increase in expression of intercellular adhesion molecule 1 (ICAM-1) by target cells. Treatment of UT-7/9 cells by STI571 (a specific inhibitor of the abl kinase) resulted in a decrease in NK susceptibility to lysis and ICAM-1 down-regulation in target cells. Furthermore, the constitutive activation of nuclear factor-κB (NF-κB) detected in BCR-ABL transfectant UT-7/9, was significantly attenuated when cells were treated by STI571. Interestingly, inhibition of NF-κB activation by BAY11-67082 (a specific NF-κB inhibitor) resulted in down-regulation of ICAM-1 expression and a subsequent decrease in NK-induced killing of UT-7/9 transfectants. Our results show that oncogenic transformation by BCR-ABL may increase susceptibility of leukemic progenitors to NK cell cytotoxicity by a mechanism involving overexpression of ICAM-1 as a consequence of NF-κB activation.
Introduction
Natural killer (NK) cells play an important role in the early defense against viral and malignant transformation. Their activity is characterized as nonadaptive and major histocompatibility complex (MHC)–unrestricted and is thought to play an important role in immune surveillance. Recent studies have shown that NK cell recognition is based on the expression of multiple cell surface receptors that bind either HLA class I or non-HLA ligands and transduce either inhibitory or activating signals.1 The balance between these signals controls NK cell activation. In addition, adhesion molecules of the β2-integrin family, such as the intercellular adhesion molecule 1 (ICAM-1) and its counterreceptor lymphocyte function–associated antigen 1 (LFA-1), also play a central role in the regulation of NK cytolysis by modulating the positive signal.2,3 These recent findings may have important implications in the role that NK cells play in the recognition and lysis of leukemic cells, particularly cells transformed by oncogeneic products such as BCR-ABL. In this context, the understanding of how this gene expression interferes with the susceptibility of these targets is of major interest. We previously described an in vitro system that allowed the differentiation and expansion from normal CD34+ progenitors of CD56brightCD16low NK cells (dNK) in presence of interleukin-15 (IL-15).4 Recently, we provided evidence that CD34+ leukemic progenitors from patients with chronic myeloid leukemia (CML) failed to differentiate into NK cells.5 We extended our studies to analyze the role of BCR-ABL expression on the target cell susceptibility to cell-mediated cytotoxicity.
Over 90% cases of CML are characterized by a reciprocal translocation between chromosomes 9 and 22 leading to the formation ofBCR-ABL chimeric gene on the derivative Philadelphia (Ph) chromosome.6 Depending of the breakpoint in theBCR gene, 3 main types of BCR-ABL genes can be formed.7,8 The predominant hybrid gene in CML encodes for a 210-kd fusion protein (p210 BCR-ABL), which exhibits deregulated protein tyrosine kinase activity compared to normal ABL.8 As a result, there is excessive tyrosine phosphorylation of many intracellular proteins including the BCR-ABL protein itself.9 Remarkably, BCR-ABL gene transfer induces growth factor independence in growth factor–dependent cell lines and antiapoptotic activity,10 which can be reversed when the tyrosine kinase activity of BCR-ABL is inhibited by the specific abl inhibitor STI571. It is also known that CML progression from chronic to transformed phase is associated with an increase of BCR-ABL messenger RNA,8 11 and results in cell escape to antileukemic treatments. Interestingly, in the present study we showed that leukemic cells expressing high levels of BCR-ABL were efficiently recognized and killed by dNK cells suggesting that these cells may constitute useful effectors for immunotherapeutic approaches in the treatment of patients with CML.
Materials and methods
Target cells, UT-7 cell line, and BCR-ABL transfectants UT-7/9 and UT-7/E8-1
The UT-7 cell line and the 3 BCR-ABL clones (UT-7/9, UT-7/E8.1, and UT-7/G6) that express high- and low-level BCR-ABL were used in the present study. All BCR/ABL-expressing clones were obtained by the use of BCR/ABL retroviral vectors. The UT-7/9 clone was generated by retroviral transduction (MPZen p210 retrovirus) without selective marker, whereas the other 2 clones were obtained by lipofection strategy using the MSCV p210 retroviral vector.10 UT-7, UT-7/E8.1, and UT-7/G6 cells proliferated in α-minimal essential medium (MEM) containing 10% fetal calf serum (FCS) and 10 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF), whereas UT-7/9 cells expanded in RPMI medium supplemented with 10% FCS. In the present studies all cell lines were grown in presence of GM-CSF and were split 3 times a week at the concentration of 105cells/mL. Cells maintained stable levels of bcr/abl in absence of selection medium.
In vitro differentiation of NK cells from cord blood CD34+ cells
Ten thousand to 20 000 CD34+ cells were implanted in α-MEM supplemented with 10% human serum (Institut Jacques Boy, Reims, France), 5% FCS (Stem Cell Technologies, Vancouver, BC, Canada), 20 ng/mL recombinant stem cell factor (SCF), and 20 ng/mL IL-15 both purchased from R & D Systems (Abingdon, United Kingdom). In some experiments, IL-2 (5 ng/mL) (Roussel Uclaf, Romainville, France) was used. Cultures were maintained for 4 to 5 weeks at 37°C with 5% CO2 atmosphere and medium was renewed weekly.4
Phenotypic analysis
Several fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, or phycoerythrin-cyanin 5 (PE-Cy5)–coupled monoclonal antibodies (mAbs) were used, allowing double fluorescence analysis: CD34 (IgG1), CD16 (IgG1), CD56 (IgG1), CD94 (IgG1), CD158 (IgG1), and CD3 (IgG1) purchased from Immunotech (Marseille, France). Each mAb was used in a volume of 8 μL to label 105 cells. Cells were incubated for 20 minutes at 4°C, washed twice with phosphate-buffered saline (PBS), and fixed before analysis on a FACS-Sort (Becton Dickinson, Pont de Claix, France). For indirect fluorescence, 105 cells were incubated with the first mAb directed against p46 (IgG1), p30 (IgG1) (from A. Moretta, Genoa, Italy), ICAM-1 (IgG2a), LFA-1 (IgG1), LFA-3 (IgG1), HLA class I W6/32 (IgG2a), and Fas (ZB4, IgG1) purchased from Immunotech, followed by an FITC-conjugated goat antimouse immunoglobulin. Background levels were measured using isotypic controls. For 2-color labeling analysis, compensation was set up with single-stained samples. Low forward-scatter elements (red cells or debris) were excluded from the analysis and 10 000 events were collected and analyzed using the Cellquest software (Becton Dickinson).
Cytotoxicity assay
Susceptibility of K562, UT-7, UT-7/9, UT-7/E8.1, and UT-7/G6 to differentiated NK cytolysis was tested in a 4-hour 51Cr release assay. Effector-target ratios ranged from 10:1 to 1:1. Determinations were performed in triplicate or quadruplicate and lysis percentages were determined as previously described.4 SDs were less than 10%. In some experiments, cell lines were treated with 1 μM (1 nM for K562) STI571 for 48 hours before the test.
Proliferation and viability assays
Cell proliferation was assessed by the dimethyl-thiazolyl-diphenyl-tetrazolium bromide (MTT) assay, and 15 × 103 cells were plated in quadruplicate in 96-well plate in α-MEM supplemented with 10% FBS, GM-CSF, and 0 or 1 μM STI. STI571 was kindly provided by Novartis (Novartis Pharma, France). Wells were assayed for uptake of MTT at hour 0 and after a 48-hour culture. Absorbance (A), which was proportional to cell viability, was measured at 570 nm. Cell viability was evaluated using the following calculation: percent of viability = 100 × A1/A2 where A1 and A2 were the absorbances obtained after 48 hours culture with and without STI, respectively.
To assess their susceptibility to Fas-mediated cell lysis, UT-7 and UT-7/9 cells (5 × 103) were incubated in quadruplicate with agonistic anti-Fas mAb (CH-11, IgM) or irrelevant IgM for 24 hours in a 96-well plate and wells were assayed for uptake of MTT as described above.
Nuclear protein extraction and electrophoretic mobility shift assay
Nuclear protein extraction for electrophoretic mobility shift assay (EMSA) was described previously.12 The palindromic κB probe was 5′-TTGGCAACGGCAGGGGAATTCCCCTCTCCTTA-3′, labeled with (γ32P) adenosine triphosphate (ATP) using T4 kinase. Nuclear extracts of UT-7 and UT-7/9 cells were performed before and 1, 4, 12, and 48 hours after treatment with 1 μM STI571.
Transient transfection and luciferase assay
To quantify NF-κB activity, the 3 cells lines were transfected with the NF-κB LUC plasmid reporter as previously described.13 Briefly, cells were transfected with 0.5 μg pCDNA3 vector containing 3 times NF-kB(pIC)-tk-LUC using the Fugene system (Roche, Meylan, France) and luciferase activity was determined as recommended by the manufacturer (Boehringer Mannheim, Germany).
Confocal microscopy analysis
For double staining of NF-κB and nuclear compartment, cells were permeabilized with ORTHOpermeafix (Ortho Diagnostic Systems, Raritan, NJ). Then, cells were incubated for 30 minutes with 1 μg/mL rabbit polyclonal purified IgG anti-NF-κB p65 (Santa Cruz Biotechnologies, Santa Cruz, CA) followed by incubation with Alexa Fluor 488 goat antirabbit conjugate (Molecular Probes, Eugene, OR). Nuclei were stained with propidium iodide (red staining). Confocal analyses were performed on UT-7 and UT-7/9 cells before and 1, 12, and 48 hours after treatment with 1 μM STI. Stained cells were washed with PBS, cytocentrifuged in a cytospin 3 (Shandon, Pittsburgh, PA), and analyzed by laser scanning confocal microscopy using a Leica TCS Confocal System (Wetzler, Germany).
Results
IL-15 dNK cells from cord blood CD34+ cells exhibit higher lytic efficiency against UT-7/9 BCR-ABL transfectants
The present studies were based on the use of dNK cells from CD34+ cord blood cells. IL-15 dNK cells express high levels of CD56 antigen (mean fluorescence intensity [MFI] > 1000) and CD16 expression is restricted to about 30% of CD56+ cells. We further showed that natural cytotoxicity receptors (NCRs), namely, p30 and p46 were expressed on most dNK cells, whereas Fas-L protein expression was low. As depicted in Table1, CD94 receptor associated with NKG2-A was strongly expressed on more than 80% of CD56+ cells, whereas less than 10% of dNK cells expressed the KIR p58, p70, and p140 receptors. Concerning coreceptors involved in lysis, 70% of dNK cells express LFA-1, whereas CD2 expression was low.
Expression of adhesion molecules and NK receptors by in vitro differentiated CD56bright CD16low NK cells from normal CD34+ progenitors
| Receptors . | % of dNK cells . | MFI (arbitrary units) . |
|---|---|---|
| CD2 | 12 | 21 |
| LFA-1 | 90 | 689 |
| CD94 | 90 | 581 |
| NKG2-A | 82 | 84 |
| p58.1 | 4 | 273 |
| p58.2 | 8 | 96 |
| p70 | 7 | 155 |
| p140 | 1 | 22 |
| p30 | 52 | 45 |
| p46 | 93 | 90 |
| Receptors . | % of dNK cells . | MFI (arbitrary units) . |
|---|---|---|
| CD2 | 12 | 21 |
| LFA-1 | 90 | 689 |
| CD94 | 90 | 581 |
| NKG2-A | 82 | 84 |
| p58.1 | 4 | 273 |
| p58.2 | 8 | 96 |
| p70 | 7 | 155 |
| p140 | 1 | 22 |
| p30 | 52 | 45 |
| p46 | 93 | 90 |
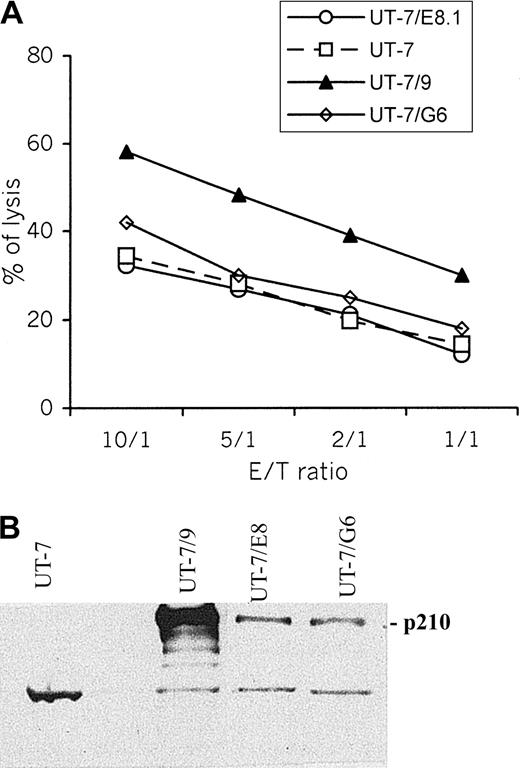
To examine the antileukemic effect of dNK cells and the influence of BCR-ABL expression on target cell lysis, the killing of UT-7, and its BCR-ABL transfectants UT-7/E8.1, UT-7/G6, and UT-7/9 by dNK cells were determined using 51Cr release assay. Data shown in Figure1A indicate that UT-7/9 cells are constantly found more susceptible to NK cell–mediated killing than UT-7 and UT-7/E8.1 cells, suggesting that the level of BCR-ABL expression is involved in the modulation of target susceptibility to NK-induced lysis. Levels of BCR/ABL expression in the 4 cell lines are depicted in Figure 1B.
UT-7/9 cells expressing high level of BCR-ABL display increased susceptibility to dNK cytolysis.
(A) Cytotoxic activity (4-hour 51Cr release assay) of dNK cells toward UT-7, UT-7/E8.1, UT-7/G6, and UT-7/9 cells was measured for the indicated effector-target (E/T) ratio. One representative experiment of 6 is shown. (B) Western blot analysis of BCR/ABL in UT-7 cell line and its BCR/ABL transfectants.
UT-7/9 cells expressing high level of BCR-ABL display increased susceptibility to dNK cytolysis.
(A) Cytotoxic activity (4-hour 51Cr release assay) of dNK cells toward UT-7, UT-7/E8.1, UT-7/G6, and UT-7/9 cells was measured for the indicated effector-target (E/T) ratio. One representative experiment of 6 is shown. (B) Western blot analysis of BCR/ABL in UT-7 cell line and its BCR/ABL transfectants.
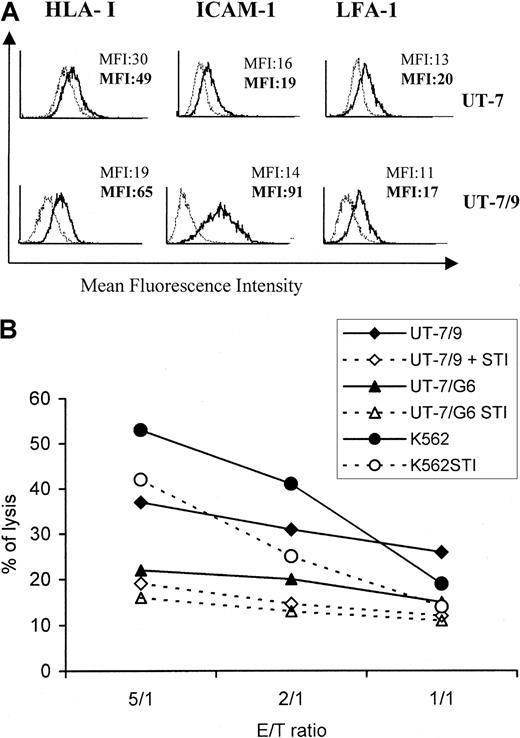
High level of BCR-ABL expression on UT-7/9 transfectants correlates with overexpression of ICAM-1 and LFA-1
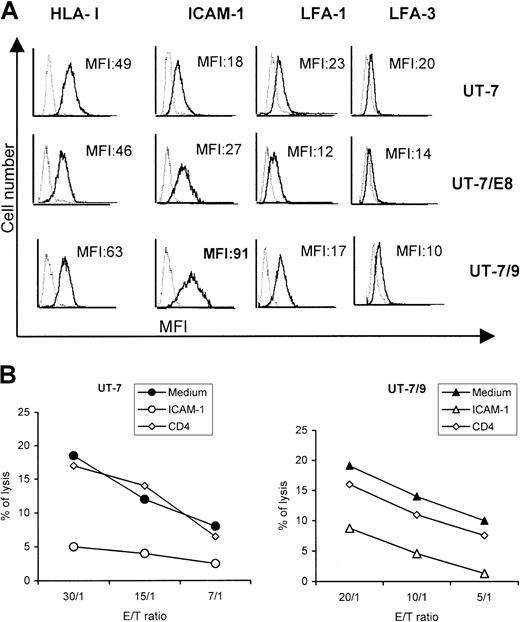
To evaluate the effect of BCR-ABL on the expression of molecules potentially involved in dNK/target cell interactions, surface expressions of HLA class I, ICAM-1, LFA-1, and LFA-3 on UT-7, UT-7/9, and UT7-E8.1 cells were assessed using FACS analysis. The expression of LFA-1 and LFA-3 was similar in UT-7 cells and in the 2 BCR-ABL transfected clones, whereas HLA-I was slightly increased in UT-7/9 cells. However, the major difference concerned the expression of ICAM-1 that was significantly higher on transfectant UT-7/9 cells (× 5), displaying the strongest BCR-ABL expression (Figure2A). In addition, incubation of UT-7 and UT-7/9 cells with anti–ICAM-1 mAb before the cytotoxicity assay resulted in a significant decrease (< 50%) of the lysis of both cell lines (Figure 2B).
UT-7/9 expressing high level of BCR-ABL overexpress ICAM-1.
UT-7 parental cells and transfectants UT-7/E8.1 and UT-7/9 were labeled with anti–HLA-I (W6/32), anti–ICAM-1 (CD54), anti–LFA-1 (CD11a), and LFA-3 (CD58), shown by bold lines. MFI indicates mean fluorescence intensity values. (B) Cytotoxic activity of dNK cells toward UT-7 (left panel) and UT-7/9 cells (right panel) in the presence or absence of anti–ICAM-1 or irrelevant anti-CD4 mAbs. One representative experiment of 3 is shown.
UT-7/9 expressing high level of BCR-ABL overexpress ICAM-1.
UT-7 parental cells and transfectants UT-7/E8.1 and UT-7/9 were labeled with anti–HLA-I (W6/32), anti–ICAM-1 (CD54), anti–LFA-1 (CD11a), and LFA-3 (CD58), shown by bold lines. MFI indicates mean fluorescence intensity values. (B) Cytotoxic activity of dNK cells toward UT-7 (left panel) and UT-7/9 cells (right panel) in the presence or absence of anti–ICAM-1 or irrelevant anti-CD4 mAbs. One representative experiment of 3 is shown.
The increase of UT-7/9 transfectant susceptibility to dNK cells involves the Ca++-dependent killing pathway
In addition to an increased expression of ICAM-1 and LFA-1 molecules, UT-7/9 transfectants that express a high level of BCR-ABL exhibit an increased membrane Fas expression (Figure3A). To determine if the Fas/Fas-L pathway is involved in the increased cytotoxicity toward UT-7/9 cells, cell-mediated lysis was assessed in the presence of EGTA that inhibits the perforin-granzyme pathway. EGTA dramatically decreased the lytic activity of dNK cells against UT-7 and UT-7/9 transfectants, suggesting that dNK cells used the classical perforin-granzyme pathway to lyse BCR-ABL targets (Figure 3B). Furthermore, UT-7 and UT-7/9 cell lines treated by anti-Fas antagonist mAb (CH-11) proliferated similarly to control IgM-treated cells (Figure 3C), indicating that despite an increased Fas expression, UT-7/9 cells were resistant to Fas-mediated apoptosis and that the susceptibility of UT-7/9 transfectants to NK cell lysis did not involve Fas/Fas-L pathway.
Fas/Fas-L pathway is not involved in the lysis of UT-7 and UT-7/9 cells.
(A) Fas expression on UT-7 (left panel) and UT-7/9 (right panel) cell lines (ZB4 mAb). (B) Calcium-dependent and calcium-independent lysis of UT-7/9 transfectants by dNK cells. Cytotoxic activity of dNK cells toward UT-7 and UT-7/9 cells was measured either in media or in the presence of MgCl2 (3 mM) and EGTA (4 mM) at the indicated E/T ratio. One representative experiment of 3 is shown. (C) Proliferation assessed by MTT assay of UT-7 and UT-7/9 cells after a 24-hour incubation with anti-Fas mAb (CH-11 indicated by black bars) or with control IgM (gray bars).
Fas/Fas-L pathway is not involved in the lysis of UT-7 and UT-7/9 cells.
(A) Fas expression on UT-7 (left panel) and UT-7/9 (right panel) cell lines (ZB4 mAb). (B) Calcium-dependent and calcium-independent lysis of UT-7/9 transfectants by dNK cells. Cytotoxic activity of dNK cells toward UT-7 and UT-7/9 cells was measured either in media or in the presence of MgCl2 (3 mM) and EGTA (4 mM) at the indicated E/T ratio. One representative experiment of 3 is shown. (C) Proliferation assessed by MTT assay of UT-7 and UT-7/9 cells after a 24-hour incubation with anti-Fas mAb (CH-11 indicated by black bars) or with control IgM (gray bars).
Down-regulation of ICAM-1 expression in UT7/9 cells by STI571 results in a decrease of their sensitivity to dNK cell cytolysis
Because oncogenic transformation by BCR-ABL is dependent on tyrosine kinase activity, selective inhibitors of the ABL tyrosine kinase were developed. Among these, STI571 is able to reduce the tyrosine phosphorylation of cellular proteins, largely affecting BCR-ABL protein level.l4 This compound was used to further investigate the involvement of BCR-ABL expression in the modulation of target susceptibility to NK cell cytolysis. Incubation of UT-7/9 cells with STI571 (1 μM) significantly inhibited their proliferation (−30%) as determined by MTT assay, whereas it had no effect on parental UT-7 cell proliferation (data not shown). As depicted in Figure 4A, treatment by STI571 for 48 hours dramatically decreased the expression of ICAM-1 by UT-7/9 cells, whereas it had no significant effect on parental UT-7 cells. It should be noted that a slight decrease in HLA class I molecule expression by UT-7/9 cells was observed following STI571 treatment (Figure 4A). Interestingly, STI571 treatment of UT-7/9 and K562 cells resulted also in a clear decrease in cell susceptibility to dNK cytolysis, whereas the lysis of UT-7 and UT-7/G6 cells was not significantly affected by STI571 treatment (data not shown and Figure4B). These data suggested a prominent role of the interaction of ICAM-1 and LFA-1 in the modulation of UT-7/9 susceptibility to NK cell lysis.
STI571 modulates the expression of ICAM-1 by UT-7/9 cells and decreases the susceptibility of UT-7/9 targets to dNK cytolysis.
(A) UT-7 and UT-7/9 cells were treated for 48 hours by STI571 (thin lines) and analyzed by cytometry for expression of HLA class I, ICAM-1, and LFA-1. Bold lines indicate untreated cells. (B) K562, UT-7/G6, and UT-7/9 cells were treated by STI571 and used as targets in a cytotoxic assay with dNK cells as effectors. One representative experiment of 4 is shown.
STI571 modulates the expression of ICAM-1 by UT-7/9 cells and decreases the susceptibility of UT-7/9 targets to dNK cytolysis.
(A) UT-7 and UT-7/9 cells were treated for 48 hours by STI571 (thin lines) and analyzed by cytometry for expression of HLA class I, ICAM-1, and LFA-1. Bold lines indicate untreated cells. (B) K562, UT-7/G6, and UT-7/9 cells were treated by STI571 and used as targets in a cytotoxic assay with dNK cells as effectors. One representative experiment of 4 is shown.
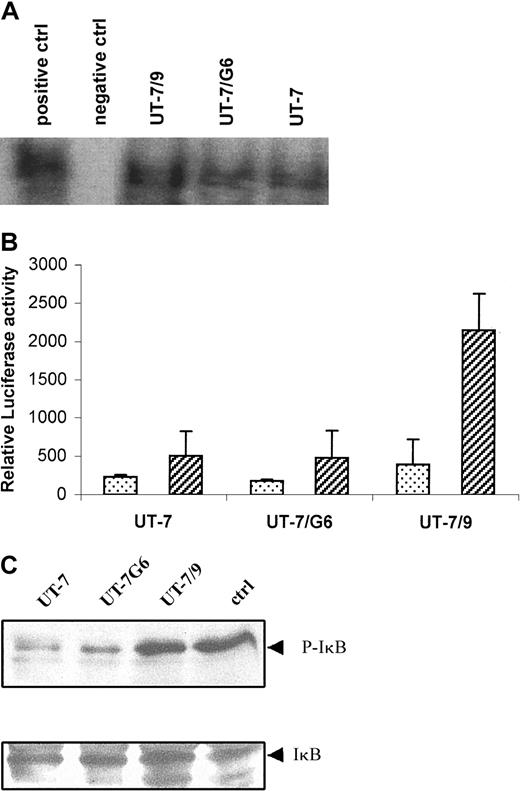
STI571 inhibits activation of NF-κB in UT-7/9 cells
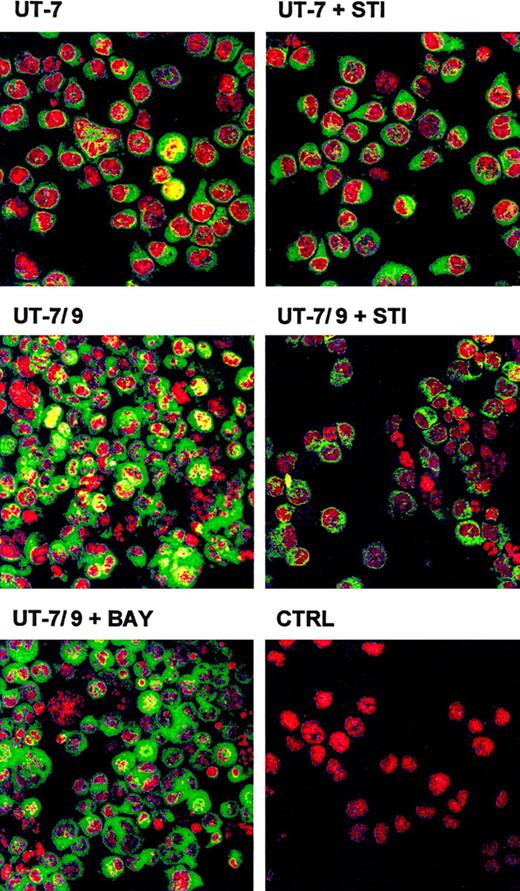
Analysis by EMSA revealed a constitutive activation of NF-κB in UT-7/9 cells (Figure 5A) and experiments using a NF-κB–dependent reporter gene confirmed the increased NF-κB activity in the UT-7/9 cells (Figure 5B). Western blot analysis evidenced an increased phosphorylation of IκB in the UT-7/9 cells (Figure 5C). Analysis by confocal microscopy of NF-κB p65 localization confirmed the nuclear localization of NF-κB, detected by a yellow nuclear staining in most of the UT-7/9 cells, whereas the parental UT-7 exhibited a green cytoplasmic staining (Figure6). STI571 treatment of UT-7/9 cells inhibited NF-κB translocation as shown by the absence of nuclear yellow staining of treated cells and the cytoplasmic expression of p65 NF-κB subunit shown by the green staining. Treatment of UT-7/9 cells by BAY 11-7082, a specific IκBα kinase inhibitor that prevents degradation of IκBα and specifically abrogates NF-κB DNA binding, resulted in a clear decrease in nuclear NF-κB staining (Figure6).
UT-7/9 cells display a constitutive activation of NF-κB that is modulated by STI571.
(A) EMSA showing NF-κB activation in UT-7, UT-7/G6, and UT-7/9 cells. (B) NF-κB activity determined by NF-κB–dependent reporter gene. Cells were transfected with a plasmid containing a NF-κB LUC reporter (3 × NF-κB(pIC)-tk-LUC) using the Fugene system and luciferase activity measured 30 hours after transfection. (C) Western blot analysis of IκB phosphorylation in the 3 cell lines.
UT-7/9 cells display a constitutive activation of NF-κB that is modulated by STI571.
(A) EMSA showing NF-κB activation in UT-7, UT-7/G6, and UT-7/9 cells. (B) NF-κB activity determined by NF-κB–dependent reporter gene. Cells were transfected with a plasmid containing a NF-κB LUC reporter (3 × NF-κB(pIC)-tk-LUC) using the Fugene system and luciferase activity measured 30 hours after transfection. (C) Western blot analysis of IκB phosphorylation in the 3 cell lines.
STI571 modulates the activation of NF-κB in UT-7/9 cells.
Confocal microscopy analyses of nuclear localization of NF-κB p65 subunit (green) in UT-7/9 and UT-7 cells in basal condition and treated for 48 hours by STI571. Yellow staining indicates nuclear localization of NF-κB p65 subunit. As control UT-7/9 cells were treated for 16 hours by BAY 11-7082 (a specific inhibitor of NF-κB) before staining. CTRL correspond to nuclear (red) staining by propidium iodide (2 μg/mL) of UT-7/9 cells.
STI571 modulates the activation of NF-κB in UT-7/9 cells.
Confocal microscopy analyses of nuclear localization of NF-κB p65 subunit (green) in UT-7/9 and UT-7 cells in basal condition and treated for 48 hours by STI571. Yellow staining indicates nuclear localization of NF-κB p65 subunit. As control UT-7/9 cells were treated for 16 hours by BAY 11-7082 (a specific inhibitor of NF-κB) before staining. CTRL correspond to nuclear (red) staining by propidium iodide (2 μg/mL) of UT-7/9 cells.
To further confirm that NF-κB activation in target cells was involved in the modulation of BCR-ABL transfectants to NK cells lysis, the effect of BAY 11-7082 was examined. UT-7/9 cells treated for 16 hours with BAY 11-7082 displayed a decreased ICAM-1 expression (Figure7A) and a significant alteration in their susceptibility to dNK cytolysis (Figure 7B). The modulation of lysis by BAY11-7082 was not as strong as that induced by STI571, suggesting that the BCR-ABL effect on target susceptibility involved additional mechanisms besides NF-κB activation.
BAY 11-7082 modulates expression of ICAM-1 by UT-7/9 cells.
(A) UT-7/9 cells were treated for 16 hours with BAY 11-7082, labeled with anti-HLA class I, anti–ICAM-1, and anti–LFA-1 mAbs, and analyzed by cytometry. Thin lines are treated cells and bold lines indicate untreated cells. (B) UT-7/9 cells treated or not with BAY-11-7082 were used as targets in cytotoxicity assay with dNK cells as effectors.
BAY 11-7082 modulates expression of ICAM-1 by UT-7/9 cells.
(A) UT-7/9 cells were treated for 16 hours with BAY 11-7082, labeled with anti-HLA class I, anti–ICAM-1, and anti–LFA-1 mAbs, and analyzed by cytometry. Thin lines are treated cells and bold lines indicate untreated cells. (B) UT-7/9 cells treated or not with BAY-11-7082 were used as targets in cytotoxicity assay with dNK cells as effectors.
Discussion
We have previously shown that in vitro IL-15 dNK cells constituted a homogeneous NK cell population with high lytic activity.4 These cells differed from peripheral NK cells because they were CD56bright cells and did not express CD16 or CD2. CD94/NKG2-A, specific for HLA-E molecules, was expressed by more than 80% of dNK cells, whereas inhibitory receptors of the Ig (KIR) superfamily were expressed only by 5% to 10% of dNK cells. Such a phenotype was similar to the phenotype of NK cells isolated from peripheral blood of patients undergoing bone marrow transplantation from unrelated matched donors15 that were potentially implicated in the antitumoral graft-versus-leukemia effect.16 Recently we provided evidence for the alteration of in vitro NK cell differentiation from CML progenitors.5We have demonstrated that this altered differentiation was related to an abnormal IL-15 transduction pathway that may account for the decreased peripheral NK activity in patients with advanced CML. The present studies were performed to gain more insights into the molecular basis of BCR-ABL–induced modulation of target susceptibility to NK lysis and to investigate the potential involvement of in vitro dNK cells from CD34+ progenitors in the lysis of leukemic cells expressing BCR-ABL oncoprotein.
It has been reported that BCR-ABL–expressing leukemic cells were highly resistant to apoptosis induced by chemotherapeutic drugs.17 One of the antiapoptotic pathways triggered by BCR-ABL involved induction of bcl-XL through signal transducers and activators of transcription (STAT5) activation.18 In this regard, inhibition of BCR-ABL kinase activity was effective in suppressing STAT5 interaction with the bcl-XL promoter and subsequently leading to bcl-XL down-regulation and increased apoptosis.19 On the other hand, several reports evidenced that human CML leukemic blasts may be killed by activated NK cells20,21 and that activated NK cells specifically suppress CML malignant hematopoı̈esis.22 This suppression was not mediated by soluble factors but was dependent on direct cell-to-cell contact and was reversed by anti–β2-integrin antibodies.22 We demonstrated here that high-level expression of BCR-ABL in the UT-7/9 cells, a model of blastic crisis, induced an increased susceptibility of target cells to NK cytolysis. Our data are in agreement with the observation of Roger et al23 who reported a greater susceptibility to cytolysis by LAK cells of UT-7/9 as compared to UT-7 cells despite their resistance to tumor necrosis factor (TNF) and drugs. We further showed that STI571 treatment of UT-7/9 cells resulted in a decreased NK susceptibility suggesting an involvement of BCR-ABL overexpression. This fits with previous observations emphasizing that leukemic cells of patients with CML in blast crisis are more susceptible to NK cytolysis than leukemic cells of patients with CML in chronic phase.24 25
It is clearly established that the regulation of NK cytolysis is controlled by several cell-to-cell contacts including interactions of KIR and NCRs with their ligands in combination with interactions of β2 integrin on NK and target cells.26 The detection of a similar expression of HLA class I on UT-7, UT-7/9, and UT-7/E8.1 as well as the absence of KIR expression on differentiated NK cells did not favor a role of KIR-ligand interaction in the increased susceptibility of UT-7/9, although down-regulation of a specific HLA allele on UT-7/9 cells may not be excluded. On the other hand, a dramatic overexpression of ICAM-1 was observed on UT-7/9 cells, which was reversed after treatment by STI571. BCR-ABL expression also induced an increased Fas expression by UT-7/9 cells found to be resistant to anti-Fas–induced lysis. Furthermore, we provided evidence that dNK cells mainly used the perforin-granzyme pathway to kill the targets and that masking of ICAM-1 on targets significantly decreased the lysis of UT-7 and UT-7/9 cells. Altogether, these results suggested that in this experimental model, interaction of ICAM-1 and LFA-1 could play a predominant role in the up-regulation of the NK cell-mediated lysis against targets expressing high-level BCR-ABL. This result mirrored recent results involving down-regulation of ICAM-1 and B7.2 in the inhibition of NK cell–mediated cytotoxicity by cells infected with Kaposi sarcoma-associated herpesvirus K5 protein27 and the effect of ICAM-1 expression in increased susceptibility to NK cells of HLA-I− melanoma cells.28
Recently, evidence has been provided that NF-κB is an important component of BCR-ABL signaling that played a role in the transformation by BCR-ABL,29 and is involved in cell survival by regulating antiapoptotic genes.30 However, we demonstrated that the constitutive expression of NF-κB had no effect on target cell death induced by killer cells, confirming our recent observations indicating that inhibition of NF-κB in target cells did not modulate their susceptibility to NK lysis.31 The constitutive activation of NF-κB observed in BCR-ABL cells, confirming previous data29,32 suggested that in these cells the activation of this transcription factor could result in increased expression of some genes including ICAM-1.29 Moreover, specific inhibition of NF-κB by the synthetic peptide BAY 11-7082 resulted in decreased expression of ICAM-1 on UT-7/9 cells confirming that ICAM-1 expression in these cells was under the control of NF-κB activation. In addition, UT-7/9 susceptibility to dNK cytolysis was also significantly decreased by BAY 11-7082 treatment. Thus, even if NF-κB activation could have a negative effect on apoptosis by decreasing the killing by some apoptosis inducers including TNF-α and cytotoxic drugs,33 34 our data nevertheless suggested that BCR-ABL, through NF-κB transcription factor activation, could induce the expression of some genes involved in the positive regulation of cell susceptibility to non–MHC-restricted cytotoxicity.
Our study evidenced that high-level BCR-ABL expression increased the susceptibility to NK cytolysis by a mechanism involving at least in part an increase of ICAM-1 expression favoring NK cell/target attachment, through activation of NF-κB. It is interesting to note that in vitro differentiated NK cells were mostly CD2− and could therefore use LFA-1/ICAM-1 in effector target interactions. Although expression of ICAM-1 overcame inhibitory controlling signals in our model, overexpression of ICAM-1 by normal cells in the absence of high level of NCR ligand expression may not be sufficient to engage the lytic pathway of dNK cells. These observations could display clinical relevance, suggesting that although the level of BCR-ABL in CML cells increases with the evolution of the disease, resulting in increased resistance to drugs and interferon α, the patients in advanced stage or blastic crisis would respond to non–MHC-restricted cytotoxic effectors. Interestingly, CML precursors obtained from patients in blastic crisis or advanced chronic phase were indeed lysed by IL-15–activated allogeneic NK cells (data not shown).
Although only a small percentage of patients benefit from this curative procedure,35 cellular therapy trials show that allogeneic marrow transplantation is firmly established as the treatment of choice for patients with CML. Current approaches to increase the graft-versus-leukemic effect is to isolate donor leukemia-specific T-cell clones using peptides spanning the BCR-ABL junction. These peptides induce cytotoxic T-cell response in vitro but it remains unclear whether the induced CTLs recognize and kill autologous CML cells. On the other hand, the opportuneness of using antineoplastic activity of allogeneic NK cells (selected for appropriate HLA-C and/or B mismatch) as an alternative to CTL, thus avoiding graft-versus-host disease, seems very promising. In addition, infusion of NK cells may lead to a rapid antileukemic activity appropriated to patients in blastic crisis. In that context, our results unexpectedly showing that oncogenic transformation by BCR-ABL improves susceptibility to non–MHC-restricted cytotoxicity by up-regulation of cellular ligands for NK cells further emphasize the interest of cellular therapy protocols using allogeneic NK cells in the treatment of patients with CML.
We would like to thank Yann Lécluse for immunofluorescence analyses. We thank Professor Alessandro Moretta for providing us with anti-NCR and anti-NKR mAbs and for helpful discussions.
J.G.-M. and A.G.T. contributed equally to the work.
Submitted January 29, 2001; accepted October 29, 2001.
Supported by grants awarded by INSERM, Institut Fédératif de Recherche IFR 54 and La Fondation de France (Nb 99003929). F.B. is postdoctoral researcher of the National Fund for Scientific Research (FNRS) Belgium.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anne Caignard, Unité INSERM 487, Cytokines et Immunologie des Tumeurs Humaines, Institut Gustave Roussy, PR1, 39, rue Camille Desmoulins, F-94805 Villejuif, France; e-mail:caignard@igr.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal