The NOD-LtSZ scid/scid (NOD/SCID) repopulation assay is the criterion for the study of self-renewal and multilineage differentiation of human hematopoietic stem cells. An important shortcoming of this model is the reported absence of T-cell development. We studied this aspect of the model and investigated how it could be optimized to support T-cell development. Occasionally, low-grade thymic engraftment was observed in NOD/SCID mice or Rag2−/−γc−/− mice. In contrast, the treatment of NOD/SCID mice with a monoclonal antibody against the murine interleukin-2Rβ, (IL-2Rβ) known to decrease natural killer cell activity, resulted in human thymopoiesis in up to 60% of the mice. T-cell development was phenotypically normal and resulted in polyclonal, mature, and functional CD1−TCRαβ+ CD4+ or CD8+single-positive T cells. In mice with ongoing thymopoiesis, peripheral T cells were observed. TREC analysis showed that T cells with a naive phenotype (CD45RA+) emerged from the thymus. In approximately half of these mice, the peripheral T cells included a pauciclonal outgrowth of CD45RO+ cells. These data suggest that all elements of a functional immune system were present in these animals.
Introduction
The NOD/SCID model, which sustains proliferation and engraftment of intravenously injected human hematopoietic stem cells (HSCs), mimics engraftment after clinical transplantation. An important shortcoming of this model is that only exceptional thymopoiesis is seen.1-5 Therefore, the multipotency of stem cells cannot be completely evaluated in this model, nor can this model be used to study T-cell reconstitution after stem cell transplantation.
Several models do exist to study the T-cell potential of HSCs. First, the SCID-Hu model enables the study of T-cell development from CD34+ HSCs after direct injection into a human thymus established from fetal material several weeks earlier.6,7Second, fetal thymus organ culture supports T-cell development from CD34+ HSCs that were introduced in fetal thymic lobes from immunodeficient mice.8 These models require fetal material or they exclusively generate T cells. Hence, functional aspects of T cells cannot be studied in these models.
To address this issue, strategies have been introduced to study T-cell development in the NOD/SCID model. Robin et al9 have identified T-cell progenitors in the bone marrow of NOD/SCID mice injected with CD34+38lo and CD34+38+ cells from human adult bone marrow and umbilical cord blood (UCB) by their capacity to form T cells when introduced into fetal thymus organ culture. Crisa et al4transplanted human fetal thymus under the kidney capsule of NOD/SCID mice that were injected with T- and B-cell–depleted human UCB 4 to 6 weeks earlier. They observed T-cell development from injected human UCB cells in the human thymus graft. These models are time consuming and technically cumbersome.
When studying T-cell development in the NOD/SCID repopulation model, we could confirm that thymopoiesis did not occur in most experiments. However, a low percentage of mice did show evidence of thymopoiesis. We wondered whether we could improve thymopoiesis by blocking the natural killer (NK) cell activity in these mice. We injected intraperitoneally TM-β1, an antibody blocking the murine interleukin-2Rβ, (IL-2Rβ) which results in lower NK activity.9 This antibody treatment could enhance the thymopoietic potential of the HSCs in irradiated (350 cGy) NOD/SCID mice. Through this modification, the NOD/SCID model generates human thymocytes in sufficient numbers to be studied phenotypically and functionally.
Materials and methods
Monoclonal antibodies
Mouse anti–human monoclonal antibodies (mAbs) used were CD1a (T6 RD1) and CD8β (2ST8.5H7) from Coulter-Immunotech (Miami, FL); CD3 (SK7), CD4 (SK3), CD7 (M-T701), CD8α (SK1), CD14 (Mφp9), CD19 (4G7), CD27 (M-T271), CD34 (8G12), CD38 (HB7), CD45 (2D1), CD45RA (HI100), CD45RO (UCHL1), CD56 (MY31), CD62L (Dreg 56), CD69 (FN50), and HLA DR (L243) from Becton Dickinson Immunocytometry Systems (BDIS, San Jose, CA); and Diversi-T (panel of mAbs to human T-cell receptor [TCR] variable regions) (T Cell Diagnostics, Cambridge, MA). For CD3 depletion OKT3 (American Type Culture Collection [ATCC], Manassas, VA) was used. Monoclonal antibodies were labeled with fluorescein isothiocyanate, phycoerythrin, or allophycocyanin or were conjugated to biotin. For flow cytometry, biotinylated antibodies were revealed by streptavidin–phycoerythrin (BDIS), and unlabeled mAbs used were revealed by GAM fluorescein isothiocyanate (BDIS). Isotypic control antibodies were immunoglobulin G1 (IgG1) (X40 from BDIS) and IgG2a (X39 from BDIS). Anti-FcγRII/III mAb (2.4G2, kind gift of Dr J. Unkeless, Mount Sinai School of Medicine, New York, NY) was used to block murine Fc receptors. The rat mAb against the murine IL-2Rβ chain was purified from supernatants of the hybridoma cell line TM-β1 (kindly provided by Dr T. Tanaka, Tokyo, Japan),10,11 grown in our laboratory.12
Mice
NOD/LtSZ-scid/scid (NOD/SCID) mice, breeding pairs originally purchased at the Jackson Laboratory (Bar Harbor, ME), and Rag2/γc double knockout (−/−) mice, breeding pairs kindly donated by Nederlands Kanker Instituut (Amsterdam, The Netherlands), were bred in our pathogen-free breeding facility. Animals were treated according to the guidelines of the Laboratory Animal Ethical Commission of the Ghent University Hospital.
Cell sources
UCB was obtained from full-term healthy newborns, and mononuclear cells were isolated within 24 hours after collection using a Lymphoprep density gradient (Nycomed Pharma, Oslo, Norway). UCB samples were obtained and used according to the guidelines of the Medical Ethical Commission of the Ghent University Hospital. Unless used fresh, cells were resuspended in 90% heat-inactivated fetal calf serum (Life Technologies, Paisley, Scotland)–10% dimethyl sulfoxide (Serva, Heidelberg, Germany) and were frozen in liquid nitrogen until use. The mononuclear cell fraction contained 53% ± 19% (n = 8) CD45+ cells (absolute number, 5.4 ± 2.3 × 106 CD45+ cells). The mononuclear cell fraction was stained with mouse anti–human CD3 mAb for immunomagnetic depletion using sheep anti–mouse immunoglobulin-coated beads (Dynabeads; Dynal AS, Oslo, Norway) with a cell-bead ratio of 1:4. After this procedure the percentage of CD3+ cells within the CD45+ cell fraction decreased 1 log, resulting in 0.63% ± 0.38% CD3+cells, representing absolute numbers of 2.8 ± 1.9 × 104. The average percentage of CD34+ cells after T-cell depletion was 2.3% ± 1% of the CD45+ cells. The total number of injected CD34+ cells was 11.7 ± 4.6 × 104. In one experiment CD34+CD3− sorted UCB cells were used. For this experiment, UCB cells were enriched in CD34+cell content using magnetic cell sorting (MACS) (Miltenyi Biotec, Bergisch Gladbach, Germany), and then CD34+CD3− cells were sorted. In experiments for T-cell receptor excision circles (TREC) analysis and TCR-Vβ repertoire analysis, MACS (Miltenyi Biotec) selected CD34+ cells were used. On average we injected per mouse 10.6 ± 9.5 × 104 cells, containing 97% ± 2% CD34+ cells (resulting in absolute numbers of 10.3 ± 9.2 × 104 CD34+ cells) and containing 0.25% ± 0.15% CD3+ cells (262 ± 145 CD3+ cells).
NOD/SCID repopulation assay
Six- to 8-week-old mice were given a sublethal dose of whole-body irradiation (350 cGy, 12-15 cGy/min) using a Cobalt radiation source and were injected intraperitoneally with 200 μg TM-β1, an antibody functionally blocking the mouse IL-2Rβ chain. Twenty-four hours later the mice were injected intravenously (in the tail vein) with human UCB cells. Eight to 15 weeks after injection, the mice were killed and peripheral blood, thymus, liver, spleen, mesenteric lymph nodes, and both femora (in some experiments together with both tibia bones) were used for analysis. Cell suspensions from these organs were filtered through a 70-μm cell strainer. Red blood cells were lysed using hypotonic lysing buffer. Cells were counted, cell viability was checked (greater than 85%), and, after blocking the Fc-receptor, cells were labeled with mAbs and analyzed on a flow cytometer.
Flow cytometry and cell sorting
Cells were analyzed or sorted on a FACSCalibur (BDIS) or a FACS Vantage (BDIS), respectively, each equipped with an argon-ion laser tuned at 488 nm and a red-diode laser tuned at 635 nm. For analysis of viable human cells, gates were set on the propidium iodide (PI)–negative, human CD45+ cells. Data acquisition and analysis were performed with CellQuest software (BDIS).
Proliferation assay
A total of 5 × 104 cells/well harvested from murine thymus or spleen were incubated in 96-well, round-bottomed microtiter plates in the presence of 2 μg/mL PHA (Wellcome Diagnostics, Beckenham, United Kingdom), 50 U/mL recombinant human IL-2 (kindly provided by M. Gately, Hoffmann-La Roche, Nutley, NJ) (first week) and feeders (irradiated [2500 cGy] human peripheral blood mononuclear cells, 106/well). Two weeks later cells were restimulated in the presence of PHA and fresh feeders for another week.
Quantitative polymerase chain reaction of TRECs
Quantification of signal-joint TRECs in sorted human CD3+ populations from repopulated mice was performed by real-time quantitative polymerase chain reaction (PCR) with the 5′ nuclease (Taqman) assay and an ABI 7700 system (Perkin-Elmer, Foster City, CA). As previously described,13 cells were lysed in 100 mg/L proteinase K (Roche Molecular Diagnostics, Rotkreuz, Switzerland) for 2 hours at 56°C, then for 15 minutes at 95°C. Each PCR reaction was performed in a 25 μL solution containing 5 μL cell lysate, 12.5 μL Taqman Universal Master Mix including AmpliTaq Gold (Applied Biosystems, Rotkreuz, Switzerland), 500 nM of each primer (signal-joint 5′ forward, CAC ATC CCT TTC AAC CAT GCT; signal-joint 3′ reverse, GCC AGC TGC AGG GTT TAG G) and 125 nM probe (FAM-ACA CCT CTG GTT TTT GTA AAG GTG CCC ACT-TAMRA) in a final volume of 25 μL. PCR conditions consisted of 1 cycle of 2 minutes at 50°C and 1 cycle of 10 minutes at 95°C, followed by 40 cycles of 30 seconds at 95°C and 1 minute at 65°C. Tenfold serial dilutions ranging from 107 to 101 copies of signal-joint internal standard, kindly provided by Dr Daniel Douek (Department of Internal Medicine, University of Texas, Southwestern Medical Center), were tested in quadruplicate in each PCR experiment. A standard curve with a linear range across 7 log DNA concentration was created that allowed calculation of the number of TRECs in a given cell population. Thus, the lowest limit of quantitation was considered to be 10 copies of the target sequence. In all PCR assays, the correlation coefficient of the curve was 0.995 or greater, whereas the slope varied between −3.54 and 3.73. TREC levels were quantified by 6 PCR replicates.
Stimulation and cytokine production measurement
A total of 5 × 104 cells/well were incubated in 96-well, flat-bottomed microtiter plates and were stimulated with 10 μg/mL anti-CD3 mAb (OKT3; ATCC) and 10 ng/mL TPA (Sigma Chemical, St Louis, MO) or 10 μg/mL concanavalin A (ConA; Serva, Heidelberg, Germany) for 24 hours. For CD3 stimulation, plates were coated with 100 μL/well of 10 μg/mL purified OKT3 in phosphate-buffered saline (1 hour incubation at 37°C) and then were washed 3 times with phosphate-buffered saline. After 24 hours of stimulation, cultures were spun down, and supernatant was harvested from each well and kept frozen at −20°C until the determination of cytokine content. Each stimulation was performed in duplicate. The content of IL-2, IL-4, and interferon-γ (IFN-γ) was determined using ELISA tests—IL-2 EASIA, IL-4 EASIA, and IFN-γ EASIA, respectively (BioSource, Nivelle, Belgium). Tests were performed according to the manufacturer's instructions.
TCR-Vβ repertoire analysis
For TCR-Vβ analysis, RNA was isolated from sorted CD45+ cell fractions from thymus and total bone marrow using TRIzol (Life Technologies). After oligo dT annealing, total RNA was subsequently reverse transcribed using Superscript II RT enzyme (Life Technologies) in the presence of dNTPs and RNAguard (Amersham Pharmacia Biotech, Uppsala, Sweden). PCR amplification and heteroduplex analysis were performed as described before.14
Statistical analysis
Statistical analysis was performed using SPSS for Windows software, version 9.0. Results are expressed as the mean ± SE. Differences were evaluated with Mann-Whitney U test where appropriate. Statistical significance was assumed forP < .05.
Results
T-cell development in NOD/SCID mice
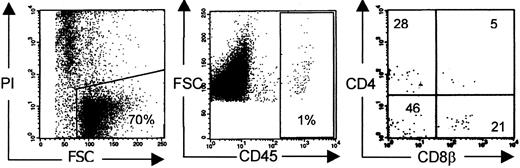
T-cell development is reported to occur exceptionally in NOD/SCID mice injected with a human stem cell source. To quantify the frequency of T-cell development, we injected human CD3 depleted UCB in NOD/SCID mice irradiated with 350 cGy at least 24 hours, and at most 48 hours, before. Twelve to 15 weeks after injection, the mice were killed and their organs were analyzed for the presence of human cells. We focused on human T-cell development in the murine thymus. We indeed found some mice (5 of 28) with a repopulated thymus, defined by the presence of CD4+CD8+ DP cells in the thymus, though the absolute number of human thymocytes was low. A typical dot of such a poorly reconstituted T-cell–containing thymus is shown in Figure 1.
Human thymus repopulation in NOD/SCID mice.
Conditioning refers to radiotherapy only. Typical phenotypic profile of a poorly reconstituted thymus of NOD/SCID mice injected with CD3-depleted UCB 12 to 15 weeks earlier. Gates were set on viable human CD45+ cells.26
Human thymus repopulation in NOD/SCID mice.
Conditioning refers to radiotherapy only. Typical phenotypic profile of a poorly reconstituted thymus of NOD/SCID mice injected with CD3-depleted UCB 12 to 15 weeks earlier. Gates were set on viable human CD45+ cells.26
Influence of IL-2Rβ blockade on engraftment of human HSCs
NK activity is known to reduce the engraftment of Hu PBL grafts in SCID and NOD/SCID mice.15 We, therefore, tested the influence of NK inactivation on engraftment in this model in 2 ways. First, we administered TM-β1, an antibody blocking the murine IL-2Rβ, to irradiated NOD/SCID mice before injection of human cells. Second, we used irradiated Rag2−/−γc−/− mice, which lack mature B, T, and NK cells,16 as hosts for human cells instead of NOD/SCID mice. The frequencies of successful thymus repopulation achieved in both adapted repopulation models are shown in Table 1. Addition of TM-β1 treatment increased significantly, from 18% to 56%, the frequency of mice with successful thymus repopulation with human DP cells. This suggests that NK cells indeed negatively influence the success rate of thymopoiesis. However, thymus repopulation observed in Rag2−/−γc−/− mice was similar to that in NOD/SCID mice. This indicates that besides eliminating T- and B-cell function, the blocking of NK activity is insufficient for increased human lymphopoiesis in the murine thymus.
Success rate of human thymopoiesis according to mouse strain and conditioning
| N (mice) . | NOD/SCID RT . | NOD/SCID RT + TM-β1 . | Rag2−/−γc−/− mice RT . |
|---|---|---|---|
| Total | 28 | 39 | 25 |
| DP in thymus (no.) | 5 | 22 | 4 |
| Success rate (%) | 18 | 56 | 6 |
| N (mice) . | NOD/SCID RT . | NOD/SCID RT + TM-β1 . | Rag2−/−γc−/− mice RT . |
|---|---|---|---|
| Total | 28 | 39 | 25 |
| DP in thymus (no.) | 5 | 22 | 4 |
| Success rate (%) | 18 | 56 | 6 |
Comparison of thymus repopulation frequency between irradiated NOD/SCID mice (RT), irradiated NOD/SCID mice pretreated with injection of TM-β1 (RT + TM-β1), and irradiated Rag2−/−γc−/− mice (RT). Of each group, the total number of mice and number/percentage of mice having DP (≥ 5%) in their thymi are shown.
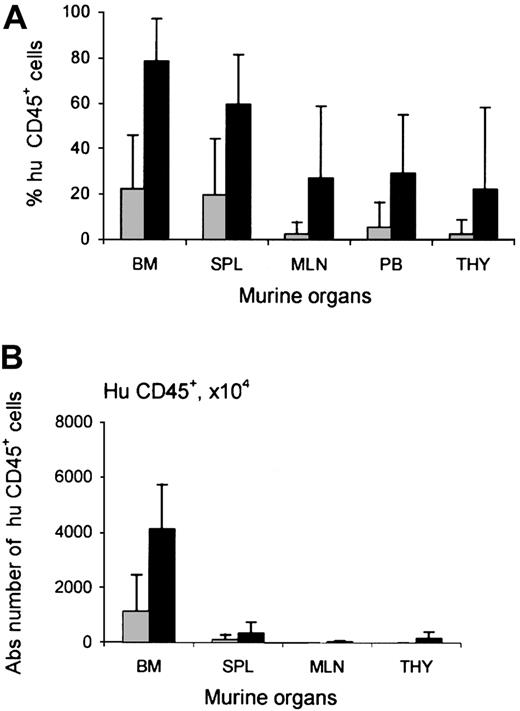
Increased chimerism after TM-β1 treatment was not restricted to the thymus. In all murine organs studied, the engraftment of human cells was higher per injected cell dose in the group pretreated with radiotherapy and TM-β1. The level of chimerism observed in mice pretreated with radiotherapy and mice pretreated with a combination of radiotherapy and TM-β1 injection was 22% ± 24% vs 79% ± 19% in murine bone marrow (P < .0005), 20% ± 25% vs 60% ± 22% in spleen, 5% ± 11% vs 29% ± 26% in peripheral blood, 2% ± 5% vs 27% ± 31% in mLN, and 2% ± 6% vs 22% ± 36% in thymus, respectively (Figure2A). In contrast, the level of chimerism in the bone marrow of NOD/SCID mice (21% ± 25%) was similar to that in Rag2−/−γc−/− mice (21% ± 16%). Figure 2B shows the absolute numbers of human cells in the bone marrow of these mice.
Influence of TM-β1 on human cell engraftment in NOD/SCID mice.
Percentages (A) and absolute numbers (B) of human CD45+cells within murine organs—bone marrow (BM), spleen (SPL), mesenteric lymph nodes (MLN), peripheral blood (PB), and thymus (THY)—of NOD/SCID mice injected with CD3-depleted human UCB 12 to 15 weeks earlier. Mouse conditioning consisted of radiotherapy alone (░) or radiotherapy and injection with TM-β1 (▪). Each bar represents the mean ± SD of 28 mice (RT) or 39 mice (RT + TM-β1), respectively. Calculation of absolute numbers of cells was performed as described before.26 Differences in engraftment between 2 groups were significant for all organs (P < .0005 for bone marrow, spleen, mesenteric lymph nodes, and peripheral blood,P = .002 for thymus).
Influence of TM-β1 on human cell engraftment in NOD/SCID mice.
Percentages (A) and absolute numbers (B) of human CD45+cells within murine organs—bone marrow (BM), spleen (SPL), mesenteric lymph nodes (MLN), peripheral blood (PB), and thymus (THY)—of NOD/SCID mice injected with CD3-depleted human UCB 12 to 15 weeks earlier. Mouse conditioning consisted of radiotherapy alone (░) or radiotherapy and injection with TM-β1 (▪). Each bar represents the mean ± SD of 28 mice (RT) or 39 mice (RT + TM-β1), respectively. Calculation of absolute numbers of cells was performed as described before.26 Differences in engraftment between 2 groups were significant for all organs (P < .0005 for bone marrow, spleen, mesenteric lymph nodes, and peripheral blood,P = .002 for thymus).
Although human cells could be observed in the bone marrow within hours of transplantation, thymopoiesis was seldom seen before 2 weeks after transplantation. Thymus repopulation increased up to week 15 (data not shown). The time delay in thymopoiesis and repopulation kinetics is compatible with the notion that stem cells from the bone marrow seed to the thymus only after homing to the mouse bone marrow rather than at the time of inoculation.
Phenotypical analysis and molecular Vβ analysis of human T cells from thymus of NOD/SCID mice injected with TM-β1
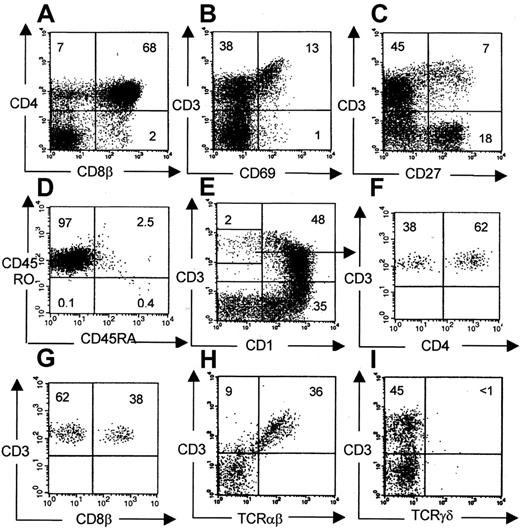
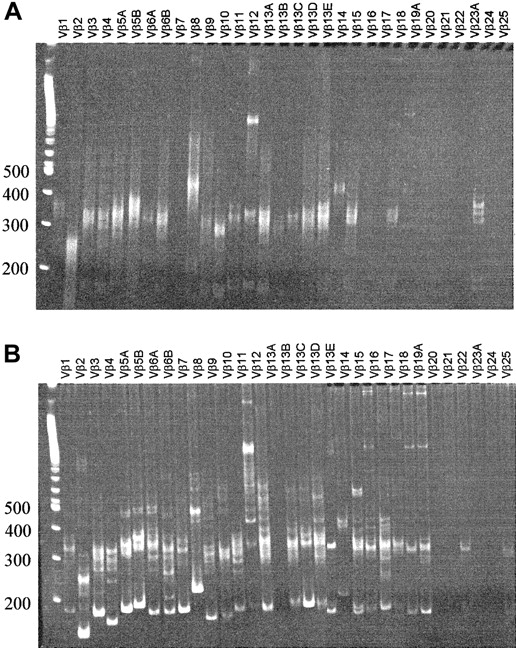
The phenotype of human thymocytes from a representative experiment is shown in Figure 3. Most of the cells are CD4CD8 double-positive, but CD4 and CD8 single-positive cells are also present. A large percentage of DN cells were usually observed, consisting mainly of CD19+ B cells, CD56+ NK cells, CD14+ monocytes, and a minor population of T-cell precursors (data not shown). In chimeric fetal thymus organ culture, a defect in terminal differentiation is observed in that CD3+CD1− terminal differentiation stage cannot develop17 or can develop only in low numbers after extended culture periods.8 Therefore, we analyzed the terminal differentiation stages more closely in our model. Phenotypically, all consecutive developmental stages were present: CD4+CD8+, CD3+CD69+, CD3+CD27+, and CD3+CD1− cells (which are TCR-αβ+) (Figure 3), suggesting that terminal differentiation occurred in these murine thymi. The CD3+CD1− cells were present in sufficient numbers to perform functional studies. The number of cells in a repopulated thymus was on average 4.7 ± 3.7 × 106(n = 22), of which 77% ± 28% were human CD45+. CD3+CD1− mature cells represent on average 4.6% ± 2.3% of the human thymocytes, which are at least 105 cells. The clonality of the thymus repopulation was addressed by molecular Vβ analysis. RNA was isolated from sorted human CD45+ fractions from a repopulated thymus. Vβ-Cβ transcripts were amplified by reverse transcription RT–PCR. As shown in Figure 4A, rearrangements were found across most human Vβ families. In addition, a clear polyclonal pattern (smear) was seen in the more prominent Vβ families in heteroduplex analysis, confirming the presence of a broad Vβ repertoire.
Phenotypical analysis of human thymocytes recovered from NOD/SCID mice.
Conditioning indicates radiotherapy + TM-β1. Unless otherwise indicated, gates were set on viable human CD45+ cells (A, B, C, E, H, I). Within this population subsequent maturational stages were identified: CD4+CD8+ (68%), CD3+CD69+ (13%), CD3+CD27+ (7%), and CD3+CD1− (2%). Virtually all CD3+cells were TCR-αβ+. (D) Gated on CD45+CD3+ cells. (F, G) Gated on CD3+CD1− cells, as indicated.
Phenotypical analysis of human thymocytes recovered from NOD/SCID mice.
Conditioning indicates radiotherapy + TM-β1. Unless otherwise indicated, gates were set on viable human CD45+ cells (A, B, C, E, H, I). Within this population subsequent maturational stages were identified: CD4+CD8+ (68%), CD3+CD69+ (13%), CD3+CD27+ (7%), and CD3+CD1− (2%). Virtually all CD3+cells were TCR-αβ+. (D) Gated on CD45+CD3+ cells. (F, G) Gated on CD3+CD1− cells, as indicated.
Vβ-Cβ RT-PCR heteroduplex analysis of thymus and bone marrow.
RNA was isolated from thymus (sorted CD45+CD3+CD27+CD69+fraction) (A) and bone marrow (total, unsorted) (B), and after reverse transcription cDNA was amplified with PCR. On heteroduplex analysis of the thymus, polyclonal smears can be observed for most Vβ families. Bone marrow shows a pauciclonal pattern superimposed on polyclonal background smears.
Vβ-Cβ RT-PCR heteroduplex analysis of thymus and bone marrow.
RNA was isolated from thymus (sorted CD45+CD3+CD27+CD69+fraction) (A) and bone marrow (total, unsorted) (B), and after reverse transcription cDNA was amplified with PCR. On heteroduplex analysis of the thymus, polyclonal smears can be observed for most Vβ families. Bone marrow shows a pauciclonal pattern superimposed on polyclonal background smears.
Human T cells in the periphery
After injection of CD3-depleted UCB cells, human cells could be detected in bone marrow, spleen, and peripheral blood within weeks after transplantation. Occasionally, contaminating T cells that were injected in a few mice expanded and could be transiently measured in the peripheral blood up to a few weeks after injection, after which these cells disappeared. Eight to 15 weeks after transplantation, T cells reappeared in the peripheral blood (Table2). However, at this time T cells were observed only in mice with repopulated thymi. This observation, together with the delay in appearance of T cells in the periphery, favors the hypothesis that these cells were derived from the thymus. To strengthen this hypothesis, rigorously sorted CD34+CD3− UCB cells were injected into NOD/SCID mice (on reanalysis, T-cell contamination was less than 0.1%). These mice were analyzed 12 weeks later for the presence of peripheral T cells. Three of 5 mice had human double-positive cells in the thymus. Two of these successfully thymic repopulated mice selectively carried human T cells in the peripheral blood. This strongly argues against the expansion of contaminating CD3+cells and indicates that the observed T cells were generated de novo in these animals (Figure 5).
Kinetics of appearance of peripheral T cells
| . | Time after injection (w) . | ||
|---|---|---|---|
| 4-7 . | 8-11 . | 12-15 . | |
| n (total) | 30 | 51 | 40 |
| n (T cells +) | 0 | 8 | 17 |
| % (T cells +/total) | 0 | 16 | 43 |
| . | Time after injection (w) . | ||
|---|---|---|---|
| 4-7 . | 8-11 . | 12-15 . | |
| n (total) | 30 | 51 | 40 |
| n (T cells +) | 0 | 8 | 17 |
| % (T cells +/total) | 0 | 16 | 43 |
Mice injected with CD3-depleted UCB were bled at several time points (4-7 weeks, 8-11 weeks, and 12-15 weeks). Percentage of mice with human T cells in the peripheral blood increased up to 15 weeks.
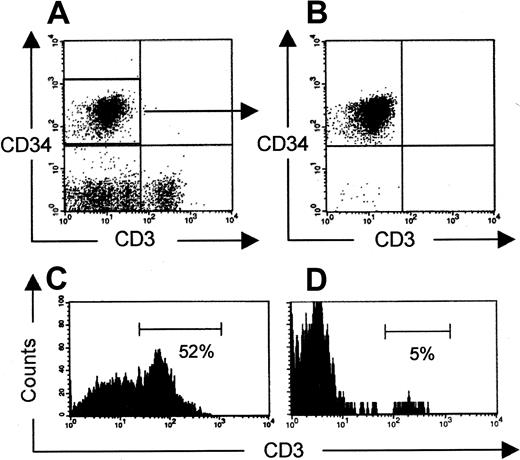
Peripheral T cells after injection of CD34+CD3− UCB cells.
(A, B) Expression of CD34 and CD3 before (CD34+-enriched UCB cells) and after cell sorting, respectively, is shown. Presence of human CD3+ T cells in murine thymus (C) and spleen (D) 12 weeks after injection of CD34+CD3− cells is shown. Plots are gated on human CD45+ cells.
Peripheral T cells after injection of CD34+CD3− UCB cells.
(A, B) Expression of CD34 and CD3 before (CD34+-enriched UCB cells) and after cell sorting, respectively, is shown. Presence of human CD3+ T cells in murine thymus (C) and spleen (D) 12 weeks after injection of CD34+CD3− cells is shown. Plots are gated on human CD45+ cells.
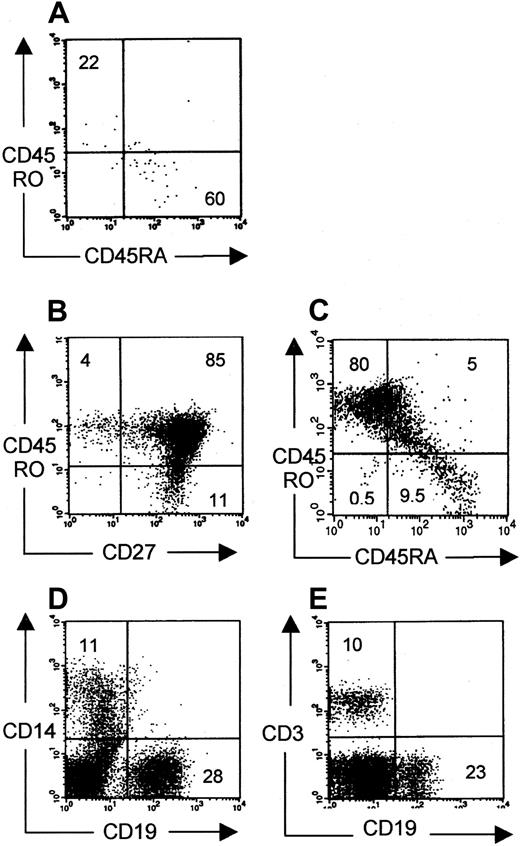
To investigate whether these peripheral CD3+ cells could be activated in vivo and could mount an immune reaction, phenotypic analysis for naive and memory cells was performed. Some animals (6 of 13) contained low percentages of human cells that were mainly CD45RA+ (Figure 6A), similar to the phenotype of thymic emigrants. However, in some mice (7 of 13), CD45RO+ cells were abundantly observed, of which both CD27− effector and CD27+ memory cells18 19 were present (Figure 6 B-C). Moreover, these mice had higher numbers of peripheral T cells than mice with mainly CD45RA+ T cells in the periphery. These data suggest T-cell activation on antigen presentation by human or murine antigen-presenting cells present in these animals. Indeed, in the periphery of repopulated mice, human CD19+ (B cells) and CD14+ (monocytes) could also be identified (Figure 6D-E). This idea of antigen-induced T-cell activation was supported by the molecular Vβ analysis data of the peripheral T cells. RNA was isolated from total bone marrow containing 5% human CD3+ T cells, of which 95% were CD45RO+. Vβ-Cβ transcripts were amplified by RT-PCR. As can be seen in Figure 4B, heteroduplex analysis showed an pauciclonal pattern of several Vβ clones in most Vβ families superimposed on a polyclonal smear. The difference in Vβ pattern between thymocytes and peripheral T cells indicates that some Vβ clones are selected in the thymus or after peripheral expansion. We suppose that the clonal expansions are associated with the CD45RO+ phenotype, but, because of the low numbers of cells in mice with mainly CD45RA+ cells, this could not be confirmed.
Phenotypical analysis of peripheral human cells.
Phenotypical profile of CD45RO and CD45RA surface markers on CD3+ cells harvested from spleen from NOD/SCID mice pretreated with radiotherapy and TM-β1. Plots representative for 6 of 13 mice (A) and 7 of 13 mice (B, C) are shown. (A-C) Gated on CD3+ cells. (D, E) Gated on CD45+cells. Monocytes (CD14+), B cells (CD19+), and T cells (CD3+) were present in the periphery of all 10 mice.
Phenotypical analysis of peripheral human cells.
Phenotypical profile of CD45RO and CD45RA surface markers on CD3+ cells harvested from spleen from NOD/SCID mice pretreated with radiotherapy and TM-β1. Plots representative for 6 of 13 mice (A) and 7 of 13 mice (B, C) are shown. (A-C) Gated on CD3+ cells. (D, E) Gated on CD45+cells. Monocytes (CD14+), B cells (CD19+), and T cells (CD3+) were present in the periphery of all 10 mice.
To investigate whether the human peripheral T cells were functional, spleen cells were cultured in the presence of PHA + IL-2. From the 3 spleens cultured, a human T-cell line was generated. On stimulation with OKT3 + TPA or ConA, significant levels of IL-2, IL-4, and IFN-γ were produced (Table 3), indicating that these cells were functional.
Cytokine production after stimulation of human T cells from spleen and thymus
| Experiment . | IL-2 (pg/mL) . | IL-4 (pg/mL) . | IFN-γ (pg/mL) . |
|---|---|---|---|
| 1 | 44.5 | 3.0 | 316.5 |
| 2 | 13.3 | 21.9 | 289.0 |
| 3 | 157.5 | 13.5 | 424.0 |
| Experiment . | IL-2 (pg/mL) . | IL-4 (pg/mL) . | IFN-γ (pg/mL) . |
|---|---|---|---|
| 1 | 44.5 | 3.0 | 316.5 |
| 2 | 13.3 | 21.9 | 289.0 |
| 3 | 157.5 | 13.5 | 424.0 |
Human cells were cultured from murine spleen by stimulation with PHA. After washing, these cells were stimulated with anti-CD3 + TPA or ConA. Supernatants were harvested 24 hours later, and cytokine content was measured. Data shown are from 3 mice injected from the same UCB donor, and numbers shown are averages from 2 stimulation methods.
TREC analysis of thymocytes and peripheral T cells
To confirm that the repopulation of the thymus results from the injected CD34+ cells, we determined the number of TRECs in the sorted human CD45+CD3+CD27/69+cells from the thymus. This CD45+CD3+CD27/69+ population was sorted from the thymus, and TREC levels were determined. TREC levels were relatively high in the thymus—on average, 97 ± 17 TRECs per 100 cells.
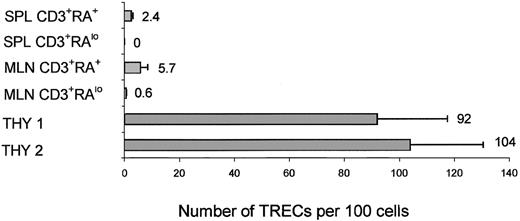
To further exclude that peripheral human T cells were the result of expansion of injected contaminating CD3+ cells, TREC levels were determined on these cells. We sorted CD3+CD45RA+ and CD3+CD45RA− cells from spleen and mesenteric lymph nodes. The number of cells we could harvest from the peripheral blood was too low for TREC analysis. A representative experiment is shown in Figure 7. TREC levels were 2.4 ± 0.6 per 100 cells in spleen CD3+CD45RA+ cells and were not detectable in spleen CD3+CD45RA− cells, 5.7 ± 2.8 in mesenteric lymph node CD3+CD45RA+ cells, and 0.6 ± 0.1 in CD3+CD45RA− cells. The number of CD3+ cells injected in this experiment was calculated to be less than 120, and the total number of CD3+CD45RA+ cells we could harvest from all peripheral organs (peripheral blood, spleen, mesenteric lymph nodes, bone marrow, and lungs) was 3.6 × 106 cells. The average number of TRECs per 100 cells in CD3+ cells from UCB was 24 ± 5 (data not shown). Therefore, after 14 to 15 cell divisions (120→3.6 × 106), the number of TRECs would be undetectable (less than 0.002 per 100 cells) if peripheral expansion of contaminating injected T cells was solely responsible for the peripheral T cells. Detection of significant levels of TRECs indicates that in most peripheral CD45RA+ cells, T cells are recent thymic emigrants and probably generate CD45RO+ cells on activation.
TREC analysis on human thymocytes and peripheral T cells harvested from mice injected with purified CD34+ cells.
Real-time PCR quantification of TRECs was performed on human CD3+ cells harvested from several organs of mice injected with purified CD34+ UCB cells 3 months earlier. CD3+CD45RAlow and CD45+CD3+CD45RA+ cells sorted from spleen and mesenteric lymph nodes (MesLN) and CD45+CD3+CD27/69+ cells sorted from the thymus.
TREC analysis on human thymocytes and peripheral T cells harvested from mice injected with purified CD34+ cells.
Real-time PCR quantification of TRECs was performed on human CD3+ cells harvested from several organs of mice injected with purified CD34+ UCB cells 3 months earlier. CD3+CD45RAlow and CD45+CD3+CD45RA+ cells sorted from spleen and mesenteric lymph nodes (MesLN) and CD45+CD3+CD27/69+ cells sorted from the thymus.
Discussion
The NOD/SCID model is widely accepted as the best-suited model to study self-renewal and multilineage differentiation capacity of human HSCs. A major drawback of the model is the inability to study T-cell development from HSCs because the frequency of thymus repopulation is extremely low. Here we show that by pretreatment of the NOD/SCID mice with irradiation and TM-β1, a thymus repopulation frequency greater than 50% can be reached. Thymopoiesis is phenotypically normal and polyclonal, and it generates functionally mature cells. The model we describe can be used to study multipotency of stem cells and T-cell reconstitution after stem cell transplantation. Experiments are now ongoing to investigate antigen-specific responses in this model.
NOD/SCID mice are relatively deficient in NK cell activity, but the administration of anti-asialo GM-1 or TM-β1, directed against the mouse IL-2Rβ, can increase engraftment of Hu-PBL-NOD/SCID mice.15 In addition, NOD/SCID/B2m−/− mice, which are severely deficient in NK cell activity, also support higher engraftment levels because of the virtually complete absence of NK cell activity.20 In line with these observations, we observed that NOD/SCID mice pretreated with TM-β1 and radiotherapy support higher levels of human overall engraftment and thymopoiesis than control NOD/SCID mice that were only irradiated. To our surprise, we found that the use of Rag2−/−γc−/− mice did not result in better overall engraftment or in better T-cell engraftment. This may be explained by the normal amount and function of macrophages in this strain.21 NOD/SCID mice, on the other hand, have a reduced ability to generate functionally mature macrophages, and they lack hemolytic complement.22 In addition, it has been suggested that the BM microenvironment of Rag2−/−γc−/− mice has a reduced ability to supply the necessary growth factors required for engraftment.21
Phenotypic analysis of the human thymocytes shows that all maturational stages are present, from the most immature to the terminally mature stage. The consecutive stages in maturation were described in the neonatal thymus and the SCID-Hu(liv/thy) model by our laboratory.23,24 We showed that functionally immature CD3+CD69+CD27− and CD3+CD69+CD27+ cells generated functionally mature CD3+CD27+CD1−cells. It has been reported that in the in vitro chimeric human–mouse fetal thymus organ culture, the latter cells either cannot develop17 or they can develop only in low numbers and after extended periods of culture.18 We were, therefore, surprised that this CD1− terminal differentiation stage was achieved in high numbers (at least 105 cells) in the mouse thymus of the in vivo model. This finding suggests that there is no need for the transplantation of a human thymus and that the murine thymus can provide all necessary signals to generate mature T cells from human HSC.
Vanhecke et al23 have shown that the capacity to proliferate on IL-2 after stimulation is absent in the most immature thymocytes and is acquired gradually on maturation. Clonal expansion and capacity to respond to stimulation with the production of cytokines are also acquired during the final stages of maturation. In our model, human cells harvested from thymus and spleen could be clonally expanded, and they produced cytokines on stimulation, proving that the phenotypically identified final maturation stages are also functionally mature.
Through molecular Vβ analysis we could show that the thymus repopulation in our model was polyclonal and involved most Vβ families because a polyclonal unskewed pattern of Vβ variant expression was observed. Not only did the mice contain human T cells in the thymus, these cells seemed to emigrate from the thymus and to migrate to the peripheral blood, spleen, and bone marrow. By molecular Vβ analysis we could demonstrate that the CD3+ T cells in these organs had an pauciclonal Vβ pattern superimposed on a polyclonal smear in most Vβ families.
These T cells resided in the periphery in the presence of human and mouse allophycocyanin. We focused our analysis on signs of T-cell activation. In 6 of 13 mice, we found mainly CD3+CD45RA+ cells and a small number of CD3+CD45RO+ cells in the periphery. In addition, in the SCID-hu model, in which T cells survive only for a few hours in the periphery, a similar pattern is seen.24 25Although we could not fully exclude that the CD45RO+ cells observed contaminated T cells instead of thymic emigrants, 7 of 13 mice had high percentages of CD45RO+ cells and virtually no CD45RA+ cells. Moreover, the number of peripheral T cells was much higher in the mice with primarily CD45RO+ cells in the periphery. Together these data suggest that T cells generated in the mouse thymus were functional in these mice and were probably activated by a foreign antigen in the context of mouse or human antigen presenting cells. The difference in Vβ pattern between thymocytes and peripheral T cells suggests a selection process in the periphery (selective expansion) that might have been antigen driven. Experiments are under way to further characterize these CD45RO+ cells and to investigate whether an antigen-specific immune response can be generated in these mice.
Because T-cell depletion of the injected cells was incomplete in most of these experiments, we could not formally exclude that the peripheral T cells observed 10 to 15 weeks after injection were not derived from a small contamination of CD3+ cells still present after CD3 depletion. It has been reported that mature circulating T cells were never detected in SCID mice, regardless of whether they were reconstituted with whole CB (n = 50) or purified CD34+cells (n = 20). This finding provides arguments against survival and proliferation of injected T cells.4 We also addressed this question experimentally by injecting highly purified CD34+CD3− UCB cells and found that 2 of 3 mice with a successfully repopulated thymus had CD3+CD45RO+ T cells in the periphery. Finally, TREC analysis demonstrated on sorted CD45RA+ and CD45RA− peripheral T cells in spleen and in mesenteric lymph nodes that thymic emigrants significantly contribute to the repopulation of the peripheral T-cell pool. Moreover, we observed different TREC levels in CD45RA+ versus CD45RA− CD3+ cells, suggesting that CD45RA+ cells might have expanded in the periphery and generated the CD45RO+ pool, though we could not exclude that these CD45RO+ cells might have been derived from the few injected mature T cells.
In conclusion, blocking the IL-2Rβ in NOD/SCID mice results in a higher overall engraftment and in a workable frequency of thymus repopulation. These human thymocytes pass all maturational stages, representing a broad Vβ repertoire, and ultimately they emigrate from the thymus and give rise to peripheral CD45RA+ T cells in these mice. The peripheral repertoire is further shaped by pauciclonal expansion of CD45RO+ cells, which could be pathogen driven and either mouse or human major histocompatibility complex restricted. All elements for a functional immune system are present in these mice, and this will have to be further evaluated.
This regimen holds great promise for a more complete experimental approach of stem cell reconstitution capacity after transplantation of fresh or long-term cultured HSCs and for the evaluation of drugs to boost human T-cell reconstitution.
We thank An De Creus and Tom Taghon for stimulating discussions; Achiel Moerman and Caroline Collier for animal care; Christian De Boever for artwork; Ingrid Wolvers-Tettero for expert technical assistance in the Vβ analysis; Jean-Charles Cerottini and Pedro Romero for the excellent collaboration; the Departments of Obstetrics, Cardiac Surgery and Hematology for the supply of human tissue; and the Department of Radiotherapy and Nuclear Medicine for use of their irradiation facility.
Supported by grants from the Gezamenlijk Overlegde Actie, Ghent University, Belgium; the Fund for Scientific Research Flanders, Belgium; and the Flanders Interuniversity Institute for Biotechnology, Belgium. T. K. is a research assistant of the Fund for Scientific Research Flanders, Belgium. A.Z. was supported by grant Zi685/1-1 from the Deutsche Forschungsgemeinschaft and M.J.P. was supported by grant KFS 633-2-1998 from the Swiss Cancer League.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tessa Kerre, Department of Clinical Chemistry, Microbiology, and Immunology, Ghent University Hospital, 4BlokA, De Pintelaan 185, B-9000 Ghent, Belgium; e-mail: tessa.kerre@rug.ac.be.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal