Outcomes after peripheral blood stem cell transplantation (PBSCT) for chronic phase chronic myeloid leukemia (n = 37) were compared with outcomes after bone marrow transplantation (BMT) (n = 54) in the HLA-compatible unrelated donor setting. Median follow-up was 17 months after PBSCT and 29 months after BMT. Both neutrophil and platelet recovery were faster after PBSCT (P < .05). PBSCT was associated with improved immune reconstitution, with higher peripheral blood naive (CD4+CD45RA+) and memory (CD4+ CD45RO+) helper T cells at 3 months and 12 months after transplantation (P < .03). The cumulative incidence of acute (grades II-IV) and chronic graft-versus-host disease (GVHD) were similar, but BMT was associated with a higher cumulative incidence of severe, acute (grade III-IV) GVHD at 24% as compared with 8% with PBSCT (P < .05). Molecular relapse, defined by 2 consecutive positive polymerase chain reaction assays for bcr-abl within a 4-week interval, occurred in 12 of 45 evaluable patients (27%) after BMT and in 4 of 37 (11%) after PBSCT (not significant). Cytogenetic relapse occurred in 5 of 54 patients after BMT (9%) and in 1 of the 37 (3%) patients after PBSCT (not significant). Seventeen of the 54 patients died after BMT (31%), as compared with 2 of 37 patients after PBSCT (5%). Deaths in the BMT group were associated mainly with infections and severe, acute GVHD. The estimated probability of transplant-related mortality (TRM) and disease-free survival at 1000 days after receiving the transplant were 30% and 64% in the BMT group and 5% and 91% in the PBSCT group (P < .03). Overall survival 1000 days after receiving the transplant was 66% after BMT and 94% after PBSCT (P < .02). In the multivariate analysis, only acute GVHD significantly influenced TRM (P < .01).
Introduction
Recombinant granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood stem cells (PBSCs) are now widely used for allogeneic transplantation from HLA-identical related donors. For transplant recipients from related donors, PBSC transplantation (PBSCT) offers improved hematologic and immune reconstitution as compared with BMT.1-7 Moreover, patients with chronic myeloid leukemia (CML) in first chronic phase may have a reduced risk of leukemic relapse after PBSCT from a related donor as compared with bone marrow transplantation (BMT).8
Although the favorable results seen after allogeneic PBSCT from related donors have not yet been documented for patients with unrelated donors, the number of PBSCTs from unrelated donors is gradually increasing. Further, given the 1-log higher T-cell dose delivered with PBSCs when compared with BM, there are potential risks for an increase in the incidence of acute and chronic graft-versus-host-disease (GVHD) along with therapy-associated mortality in the unrelated donor setting.1-4
In the present retrospective single-center study, we compare our experience with the use of PBSCs or BM for allogeneic transplantation from unrelated donors in patients with chronic phase CML.
Patients and methods
Patients
All patients (n = 91) undergoing BMT (n = 54) or PBSCT (n = 37) in the first chronic phase of CML from phenotypically HLA-identical unrelated donors at the University Hospital of Essen (Germany) between September 1995 and March 2001 were consecutively included in the present study. All aspects of this study were approved by the Institutional Review Board on Medical Ethics at the Essen University Hospital. Informed consent was provided according to the Declaration of Helsinki. The observation period of this study began with the first PBSCT from an unrelated donor in September 1995. Grafts were received from donor collection centers of European countries (n = 81), the United States (n = 8), Canada (n = 1), and Australia (n = 1). In almost all cases, the donor collection center decided the method of collection (BM or PBSCs). Differences in the observation period between study groups resulted from difficulties in obtaining unrelated donor PBSCs during the first 2 years.
All transplantations were performed in reverse isolation rooms equipped with high-efficiency particle air filtration systems, and all patients received prophylactic metronidazole, ciprofloxacin, and fluconazole. Patients who were discharged after transplantation were enrolled in our long-term follow-up program. Outpatient visits were performed at least monthly during the first 6 months and at 3-month intervals during the first 2 years after transplantation. After 2 years, patients were usually seen at yearly intervals.
Conditioning regimen
The conditioning regimen consisted of intravenous cyclophosphamide (60 mg/kg of body weight per day × 2) in combination with fractioned total body irradiation (TBI) delivered by a 60-cobalt source in 4 daily fractions of 2.5 Gy (n = 87) or oral busulfan (1 mg/kg of body weight every 6 hours over 4 days) in combination with intravenous cyclophosphamide (60 mg/kg of body weight per day × 2) (n = 3). One patient who suffered a myocardial infarction at day 4 of TBI received fludarabine (30 mg/m2 body surface) for 3 days instead of cyclophosphamide. All grafts were infused without manipulation or T-cell depletion. All blood products were irradiated (30 Gy) and leukocyte-depleted throughout the posttransplantation course. GVHD prophylaxis consisted of intravenous methotrexate (15 mg/m2 on day 1; 10 mg/m2 on days 3, 6, and 11) in combination with continuous intravenous cyclosporine in all patients. All except 3 patients received all 4 methotrexate doses.
Patient demographics are summarized in Table1.
Demographic and treatment characteristics of patients
| . | BMT . | PBSCT . | P . |
|---|---|---|---|
| No. patients | 54 | 37 | |
| Median age (range), y | |||
| Patients | 40 (18-56) | 40 (17-58) | NS |
| Donors | 37 (23-55) | 34 (20-52) | NS |
| Sex, no. (%) | |||
| Male, with male donor | 27 (50) | 19 (51) | NS |
| Male, with female donor | 8 (15) | 3 (8) | NS |
| Female, with female donor | 15 (28) | 11 (30) | NS |
| Female, with male donor | 4 (7) | 4 (11) | NS |
| Median time from diagnosis to transplantation (range), mo. | 24 (4-86) | 16 (3-71) | .05 |
| Median graft size (range) | |||
| Nucleated cell dose (× 108/kg) | 3.11 (1.58-5.02) | 13.8 (4.35-29.61) | .001 |
| CD34+ cell dose (× 106/kg) | 3.35 (1.5-6.83) | 8.40 (4.2-42.8) | .001 |
| GVHD prophylaxis, no. | |||
| Methotrexate and cyclosporine A | 54 | 37 | |
| Without day 11 methotrexate | 3 | 0 | NS |
| Myeloablative regimen, no. (%) | |||
| TBI and cyclophosphamide | 52 (96) | 35 (94) | NS |
| Busulfan and cyclophosphamide | 2 (4) | 1 (3) | NS |
| TBI and fludarabine | — | 1 (3) | |
| Cytomegalovirus status, no. (%) | |||
| Seropositive, seropositive donor | 14 (26) | 7 (19) | NS |
| Seropositive, seronegative donor | 11 (20) | 10 (27) | NS |
| Seronegative, seronegative donor | 26 (48) | 20 (54) | NS |
| Seronegative, seropositive donor | 3 (6) | 0 (0) | NS |
| Patients receiving G-CSF, no. (%) | 4 (7) | 2 (5) | NS |
| Median follow-up (range), mo. | 29 (2-68) | 17 (2-41) | .01 |
| . | BMT . | PBSCT . | P . |
|---|---|---|---|
| No. patients | 54 | 37 | |
| Median age (range), y | |||
| Patients | 40 (18-56) | 40 (17-58) | NS |
| Donors | 37 (23-55) | 34 (20-52) | NS |
| Sex, no. (%) | |||
| Male, with male donor | 27 (50) | 19 (51) | NS |
| Male, with female donor | 8 (15) | 3 (8) | NS |
| Female, with female donor | 15 (28) | 11 (30) | NS |
| Female, with male donor | 4 (7) | 4 (11) | NS |
| Median time from diagnosis to transplantation (range), mo. | 24 (4-86) | 16 (3-71) | .05 |
| Median graft size (range) | |||
| Nucleated cell dose (× 108/kg) | 3.11 (1.58-5.02) | 13.8 (4.35-29.61) | .001 |
| CD34+ cell dose (× 106/kg) | 3.35 (1.5-6.83) | 8.40 (4.2-42.8) | .001 |
| GVHD prophylaxis, no. | |||
| Methotrexate and cyclosporine A | 54 | 37 | |
| Without day 11 methotrexate | 3 | 0 | NS |
| Myeloablative regimen, no. (%) | |||
| TBI and cyclophosphamide | 52 (96) | 35 (94) | NS |
| Busulfan and cyclophosphamide | 2 (4) | 1 (3) | NS |
| TBI and fludarabine | — | 1 (3) | |
| Cytomegalovirus status, no. (%) | |||
| Seropositive, seropositive donor | 14 (26) | 7 (19) | NS |
| Seropositive, seronegative donor | 11 (20) | 10 (27) | NS |
| Seronegative, seronegative donor | 26 (48) | 20 (54) | NS |
| Seronegative, seropositive donor | 3 (6) | 0 (0) | NS |
| Patients receiving G-CSF, no. (%) | 4 (7) | 2 (5) | NS |
| Median follow-up (range), mo. | 29 (2-68) | 17 (2-41) | .01 |
BMT indicates bone marrow transplantation; PBSCT, peripheral blood stem cell transplantation; GVHD, graft-versus-host disease; TBI, total body irradiation; G-CSF, granulocyte colony-stimulating factor.
Donors
All donors were HLA-identical at the A, B, DRB1, and DQB1 loci with their respective recipients. DNA-based typing at the HLA-A and HLA-B levels was performed in 76 of 91 patients (80%) (polymerase chain reaction–sequence specific primers [PCR-SSP]). In the remaining 15 patients (20%), HLA-A and HLA-B antigens were determined by conventional serologic methods confirmed by 1-dimensional isoelectric focusing (1D-IEF). DRB1, DQB1 antigens were assessed in all patients without exception by high-resolution molecular genetic typing (PCR-SSP). HLA typing of all donors was originally performed at the donor centers and was confirmed by a second analysis prior to transplantation.
All PBSC donors received a G-CSF at a dose ranging from 9 to 12.5 μg/kg/d, administered subcutaneously once daily for 5 consecutive days. If the first apheresis procedure resulted in the collection of fewer than 5 × 106 CD34+ cells per kilogram of the recipient's body weight, a second apheresis procedure was performed the following day.
Quantitation of CD34+ cells and peripheral blood cell subsets
CD34+ cell determinations were performed on bone marrow and leukapheresis products. Peripheral blood cell subsets were determined by flow cytometry, as previously described.4
Clinical evaluation
Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count exceeding 0.5 × 109/L. Platelet engraftment was defined as the first day of a platelet count exceeding 20 × 109/L or 50 × 109/L without platelet transfusions. Acute GVHD was graded according to standard criteria.9 Chronic GVHD was assessed in patients alive after day 90. Cytomegalovirus (CMV) reactivation was determined weekly by the pp 65 antigen in blood leukocytes. Treatment-related mortality (TRM) was defined as death with no relapse.
Minimal residual disease detection
Isolation of messenger RNA and PCR for the bcr-abl transcript were performed from peripheral blood cells and bone marrow buffy coat cells as described earlier.8 The PCR assay is reported to have a sensitivity of 0.0001%.8 Blood samples were evaluated monthly in the first 6 months after transplantation and in 3-month intervals during the first 2 years after transplantation. After 2 years, patients without evidence of molecular or cytogenetic disease were evaluated once per year. A total of 430 samples (blood or bone marrow) were evaluable.
Definition of relapse
Hematologic relapse was diagnosed on the basis of standard hematologic criteria. An isolated cytogenetic relapse was assumed if after a period of negativity, Philadelphia-chromosome–positive metaphases were detected in repeated cytogenetic analyses without evidence of hematologic disease. For the diagnosis of molecular relapse or persistence, only those positive bcr-abl–PCR results that were confirmed on a consecutive PCR assay within a 4-week interval were considered positive.
Statistics
Cumulative estimates (± SE) were calculated with the use of the Kaplan-Meier method.10 Differences between time-to-event distribution functions were compared by a log-rank test (Mantel-Haenszel).11 A stepwise proportional hazards general linear model (PHGLM) analysis was used to evaluate interactions of different covariates on the analytical endpoint of TRM. Covariates in PHGLM analyses were stratified according to time between diagnosis and transplantation (12 months or fewer versus more than 12 months); patient age (40 years or fewer versus more than 40 years); gender constellation (male recipient with female donor or other); and occurrence of acute (grades II-IV) and chronic GVHD. Conditional risk ratios and their 95% confidence intervals were derived from PHGLM analyses after adjustment for significant covariates in the model. Differences between the 2 groups in the number of cells administered, the time to engraftment, the number of transfusions, and the number of peripheral blood mononuclear cell subsets were evaluated with the use of a 2-sample t test and the Mann-Whitney Utest.
Only patients surviving more than 30 days were included in the analysis of acute GVHD. A minimum of 90 days of follow-up was the criterion for chronic GVHD.
Results
Cell yields
Mononuclear cells and CD34+ cells were significantly higher in the PBSC leukapheresis products than in the bone marrow products (Table 1) (P < .001).
Time to engraftment and transfusion requirements
Absolute neutrophil counts exceeded 500/μL 5 days earlier in the PBSC recipients than in the BM recipients (P < .01) (Table 2). Similarly, platelet counts exceeded 20 000/μL, without the need for transfusions, 5 days earlier in the PBSC recipients than in the BM recipients (P < .01). Fewer units of platelets and red cells were transfused in the PBSC group than in the bone marrow group (P < .02 and P < .04, respectively).
Time to engraftment, transfusion requirements, graft-versus-host disease
| . | BMT . | PBSCT . | P . |
|---|---|---|---|
| Time to engraftment, median (range), d | |||
| Neutrophils > 500/μL | 22 (13-52) | 17 (8-28) | < .01 |
| Platelets > 20 000/μL | 22 (12-54) | 17 (10-60) | < .01 |
| Platelets > 50 000/μL | 26 (17-174) | 22 (12-129) | < .05 |
| Transfusions, no. units (range) | |||
| Red cells, median | 12 (4-166) | 8 (4-24) | < .04 |
| Platelets, median | 19 (4-326) | 8 (2-27) | < .02 |
| Acute GVHD by grade, no. (%) | |||
| 0 | 8 (15) | 7 (19) | NS |
| I | 23 (43) | 15 (41) | NS |
| II | 10 (19) | 12 (32) | NS |
| III | 7 (13) | 2 (5) | NS |
| IV | 6 (11) | 1 (3) | NS |
| II − IV | 23 (43) | 15 (41) | NS |
| III + IV | 13 (24) | 3 (8) | < .05 |
| Chronic GVHD,* no. (%) | |||
| No GVHD | 9 (19) | 4 (17) | NS |
| Limited | 27 (55) | 22 (59) | NS |
| Extensive | 13 (26) | 10 (28) | NS |
| . | BMT . | PBSCT . | P . |
|---|---|---|---|
| Time to engraftment, median (range), d | |||
| Neutrophils > 500/μL | 22 (13-52) | 17 (8-28) | < .01 |
| Platelets > 20 000/μL | 22 (12-54) | 17 (10-60) | < .01 |
| Platelets > 50 000/μL | 26 (17-174) | 22 (12-129) | < .05 |
| Transfusions, no. units (range) | |||
| Red cells, median | 12 (4-166) | 8 (4-24) | < .04 |
| Platelets, median | 19 (4-326) | 8 (2-27) | < .02 |
| Acute GVHD by grade, no. (%) | |||
| 0 | 8 (15) | 7 (19) | NS |
| I | 23 (43) | 15 (41) | NS |
| II | 10 (19) | 12 (32) | NS |
| III | 7 (13) | 2 (5) | NS |
| IV | 6 (11) | 1 (3) | NS |
| II − IV | 23 (43) | 15 (41) | NS |
| III + IV | 13 (24) | 3 (8) | < .05 |
| Chronic GVHD,* no. (%) | |||
| No GVHD | 9 (19) | 4 (17) | NS |
| Limited | 27 (55) | 22 (59) | NS |
| Extensive | 13 (26) | 10 (28) | NS |
For abbreviations, see Table 1.
Number of evaluated patients was 49 in the BMT group and 36 in the PBSCT group.
Immune reconstitution 3 and 12 months after transplantation
Peripheral blood CD3+CD4+ T cells were significantly higher 3 and 12 months after PBSCT as compared with BMT (P < .001 and P < .01, respectively) (Table 3). This difference was also seen for CD3+CD4+ subsets (naive T-helper cells and memory T-helper cells) 3 months after transplantation (P < .002 and P < .03). In contrast, the levels of monocytes, CD8+ cells, and CD19+ cells did not differ significantly after PBSCT and BMT (Table 3). Although the CD4-to-CD8 ratio was higher after PBSCT (mean 3 months after transplantation, 0.66 after PBSCT versus 0.48 after BMT), the difference was not significant.
Phenotypic reconstitution of mononuclear cell subsets 3 months and 12 months after transplantation
| . | BMT, cells/μL . | PBSCT, cells/μL . | P . |
|---|---|---|---|
| 3 months after transplantation3-150 | |||
| CD3+CD4+T cells | 71.3 ± 49 | 182.2 ± 111 | < .001 |
| CD3+CD4+CD45RA+ T cells | 11.2 ± 13.4 | 44.6 ± 41.0 | < .002 |
| CD3+CD4+CD45R0+ T cells | 59.8 ± 43.7 | 124.3 ± 75.7 | < .03 |
| CD3+CD8+ T cells | 274 ± 442 | 515 ± 563 | NS |
| CD4/CD8 ratio | 0.48 ± 0.8 | 0.66 ± 0.6 | NS |
| CD19+ B cells | 18.1 ± 86 | 20.4 ± 18 | NS |
| Monocytes | 483 ± 322 | 569 ± 286 | NS |
| NK cells | 164 ± 118 | 167 ± 131 | NS |
| 12 months after transplantation3-151 | |||
| CD3+CD4+T cells | 191 ± 138 | 332 ± 188 | < .01 |
| CD3+CD4+CD45RA+ T cells | 37.8 ± 42.4 | 85.6 ± 72.8 | < .07 |
| CD3+CD4+CD45R0+ T cells | 181 ± 209 | 223 ± 116 | NS |
| CD3+CD8+ T cells | 500 ± 517 | 548 ± 425 | NS |
| CD4/CD8 ratio | 0.75 ± 0.8 | 0.82 ± 0.8 | NS |
| CD19+ B cells | 91.2 ± 119 | 87.2 ± 105 | NS |
| Monocytes | 475 ± 182 | 640 ± 436 | NS |
| NK cells | 137 ± 77 | 186 ± 132 | < .03 |
| . | BMT, cells/μL . | PBSCT, cells/μL . | P . |
|---|---|---|---|
| 3 months after transplantation3-150 | |||
| CD3+CD4+T cells | 71.3 ± 49 | 182.2 ± 111 | < .001 |
| CD3+CD4+CD45RA+ T cells | 11.2 ± 13.4 | 44.6 ± 41.0 | < .002 |
| CD3+CD4+CD45R0+ T cells | 59.8 ± 43.7 | 124.3 ± 75.7 | < .03 |
| CD3+CD8+ T cells | 274 ± 442 | 515 ± 563 | NS |
| CD4/CD8 ratio | 0.48 ± 0.8 | 0.66 ± 0.6 | NS |
| CD19+ B cells | 18.1 ± 86 | 20.4 ± 18 | NS |
| Monocytes | 483 ± 322 | 569 ± 286 | NS |
| NK cells | 164 ± 118 | 167 ± 131 | NS |
| 12 months after transplantation3-151 | |||
| CD3+CD4+T cells | 191 ± 138 | 332 ± 188 | < .01 |
| CD3+CD4+CD45RA+ T cells | 37.8 ± 42.4 | 85.6 ± 72.8 | < .07 |
| CD3+CD4+CD45R0+ T cells | 181 ± 209 | 223 ± 116 | NS |
| CD3+CD8+ T cells | 500 ± 517 | 548 ± 425 | NS |
| CD4/CD8 ratio | 0.75 ± 0.8 | 0.82 ± 0.8 | NS |
| CD19+ B cells | 91.2 ± 119 | 87.2 ± 105 | NS |
| Monocytes | 475 ± 182 | 640 ± 436 | NS |
| NK cells | 137 ± 77 | 186 ± 132 | < .03 |
CD3+CD4+ T cells are T-helper cells; CD3+CD4+CD45RA+ T cells, naive T-helper cells; CD3+CD4+CD45R0+ T cells, memory T-helper cells.
NK cells indicate natural killer cells; for other abbreviations, see Table 1.
Number of evaluated patients was 37 in the BMT group and 23 in the PBSCT group.
Number of evaluated patients was 32 in the BMT group and 19 in the PBSCT group.
Acute and chronic GVHD
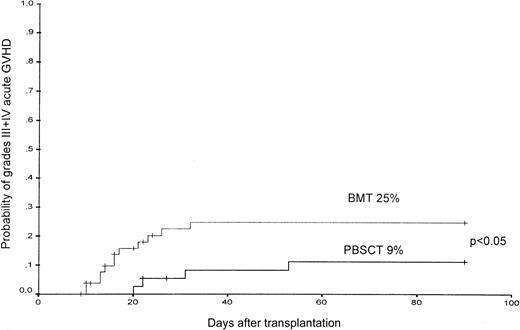
The cumulative incidence of grades II-IV acute GVHD at day 90 was similar, with 41% after PBSCT and 43% after BMT; however, the cumulative incidence of severe, acute GVHD (grades III-IV) was higher after BMT and was 24% as compared with 8% (P < .05) (Figure 1). The estimated probability of grade III or IV acute GVHD at day 90 was 25% ± 7% for the BMT group as compared with 9% ± 7% for the PBSC group (P < .05) (Figure 2).
The probability of the occurrence of grades II-IV acute GVHD in patients after PBSCT or BMT from unrelated donors.
The probability of the occurrence of grades II-IV acute GVHD in patients after PBSCT or BMT from unrelated donors.
The probability of the occurrence of grades III-IV acute GVHD in patients after PBSCT or BMT from unrelated donors.
The probability of the occurrence of grades III-IV acute GVHD in patients after PBSCT or BMT from unrelated donors.
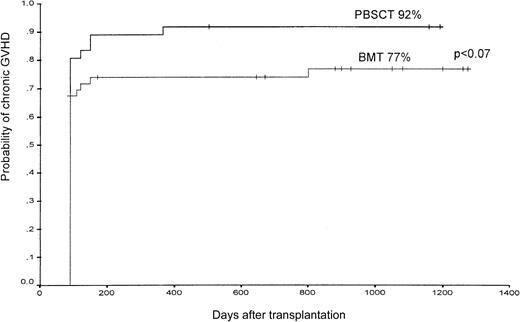
Extensive chronic GVHD occurred in 10 patients assigned to receive PBSCs compared with 13 of those assigned to receive bone marrow (not significant) (Table 2). The estimated probability of chronic GVHD (limited and extensive grade) at 1000 days after undergoing transplantation was 92% in the PBSC group and 77% in the BMT group (P < .07) (Figure 3).
The probability of the occurrence of chronic GVHD (limited and extensive) in patients after PBSCT or BMT from unrelated donors.
The probability of the occurrence of chronic GVHD (limited and extensive) in patients after PBSCT or BMT from unrelated donors.
Molecular and cytogenetic relapse
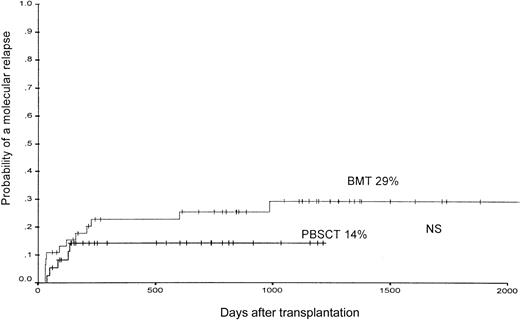
According to the applied definition of molecular relapse, a repeatedly positive bcr-abl–PCR assay within a 4-week interval was detectable in 12 of 45 evaluable patients (27%) after BMT and in 4 of 37 (11%) after PBSCT (not significant). The 1000-day estimated probability of molecular relapse was 29% ± 8% after BMT and 14% ± 7% after PBSCT (not significant) (Figure4).
The probability of the occurrence of a molecular relapse, defined by 2 consecutive positive PCR assays for bcr-abl within a 4-week interval in patients after PBSCT or BMT from unrelated donors.
The probability of the occurrence of a molecular relapse, defined by 2 consecutive positive PCR assays for bcr-abl within a 4-week interval in patients after PBSCT or BMT from unrelated donors.
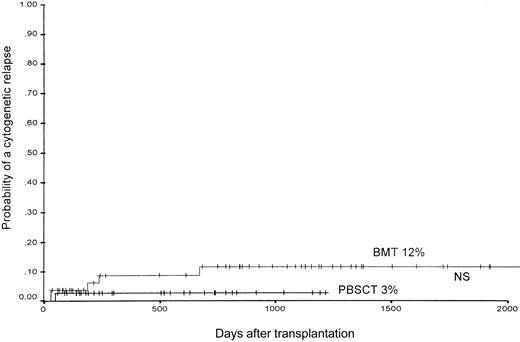
Cytogenetic relapse developed in 5 of 54 patients after BMT (9%) and in 1 of the 37 patients after PBSCT (3%). Similarly, the 1000-day estimated probability of cytogenetic relapse was 12% ± 5% after BMT and 3% ± 4% after PBSCT (not significant) (Figure5). Of the 6 patients with a cytogenetic relapse, 5 patients also developed a hematologic relapse. One patient with a cytogenetic relapse achieved a molecular remission after withdrawal of immunosuppression.
The probability of the occurrence of a cytogenetic relapse in patients after PBSCT or BMT from unrelated donors.
The probability of the occurrence of a cytogenetic relapse in patients after PBSCT or BMT from unrelated donors.
Rates of death and survival
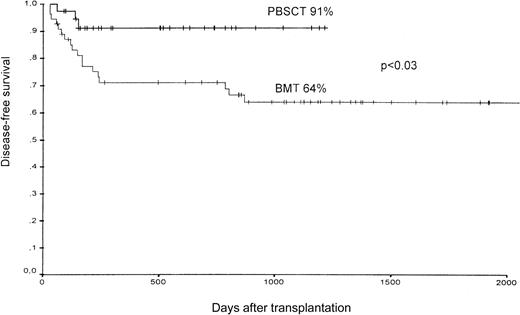
Of the 37 patients assigned to receive PBSCT, 2 died compared with 17 of the 54 patients in the BMT group (31%). In the PBSCT group, one patient died from acute GVHD and the other from cerebral hemorrhage at day 140 after receiving the transplant. The predominant causes of death in the bone marrow group were infections and severe, acute GVHD. Most of the patients died within the first 250 days after transplantation (13 of 17 patients) (Table 4). The estimated probability of transplantation-related death at 1000 days after receiving the transplant was 30% ± 7% (mean ± SE) in the BMT group and 5% ± 3% in the PBSCT group. The estimated probability of disease-free survival for all patients 1000 days after transplantation was 91% in the PBSCT group, as compared with 64% in the BMT group (P < .03) (Figure6).
Causes of death
| . | BMT, no. (days after transplantation for each patient) . | PBSCT, no. (days after transplantation) . |
|---|---|---|
| Acute GVHD | 3 (35, 82, and 94) | 1 (60) |
| Relapse | 2 (646 and 868) | — |
| Invasive fungi | 2 (171 and 242) | — |
| Septicemia | 4 (65, 80, 171, and 175) | — |
| CMV-IP | 2 (58 and 118) | — |
| Pneumocystis pneumonia | 1 (784) | — |
| Encephalitis | 1 (149) | — |
| Toxoplasmosis | 1 (245) | — |
| Acute cardiac failure | 1 (802) | — |
| Cerebral bleeding | — | 1 (140) |
| Total | 17 | 2 |
| . | BMT, no. (days after transplantation for each patient) . | PBSCT, no. (days after transplantation) . |
|---|---|---|
| Acute GVHD | 3 (35, 82, and 94) | 1 (60) |
| Relapse | 2 (646 and 868) | — |
| Invasive fungi | 2 (171 and 242) | — |
| Septicemia | 4 (65, 80, 171, and 175) | — |
| CMV-IP | 2 (58 and 118) | — |
| Pneumocystis pneumonia | 1 (784) | — |
| Encephalitis | 1 (149) | — |
| Toxoplasmosis | 1 (245) | — |
| Acute cardiac failure | 1 (802) | — |
| Cerebral bleeding | — | 1 (140) |
| Total | 17 | 2 |
CMV-IP indicates cytomegalovirus-induced interstitial pneumonia. For other abbreviations, see Table 1.
Disease-free survival of patients after PBSCT or BMT from unrelated donors.
Overall survival 1000 days after receiving the transplant was 66% after BMT and 94% after PBSCT (P < .02).
Multivariate analysis for TRM
For TRM and molecular relapse, the following were analyzed: the time from diagnosis to transplantation (not more than 12 months or more than 12 months); age (not more than 40 or more than 40 years); gender constellation (male recipient with female donor or other); and acute and chronic GVHD. Only acute GVHD significantly influenced TRM (P < .01). Chronic GVHD (P < .006) and time from diagnosis to transplantation (P < .002) influenced the occurrence of a molecular relapse after the transplantation.
Influence of HLA-testing method on the occurrence of acute or chronic GVHD
The method of HLA testing for class I (PCR-SSP [n = 76] versus 1D-IEF [n = 15]) had no influence on the occurrence of acute (grades II-IV or grades III-IV) or chronic GVHD in univariate testing.
Discussion
Our study is the first to compare the transplantation of peripheral blood stem cells with bone marrow from unrelated HLA-A–, HLA-B–, and HLA-DR–compatible donors in patients with chronic phase CML.
In our study, both neutrophil and platelet recovery were faster in the PBSCT group. Furthermore, PBSCT was associated with the transfusion of fewer units of platelets and red blood cells compared with the bone marrow group. However, these results are similar to those of previous studies comparing PBSCT with BMT from HLA-identical siblings1-7 and are also in line with previously published studies using PBSCs for various diseases and disease stages from unrelated donors.12-14 It is generally thought that the 3 to 4 times higher number of CD34+ cells and the approximately 10 times higher number of CD3+ cells in PBSC grafts compared with bone marrow grafts may be responsible for the faster recovery in patients after PBSCT.
We have also documented superior immune reconstitution in patients receiving PBSCs compared with those receiving bone marrow from unrelated donors. PBSC recipients had peripheral blood naive (CD4+CD45RA+) and memory (CD4+CD45RO+) helper T cells that were significantly higher at 3 and 12 months after transplantation. The accelerated immune reconstitution after PBSCT may be associated with a reduced risk for infectious complications. Indeed, the risk for interstitial pneumonia caused by cytomegalovirus following allogeneic PBSCT is reduced as compared with BMT.15 The faster immune reconstitution after PBSCT has been already described in patients with HLA-identical sibling donors4 but has not been previously described in the unrelated transplant setting.
Although the number of CD3+ cells in the PBSC grafts was markedly higher than in the marrow grafts, the rates of acute and chronic GVHD were not significantly higher in the group that received PBSCs. Moreover, we found a slightly decreased incidence of severe, acute (grades III-IV) GVHD in patients receiving PBSCT as compared with patients receiving BMT. The estimated probability of acute grades III-IV GVHD was 25% after BMT as compared with 9% after PBSCT (P < .05), whereas the estimated probability for acute GVHD grades II-IV was similar in the 2 groups. The incidence of acute grades III-IV GVHD in patients after BMT in our study was lower than in another study of CML patients in chronic phase after BMT from unrelated donors (25% versus 35%).16
HLA typing at A and B loci was performed in the first 15 study patients by conventional serology combined with 1D-IEF, whereas the majority (76) of study patients were tested by PCR-SSP. We therefore analyzed the influence of the typing method by univariate analysis and found no influence on the occurrence of acute or chronic GVHD.
The lower incidence of acute grades III-IV GVHD after PBSCT (P < .05) observed in our study has not been reported in studies comparing PBSCT with BMT from HLA-identical siblings; those studies also included patients in their analyses with diseases other than CML.5-7 Donor pretreatment with G-CSF may have some advantageous effects on the occurrence of severe, acute GVHD, as has been shown in murine models.17,18 Furthermore, it seems important that in comparison with other studies addressing PBSCT from unrelated donors, this study analyzed only CML patients in chronic phase. Therefore, the higher incidence of severe, acute GVHD seen in other studies might be associated with a higher proportion of patients with more advanced disease stages.
The most importing finding of our study is the surprisingly low rate of transplantation-related mortality in patients receiving PBSCs compared with patients receiving bone marrow. Of 37 analyzed patients receiving PBSCTs, only 2 have died at the time of this analysis, whereas the transplantation-related mortality in patients after BMT was in the expected range of 30%, which results in a overall survival rate of 66% at 1000 days after transplantation.5-7,12-14,16 The longer follow-up of the BMT group (median 29 months) as compared with the PBSCT group (median 17 months) is due to difficulties in obtaining PBSCs in the first 2 years of the study and does not explain the higher cumulative death rate in the BMT group since 13 of 17 observed deaths occurred before day 250 after transplantation. The estimated probability of 66% survival in patients after BMT in this study is in agreement with the results of another study in CML patients in chronic phase undergoing transplantation from unrelated donor; in that study, survival rates between 57% and 74% have been described depending on the age of the patient and time from diagnosis to transplantation.16
Most of the patients who died after BMT died from infectious complications (65%) or from severe, acute GVHD (18%). We conclude that the low rate of TRM in patients receiving PBSCs may be due both to the superior immune reconstitution compared with patients receiving bone marrow grafts and to a lower incidence of severe, acute GVHD (grades III and IV). Indeed, a multivariate analysis for TRM identified the occurrence of severe grades III-IV acute GVHD as an independent predictor for TRM.
No significant differences in the occurrence of molecular and cytogenetic relapse of CML were seen between patients receiving PBSCT and those receiving BMT. In contrast to the data for transplants from sibling donors, differences in leukemic relapse rates are unlikely in patients receiving transplants from unrelated donors owing to the lower rate of hematological relapses of only 6% to 10% in this transplantation setting, as compared with 20% to 25% after transplants from HLA-identical sibling donors.16 19-21Nevertheless, we found a slight tendency toward a higher molecular relapse rate in patients after BMT than in patients after PBSCT, with a calculated cumulative incidence of a molecular relapse 1500 days after BMT of 29% versus 14% after PBSCT.
Although there are encouraging early results with the tyrosine kinase inhibitor STI571 in patients with CML,22 allogeneic PBSCT from HLA-identical unrelated donors remains the therapeutic option, with proven, durable antileukemic effects for those lacking a suitable matched sibling donor, which may also result in improved disease-free survival. Our data support the notion that CML patients with a compatible donor for whom cure is the chief objective should undergo allogeneic stem cell transplantation, as stated earlier by Goldman and Melo.23
In conclusion, PBSCT is associated with faster hematopoietic recovery, improved immune reconstitution, improved survival, lower molecular and cytogenetic relapse rates (although not significant), and decreased rates of severe grades III-IV GVHD in patients undergoing allogeneic transplantation from matched unrelated donors when compared with BMT. The improved immune reconstitution might explain the decreased rate of fatal infections seen after PBSCT from unrelated donors when compared with BMT from unrelated donors.
In contrast to the reported experience in the HLA-matched sibling donor setting, our patients had more favorable pretransplantation characteristics. There were fewer donors with positive CMV serology and fewer unfavorable gender constellations between recipients and donors (here a low rate of male recipients with female donors at about 10%, as compared with 25% to 35% in other studies from HLA-identical sibling donors3,5), which is associated with a lower TRM.24 Prospective randomized trials comparing PBSCT and BMT in patients with first chronic phase CML are required to further substantiate the differences in the evaluation of survival and TRM.
The authors thank Katja Ahrens, Melanie Kroll, and Ines Riepenhoff for their excellent technical performance of the PCR analyses.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ahmet H. Elmaagacli, Department of Bone Marrow Transplantation, University Hospital Essen, Hufelandstr 55, 45122 Essen, Germany; e-mail: ahmet.elmaagacli@uni-essen.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal