The International Prognostic Index (IPI) identifies poor- and good-risk patients with diffuse large B cell lymphoma (DLBCL); however, the majority of patients have an intermediate IPI, with an uncertain prognosis. To determine whether cellular factors can be combined with the IPI to more accurately predict outcome, we have analyzed 177 presentation nodal DLBCLs for the expression of bcl-2 and a germinal center (GC) phenotype (defined by expression of bcl-6 and CD10). P53 gene band shifts were detected using single-stranded conformational polymorphism polymerase chain reaction analysis of exons 5-9 and were correlated with protein expression. In a Cox regression analysis, IPI (R = 0.22, P < .0001) and bcl-2 (R = 0.14, P = .0001) were independent poor prognostic factors and a GC phenotype predicted a favorable outcome (R = −0.025, P = .02). Neither p53 expression nor band shifts had a significant effect on survival. Using the IPI alone, 8% of patients were identified as high risk. Expression of bcl-2 in the intermediate IPI group identified a further 28% of patients with an overall survival comparable to the high IPI group. In the intermediate IPI, bcl-2− group, the presence of a GC phenotype improved overall survival to levels approaching the IPI low group. Following this analysis only 15% of patients failed to be assigned to a favorable- or poor-risk group. Sequential addition of bcl-2 expression and GC phenotype into the IPI significantly improves risk stratification in DLBCL. For the 36% of high-risk patients with a 2-year overall survival of 19%, alternative treatment strategies should be considered in future trials.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a generic term for a clinically and biologically heterogeneous group of tumors. Approximately half of all patients with DLBCL can be cured by conventional CHOP-type chemotherapy. The remainder have tumors that are either refractory to currently available treatment or have a relapse after a period of remission, and most of these patients will die of the disease. It is possible that alternative therapeutic strategies, such as the use of antibody and chemotherapy combinations or more intensive primary therapy with stem cell rescue, may be effective in some of these patients. It is therefore important to be able to identify these patients at presentation to allow effective trials to be designed.
The most effective tool for predicting outcome of patients with DLBCL is the International Prognostic Index (IPI), which has been validated in many clinical trials.1-5 The IPI is a clinical index, based on extent of disease, as determined by stage and lactate dehydrogenase (LDH), and on the patient's age and Eastern Cooperative Oncology Group performance status (ECOG score).1 The IPI successfully identifies subgroups of patients with a very poor (16%) or a good (35%) outcome. Patients with a low IPI (0-1) have an overall survival (OS) of 73% at 5 years, compared to 26% in patients with a high IPI.4,5 Half of all patients are assigned to the high-intermediate (27% of all patients) or low-intermediate (22%) categories,2,3 having survivals at 5 years of 51% and 43%, respectively,1 which is little different from the group as a whole. Therefore, the IPI alone is not sufficiently powerful to separate patients who will be cured by conventional therapy from those who have refractory or relapsing disease.
A number of cellular factors have been identified that have prognostic significance in DLBCL. It is uncertain how these variables interact in their effect on prognosis and there is as yet no consensus as to how this additional information can be used, in combination with the IPI, to improve the risk stratification of patients at presentation. A proportion of DLBCLs have a phenotype similar to normal germinal center (GC) cells and follicular lymphomas. The remaining cases are likely to be post-GC–derived tumors, based on the observation that DLBCLs with unmutated immunoglobulin genes are rare6 and that non-GC B-like DLBCLs have mutated immunoglobulin genes with no ongoing somatic mutations.7 This observation is strengthened by the application of microarray technology, which has been used to classify DLBCL into GC and non-GC subtypes. Those with a GC pattern of gene expression had a more favorable outcome.8 In addition to the stage of differentiation defined by patterns of gene expression, other cellular prognostic factors have been identified. The expression of bcl-2 protein has been shown to have an adverse effect on outcome, independent of the IPI, in a number of large scale clinical trials,9-11 and is expressed in a number of cases irrespective of the presence of the t(14;18).10,11 The data on the effect of expression of p53 or the presence ofP53 mutations is more ambiguous with some studies indicating an adverse effect on outcome,12-16 whereas others appear to show no effect.9,17 18 Many of these studies are relatively small with low statistical power, or they have included various types of lymphoma.
The aim of this study was to investigate whether a GC phenotype, bcl-2 expression, and P53 gene mutation and expression can be combined with IPI to more accurately predict outcome in individual patients with DLBCL and thereby increase the proportion of patients who can be confidently assigned to favorable or adverse prognostic groups at presentation.
Patients, materials, and methods
Patient selection and clinical information
Group 1.
Patients were selected only on the basis of availability of clinical information and histologic material. Formalin-fixed, paraffin wax–embedded lymph nodes from a cohort of nodal DLBCLs were investigated. The 177 patients presented through the Yorkshire regional hematological malignancy diagnostic service and Nottingham City Hospital between 1985 and 1997; the diagnoses were confirmed by pathologic review using the diagnostic criteria defined in the Revised European-American Lymphoma classification.19 The cases analyzed represent approximately 30% of the total number of DLBCL cases of the catchment area for the laboratory, with approximately 90 new cases in the region per year. Cases were excluded if clinical information was not available. Mediastinal B-cell lymphoma, Burkitt lymphoma, DLBCL with evidence of an underlying follicular lymphoma, anaplastic variants, and primary extranodal disease (ie, where the extranodal site is the main site of disease, with clinical stage 1E or IIE) were excluded from the analysis, because these are distinct disease entities, with diverse natural histories. This study was therefore restricted to primary nodal de novo DLBCL with maximum uniformity of the patient cohort. All patients were previously untreated and received standard primary anthracycline-containing combination chemotherapy (predominantly CHOP), with the exception of 9 patients with localized disease who were treated with radiotherapy alone. A further 11 patients received no treatment; 1 patient refused and the remainder presented with advanced disease and died before commencing treatment.
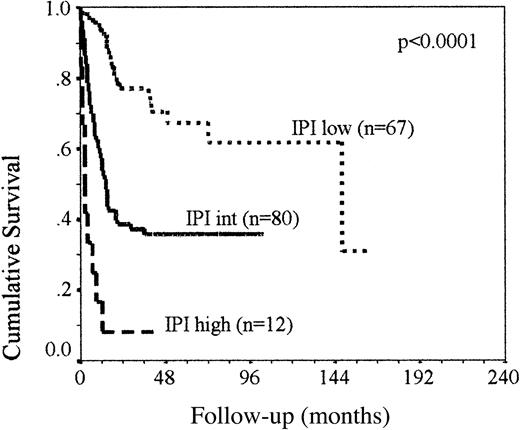
Clinical information was available in 177 cases and the features of the patient cohort are summarized in Table 1(group 1). It was possible to calculate the IPI1 in 143 patients: 67 had a low-risk IPI (0-1), 38 were low-intermediate (2), 26 were high-intermediate (3), and 12 had a high IPI (4-5). A further 16 patients with missing clinical data were assigned an IPI group, when patients were classified as low (0-1), intermediate (2-3), or high (4-5) IPI, because the IPI was 2 or 3 depending on the score from the missing variable. The survival curve comparing IPI groups low, intermediate, and high is shown in Figure1. The low-intermediate and high-intermediate patients were grouped for the purpose of the survival analysis because the primary objective of the study was to increase the proportion of intermediate-risk IPI cases that could be reassigned to high or low risk on the basis of cellular prognostic factors.
Clinical features of the group 1 patient cohort
| Clinical parameter . | Frequency . | 2-y OS . | Log-rank . |
|---|---|---|---|
| Sex | |||
| M/F ratio | 1.1:1 | ||
| Age (y) | |||
| Median (range) | 66 (11-90) | ||
| No more than 60 | 71 | 65% | |
| More than 60 | 106 | 43% | P = .0001 |
| Ann Arbor stage | |||
| I | 48 (27%) | ||
| II | 39 (22%) | 68% (I-II) | |
| III | 52 (29%) | ||
| IV | 38 (22%) | 38% (III-IV) | P = .0001 |
| No. of extranodal sites | |||
| 0 to 1 | 164 | 55% | |
| At least 2 | 11 | 65% | NS |
| N/A | 2* | ||
| LDH | |||
| Normal | 80 | 69% | |
| High | 53 | 26% | P< .0001 |
| N/A | 44* | ||
| Performance status | |||
| 0 to 1 | 133 | 60% | |
| At least 2 | 44 | 32% | P = .0004 |
| IPI group† | |||
| Low (0-1) | 67 (42%) | 78% | |
| Low-intermediate (2) | 38 (24%) | 54% | |
| High-intermediate (3) | 26 (16%) | 22% | |
| High (4-5) | 12 (8%) | 8% | P< .0001 |
| Unclassifiable (no LDH) | |||
| 1 or 2 | 11 | ||
| 2 or 3 | 16 (10%)* | ||
| 3 or 4 | 7 |
| Clinical parameter . | Frequency . | 2-y OS . | Log-rank . |
|---|---|---|---|
| Sex | |||
| M/F ratio | 1.1:1 | ||
| Age (y) | |||
| Median (range) | 66 (11-90) | ||
| No more than 60 | 71 | 65% | |
| More than 60 | 106 | 43% | P = .0001 |
| Ann Arbor stage | |||
| I | 48 (27%) | ||
| II | 39 (22%) | 68% (I-II) | |
| III | 52 (29%) | ||
| IV | 38 (22%) | 38% (III-IV) | P = .0001 |
| No. of extranodal sites | |||
| 0 to 1 | 164 | 55% | |
| At least 2 | 11 | 65% | NS |
| N/A | 2* | ||
| LDH | |||
| Normal | 80 | 69% | |
| High | 53 | 26% | P< .0001 |
| N/A | 44* | ||
| Performance status | |||
| 0 to 1 | 133 | 60% | |
| At least 2 | 44 | 32% | P = .0004 |
| IPI group† | |||
| Low (0-1) | 67 (42%) | 78% | |
| Low-intermediate (2) | 38 (24%) | 54% | |
| High-intermediate (3) | 26 (16%) | 22% | |
| High (4-5) | 12 (8%) | 8% | P< .0001 |
| Unclassifiable (no LDH) | |||
| 1 or 2 | 11 | ||
| 2 or 3 | 16 (10%)* | ||
| 3 or 4 | 7 |
Components of the IPI were unavailable (N/A) in a number of patients. In 44 cases LDH at presentation was not recorded. Of these, the IPI could not be determined in 34 cases; however, in 15 cases an extra score (in the event of the LDH being greater than normal) would have resulted in a reclassification from 2 to 3; therefore, these patients were classified in the intermediate IPI group. In the remaining 11 of 44 cases lacking LDH data, the score was either 0 to 1 or 4 to 5 and the IPI classification was not affected. Similarly, 2 cases lacked details of extranodal involvement; in one case the IPI score was either 2 or 3 and in the other case the score was 4 or 5.
In the IPI categories, percentages are calculated using the number of cases with a classifiable IPI group (159) as the denominator.
The OS of the 159 patients, group 1, classified as IPI low, intermediate, and high.
Kaplan-Meier analysis demonstrates that the IPI adequately classifies patients as poor and good risk; however, half of all patients have an intermediate IPI with an unpredictable outcome.
The OS of the 159 patients, group 1, classified as IPI low, intermediate, and high.
Kaplan-Meier analysis demonstrates that the IPI adequately classifies patients as poor and good risk; however, half of all patients have an intermediate IPI with an unpredictable outcome.
The median follow-up was 26 months (range, 0-180 months) and median OS was 33.3 months. Patients with an intermediate-risk IPI had a median OS of 14.3 months, with 35% OS at 5 years. High-risk patients had a median survival of 2.2 months, and in the low IPI groups the median survival has not yet been reached, but OS at 5 years was 68% in this group. This is comparable to published survival data based on IPI group, suggesting that the group of patients analyzed in this series are representative of the whole population of patients with this disorder. The survival of the intermediate-risk patients in this series is slightly worse than previously published data, but may reflect the exclusion of patients with primary extranodal disease.
Group 2.
To validate the results observed in patient group 1, we have analyzed an additional series of 59 patients (group 2) in which all the patients were tested for each variable. The majority of these patients presented sequentially through the Yorkshire regional diagnostic laboratory between 1998 and 2000, with additional cases from a source not previously in the catchment area for the laboratory. The median follow-up was 15.6 months (range, 0-44 months). The selection and exclusion criteria were as described for group 1.
This study did not involve additional investigations or contact with patients beyond that required for clinical care and treatment and was not modified as a consequence of the study. The study conforms to local ethical standards.
Immunohistochemistry
All cases in which paraffin blocks were available were stained for bcl-2, p53, bcl-6 protein, and CDw75 using the Dako Duet (K0492) kit in standard immunocytochemistry,20,21 and biotinyl tyramine amplification (tryamine signal amplification [TSA] method,22) was used for CD10 and CD23. Antibody dilutions and pretreatments used to achieve optimum staining are given in Table2. Cases were classed as bcl-2 expressing if the protein was detected in more than 50% of tumor cells, based on preliminary studies, which identified this as the most effective cut-off in relation to the prediction of outcome (data not shown). The staining pattern of CD23, CD10, and CDw75 was generally 100% positive or completely negative and was scored as such. Preliminary morphometry data identified 3 distinct groups of p53 positivity with mean expressions of more than 50%, 15% to 49%, and less than 15%. Expression of bcl-6 protein showed a similar staining pattern to p53, and both were classed as positive if more than 15% of the nuclei expressed the proteins. Classification according to a cut-off of 15% compared to 50% did not have a significant effect on the overall analysis (data not shown). A GC phenotype was defined using a combination of CD10, bcl-6, CDw75, and CD23. bcl-6,23,24CD1025 and CDw7526 are well characterized for their expression on GC B cells and CD23 is expressed in the reactive GC,27 particularly on non–class-switched GC B cells,28 but is more commonly associated with activated pregerminal center B cells.27 CD10, CDw75, and CD23 are also known to react with non-GC cells, and rearrangement or mutation of the gene at 3q27 may alter bcl-6 expression. The most effective operational definition of a GC phenotype was evaluated with respect of the ability to predict clinical outcome.
Antibodies used along with pretreatments and dilutions
| Antibody and clone . | Source . | Pretreatment . | Dilution . |
|---|---|---|---|
| bcl-2 clone 124 | DAKO M887 | M/W | 1:50 |
| Anti-ki-67, clone MIB-1 | Immunotech 0505 | M/W | 1:50 |
| Anti-P53 protein, clone DO7 | HMDS, hybridoma kindly donated by Prof. D. Lane | M/W | Neat |
| NCL-CD10-270 | Novacastra | M/W (TSA) | 1:50 |
| CD23 | Dako | M/W (TSA) | 1:20 |
| CDw75 (NCL-LN1) | Novacastra | M/W × 2 | 1:20 |
| Anti-bcl6 N-3 | Santa Cruz | M/W × 2 | 1:10 |
| Antibody and clone . | Source . | Pretreatment . | Dilution . |
|---|---|---|---|
| bcl-2 clone 124 | DAKO M887 | M/W | 1:50 |
| Anti-ki-67, clone MIB-1 | Immunotech 0505 | M/W | 1:50 |
| Anti-P53 protein, clone DO7 | HMDS, hybridoma kindly donated by Prof. D. Lane | M/W | Neat |
| NCL-CD10-270 | Novacastra | M/W (TSA) | 1:50 |
| CD23 | Dako | M/W (TSA) | 1:20 |
| CDw75 (NCL-LN1) | Novacastra | M/W × 2 | 1:20 |
| Anti-bcl6 N-3 | Santa Cruz | M/W × 2 | 1:10 |
M/W indicates microwave antigen retrieval (400 mL of citrate buffer pH 6.0 on high power (800 W) for 12 minutes); M/W × 2, M/W plus an additional 5 minutes following a 5-minute rest.
P53 polymerase chain reaction–single-stranded conformational polymorphism
Genomic DNA was extracted from the paraffin wax–embedded tissue samples using the Nucleon Kit (Scotlab, Coatbridge, Scotland). Polymerase chain reaction–single-stranded conformational polymorphism (PCR-SSCP) has been validated as an effective and sensitive method for demonstrating point mutations,29 and was used to analyze the P53 gene at exons 5-9. PCR primers (sequences as published30) were 5′-end labeled with different fluorochromes (TET, HEX, and 6-FAM) and PCRs were undertaken on 200 ng genomic DNA using 1 U Dynayme II (Flowgen, Leics, United Kingdom) in a reaction volume of 25 μL. Denatured PCR products were electrophoresed with GS-500 (TAMRA) size standard through nondenaturing 7.5% polyacrylamide (37:1) gels at 22°C using an ABI 377 sequencer with external temperature control. Runs were 40 W for up to 8 hours depending on the size of the product. Genescan analysis software was used to normalize mobilities against the internal size standard and to facilitate the detection of small band shifts. A significant band shift was defined when the calculated migration of the band (in scans) was outside the mean ± 2 SDs of variation calculated for the corresponding wild-type allele, and a mutation was implied.31 The sensitivity of the assay was between 5% and 10%. PCR products (both strands) were directly sequenced in 8 cases using dye terminator chemistry (Amersham Thermosequenase kit) on the ABI prism. Sequencing primers are given in Table 3.
P53 sequencing primers
| P53 sequencing primer . | Sequence (5′-3′) . | Product size (bp) . |
|---|---|---|
| Exon 5/6 (forward) | cctcttcctacagtac | |
| Exon 5/6 (reverse) | agttgcaaaccagacctc | 406 |
| Exon 7 (forward) | ctcctaggttggctctgac | |
| Exon 7 (reverse) | caagtggctcctgacctgg | 148 |
| Exon 8/9 (forward) | cctatcctgagtagtgg | |
| Exon 8/9 (reverse) | aagacttagtacctgaagggt | 324 |
| P53 sequencing primer . | Sequence (5′-3′) . | Product size (bp) . |
|---|---|---|
| Exon 5/6 (forward) | cctcttcctacagtac | |
| Exon 5/6 (reverse) | agttgcaaaccagacctc | 406 |
| Exon 7 (forward) | ctcctaggttggctctgac | |
| Exon 7 (reverse) | caagtggctcctgacctgg | 148 |
| Exon 8/9 (forward) | cctatcctgagtagtgg | |
| Exon 8/9 (reverse) | aagacttagtacctgaagggt | 324 |
Statistical analysis
The χ2 test was used to examine relationships between variables. OS time was calculated from the date of diagnosis until death or date of last follow-up, and relapse-free survival (RFS) was until the date of relapse or death. Survival curves were estimated by the Kaplan-Meier analysis, using the log-rank test to analyze the statistical differences between the groups. Multivariate regression analysis of OS was carried out according to the Cox model to test the variables analyzed in the study as potential independent prognostic factors. P < .05 was considered to indicate statistical significance. Statistical analysis, Kaplan-Meier survival functions, and Cox regression analysis were carried using Microsoft Excel and SSCP software.
Results
Patient group 1
GC and bcl-2 expression have prognostic significance independent of IPI.
Cox regression analysis of OS showed that bcl-2 expression was a poor prognostic factor and that a GC phenotype predicted a favorable outcome, and both were independent of IPI (Table4). Preliminary multivariate analysis data demonstrated that the definition of the GC phenotype based on the combined expression of CD10 and bcl-6 was the most effective method to identify the favorable prognostic group, compared to using the full panel of markers, defined by CD10, CDw75, CD23, and bcl-6 (data not shown). Expression of p53 had no effect on survival. P53SSCP data were not included in the Cox model because only a proportion of cases were analyzed. No association was found between any of the variables analyzed using the χ2 test (IPI or components of IPI, bcl-2 expression, GC phenotype, or P53 status), with the exception of a weak relationship between bcl-2 expression and a GC phenotype (Pearson χ2 value = 7.15,P = .006). Thirty-nine of the 59 cases (66%) that expressed a GC phenotype also expressed bcl-2, compared to 49 of the 110 GC phenotype-negative cases (45%).
Cox regression analysis of OS
| Variable . | B . | SE . | exp(b) . | P(log-rank) . |
|---|---|---|---|---|
| IPI | 1.34 | 0.23 | 3.83 | <.0001 |
| bcl-2 | 1.02 | 0.26 | 2.79 | .0001 |
| GC phenotype | − 0.19 | 0.09 | 0.83 | .025 |
| p53 expression | 0.16 | 0.25 | 1.17 | .51 |
| Variable . | B . | SE . | exp(b) . | P(log-rank) . |
|---|---|---|---|---|
| IPI | 1.34 | 0.23 | 3.83 | <.0001 |
| bcl-2 | 1.02 | 0.26 | 2.79 | .0001 |
| GC phenotype | − 0.19 | 0.09 | 0.83 | .025 |
| p53 expression | 0.16 | 0.25 | 1.17 | .51 |
IPI was stratified as low, intermediate, or high. GC phenotype was defined by CD10 and bcl-6 expression.
B indicates Cox regression coefficient.
DLBCL can be classified according to GC phenotype and bcl-2 expression.
Fifty-nine of 169 (35%) of the lymph nodes studied expressed a GC phenotype as defined by expression of CD10 and bcl-6. Eighty-eight of 169 (52%) cases of DLBCL expressed bcl-2 protein in more than 50% of the neoplastic cells and were classed as positive. Analysis of the combined effect of GC phenotype and expression of bcl-2 demonstrated that the presence of a GC phenotype was associated with an improved survival of patients in both the bcl-2+ and bcl-2− groups (P = .0004). Median survival of the bcl-2+, GC+ patients was 49.3 months compared to 12.3 months in the bcl-2+, GC−patients, with 5-year survival rates of 47% and 22%, respectively. The median survival was not reached in either of the bcl-2− groups, with a maximum follow-up of 12 years, but 5-year survival rates were 68% and 58% in the GC+ and GC− patients, respectively.
All cases with a detectable band shift of the P53 gene overexpressed the protein.
Immunocytochemistry for p53 protein was analyzed in 166 cases, and 32% of these were positive. Of analyzable cases 19% (17 of 91) had a detectable band shift at one or more P53 exons by SSCP. Two cases had band shifts at exon 5, 2 at exon 6, 10 at exon 7, 2 at exon 8, and none at exon 9. One case had band shifts both in exons 5 and 7. There was a significant correlation between P53 band shifts and expression (Pearson χ2 value = 26.4,P < .0001). In all cases where a band shift was detected, p53 protein was overexpressed; however, 16 cases expressed the protein in the absence of a detectable band shift. Mutations were confirmed by sequencing of PCR products in 5 cases with SSCP band shifts. There were 4 transversions (3 G > A and 1 T > C) and one transition (G > T). In each of these cases the mutation resulted in amino acid substitution.
P53 band shifts or expression of the protein in bcl-2+cases identifies a poor prognostic group.
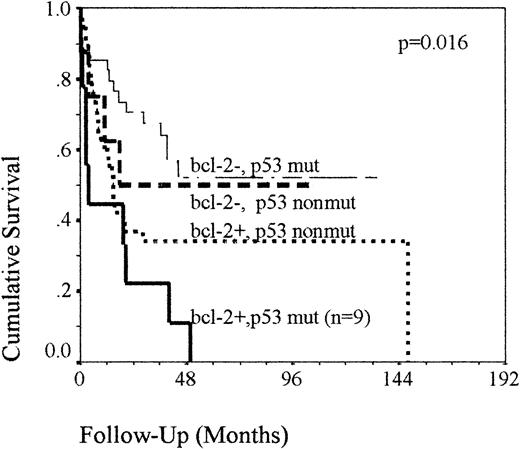
When the prognostic effect of p53 was analyzed using the univariate Kaplan-Meier model, no difference in survival was seen between p53 protein-expressing and protein-nonexpressing cases. Patients with a detectable P53 band shift had a median OS of 17.5 months compared to 20 months in those without detectable band shifts (P = NS), and RFSs were 9 and 17 months (P = .09). In the cases in which P53 SSCP was carried out, the presence of a band shift at P53 in addition to bcl-2 expression had an adverse prognostic effect (P = .016). This small group of patients (n = 9) had a median OS of 3.8 months compared to 14.7 months in the bcl-2+, germline P53 group (n = 38). In the bcl-2− group, a P53 band shift had apparently no effect on OS (Figure 2). A more marked effect was seen when examining RFS. Two-year RFSs were reduced from 64% to 45% in the bcl-2− and 39% to 21% in the bcl-2+ groups in the presence of a band shift (P = .009). Analysis of p53 protein expression in combination with bcl-2 resulted in a similar survival analysis, with comparable levels of statistical significance (data not shown).
OS of the 86 patients classified according to their combined
P53 and bcl-2 status. Kaplan-Meier analysis demonstrating that the detection of a band shift at P53 in patients with bcl-2–expressing tumors identifies a small group of patients with a very poor outcome. Mut cases are defined as cases with a detectable band shift by SSCP-PCR. Nonmut cases are defined as cases with no detectable band shift.
OS of the 86 patients classified according to their combined
P53 and bcl-2 status. Kaplan-Meier analysis demonstrating that the detection of a band shift at P53 in patients with bcl-2–expressing tumors identifies a small group of patients with a very poor outcome. Mut cases are defined as cases with a detectable band shift by SSCP-PCR. Nonmut cases are defined as cases with no detectable band shift.
Analysis of cellular factors in combination with the IPI improves patient risk stratification.
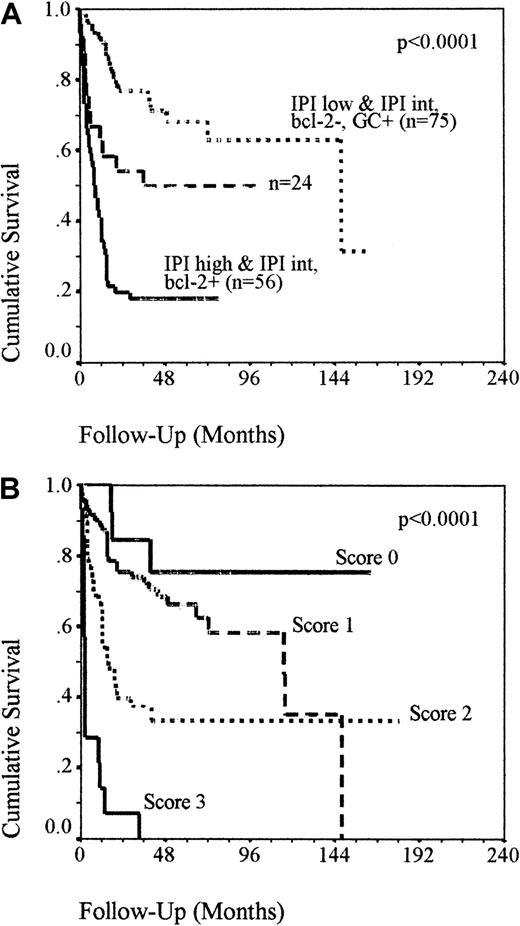
When the IPI was used alone, only 8% of high IPI patients with a very poor prognosis were identified and 42% of patients were in the low IPI group with a relatively good prognosis. Fifty percent of patients were classed as intermediate risk, with a 36% chance of survival at 5 years (Figure 1). To test whether these patients could be more accurately categorized into favorable- and poor-risk groups using additional factors, the cellular factors were sequentially added to the analysis in order of strength of prognostic effect, as determined by exp(b) (Table 4). Patients with an intermediate IPI that were bcl-2+ had a 2-year OS of 24% compared to 65% in the bcl-2− patients (P = .006), which was comparable to that seen in the high IPI group, and increased the number of poor-risk patients to 36%. The presence of a GC phenotype in the intermediate IPI, bcl-2− group identified a favorable prognostic group with all patients still alive at 78 months (P = .03). Combining these patients with the IPI low group increased the number of low-risk patients to 48%. Following this sequential analysis only 24 patients failed to be assigned to a favorable- or poor-risk group. Reassigning patients into risk groups using this approach confirmed this as a valid method for stratification of patients at diagnosis (Figure 3A). Low-risk patients (ie, IPI low, regardless of bcl-2 expression or IPI intermediate without bcl-2 but with a GC phenotype) have a 2-year OS of 68% and high- risk patients (ie, IPI high or IPI intermediate with bcl-2 expression, regardless of GC status) have a 2-year OS of 20%. The remaining 24 patients (ie, IPI intermediate without bcl-2 or GC expression) retained an indeterminate outcome, with a 50% chance of survival. Neither bcl-2 expression in the IPI low patients or a GC phenotype expression in the IPI intermediate, bcl-2–expressing patients had a significant effect on survival. Assigning patients with a ‘score’ of 1 each for an IPI more than 2, bcl-2 in more than 50% of tumor cells, and the lack of a GC phenotype illustrates that these biologic factors can be added to the IPI to improve the risk stratification of patients with nodal DLBCL patients at presentation (Figure 3B). Two-year survival rates were 7%, 40%, 75%, and 84%, respectively, in patients scoring 3, 2, 1, or 0 (P < .0001).
OS of group 1 patients as reclassified.
(A) OS of the group 1 patients reclassified into risk groups using bcl-2 and GC phenotype in combination with IPI. Kaplan-Meier analysis demonstrating that the sequential addition of bcl-2 and GC status into the IPI allows effective risk stratification of 85% of patients. Patients with IPI high or IPI intermediate with bcl-2 expression have a poor outcome and patients with IPI low or IPI intermediate without bcl-2 but with GC phenotype have a favorable prognosis. Twenty-four patients do not fit either category and retain an undetermined outcome. (B) OS of the group 1 patients assigned with a “score” of 1 each for an IPI more than 2, bcl-2 in more than 50% of tumor cells and the lack of a GC phenotype. Kaplan-Meier analysis demonstrating that cellular factors can potentially be added to the IPI to improve risk stratification in nodal DLBCL. Patients scoring 0 or 1 have a more than 60% chance of survival at 5 years compared to a less than 35% chance with a score of 2 or 3.
OS of group 1 patients as reclassified.
(A) OS of the group 1 patients reclassified into risk groups using bcl-2 and GC phenotype in combination with IPI. Kaplan-Meier analysis demonstrating that the sequential addition of bcl-2 and GC status into the IPI allows effective risk stratification of 85% of patients. Patients with IPI high or IPI intermediate with bcl-2 expression have a poor outcome and patients with IPI low or IPI intermediate without bcl-2 but with GC phenotype have a favorable prognosis. Twenty-four patients do not fit either category and retain an undetermined outcome. (B) OS of the group 1 patients assigned with a “score” of 1 each for an IPI more than 2, bcl-2 in more than 50% of tumor cells and the lack of a GC phenotype. Kaplan-Meier analysis demonstrating that cellular factors can potentially be added to the IPI to improve risk stratification in nodal DLBCL. Patients scoring 0 or 1 have a more than 60% chance of survival at 5 years compared to a less than 35% chance with a score of 2 or 3.
Patient group 2: Validation of risk assessment model
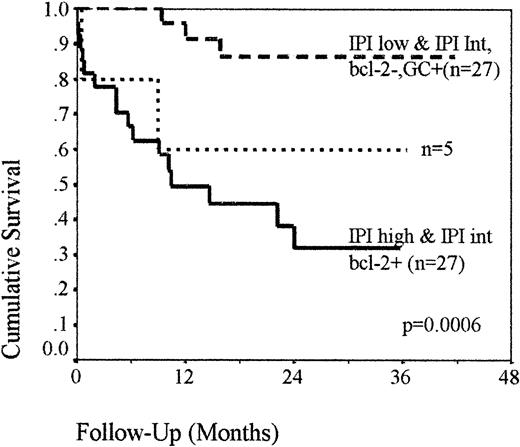
The described model of risk assessment, which combines cellular factors with the IPI, was further validated by analysis of a second similar group of patients (n = 59), with a median age of 66 (range, 27-89). The 2-year OS of the group was 52%, and 28 of 59 patients had an intermediate-risk IPI, with a median OS of 24 months. The prognostic model was applied to these patients, and the resulting survival analysis was comparable to that demonstrated in group 1 (Figure 4). Twenty-seven patients (46%) were low risk (ie, IPI low, regardless of bcl-2 expression or IPI intermediate without bcl-2 but with a GC phenotype), with a 2-year OS of 87% and 27 (46%) were high risk (ie, IPI high or IPI intermediate with bcl-2 expression, regardless of GC status), with a 2-year OS of 31%. The remaining 5 patients (8%; ie, IPI intermediate without bcl-2 or GC expression) retained an indeterminate outcome, with a 60% chance of survival (P = .0006).
OS of the group 2 patients classified into risk groups using bcl-2 and GC phenotype in combination with IPI.
Kaplan-Meier analysis demonstrating that the risk stratification model is effective in an additional, similar group of patients.
OS of the group 2 patients classified into risk groups using bcl-2 and GC phenotype in combination with IPI.
Kaplan-Meier analysis demonstrating that the risk stratification model is effective in an additional, similar group of patients.
Discussion
The results of this study show that bcl-2 and a GC phenotype are independent prognostic factors, and when used in combination with IPI, the majority of patients with primary nodal DLBCL can be subdivided into favorable and adverse prognostic groups. By sequential addition of these factors into the IPI only 15% of patients remained in the group with an indeterminate outcome, compared to 50% using the IPI alone. The analysis of a second, similar group of patients confirmed this risk stratification approach.
As in other studies, in this series of patients bcl-2 was associated with decreased survival9-11,32 and was the most powerful of the cellular prognostic factors tested. In patients with an intermediate IPI, overexpression of bcl-2 reduced the 2-year survival to the same level as the IPI high-risk category. The function of bcl-2 as an inhibitor of apoptosis is well known,33,34 and it is reasonable to assume that the adverse effect on prognosis may be due to a reduction in the level of apoptosis induced by chemotherapy.35 The mechanism of expression of bcl-2 in DLBCL is less clear. Although transformed lymphomas were specifically excluded from this study, it is likely that in some of the tumors bcl-2 expression was a consequence of a t(14;18), and in these cases expression of a GC phenotype would also be expected. A weak association between bcl-2 expression and a GC phenotype was demonstrated in the present study, and this may be explained by the presence of an occult follicular lymphoma in some or all of these cases. An alternative explanation is that the t(14;18) is a feature of true primary DLBCL; however, this is currently not clear. No association was demonstrated between bcl-2 expression and GC features by Alizadeh et al,8 with only 29% of the GC-like DLBCLs expressing bcl-2 messenger RNA (mRNA), compared to 71% of the activated group; however, the numbers of patients investigated in this series was small. The pattern of staining of bcl-2 shows a continuous distribution from 0% to 100% and does not fall into distinct groups, suggesting that factors other than or additional to the t(14;18) are responsible for the aberrant expression of bcl-2. It has been shown that the bcl-2 overexpression in some DLBCLs may be due to amplification of the bcl-2 locus.36 Although it has not been shown that the t(14;18) has a prognostic effect in DLBCL separate from bcl-2 expression, some of the studies have relied on MBR10 or MBR and mcr11 PCR carried out on paraffin blocks, which is a relatively insensitive technique and there remains a need for definitive studies based on large series of cases. In particular, it is important to determine whether DLBCL patients with t(14;18) have an increased risk of relapse as would be expected by analogy with follicular lymphoma.
This study has confirmed the favorable effect of a GC phenotype in DLBCL, suggested by analysis of BCL6expression37 and using microarray technology, in which patients with a GC-like pattern of gene expression had a significant survival advantage compared to patients with an activated gene profile.8 However, there are important differences in the magnitude of the observed effect. In our series, a GC phenotype had only a weak effect on OS, particularly when analyzed by a univariate survival model (results not shown). This may be explained by the observed association between bcl-2 expression and a GC phenotype, and the very strong adverse prognostic effect of bcl-2, but is in contrast to the very powerful effect reported by Alizadeh et al.8The most likely explanation for this difference is the proportion of bcl-2–expressing tumors included in each series. In the present study we analyzed a pure cohort of 177 patients with nodal DLBCL and the proportion of bcl-2+ cases demonstrated in the present study is comparable to similar published series.10,38Approximately half of all cases overexpressed bcl-2 protein, including 66% of cases with a GC phenotype. Alizadeh et al8 analyzed 40 patients and demonstrated increased levels of bcl-2 mRNA in 29% of the GC-like group and in 71% of those with an activated profile. This skewed expression of bcl-2 alone could account for the significant differences in survival between the 2 groups. Furthermore, in the present study, a GC phenotype did not significantly improve the survival of patients with a low-risk IPI; however, using gene expression profiling, a significant survival advantage was demonstrated in low-risk patients, which included IPI low and low-intermediate. The survival of patients with low-intermediate risk is significantly worse than survival for those with low IPI and therefore this survival difference may be partly explained by the proportion of patients of low-intermediate risk included.
In this study, CD10, bcl-6, CDw75, and CD23 were initially used to define a GC phenotype. CD10 and bcl-6 have been identified by gene expression profiling to be strongly associated with GC B cells8 and were highly expressed in all of the GC-like DLBCLs. CD10 was also shown to be expressed in all of these cases by immunocytochemistry.8 When cases that coexpressed CD10 and bcl-6 were compared with those defined using the full panel of antibodies, it was found that using CD10 and bcl-6 alone was the most effective for the identification of good risk. The small proportion of cases that were CD23+ may have represented cases with an activated profile, suggesting that CD23 is not a suitable marker for determining stage of differentiation in this group of disorders. Gene expression profiling has the advantage of taking a global view of the pattern of gene expression and is not likely to be affected by aberrant expression of individual proteins. The main disadvantages are the complexity of the method and analysis and, unless cell sorting is used, malignant and nonmalignant cells are analyzed, resulting in highly complex array data. DLBCL samples contain variable proportions of reactive cells, and in cases with a very high percentage this would be expected to affect the results. In this study immunocytochemistry was used to demonstrate protein expression. This technique is relatively simple, is readily applicable, and has the advantage that only the tumor cells are evaluated.
It is not known why the presence of a GC phenotype should have a favorable effect on outcome of DLBCL. Normal GC cells are highly susceptible to apoptosis and die rapidly in culture unless bcl-2 is induced by stimulation of CD40.34 It is possible that susceptibility to apoptosis is retained by neoplastic GC cells, making them more sensitive to chemotherapy. As was found in this study, this effect would be modified by the presence of bcl-2.
If resistance to apoptosis were a key determinant of outcome in DLBCL it would be expected that inactivation of P53 would correlate with a poor outcome. Wild-type p53 protein has a short half-life and mutated forms of the protein have an increased half-life,39 allowing detection by immunocytochemistry. In this study P53 band shifts were associated with overexpression of the protein, as has been shown by others.12,40,41 The incidence of detectable mutations was 19% in this study, which is comparable to previous studies of non-Hodgkin lymphoma (NHL), in which mutations have been demonstrated at exons 5-9 in 6% to 33% of DLBCL cases.12,13,16,41-48p53 was demonstrated in 32% of cases, with a number of cases expressing the protein in the absence of a detectable band shift. This may have been due to the sensitivity of the PCR-SSCP technique in archival tissue, or to mutations outside exons 5-9.48-50Expression of p53 protein has previously been demonstrated in 13% to 70% of DLBCLs,9,12,50-52 and has been frequently demonstrated in the absence of a mutation.49,53,54 This may alternatively be explained by p53 stabilization by mechanisms such as mdm2 interaction.55-57 Wild-type p53 activity induces mdm2,58 functioning to degrade p53 as a negative feedback control mechanism,59 and high levels of mdm2 have been demonstrated in association with p53 expression in high-grade NHL.60,61 P21 is transcriptionally activated by wild-type but not mutant p53 protein and serves as a mediator of the cell cycle arrest function of p5362 via the inhibition of cyclin dependent kinases (CDKs).63 In tumor cells that have lost p53 or contain a mutated form, p21 is absent. The combined expression of p53 and p21 can be used to predict P53mutation,15,41,64 and this was confirmed by this study (data not shown). In a univariate analysis p53 expression did not correlate with outcome, irrespective of p21 expression or whether a band shift was present. This is consistent with the results of a number of published studies9,18,65 although a poor prognostic effect of both mutations and protein expression has also been reported.12,45 One explanation for the differences between studies may be the inclusion of some patients with relapsed DLBCL. These patients are more likely to have a mutation18 and would also be expected to have a less favorable outcome. In this study we examined only lymph nodes from patients with DLBCL at presentation. The median time to relapse in patients with a P53 band shift was shorter than in those with germline P53 genes (data not shown), suggesting that P53 mutation may be predictive of early relapse. In this study the small group of patients with bcl-2 expression and P53 band shifts or expression had a very poor outcome, and this effect has been previously reported.14This may be explained by a possible interaction between bcl-2 andP53. In cultured cells bcl-2 expression has been shown to inhibit the induction of apoptosis via the p53 pathway, without altering intracellular levels of p53 or localization to the nucleus.66 This suggests that bcl-2 interferes with the apoptotic pathway downstream rather than by direct interaction with p53; however, bcl-2 can also inhibit apoptosis via p53-independent mechanisms.67
This study has demonstrated the feasibility of combining cellular prognostic factors with the IPI to improve the prediction of outcome in patients with nodal DLBCL. This method of risk stratification can be justified because the biologic variables were not associated with the components of the IPI by χ2 analysis. Biologic variables did not significantly alter the survival of the patients with a high or low IPI (data not shown), but the outcome of the intermediate group was more accurately determined by analysis of the biologic variables in these patients. Patients with IPI high or IPI intermediate with bcl-2 expression had a very poor outcome and patients with IPI low or IPI intermediate without bcl-2, but with a GC phenotype, had a favorable survival. The methods of risk assessment described in this study are detected by routine immunocytochemistry, which can be readily applied to the routine setting and to retrospective clinical trials. At present more sophisticated technologies such as gene expression profiling cannot be used in this way. Although definitions of favorable or poor outcome are to some extent subjective, it could be argued that a probability of survival of 19% at 5 years is sufficiently low that alternative primary therapies should be considered for the 36% of patients in this category.
Supported by a grant from the United Kingdom Leukaemia Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andrew S. Jack, Hematological Malignancy Diagnostic Service, Academic Unit of Hematology and Oncology, Leeds General Infirmary, Leeds, LS1 3EX, United Kingdom; e-mail:a.jack@hmds.org.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal