The stromal cell–derived factor 1 (SDF-1) chemokine has various effects on hematopoietic cell functions. Its role in migration and homing of hematopoietic progenitors is currently well established. Previously it was shown that SDF-1 stimulates myeloid progenitor proliferation in synergy with cytokines. Results of this study indicate that SDF-1 alone promotes survival of purified CD34+ cells from human unmobilized peripheral blood (PB) by counteracting apoptosis as demonstrated by its capacity to reduce DNA fragmentation, annexin-V+ cell number, and APO2.7 detection and to modulate bcl-2 homolog protein expression. The study demonstrates that SDF-1, produced by sorted CD34+CD38+ cells and over-released in response to cell damage, exerts an antiapoptotic effect on CD34+ cells through an autocrine/paracrine regulatory loop. SDF-1 participates in the autonomous survival of circulating CD34+ cells and its effect required activation of the phosphotidyl inositol 3 kinase (PI3-K)/Akt axis. Cell sorting based on Hoechst/pyroninY fluorescences shows that SDF-1 production is restricted to cycling CD34+ cells. SDF-1 triggers G0 quiescent cells in G1 phase and, in synergy with thrombopoietin or Steel factor, makes CD34+ cells progress through S+G2/M phases of cell cycle. By assessing sorted CD34+CD38− and CD34+CD38+ in semisolid culture, the study demonstrates that SDF-1 promotes survival of clonogenic progenitors. In conclusion, the results are the first to indicate a role for endogenous SDF-1 in primitive hematopoiesis regulation as a survival and cell cycle priming factor for circulating CD34+ cells. The proposal is made that SDF-1 may contribute to hematopoiesis homeostasis by participating in the autonomous survival and cycling of progenitors under physiologic conditions and by protecting them from cell aggression in stress situations.
Introduction
Hematopoiesis consists of a complex process in which hematopoietic progenitors (HPs) migrate, proliferate, and generate a large number of lineage-committed blood cells. This process is closely dependent on stromal cells and their secreted cytokines and chemokines. An adjusted balance between self-renewal and differentiation is necessary to maintain an adequate number of HPs, which have long-term multilineage repopulating potential, and of mature blood cells.1,2 Apoptosis is a naturally occurring process involved in the homeostasis regulation of hematopoiesis.3Whereas its role in control of mature compartment homeostasis is well documented, molecular mechanisms underlying HP survival are still unclear.4,5 Endogenous cell death regulatory proteins and exogenous growth factors have been reported to participate in apoptosis regulation.3 Actually, cytokines such as interleukin-3 (IL-3), thrombopoietin (Tpo), granulocyte-macrophage colony-simulating factor (GM-CSF), Flt-3, and Steel factor (SF) have been described as survival factors,6-10 but less is known about a possible role for chemokines in HP survival.2,11 12
Stromal cell–derived factor 1 (SDF-1) is a CXC chemokine known to be an effective chemotactic factor for progenitors and mature blood cells.13-15 It is constitutively produced by bone marrow (BM) stromal cells and by other cells including CD34+cells.16 SDF-1 was initially characterized as a pre-B cell-stimulating factor. SDF1 gene knockout experiments have demonstrated several defects including impaired myelopoiesis.17,18 Recent studies have also reported a crucial role for this chemokine in stem cell engraftment and myelopoiesis.19-21 We previously demonstrated that SDF-1 enhanced proliferation and differentiation of unmobilized peripheral blood (PB) CD34+ cells in synergy with cytokines. From these results, we have proposed that exogenous SDF-1 could act as a survival factor for CD34+ cells.22 Recently, studies reporting the protecting effect of SDF-1 on normal hematopoietic cells or leukemic B lymphocytes strengthened this hypothesis.23-25
In the present study, we attempted to determine the mechanisms whereby SDF-1 participated in CD34+ cell survival. For this purpose, we investigated whether SDF-1 protected HPs from spontaneous apoptosis and whether it interfered with cell cycle progression. Our results showed that SDF-1 reduced DNA fragmentation, annexin-V+ cell number, and APO2.7 expression, and modulated apoptotic regulatory proteins of bcl-2 family in CD34+ cells undergoing spontaneous apoptosis.3,26 27 We showed that SDF-1 produced by PB CD34+CD38+ cells played a role in their autonomous survival through phosphotidyl inositol 3 kinase (PI3-K)/Akt signaling. We also demonstrated the survival effect of SDF-1 on sorted CD34+CD38− and CD34+CD38+ cells in a clonogenic progenitor assay. Finally, we showed that SDF-1 alone triggered resting G0 CD34+ cells into cycle and stimulated their progression through cell cycle in synergy with Tpo or SF. Our present results suggest that SDF-1 could contribute to maintaining hematopoiesis homeostasis by acting as a survival and cell cycle priming factor for circulating CD34+ progenitors.
Materials and methods
Mononuclear cell preparation and purification of CD34+ cells
Mononuclear cells expressing the CD34 antigen were immunomagnetically selected directly (Inc−) or after an overnight incubation (Inc+) from PB of unmobilized healthy adults with their informed consent, as previously described.22 This procedure gave rise to a 92% to 98% pure CD34+ cells. In some experiments, highly purified CD34+ cells (up to 99%) were obtained by either a second column step or cell sorting.
CD34+ cell culture conditions for analysis of apoptosis, survival, and clonogenicity
To determine the appropriate culture conditions for studying spontaneous apoptosis in CD34+ cells, freshly purified CD34+ cells (1 × 105/mL) were incubated in a serum- and cytokine-free medium (Iscoves modified Dulbecco medium [IMDM]). After 6 to 96 hours, cells were processed for viability by trypan blue exclusion, apoptosis detection, and SDF-1 secretion by Quantikine enzyme-linked immunosorbent assay (ELISA; R & D Systems, Abingdon, United Kingdom).
The effect of exogenous or endogenous SDF-1 on apoptosis, cell survival, and clonogenicity was evaluated by adding either recombinant human SDF-1α (rhSDF-1α; 0.01-0.5 ng/mL; R & D Systems) or anti-rhSDF-1 neutralizing antibody (10 ng/mL; R & D Systems) to highly purified or sorted CD34+CD38+ and CD34+CD38− cells for 72 hours. Semisolid progenitor assay was performed in methylcellulose medium as previously described.22
Involvement of the PI3-K/Akt and mitogen-activated protein (MAP) kinase/MEK pathways in the antiapoptotic effect of SDF-1 on CD34+ cells was examined by using LY294002 and PD98059 specific inhibitors (10-100 μM), respectively (France Biochem, Meudon, France).28 29 Equivalent dilutions of dimethyl sulfoxide (DMSO) were used as controls.
Assessment of DNA fragmentation by “sub-G1peak” detection
CD34+ cells (1 × 105/mL) were cultured for 4 days under apoptosis-inducing conditions with or without SDF-1 (0.01-0.5 ng/mL) and prepared for cell cycle analysis. Cells (5 × 104) were fixed in 70% ice-cold ethanol at −20°C for 30 minutes and were incubated for 15 minutes in the dark at 4°C in phosphate-buffered saline (PBS) containing propidium iodide (PI, 0.4 mg/mL; Molecular Probes Europe, Leiden, The Netherlands). The PI fluorescence from fractional DNA content of apoptotic cells produced a “sub-G1 peak” on the DNA content frequency distribution histogram that defined the proportion of apoptotic cells.30
Detection of membrane and mitochondrial apoptosis markers
The percentage of cells undergoing apoptosis was determined using annexin-V and mitochondrial APO2.7 antigen detection assays.31 32 Briefly, CD34+ cells were incubated for 6 to 24 hours under apoptosis-inducing conditions with or without SDF-1 (0.01-0.5 ng/mL). In some experiments, an anti-rhSDF-1α antibody (5 ng/mL) was added to the medium. Cells (1 × 105) were incubated at room temperature in the dark for 15 minutes in 200 μL buffer containing either fluorescein-conjugated human annexin-V (5 μL, Pharmingen, BD Biosciences, Le Pont de Claix, France) plus PI (5 μg/mL, Molecular Probes) or R-phycoerythrin (PE)–conjugated APO2.7 antibody (Beckman Coulter, Le Pont de Claix, France). Annexin-V+PI− and annexin-V+PI+ cells correspond to apoptotic and necrotic cells, respectively.
Cell cycle fractionation with PI and Ki67
The effect of SDF-1 on cell cycle progression was evaluated by culturing PB CD34+ cells in serum- and cytokine-free Stemα-A medium (Stem-Alpha, St Clément les Places, France) in the presence or absence of SDF-1 (0.01-1 ng/mL), Tpo, SF, and Flt-3 ligand (R & D Systems). At different time points up to 72 hours, cells were analyzed for simultaneous expression of Ki67 proliferation-associated nuclear antigen and DNA content. The Ki67 antigen is expressed in cells entering G1 with increasing expression during cell cycle but was undetectable in G0 resting cells.33 Cells (1 × 105) were fixed in 70% ice-cold ethanol, permeabilized (OrthoPermeafix, OrthoDiagnostic System, Roissy en France, France), immunostained using fluorescein isothiocyanate (FITC)–labeled anti-Ki67 (Beckman Coulter) or its isotype IgG control and washed before staining with PI. Specificity of the Ki67 antibody binding was evaluated by using a FluoroTrol-DNAplus Cell Cycle control kit (Bioergonomics, Clinisciences, Montrouge, France).
Intracellular detection of SDF-1, cyclins, and apoptosis-related Bcl-2 family proteins
Intracellular expression of SDF-1, cyclins (D1-3 and E), and Bcl-2 family proteins (Bad, Bax, Bcl-2, and Bcl-xL) was detected by flow cytometry. CD34+ cells were cultured in serum- and cytokine-free medium (Stemα-A or IMDM) in the presence or absence of SDF-1 (0.05 and 0.5 ng/mL). At various time points up to 72 hours, 1 × 105 cells were harvested and incubated with FITC- or PE-conjugated anti-CD38 monoclonal antibody (mAb) (clone T16, Beckman Coulter) and with allophycocyanin (APC)–conjugated anti-CD34 mAb (clone 581, Beckman Coulter) for 30 minutes at 4°C in the dark. Cells were washed with PBS/2% human serum albumin (HSA)/0.5% Ig, incubated in OrthoPermeafix, and labeled with the following conjugated mouse antihuman antibodies: bcl-2-FITC (clone 124, Dako SA, Trappes, France), Bcl-xL-PE (clone 7B2.5, Chemicon, Euromedex, Souffelweyersheim, France), Bad-FITC (clone 48, Transduction Laboratories, BD Biosciences, Le Pont de Claix, France), cyclins D1-, D2-, D3-FITC (clone G124-326, G132-43, G107-565, from Pharmingen, BD Biosciences, respectively) or unconjugated mouse antihuman antibodies: Bax (clone 4F11, Beckman Coulter), cyclin E (clone HE12, Pharmingen, BD Biosciences), or goat antihuman SDF-1 antibody (R & D Systems). To avoid SDF-1 release during permeabilization, cells were preincubated for 4 hours with 10 μg/mL brefeldin-A (Sigma, Saint-Quentin Fallavier, France). Cells labeled with anti-Bax, anticyclin E, anti–SDF-1 were subsequently labeled with either a PE-conjugated goat antimouse (Caltag Laboratories, Tebu, Saint-Quentin en Yvelines, France) or an FITC-conjugated rabbit antimouse (Dako SA) or a PE- or FITC-conjugated swine antigoat (Caltag Laboratories), respectively. Cells were washed and analyzed by flow cytometry. Membrane-bound SDF-1 was estimated by omitting permeabilization before SDF-1 staining. Background fluorescence was assessed by using isotype-matched irrelevant mouse or goat immunoglobulins.
Flow cytometry analysis and cell sorting
Analysis was performed using a Coulter Elite flow cytometer (Beckman Coulter). Cell debris was excluded from the analysis by gating on apoptotic and live cells on forward light scatter (FSC) versus side light scatter (SSC) dot plots. Stored events (5000-10 000) were analyzed with WinMDI (J. Trotter J, The Scripps Research Institute [TSRI], La Jolla, CA) or WinCycle software (Phoenix Flow Systems, San Diego, CA). Mean fluorescence intensity (MFI) was expressed in arbitrary units (AU).
In some experiments, CD34+ subpopulations were sorted according to their CD38 expression.22 Resting (G0) or cycling (G1+S+G2/M) CD34+ cells were sorted based on Hoechst/pyroninY fluorescences as described by Gothot et al.34 35 Figure1 shows the gating strategy of cell sorting and the purity of sorted fractions.
Cell sorting strategy and purity of sorted fractions.
Stained cells were sorted according to CD34 and CD38 expression (A) or DNA/RNA staining (B) by using a Coulter Elite flow cytometer. For cell sorting, immunomagnetic-purified CD34+ cells were gated on a first R1 region by selecting lymphomononuclear cells and excluding dead cells (FSC/SSC dot plot). A second region R2 corresponding to CD34+ cells was drawn on a second dot plot (CD34/SSC) gated on R1. (A) The CD34+CD38−fraction was arbitrarily defined according to the CD38 expression level as the 10% of CD34+ cells with the lowest CD38 labeling. Two gates corresponding to CD34+CD38− and to CD34+CD38+ fractions were defined (Ai and Aii, respectively). Five percent of cells at the limits between these populations were excluded from the sort. (B) CD34+ cells were also sorted according to simultaneous DNA/RNA staining with Hoechst 33342 and pyronin Y. This cell cycle fractionation allowed isolation of viable [G0] CD34+ cells from [G1+S+G2/M] cells. Cells residing in G0 exhibit a low RNA content and appear at the bottom of the Hoechst/pyronin Y dot plot identified by a low pyronin Y and a low Hoechst staining (Bi). Cells in G1 show a brighter pyronin Y signal associated with a low Hoechst staining and cells in S+G2/M display both high Hoechst and pyronin Y staining. Two gates were defined corresponding to [G0] CD34+cells and to [G1+S+G2/M] CD34+ cells (Bi and Bii, respectively). The purity of the CD34+CD38−, CD34+CD38+, [G0] CD34+, and [G1+S+G2/M] CD34+ sorted fractions was more than 99% as established by the flow cytometry analysis following sorting. A representative analysis of each sorted fraction cell purity was shown on histograms of CD38 and pyronin Y fluorescences (Ai, Aii and Bi, Bii).
Cell sorting strategy and purity of sorted fractions.
Stained cells were sorted according to CD34 and CD38 expression (A) or DNA/RNA staining (B) by using a Coulter Elite flow cytometer. For cell sorting, immunomagnetic-purified CD34+ cells were gated on a first R1 region by selecting lymphomononuclear cells and excluding dead cells (FSC/SSC dot plot). A second region R2 corresponding to CD34+ cells was drawn on a second dot plot (CD34/SSC) gated on R1. (A) The CD34+CD38−fraction was arbitrarily defined according to the CD38 expression level as the 10% of CD34+ cells with the lowest CD38 labeling. Two gates corresponding to CD34+CD38− and to CD34+CD38+ fractions were defined (Ai and Aii, respectively). Five percent of cells at the limits between these populations were excluded from the sort. (B) CD34+ cells were also sorted according to simultaneous DNA/RNA staining with Hoechst 33342 and pyronin Y. This cell cycle fractionation allowed isolation of viable [G0] CD34+ cells from [G1+S+G2/M] cells. Cells residing in G0 exhibit a low RNA content and appear at the bottom of the Hoechst/pyronin Y dot plot identified by a low pyronin Y and a low Hoechst staining (Bi). Cells in G1 show a brighter pyronin Y signal associated with a low Hoechst staining and cells in S+G2/M display both high Hoechst and pyronin Y staining. Two gates were defined corresponding to [G0] CD34+cells and to [G1+S+G2/M] CD34+ cells (Bi and Bii, respectively). The purity of the CD34+CD38−, CD34+CD38+, [G0] CD34+, and [G1+S+G2/M] CD34+ sorted fractions was more than 99% as established by the flow cytometry analysis following sorting. A representative analysis of each sorted fraction cell purity was shown on histograms of CD38 and pyronin Y fluorescences (Ai, Aii and Bi, Bii).
Western blotting analysis
Statistical analysis
Data were expressed as means ± SD. P < .05 was considered statistically significant (Student t test for paired samples).
Results
Exogenous SDF-1 acts as a survival factor in counteracting PB CD34+ cell apoptosis
We have previously reported that SDF-1 displayed a stimulatory effect on PB CD34+ proliferation in synergy with cytokines and have suggested that SDF-1 might act as a survival factor.22 This promoting effect was mainly demonstrated on CD34+ cells purified after an overnight incubation on a plastic support (Inc+CD34+). In the present study, we investigated whether SDF-1 could counteract CD34+cell apoptosis. We first determined the appropriate experimental conditions inducing optimum spontaneous apoptosis in PB CD34+ cells.8 We quantified the percentage of subdiploid cells37 and analyzed the expression of annexin-V and APO2.7 in response to SDF-1.38 We studied whether SDF-1 altered the expression of Bcl-2 family proteins involved in apoptosis regulation.1,3 27
SDF-1 decreases the sub-G1 peak, annexin-V, and APO2.7 expression.
Freshly isolated Inc− and Inc+CD34+ cells showed a low proportion of apoptotic cells as indicated by the “sub-G1 peak” detection (1.2% ± 0.8% and 5.6% ± 2.3%, respectively; Figure2A) and the annexin-V+PI− cell percentage (1.2% ± 0.2% and 3.8% ± 1.4%, respectively; Figure3A). The percentage of apoptotic cells, as demonstrated by the sub-G1 and annexin-V+PI− cell detection, was increased after a short incubation in IMDM (1-4 days). Interestingly, these percentages were higher in Inc+ (53.5% ± 4.8% and 10.7% ± 2.1%) than in Inc− cells (30.5% ± 3.1% and 1.8% ± 0.2%; Figures 2B and 3B). Addition of SDF-1 (0.01-0.5 ng/mL) significantly prevented spontaneous DNA degradation in Inc+CD34+ cells because their proportion in sub-G1 was decreased from 53.5% ± 4.8% to 22% ± 3.1% (n = 3; P = .002; Figure 2B). The antiapoptotic effect of SDF-1 was also illustrated by a reduction of the apoptotic annexin-V+PI− CD34+cell percentage (Figure 3B) and was predominant on Inc+ as compared to Inc− cells (from 10.7% ± 2.1% to 3.2% ± 0.6% and 1.8% ± 0.2% to 0.2% ± 0.7%, respectively, n = 3; P = .02 and P = .03). The preventing effect of SDF-1 on CD34+ cell apoptosis was dose dependent (data not shown) and was maximal for 0.05 ng/mL and 0.5 ng/mL in Inc+ and Inc− cells, respectively (Figures 2 and 3).
SDF-1 counteracts apoptosis-associated nuclear DNA degradation in PB Inc+CD34+ cells.
PB CD34+ cells purified immediately after density gradient separation (Inc−) or after incubation on a plastic support (Inc+) were incubated (1 × 105 cells/mL) under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]). Freshly isolated cells (A) or cells harvested after a 4-day incubation (B) were processed for apoptosis assessment by “sub-G1 peak” detection as indicated in “Materials and methods.” The biologic effect of SDF-1 on apoptosis was evaluated by adding rhSDF-1α at various concentrations (0.01, 0.05, 0.1, 0.5 ng/mL) to the culture medium. The percentages of sub-G1apoptotic cells shown for each histogram are for one experiment representative of the 3 performed. The logarithm fluorescence of PI is expressed in channel numbers. Maximal effect of SDF-1 was obtained for 0.5 ng/mL in Inc− cells and for 0.05 ng/mL in Inc+ cells.
SDF-1 counteracts apoptosis-associated nuclear DNA degradation in PB Inc+CD34+ cells.
PB CD34+ cells purified immediately after density gradient separation (Inc−) or after incubation on a plastic support (Inc+) were incubated (1 × 105 cells/mL) under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]). Freshly isolated cells (A) or cells harvested after a 4-day incubation (B) were processed for apoptosis assessment by “sub-G1 peak” detection as indicated in “Materials and methods.” The biologic effect of SDF-1 on apoptosis was evaluated by adding rhSDF-1α at various concentrations (0.01, 0.05, 0.1, 0.5 ng/mL) to the culture medium. The percentages of sub-G1apoptotic cells shown for each histogram are for one experiment representative of the 3 performed. The logarithm fluorescence of PI is expressed in channel numbers. Maximal effect of SDF-1 was obtained for 0.5 ng/mL in Inc− cells and for 0.05 ng/mL in Inc+ cells.
SDF-1 decreases the expression of apoptosis-associated cell membrane proteins in PB CD34+ cells.
PB CD34+ cells purified immediately after density gradient separation (Inc−) or after incubation on a plastic support (Inc+) were incubated (1 × 105 cells/mL) under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]). Freshly isolated cells (A) or cells harvested after a 24-hour incubation program (B) were processed for apoptosis assessment by using the annexin-V apoptosis detection kit as indicated in “Materials and methods.” The biologic effect of SDF-1 on apoptosis was evaluated by adding rhSDF-1α at various concentrations (0.01, 0.05, 0.1, 0.5 ng/mL) to the culture medium. The percentages of annexin-V+PI− apoptotic cells and annexin-V+PI+ necrotic cells shown for each histogram are for one experiment representative of the 3 performed. Maximal effect of SDF-1 was obtained for 0.5 ng/mL in Inc− cells and for 0.05 ng/mL in Inc+ cells.
SDF-1 decreases the expression of apoptosis-associated cell membrane proteins in PB CD34+ cells.
PB CD34+ cells purified immediately after density gradient separation (Inc−) or after incubation on a plastic support (Inc+) were incubated (1 × 105 cells/mL) under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]). Freshly isolated cells (A) or cells harvested after a 24-hour incubation program (B) were processed for apoptosis assessment by using the annexin-V apoptosis detection kit as indicated in “Materials and methods.” The biologic effect of SDF-1 on apoptosis was evaluated by adding rhSDF-1α at various concentrations (0.01, 0.05, 0.1, 0.5 ng/mL) to the culture medium. The percentages of annexin-V+PI− apoptotic cells and annexin-V+PI+ necrotic cells shown for each histogram are for one experiment representative of the 3 performed. Maximal effect of SDF-1 was obtained for 0.5 ng/mL in Inc− cells and for 0.05 ng/mL in Inc+ cells.
The antiapoptotic effect of SDF-1 was further confirmed by evaluating the expression of APO2.7 protein, a mitochondrial marker of early apoptotic events.32 As shown in Figure4, APO2.7 expression level was strongly reduced from 450.5 ± 62.4 AU to 110.3 ± 24.6 AU (n = 3;P = .001) after 6 hours in the presence of SDF-1; this effect was specific because it was totally abolished by addition of a neutralizing anti–SDF-1 antibody (Figure 4).
SDF-1 decreases the expression of APO2.7 apoptosis-associated mitochondrial protein in PB Inc+CD34+ cells.
PB CD34+ cells freshly purified after incubation on a plastic support (Inc+) were incubated (1 × 105 cells/mL) under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]). Cells harvested after a 6-hour incubation program were processed for apoptosis assessment by using the mitochondrial APO2.7 antigen detection assay as indicated in “Materials and methods.” The biologic effect of SDF-1 on apoptosis was evaluated by adding 0.5 ng/mL rhSDF-1α to the culture medium. Biologic specificity of SDF-1 was demonstrated by using an anti–SDF-1 antibody (5 ng/mL) or its isotype control (5 ng/mL). A histogram from a typical donor is presented. The MFI (AU) of cells positive for APO2.7 is shown for each condition.
SDF-1 decreases the expression of APO2.7 apoptosis-associated mitochondrial protein in PB Inc+CD34+ cells.
PB CD34+ cells freshly purified after incubation on a plastic support (Inc+) were incubated (1 × 105 cells/mL) under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]). Cells harvested after a 6-hour incubation program were processed for apoptosis assessment by using the mitochondrial APO2.7 antigen detection assay as indicated in “Materials and methods.” The biologic effect of SDF-1 on apoptosis was evaluated by adding 0.5 ng/mL rhSDF-1α to the culture medium. Biologic specificity of SDF-1 was demonstrated by using an anti–SDF-1 antibody (5 ng/mL) or its isotype control (5 ng/mL). A histogram from a typical donor is presented. The MFI (AU) of cells positive for APO2.7 is shown for each condition.
Expression of Bcl-2 family proteins is modulated by SDF-1.
We evaluated whether the antiapoptotic effect of SDF-1 went through modulation of Bad, Bax, Bcl-2, and Bcl-xLexpression. We showed that freshly isolated PB Inc+CD34+ cells expressed Bcl-2 and Bcl-xL proteins (42.7 ± 5.6 AU and 21.6 ± 4.8 AU, respectively; Figure 5A). We observed a bimodal expression profile in which a small proportion of CD34+ cells expressed low level of Bcl-2 (15% ± 6.3%, 12.7 ± 2.6 AU) and Bcl-xL (29.3% ± 5.2%, 7.1 ± 3.4 AU), whereas the majority of CD34+ cells displayed high levels of these proteins (84.8% ± 4.2%, 48.1 ± 3.8 AU and 72.6% ± 6.1%, 28.9 ± 4.6 AU). After 72 hours under apoptosis-inducing conditions, the global expression of Bcl-2 and Bcl-xL cell death suppressors was increased up to 90.6 ± 5.3 AU (P = .03) and 83.5 ± 3.8 AU (P = .001), respectively, with disappearance of the bimodal profile (Figure 5A). Addition of SDF-1 (0.05 ng/mL) maintained the global expression levels of Bcl-2 and Bcl-xL at their initial values (39.2 ± 5.1 AU and 28.5 ± 5.6 AU, respectively, n = 3; Figure 5A). Expression of Bad and Bax cell death promoters, undetectable in freshly isolated Inc+CD34+cells, increased up to 41.2 ± 6.1 AU and 150.6 ± 5.8 AU, respectively, after 72 hours under apoptosis-inducing condition (P < .001; n = 3; Figure 5B). Addition of SDF-1 (0.05 ng/mL) reduced their expression levels to 25.5 ± 2.8 AU,P = .03 and 36.5 ± 5.2 AU, P = .006, respectively (n = 3; Figure 5B).
SDF-1 modulates apoptosis-regulatory gene products in PB Inc+CD34+ cells.
PB CD34+ cells freshly purified after incubation on a plastic support (Inc+) were incubated (1 × 105 cells/mL) under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]). Freshly isolated cells or cells harvested after a 72-hour incubation program were processed for intracellular detection of (A) antiapoptotic regulatory proteins (Bcl-2 and Bcl-xL) and (B) proapoptotic regulatory proteins (Bad and Bax) as indicated in “Materials and methods.” Modulation of Bcl-2 homolog protein expressions was evaluated in the absence or presence of SDF-1 (0.05 ng/mL). Data from at least 3 different donors with similar results were analyzed. Histograms from a typical donor are presented. The percentage or MFI (AU) or both of positive cells for Bcl-2, Bcl-xL, Bad, and Bax are shown within the histograms. The left-hand histogram represents the negative control (IgG).
SDF-1 modulates apoptosis-regulatory gene products in PB Inc+CD34+ cells.
PB CD34+ cells freshly purified after incubation on a plastic support (Inc+) were incubated (1 × 105 cells/mL) under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]). Freshly isolated cells or cells harvested after a 72-hour incubation program were processed for intracellular detection of (A) antiapoptotic regulatory proteins (Bcl-2 and Bcl-xL) and (B) proapoptotic regulatory proteins (Bad and Bax) as indicated in “Materials and methods.” Modulation of Bcl-2 homolog protein expressions was evaluated in the absence or presence of SDF-1 (0.05 ng/mL). Data from at least 3 different donors with similar results were analyzed. Histograms from a typical donor are presented. The percentage or MFI (AU) or both of positive cells for Bcl-2, Bcl-xL, Bad, and Bax are shown within the histograms. The left-hand histogram represents the negative control (IgG).
Endogenous SDF-1 participates in the autonomous survival of PB CD34+ cells
Inc−CD34+CD38+ cells express intracellular SDF-1 and CXCR-4.
When freshly isolated, 84.2% ± 8.2% of Inc−CD34+CD38+ cells expressed intracellular SDF-1 (28.5 ± 3.6 AU; Figure6A) and 18.3% ± 6.8% of them expressed CXCR-4 (Table 1). We showed that 25.8% ± 5.2% of Inc−CD34+CD38+ coexpressed intracellular SDF-1 and CXCR-4 and that 90% of CXCR-4–expressing cells also expressed a high level of intracellular SDF-1 (data not shown). In contrast, only a low proportion of PB Inc+CD34+CD38+ cells expressed SDF-1 (12.5% ± 1.2%, 6.8 ± 4.3 AU; Figure 6A). After a 48-hour apoptosis-inducing incubation, CXCR-4 was overexpressed in both Inc−CD34+CD38− and Inc−CD34+CD38+ cells (Table 1), whereas expression of intracellular SDF-1 by the Inc−CD34+CD38+ fraction was decreased from 28.5 ± 3.6 AU to 9.5 ± 3.6 AU (P = 0.02, n = 3; Figure 6), suggesting that SDF-1 was released during incubation. This hypothesis was strongly supported by detection of SDF-1 in the supernatant of sorted Inc−CD34+CD38+ cells undergoing apoptosis (Table 2). The low amount of SDF-1 secreted by Inc+CD34+CD38+cells was in agreement with its low intracellular level (Figure 6). No SDF-1 was detected in CD34+CD38− cells (Figure6 and Table 2).
PB Inc− CD34+CD38+cells express intracellular SDF-1.
Representative flow cytometry profiles for Inc− and Inc+ cells (1 × 105 cells/mL) isolated from freshly purified PB CD34+ cells (A) and apoptosis-induced cells (B) after staining for CD34 and CD38 antigens and intracellular SDF-1 as indicated in “Materials and methods.” The logarithm fluorescence of intracellular SDF-1 is expressed in channel numbers. The shaded histogram shows results from staining with irrelevant isotype-matched control antibody, the open histogram, results from staining with SDF-1 mAb.
PB Inc− CD34+CD38+cells express intracellular SDF-1.
Representative flow cytometry profiles for Inc− and Inc+ cells (1 × 105 cells/mL) isolated from freshly purified PB CD34+ cells (A) and apoptosis-induced cells (B) after staining for CD34 and CD38 antigens and intracellular SDF-1 as indicated in “Materials and methods.” The logarithm fluorescence of intracellular SDF-1 is expressed in channel numbers. The shaded histogram shows results from staining with irrelevant isotype-matched control antibody, the open histogram, results from staining with SDF-1 mAb.
CXCR-4 expression is up-regulated in sorted CD34+ cells undergoing apoptosis
| Source of cells . | Apoptosis-inducing incubation time, h . | CXCR-4 expression (mean ± SD) . | |
|---|---|---|---|
| % of positive cells . | MFI (AU) . | ||
| Inc−CD34+CD38− | 0 | 6.1 ± 2.3 | 20 ± 8.5 |
| 48 | 24.2 ± 6.1* | 27 ± 9.8 | |
| Inc−CD34+CD38+ | 0 | 18.3 ± 6.8 | 38 ± 12.5 |
| 48 | 44.2 ± 9.9* | 77.2 ± 18.8 | |
| Inc−CD34+[G0] | 0 | 8.8 ± 3.4 | 12.5 ± 8.6 |
| 48 | 31.5 ± 8.2* | 28.6 ± 9.6 | |
| Inc−CD34+[G1 + S + G2/M] | 0 | 19.8 ± 2.6 | 32.6 ± 9.1 |
| 48 | 54.8 ± 11* | 65.3 ± 12.5 | |
| Source of cells . | Apoptosis-inducing incubation time, h . | CXCR-4 expression (mean ± SD) . | |
|---|---|---|---|
| % of positive cells . | MFI (AU) . | ||
| Inc−CD34+CD38− | 0 | 6.1 ± 2.3 | 20 ± 8.5 |
| 48 | 24.2 ± 6.1* | 27 ± 9.8 | |
| Inc−CD34+CD38+ | 0 | 18.3 ± 6.8 | 38 ± 12.5 |
| 48 | 44.2 ± 9.9* | 77.2 ± 18.8 | |
| Inc−CD34+[G0] | 0 | 8.8 ± 3.4 | 12.5 ± 8.6 |
| 48 | 31.5 ± 8.2* | 28.6 ± 9.6 | |
| Inc−CD34+[G1 + S + G2/M] | 0 | 19.8 ± 2.6 | 32.6 ± 9.1 |
| 48 | 54.8 ± 11* | 65.3 ± 12.5 | |
PB CD34+ cells were purified immediately after density gradient separation (Inc−) and were sorted according to CD38 antigen expression or to their cell cycle status based on Hoechst and pyronin Y staining. Cells (1 × 105/mL) were incubated under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]) for 48 hours prior to the harvesting for CD34 and CXCR-4 labeling and flow cytometer analysis. Cell debris was excluded from the analysis by gating on apoptotic and live cells on forward light scatter versus side light scatter dot plots. Percentage of CD34+CD38−, CD34+CD38+, CD34+[G0] and CD34+[G1 + S + G2/M] cells coexpressing CXCR-4 was calculated after gating on CD34+ cells.
Results are based on 3 independent experiments.
P < .05 versus 0 hours incubation.
Production of SDF-1 by CD34+ cells is increased under apoptosis-inducing conditions
| Source of cells . | Apoptosis-inducing incubation time, h . | SDF-1 levels, pg/mL . |
|---|---|---|
| Inc+ | ||
| CD34+CD38− | 2 | bsd |
| 72 | bsd | |
| CD34+CD38+ | 2 | bsd |
| 72 | 28.2 ± 12.6* | |
| Inc− | ||
| CD34+CD38− | 2 | bsd |
| 72 | bsd | |
| CD34+CD38+ | 2 | 25.5 ± 12.6 |
| 72 | 164.6 ± 19.4* | |
| CD34+[G0] | 2 | bsd |
| 72 | bsd | |
| CD34+[G1 + S + G2/M] | 2 | 40 ± 10.2 |
| 72 | 206.6 ± 49.8* |
| Source of cells . | Apoptosis-inducing incubation time, h . | SDF-1 levels, pg/mL . |
|---|---|---|
| Inc+ | ||
| CD34+CD38− | 2 | bsd |
| 72 | bsd | |
| CD34+CD38+ | 2 | bsd |
| 72 | 28.2 ± 12.6* | |
| Inc− | ||
| CD34+CD38− | 2 | bsd |
| 72 | bsd | |
| CD34+CD38+ | 2 | 25.5 ± 12.6 |
| 72 | 164.6 ± 19.4* | |
| CD34+[G0] | 2 | bsd |
| 72 | bsd | |
| CD34+[G1 + S + G2/M] | 2 | 40 ± 10.2 |
| 72 | 206.6 ± 49.8* |
PB CD34+ cells were purified immediately after density gradient separation (Inc−) or after incubation on a plastic support (Inc+) and were sorted according to CD38 antigen expression or to their cell cycle status based on Hoechst and pyronin Y staining. Cells (1.5 × 105 cells/mL) were incubated under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]) for 72 hours before harvesting the conditioned media. Secretion of SDF-1 by the different fractions of sorted cells undergoing apoptosis was evaluated by Quantikine human immunoassay according to the manufacturer's protocol. Analysis of the medium collected after 2 hours of incubation was used as control. Levels of SDF-1 are presented as the means ± SD of 3 independent experiments.
The sensitivity of ELISA for SDF-1 was > 18 pg/mL.
Bsd indicates below sensitivity detection.
P < .002 versus 2 hours incubation.
Endogenous SDF-1 maintains PB CD34+ cell survival.
We further investigated whether intracellular SDF-1 played a role in the control of CD34+ cell autonomous survival by adding an anti–SDF-1 neutralizing antibody to the culture. Table3 shows that the percentage of surviving cells was higher in Inc− than in Inc+CD34+ cells after 96 hours under apoptosis conditions (80.4% ± 5.8% and 68.4% ± 9.2%, respectively;P = .02). Treatment with an anti–SDF-1 antibody significantly reduced the number of Inc−CD34+surviving cells from 80.4 ± 5.8 × 103 to 50.2 ± 5.1 × 103 (P = .003, n = 3), whereas it had no effect on Inc+CD34+ cell survival. The survival effect of endogenous SDF-1 was further confirmed on sorted Inc−CD34+ cells and was demonstrated to be restricted to the CD34+CD38+ fraction (Table 3). Addition of isotype control did not alter CD34+cell survival (data not shown).
Neutralization of endogenous SDF-1 affects PB Inc−CD34+ progenitor survival
| Source of cells . | Incubation time, h . | Total cell number (× 103) Anti-SDF-1 treatment . | |
|---|---|---|---|
| − . | + . | ||
| Inc+ | |||
| Total CD34+ | 0 | 100 | 100 |
| 48 | 96 ± 2.5 | 98.5 ± 1.2 | |
| 96 | 68.4 ± 9.23-151 | 65.8 ± 12.7 | |
| Inc− | |||
| Total CD34+ | 0 | 100 | 100 |
| 48 | 95.7 ± 2.8 | 88.4 ± 3.7 | |
| 96 | 80.4 ± 5.8 | 50.2 ± 5.13-150 | |
| Sorted CD34+CD38+ | 0 | 100 | 100 |
| 72 | 78.1 ± 7.2 | 47.6 ± 4.83-150 | |
| Sorted CD34+CD38− | 0 | 100 | 100 |
| 72 | 48.6 ± 6.6 | 45.4 ± 5.8 | |
| Source of cells . | Incubation time, h . | Total cell number (× 103) Anti-SDF-1 treatment . | |
|---|---|---|---|
| − . | + . | ||
| Inc+ | |||
| Total CD34+ | 0 | 100 | 100 |
| 48 | 96 ± 2.5 | 98.5 ± 1.2 | |
| 96 | 68.4 ± 9.23-151 | 65.8 ± 12.7 | |
| Inc− | |||
| Total CD34+ | 0 | 100 | 100 |
| 48 | 95.7 ± 2.8 | 88.4 ± 3.7 | |
| 96 | 80.4 ± 5.8 | 50.2 ± 5.13-150 | |
| Sorted CD34+CD38+ | 0 | 100 | 100 |
| 72 | 78.1 ± 7.2 | 47.6 ± 4.83-150 | |
| Sorted CD34+CD38− | 0 | 100 | 100 |
| 72 | 48.6 ± 6.6 | 45.4 ± 5.8 | |
PB CD34+ cells were purified immediately after density gradient separation (Inc−) or after incubation on a plastic support (Inc+). In some experiments, Inc− cells were sorted according to their CD38 antigen expression. Cells (1 × 105/mL) were cultured under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]). The role of intracellular SDF-1 in the control of CD34+ cell autonomous survival was evaluated by adding an anti–SDF-1 neutralizing antibody (10 ng/mL) to the culture. At various time points, cells were harvested, counted, and processed for viability by trypan blue exclusion. Total cell numbers are presented as the means ± SD of 3 independent experiments.
P < .003 versus anti–SDF-1 untreated control.
P = .02 versus Inc− cells.
The role of endogenous SDF-1 in CD34+ cell survival was also established by studying the effect of an anti–SDF-1 antibody on annexin-V expression under apoptosis-inducing conditions. Such treatment significantly increased the percentage of annexin-V+ Inc− cells from 25.5% ± 5.1% to 48.4% ± 6.8% (P = .002; n = 3), whereas no difference was observed in Inc+cells (Figure 7).
Endogenous SDF-1 improves PB Inc−CD34+ autonomous cell survival.
(A) PB CD34+ cells purified immediately after density gradient separation (Inc−) or (B) after incubation on a plastic support (Inc+) were incubated (1 × 105 cells/mL) for 48 hours under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]). The role of endogenous SDF-1 was evaluated by adding an anti–SDF-1 neutralizing antibody (10 ng/mL) to the culture. Freshly isolated cells and cells harvested after a 48-hour incubation were processed for apoptosis assessment by using the annexin-V apoptosis detection kit as indicated in “Materials and methods.” The percentages of annexin-V+ PI− apoptotic cells and annexin-V+ PI+ necrotic cells shown for each histogram are for one experiment representative of the 3 performed.
Endogenous SDF-1 improves PB Inc−CD34+ autonomous cell survival.
(A) PB CD34+ cells purified immediately after density gradient separation (Inc−) or (B) after incubation on a plastic support (Inc+) were incubated (1 × 105 cells/mL) for 48 hours under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]). The role of endogenous SDF-1 was evaluated by adding an anti–SDF-1 neutralizing antibody (10 ng/mL) to the culture. Freshly isolated cells and cells harvested after a 48-hour incubation were processed for apoptosis assessment by using the annexin-V apoptosis detection kit as indicated in “Materials and methods.” The percentages of annexin-V+ PI− apoptotic cells and annexin-V+ PI+ necrotic cells shown for each histogram are for one experiment representative of the 3 performed.
SDF-1 antiapoptotic effect is mediated by the PI3-K pathway
The PI3-K/Akt and MAP kinase proteins are reported to be the 2 main pathways involved in hematopoietic cell survival.28 39-41 We examined whether SDF-1 could prevent CD34+ cells from undergoing apoptosis through these signaling pathways by using LY294002- and PD98059-specific inhibitors (Figure 8). When Inc+ or Inc−CD34+ cells were cultured for 24 hours under apoptosis-inducing conditions, the percentage of annexin-V+ cells was 31% ± 3.6% and 10.5% ± 3.2%, respectively.➁ In presence of SDF-1 (0.5 ng/mL), this percentage decreased from 31% ± 3.6% to 8.1% ± 3.4% for Inc+(P = .002; n = 5) and from 10.5% ± 3.2% to 5.4% ± 1.8% for Inc− cells (P = .05; n = 5), confirming the antiapoptotic effect of exogenous SDF-1 (➁/➀). Addition of LY294002 (25 μM) alone significantly increased the percentage of annexin-V+ cells to 58.7% ± 6.8% and 46.5% ± 4.8%, respectively, for Inc+ and Inc− cells, as compared to untreated control cells (P < .02, n = 3; ③/➁), demonstrating the role of the PI3-K in the autonomous survival of CD34+cells. Addition of LY294002 to SDF-1 inhibited the survival effect of SDF-1 because it increased the percentage of annexin-V+cells from 8.1% ± 3.4% and 6.4% ± 3.8% to 59.1% ± 6.4% and 47.5% ± 2.3% for Inc+ and Inc− cells, respectively (➀/④), demonstrating that exogenous SDF-1 acts on cell survival through the PI3-K/Akt pathway. When anti–SDF-1 alone was added to the culture, the percentage of annexin-V+ Inc− cells significantly increased from 10.5% ± 3.2% to 37.8% ± 5.8% (P < .002; n = 3; ➁/⑤), whereas it did not in Inc+ cells (➁/⑤). These results confirmed the antiapoptotic effect of endogenous SDF-1 produced by Inc−CD34+ cells. Addition of LY294002 to anti–SDF-1 did not modify the percentage of annexin-V+ cells as compared to LY294002 treated cells (⑥/③), suggesting that the survival effect of endogenous SDF-1 did not go through a pathway different from PI3-K. In contrast, PD98059 (20-100 μM) alone had no effect on the percentage of annexin-V+ cells as compared to untreated control cells (⑦/➁). Addition of PD98059 to SDF-1 or anti–SDF-1 did not modify the percentage of apoptotic cells (⑧/⑨, ➀/⑤). This suggested that, in our system, the MAP kinase pathway is not involved in the autonomous survival of CD34+ cells and that this pathway is not required for the antiapoptotic activity of SDF-1. Equivalent amounts of DMSO did not affect CD34+ cell survival (data not shown).
SDF-1 protects PB CD34+ cells from apoptosis through the PI3-K pathway.
PB CD34+ cells purified immediately after density gradient separation (Inc−, ░) or after incubation on a plastic support (Inc+, ■) were incubated (1 × 105cells/mL) for 24 hours under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]) in presence or absence of SDF-1 (0.05 and 0.5 ng/mL) and processed for apoptosis assessment by using the annexin-V apoptosis detection kit as indicated in “Materials and methods.” The role of endogenous SDF-1 was evaluated by adding an anti–SDF-1 neutralizing antibody (10 ng/mL) to the culture. Involvement of PI3-K/AKT and MAP kinase pathways was evaluated by using LY294002- and PD98059-specific inhibitors at concentrations varying from 10 to 100 μM. Data from 3 to 5 independent experiments are presented as mean percentage of annexin-V+ cells ± SD. The antiapoptotic effect of exogenous SDF-1 is shown in conditions ➁/➀. The role of PI3-K in the autonomous survival of CD34+ cells is shown in conditions ③/➁. Requirement of PI3-K axis for the antiapoptotic effect of exogenous SDF-1 is shown in conditions ➀/④. The antiapoptotic effect of endogenous SDF-1 produced by Inc−cells is shown in conditions ➁/⑤. Requirement of PI3-K axis for the antiapoptotic effect of endogenous SDF-1 is shown in conditions ⑥/③. No requirement of MAP kinase pathway in the autonomous survival of CD34+ cells and in the antiapoptotic effect of SDF-1 is shown in conditions ⑦/➁, and ⑧/⑨, ➀/⑤, respectively.
SDF-1 protects PB CD34+ cells from apoptosis through the PI3-K pathway.
PB CD34+ cells purified immediately after density gradient separation (Inc−, ░) or after incubation on a plastic support (Inc+, ■) were incubated (1 × 105cells/mL) for 24 hours under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]) in presence or absence of SDF-1 (0.05 and 0.5 ng/mL) and processed for apoptosis assessment by using the annexin-V apoptosis detection kit as indicated in “Materials and methods.” The role of endogenous SDF-1 was evaluated by adding an anti–SDF-1 neutralizing antibody (10 ng/mL) to the culture. Involvement of PI3-K/AKT and MAP kinase pathways was evaluated by using LY294002- and PD98059-specific inhibitors at concentrations varying from 10 to 100 μM. Data from 3 to 5 independent experiments are presented as mean percentage of annexin-V+ cells ± SD. The antiapoptotic effect of exogenous SDF-1 is shown in conditions ➁/➀. The role of PI3-K in the autonomous survival of CD34+ cells is shown in conditions ③/➁. Requirement of PI3-K axis for the antiapoptotic effect of exogenous SDF-1 is shown in conditions ➀/④. The antiapoptotic effect of endogenous SDF-1 produced by Inc−cells is shown in conditions ➁/⑤. Requirement of PI3-K axis for the antiapoptotic effect of endogenous SDF-1 is shown in conditions ⑥/③. No requirement of MAP kinase pathway in the autonomous survival of CD34+ cells and in the antiapoptotic effect of SDF-1 is shown in conditions ⑦/➁, and ⑧/⑨, ➀/⑤, respectively.
SDF-1 promotes survival of sorted clonogenic CD34+progenitors
We further evaluated the effect of exogenous or endogenous SDF-1 on clonogenic hematopoietic progenitor survival. This was performed by preincubating Inc+ or Inc− sorted CD34+ cells with either SDF-1 or anti–SDF-1 antibody for 72 hours under apoptosis conditions before plating in methylcellulose. SDF-1 pretreatment (0.1 ng/mL) significantly gave 165.2% ± 21.3% more erythroid burst-forming units (BFU-Es), 253% ± 18.4% more granulocyte colony-forming units (CFU-Gs), 410% ± 26.5% more macrophage colony-forming units (CFU-Ms), 225% ± 48.2% more granulocyte-macrophage colony-forming units (CFU-GMs), and 100% ± 39.4% more CFU-Mix colony formation by Inc+-sorted CD34+CD38− and 375.2% ± 36.3% more BFU-Es, 125.8% ± 31.1% more CFU-Ms, 150% ± 26.3% more CFU-GMs, and 350% ± 86.3% more CFU-Mix colony formation by Inc+-sorted CD34+CD38+ cells than was recorded for untreated control cells (P < .05, n = 3; Figure9A,B).
SDF-1 promotes survival of sorted clonogenic CD34+ progenitors.
PB CD34+ cells were purified after overnight incubation on a plastic support (Inc+) (A,B) or directly after density gradient separation (Inc−) (C,D) and sorted according to CD38 expression: CD34+CD38− cells (A,C) and CD34+CD38+ cells (B,D). The role of exogenous SDF-1 was evaluated on Inc+ cells. The role of intracellular SDF-1 was evaluated by adding an anti–SDF-1 neutralizing antibody on Inc− cells. Inc+ or Inc− cells (1.5 × 105/mL) were incubated for 72 hours under apoptosis-inducing culture conditions in the presence or not of SDF-1 (0.05 ng/mL) or anti–SDF-1 (10 ng/mL). Cells were harvested, counted, and processed for viability by trypan blue exclusion before plating in duplicate at a density of 2000 cells/mL on semisolid medium. Colonies were scored on day 14. Results are expressed as mean percentages of SDF-1 or anti–SDF-1 untreated control cells (CT) ± SD. Numbers of control colonies, derived from untreated CD34+ incubated under apoptosis conditions for 72 hours, were 11.25 ± 4.4 and 37.25 ± 10.2 (BFU-E); 6.50 ± 3.25 and 29 ± 8.5 (CFU-G); 1.5 ± 0.5 and 7.75 ± 2.2 (CFU-M); 2 ± 0.5 and 1.5 ± 0.5 (CFU-GM); 2 ± 1.5 and 0.75 ± 0.25 (CFU-Mix) from Inc+CD34+CD38− and Inc+CD34+CD38+ cells, respectively, and 31.5 ± 7 and 119.75 ± 50.2 (BFU-E); 33 ± 7.2 and 51 ± 7.3 (CFU-G); 11.5 ± 2 and 23.75 ± 3.6 (CFU-M); 2 ± 1.4 and 5.5 ± 1.5 (CFU-GM); 2.5 ± 0.8 and 2.25 ± 0.4 (CFU-Mix) from Inc−CD34+CD38− and Inc−CD34+CD38+ cells, respectively. The control plating efficiency (calculated for the total number of colonies) was 1% ± 0.85% and 4.6% ± 2.1% for Inc+ and Inc−CD34+CD38− cells, respectively, and 3.8% ± 0.7% and 11.5% ± 4.4% for Inc+ and Inc−CD34+CD38+ cells, respectively (n = 3 independent experiments). The asterisk indicates significant difference from control values, P < .05.
SDF-1 promotes survival of sorted clonogenic CD34+ progenitors.
PB CD34+ cells were purified after overnight incubation on a plastic support (Inc+) (A,B) or directly after density gradient separation (Inc−) (C,D) and sorted according to CD38 expression: CD34+CD38− cells (A,C) and CD34+CD38+ cells (B,D). The role of exogenous SDF-1 was evaluated on Inc+ cells. The role of intracellular SDF-1 was evaluated by adding an anti–SDF-1 neutralizing antibody on Inc− cells. Inc+ or Inc− cells (1.5 × 105/mL) were incubated for 72 hours under apoptosis-inducing culture conditions in the presence or not of SDF-1 (0.05 ng/mL) or anti–SDF-1 (10 ng/mL). Cells were harvested, counted, and processed for viability by trypan blue exclusion before plating in duplicate at a density of 2000 cells/mL on semisolid medium. Colonies were scored on day 14. Results are expressed as mean percentages of SDF-1 or anti–SDF-1 untreated control cells (CT) ± SD. Numbers of control colonies, derived from untreated CD34+ incubated under apoptosis conditions for 72 hours, were 11.25 ± 4.4 and 37.25 ± 10.2 (BFU-E); 6.50 ± 3.25 and 29 ± 8.5 (CFU-G); 1.5 ± 0.5 and 7.75 ± 2.2 (CFU-M); 2 ± 0.5 and 1.5 ± 0.5 (CFU-GM); 2 ± 1.5 and 0.75 ± 0.25 (CFU-Mix) from Inc+CD34+CD38− and Inc+CD34+CD38+ cells, respectively, and 31.5 ± 7 and 119.75 ± 50.2 (BFU-E); 33 ± 7.2 and 51 ± 7.3 (CFU-G); 11.5 ± 2 and 23.75 ± 3.6 (CFU-M); 2 ± 1.4 and 5.5 ± 1.5 (CFU-GM); 2.5 ± 0.8 and 2.25 ± 0.4 (CFU-Mix) from Inc−CD34+CD38− and Inc−CD34+CD38+ cells, respectively. The control plating efficiency (calculated for the total number of colonies) was 1% ± 0.85% and 4.6% ± 2.1% for Inc+ and Inc−CD34+CD38− cells, respectively, and 3.8% ± 0.7% and 11.5% ± 4.4% for Inc+ and Inc−CD34+CD38+ cells, respectively (n = 3 independent experiments). The asterisk indicates significant difference from control values, P < .05.
Addition of anti–SDF-1 (10 ng/mL) significantly gave 64% ± 12.3% fewer BFU-Es, 24.5% ± 9.6% fewer CFU-Gs, 52.6% ± 5.6% fewer CFU-Ms, 86.4% ± 4.6% fewer CFU-GMs, and 88.9% ± 6.5% fewer CFU-Mix colony formations by Inc−-sorted CD34+CD38+ cells than was recorded for untreated control cells (P < .05; n = 3), but had no effect on CD34+CD38− cells (Figure 9C,D). SDF-1 antibody specificity was attested by the inability of its isotype control to alter colony formation (data not shown). Interestingly, the plating efficiency was higher in control untreated Inc−than in Inc+ cells for both CD34+CD38− and CD34+ CD38+ populations (Figure 9), suggesting that endogenous growth factors, of which SDF-1, could participate in the autonomous survival of Inc−cells.
SDF-1 triggers G0 CD34+ cells into cycle
Our demonstration of a role for SDF-1 as an antiapoptotic factor incited us to investigate its effect on cell cycle progression. We have previously reported that SDF-1 increased the percentage of PB Inc+CD34+ cells in S+G2/M phases22; this effect was not observed in Inc−CD34+ cells. To analyze mechanisms underlying such a difference, we studied the role of SDF-1 in the early phases of the cell cycle. Therefore, we attempted to discriminate G0 from G1 phases by using the Ki67 expression assay. When freshly isolated, 28.3% ± 5.8% of Inc−CD34+ cells did not express Ki67 (Figure10A) and therefore were considered to be in the G0 phase. In contrast, the majority of Inc+CD34+ cells were in the G1phase because 93.9% ± 2.1% of them expressed Ki67 (Figure11A). As expected, the percentage of cells in S+G2/M phases was low in both populations, with a higher proportion in Inc+ cells as compared to Inc− cells (5.2% ± 0.4% and 1.9% ± 0.9%, respectively, n = 3; Figures 10 and 11). Treatment with SDF-1 decreased the percentage of Inc− cells in G0from 22.3% ± 2.8% to 5% ± 0.7% (P < .0001, n = 4; SDF-1: 0.5 ng/mL) and triggered them into cycle because their percentage in G1+S+G2/M phases increased from 78% ± 3.8% to 93.8% ± 1.6% (P < .0005, n = 4; Figure 10B). The SDF-1 effect was dose dependent and maximal for 0.5 ng/mL (Figure 10B). SDF-1 increased the percentage of Inc+CD34+ cells in S+G2/M phases from 14% ± 0.2% to 28.8% ± 0.7% (P < .0001, n = 4; SDF-1: 0.05 ng/mL; Figure 11B). Therefore, SDF-1 triggered the progression of CD34+ cells from G0 into cycle when cells were initially quiescent, whereas it stimulated the transition of cells already engaged in G1 to S+G2/M phases.
SDF-1 triggers G0 PB Inc−CD34+ cells into cycle.
PB CD34+ cells purified immediately after density gradient separation (Inc−) were incubated (1 × 105cells/mL) in a serum- and cytokine-free Stemα-A medium in the presence or absence of SDF-1 (0.01 ng/mL to 0.5 ng/mL). Freshly isolated cells (A) and cells harvested after a 48-hour incubation (B) were processed for cell cycle fractionation by using simultaneous staining for DNA content (PI) and for Ki67 expression as indicated in “Materials and methods.” This assay allowed discrimination of G0 from G1 and S+G2/M cells as shown in each histogram. Arbitrary quadrants were drawn on the basis of isotype-matched negative control profiles (first dot plot of each panel). The results shown are for one experiment representative of the 3 to 4 performed.
SDF-1 triggers G0 PB Inc−CD34+ cells into cycle.
PB CD34+ cells purified immediately after density gradient separation (Inc−) were incubated (1 × 105cells/mL) in a serum- and cytokine-free Stemα-A medium in the presence or absence of SDF-1 (0.01 ng/mL to 0.5 ng/mL). Freshly isolated cells (A) and cells harvested after a 48-hour incubation (B) were processed for cell cycle fractionation by using simultaneous staining for DNA content (PI) and for Ki67 expression as indicated in “Materials and methods.” This assay allowed discrimination of G0 from G1 and S+G2/M cells as shown in each histogram. Arbitrary quadrants were drawn on the basis of isotype-matched negative control profiles (first dot plot of each panel). The results shown are for one experiment representative of the 3 to 4 performed.
SDF-1 induces PB Inc+ CD34+cell cycle progression from G1 to S+G2/M.
PB CD34+ cells purified after incubation on a plastic support (Inc+) were incubated (1 × 105cells/mL) in a serum- and cytokine-free Stemα-A medium in presence or absence of SDF-1 (0.01 ng/mL to 0.5 ng/mL). Freshly isolated cells (A) and cells harvested after a 48-hour incubation (B) were processed for cell cycle fractionation by using simultaneous staining for DNA content (PI) and for Ki67 expression as indicated in “Materials and methods.” Arbitrary quadrants were drawn on the basis of isotype-matched negative control profiles (first dot plot of each panel). Maximal effect of SDF-1 was obtained for 0.05 ng/mL. Cell cycle fractionation percentages shown in each histogram were obtained from one experiment representative of the 3 to 4 performed.
SDF-1 induces PB Inc+ CD34+cell cycle progression from G1 to S+G2/M.
PB CD34+ cells purified after incubation on a plastic support (Inc+) were incubated (1 × 105cells/mL) in a serum- and cytokine-free Stemα-A medium in presence or absence of SDF-1 (0.01 ng/mL to 0.5 ng/mL). Freshly isolated cells (A) and cells harvested after a 48-hour incubation (B) were processed for cell cycle fractionation by using simultaneous staining for DNA content (PI) and for Ki67 expression as indicated in “Materials and methods.” Arbitrary quadrants were drawn on the basis of isotype-matched negative control profiles (first dot plot of each panel). Maximal effect of SDF-1 was obtained for 0.05 ng/mL. Cell cycle fractionation percentages shown in each histogram were obtained from one experiment representative of the 3 to 4 performed.
We further analyzed the synergistic effect of SDF-1 with early acting cytokines, such as Tpo, SF, and Flt-3 ligand, on CD34+ cell cycle progression. Tpo or SF alone increased the percentage of cells in S+G2/M without affecting the G0 compartment. Interestingly, although SDF-1 triggered G0 quiescent cells in G1, it made them progress through the S+G2/M phases in combination with Tpo or SF (Table 4). In contrast, Flt-3 ligand alone neither affected CD34+ cell cycle status nor synergized with SDF-1.
SDF-1 triggers G0Inc−CD34+ cells into G1 and makes them progress through S + G2/M phases in synergy with Tpo and SF
| Cytokines . | % of PB Inc−CD34+ cells in cell cycle phases4-150 (mean ± SD) . | ||
|---|---|---|---|
| G0 . | G1 . | S + G2/M . | |
| 0 h culture time | 28.3 ± 5.8 | 69.4 ± 6.2 | 1.9 ± 0.9 |
| 48 h culture time | |||
| SDF-1− | |||
| No cytokine | 21.5 ± 5.1 | 72.2 ± 3.1 | 6.8 ± 1.4 |
| SF | 25.3 ± 5.1 | 52.4 ± 2.5 | 20.1 ± 3.4‡ |
| Tpo | 20.8 ± 4.2 | 67.4 ± 2.8 | 13.1 ± 3.24-151 |
| Flt-3 ligand | 28.8 ± 4.8 | 63.8 ± 4.8 | 8.2 ± 3.5 |
| SDF-1+ | |||
| No cytokine | 6.4 ± 1.8‡ | 81.8 ± 4.1 | 11.2 ± 2.1 |
| SF | 10.5 ± 3.24-151 | 66.3 ± 2.9 | 22.3 ± 2.44-153 |
| Tpo | 8.4 ± 2.1‡ | 63.8 ± 3.1 | 24.1 ± 3.94-153 |
| Flt-3 ligand | 9.1 ± 2.8 | 79.7 ± 3.5 | 12.4 ± 2.8 |
| Cytokines . | % of PB Inc−CD34+ cells in cell cycle phases4-150 (mean ± SD) . | ||
|---|---|---|---|
| G0 . | G1 . | S + G2/M . | |
| 0 h culture time | 28.3 ± 5.8 | 69.4 ± 6.2 | 1.9 ± 0.9 |
| 48 h culture time | |||
| SDF-1− | |||
| No cytokine | 21.5 ± 5.1 | 72.2 ± 3.1 | 6.8 ± 1.4 |
| SF | 25.3 ± 5.1 | 52.4 ± 2.5 | 20.1 ± 3.4‡ |
| Tpo | 20.8 ± 4.2 | 67.4 ± 2.8 | 13.1 ± 3.24-151 |
| Flt-3 ligand | 28.8 ± 4.8 | 63.8 ± 4.8 | 8.2 ± 3.5 |
| SDF-1+ | |||
| No cytokine | 6.4 ± 1.8‡ | 81.8 ± 4.1 | 11.2 ± 2.1 |
| SF | 10.5 ± 3.24-151 | 66.3 ± 2.9 | 22.3 ± 2.44-153 |
| Tpo | 8.4 ± 2.1‡ | 63.8 ± 3.1 | 24.1 ± 3.94-153 |
| Flt-3 ligand | 9.1 ± 2.8 | 79.7 ± 3.5 | 12.4 ± 2.8 |
PB CD34+ cells purified immediately after density gradient separation (Inc−) were incubated (1 × 105 cells/mL) in a serum-free StemαA medium in presence or absence of Tpo (10 ng/mL), SF (50 ng/mL), Flt-3 ligand (50 ng/mL), SDF-1 (0.5 ng/mL) used alone, or in combination. At various time points, cells were harvested, counted, and dually stained with PI and anti-Ki67-FITC mAb before flow cytometry analysis.
Based on 3 independent experiments.
P < .003 and
P < .0001 versus no factor.
P < .003 versus SDF-1 alone.
SDF-1 up-regulates cyclin E and cyclin D1 expression in CD34+ cells.
Cyclins D1 to D3 are known to be restricted to G0/G1 phases, whereas cyclin E is mainly expressed during late G1 and during G1/S transition.42 We investigated the effect of SDF-1 on cyclin expression in Inc−CD34+ cells, a proportion of which was in G0. Steady-state Inc−CD34+ cells only expressed low levels of cyclin D1 (Figure 12A). Addition of SDF-1 significantly induced cyclin E and up-regulated cyclin D1 expression in Inc−CD34+cells as compared to untreated cells (Figure 12B); however, it neither altered cyclin D2 nor cyclin D3 expression. SDF-1 activity was dose-dependent (data not shown) and was maximal for 0.5 ng/mL. Cyclin D1 overexpression was slight and therefore was confirmed by Western blot analysis (Figure13). SDF-1 specificity was demonstrated by a reduction in cyclin D1 signal in response to anti–SDF-1 treatment.
SDF-1 overexpresses cyclin D1 and cyclin E in PB Inc−CD34+ cells.
PB CD34+ cells purified immediately after density gradient separation (Inc−) were incubated (1 × 105 cells/mL) in a serum- and cytokine-free Stemα-A medium in the presence or absence of SDF-1 (0.5 ng/mL). Freshly isolated cells (A) and cells harvested after a 48-hour incubation (B) were processed for intracellular detection of cyclin proteins as indicated in “Materials and methods.” The logarithm fluorescence of intracellular cyclin proteins is expressed in channel numbers. In panel A, histograms show results from staining with cyclin mAb (open histogram) and results from staining with irrelevant isotype-matched control antibody (solid histogram). Panel B shows histograms from cells stained with cyclin mAb after incubation in presence (open histogram) or absence (solid histogram) of SDF-1. Histograms from a typical donor are presented. The MFI (AU) of positive cells shown for each histogram are for one experiment representative of the 3 performed. Asterisk indicates P < .002 versus untreated cells.
SDF-1 overexpresses cyclin D1 and cyclin E in PB Inc−CD34+ cells.
PB CD34+ cells purified immediately after density gradient separation (Inc−) were incubated (1 × 105 cells/mL) in a serum- and cytokine-free Stemα-A medium in the presence or absence of SDF-1 (0.5 ng/mL). Freshly isolated cells (A) and cells harvested after a 48-hour incubation (B) were processed for intracellular detection of cyclin proteins as indicated in “Materials and methods.” The logarithm fluorescence of intracellular cyclin proteins is expressed in channel numbers. In panel A, histograms show results from staining with cyclin mAb (open histogram) and results from staining with irrelevant isotype-matched control antibody (solid histogram). Panel B shows histograms from cells stained with cyclin mAb after incubation in presence (open histogram) or absence (solid histogram) of SDF-1. Histograms from a typical donor are presented. The MFI (AU) of positive cells shown for each histogram are for one experiment representative of the 3 performed. Asterisk indicates P < .002 versus untreated cells.
Western blot analysis of cyclin D1 in PB Inc− CD34+ cells.
PB CD34+ cells immediately purified after density gradient separation (Inc−) were incubated (1 × 105cells/mL) in a serum- and cytokine-free Stemα-A medium in the presence or absence of SDF-1 (0.5 ng/mL). After a 48-hour incubation, cells were harvested and processed for Western blot analysis of cyclin D1 as indicated in “Materials and methods.” Biologic specificity of SDF-1 was demonstrated by using an anti–SDF-1 antibody (5 ng/mL). Results from a typical donor are presented.
Western blot analysis of cyclin D1 in PB Inc− CD34+ cells.
PB CD34+ cells immediately purified after density gradient separation (Inc−) were incubated (1 × 105cells/mL) in a serum- and cytokine-free Stemα-A medium in the presence or absence of SDF-1 (0.5 ng/mL). After a 48-hour incubation, cells were harvested and processed for Western blot analysis of cyclin D1 as indicated in “Materials and methods.” Biologic specificity of SDF-1 was demonstrated by using an anti–SDF-1 antibody (5 ng/mL). Results from a typical donor are presented.
Endogenous SDF-1 and CXCR-4 expression are related to CD34+ cell cycle status.
We further explored whether endogenous SDF-1 expression was associated with a precise phase of the cell cycle and whether it might control CD34+ cell cycling. SDF-1 and its receptor were expressed in cycling G1+S/G2M cells; in contrast, quiescent G0 Inc− cells did not express (Figure 14) nor produce SDF-1 (Table 2) but weakly expressed CXCR-4 (Table 1). Under apoptosis-inducing conditions, both SDF-1 secretion (Table 2) and CXCR-4 expression were increased in sorted cycling cells, whereas only CXCR-4 expression was up-regulated in quiescent cells (Table1).
PB Inc−CD34+ cells that express SDF-1 are in cycle.
Freshly isolated PB CD34+ cells were purified immediately after density gradient separation (Inc−). Cell cycle status of SDF-1–expressing cells was determined by using a dual staining for Ki67 expression and SDF-1 intracellular expression as indicated in “Materials and methods.” (A) The Ki67 expression allowed discrimination of G0 (Ki67−) from G1+S+G2/M (Ki67+) cells. Arbitrary quadrants were drawn on the basis of isotype-matched negative control. (B) The logarithm fluorescence of intracellular SDF-1 is expressed in channel numbers. Histograms show results from staining with SDF-1 mAb (solid histogram) and results from staining with irrelevant isotype-matched control antibody (open histogram). The histograms shown are for one experiment representative of the 3 performed.
PB Inc−CD34+ cells that express SDF-1 are in cycle.
Freshly isolated PB CD34+ cells were purified immediately after density gradient separation (Inc−). Cell cycle status of SDF-1–expressing cells was determined by using a dual staining for Ki67 expression and SDF-1 intracellular expression as indicated in “Materials and methods.” (A) The Ki67 expression allowed discrimination of G0 (Ki67−) from G1+S+G2/M (Ki67+) cells. Arbitrary quadrants were drawn on the basis of isotype-matched negative control. (B) The logarithm fluorescence of intracellular SDF-1 is expressed in channel numbers. Histograms show results from staining with SDF-1 mAb (solid histogram) and results from staining with irrelevant isotype-matched control antibody (open histogram). The histograms shown are for one experiment representative of the 3 performed.
Addition of anti–SDF-1 to Inc−CD34+cells inhibited their progression into cycle and maintained the percentage of G0 cells at its steady-state value as compared to untreated cells (P = .001, n = 5; Table5), suggesting that SDF-1 released by cycling cells triggered G0 cells into cycle through an autocrine/paracrine mechanism.
Neutralization of endogenous SDF-1 maintains PB Inc−CD34+ cells in G0 phase
| Incubation time, h . | Anti-SDF-1 treatment . | % of Inc−CD34+ cells in cell cycle phases5-150 . | ||
|---|---|---|---|---|
| G0 . | G1 . | S + G2/M . | ||
| 0 | 28.3 ± 5.8 | 69.4 ± 6.2 | 1.9 ± 0.9 | |
| 48 | − | 15.8 ± 2.45-151 | 78.5 ± 2.9 | 3.9 ± 0.7 |
| + | 29.4 ± 2.15-152 | 68.7 ± 2 | 1.8 ± 0.045-152 | |
| Incubation time, h . | Anti-SDF-1 treatment . | % of Inc−CD34+ cells in cell cycle phases5-150 . | ||
|---|---|---|---|---|
| G0 . | G1 . | S + G2/M . | ||
| 0 | 28.3 ± 5.8 | 69.4 ± 6.2 | 1.9 ± 0.9 | |
| 48 | − | 15.8 ± 2.45-151 | 78.5 ± 2.9 | 3.9 ± 0.7 |
| + | 29.4 ± 2.15-152 | 68.7 ± 2 | 1.8 ± 0.045-152 | |
PB CD34+ cells purified immediately after density gradient separation (Inc−) were incubated (1 × 105 cells/mL) in a serum-free Stemα-A medium for 48 hours. The role of autocrine/paracrine SDF-1 in the cell cycle was evaluated by adding an anti–SDF-1 neutralizing antibody (10 ng/mL) to the culture. Cells were harvested after 0 and 48 hours, counted, and dually stained with PI and anti-Ki67-FITC mAb before flow cytometry analysis.
Based on 5 independent experiments.
P = .001 versus 0 hours.
P = .001 versus untreated cells.
Discussion
Hematopoiesis is supported by a network of growth factors mainly produced by stromal cells within the hematopoietic microenvironment and by hematopoietic cells.43 Whereas a number of studies have dissected the ability of these factors to support proliferation and differentiation, less is known about their capacity to promote cell survival. Among a myriad of growth factors, only a few, such as Tpo, SF, and Flt-3, display survival activity by suppressing apoptosis and stimulating cell cycling.6-9,44,45 Chemokines are also reported to be involved in hematopoiesis. They promote cell migration and participate in the negative regulation of progenitor proliferation.11,46 In our group, we were particularly concerned to study the role of SDF-1 in hematopoiesis because it induced CD34+ progenitor mobilization.14 Our previous studies demonstrating that SDF-1 stimulated the proliferation of primitive circulating CD34+ cells suggested a role for this chemokine as a survival factor.22 To determine the mechanisms whereby SDF-1 acts on cell survival, we investigated whether SDF-1 protected CD34+ cells from spontaneous apoptosis and whether it interfered with cell cycle progression.
Apoptosis is a complex process characterized by a series of controlled sequential events. Therefore, spontaneous apoptotic CD34+cells resulting from a short-term incubation in serum- and cytokine-free medium were analyzed by using different appropriate methods.38 We showed that SDF-1 counteracted spontaneous apoptosis of CD34+ cells, as demonstrated by the reduction of APO2.7 expression and of annexin-V+ and sub-G1 percentages, according to the kinetics of the apoptotic process.32 We also investigated the effect of SDF-1 on Bcl-2 family proteins reported to be involved in the regulation of hematopoietic cell survival.1,7,27 Our results showed that freshly isolated PB CD34+ cells expressed Bcl-2 and Bcl-xL proteins, whereas Bad and Bax were not detected in these cells. These results fit with those reported in CD34+ isolated from BM or cord blood.3,47When CD34+ cells underwent spontaneous apoptosis, expression of Bcl-2 and Bcl-xL was up-regulated. Whereas these results could not fit with some data showing that antiapoptotic cytokines such as IL-10, Flt-3, and SF increased Bcl-2 expression,7,10,12 they are in agreement with data from other groups suggesting that such Bcl-2 up-regulation could be one of the mechanisms allowing cells to escape from apoptosis.3 26 Therefore, prevention of Bcl-2 and Bcl-xL modulation in response to SDF-1 could indicate that CD34+ cells did not undergo apoptosis under these conditions. Lastly, our results showing a reduction in Bad and Bax cell death promoter expression in response to SDF-1 argued for the antiapoptotic effect of this chemokine on CD34+ cells.
Inc+ cells were more responsive to exogenous SDF-1 than Inc− cells, because the antiapoptotic effect was observed at 10-fold lower concentration in Inc+ than in Inc− cells. Overexpression of CXCR-4 in Inc+as compared to Inc−CD34+ cells22could account for such a difference. The higher secretion of SDF-1 by Inc−CD34+CD38+ cells might also participate in their lower responsiveness by a mechanism of desensitization of the CXCR-4 receptor.48
Under apoptosis-inducing conditions, the up-regulation of CXCR-4 expression on both Inc−CD34+CD38+and Inc−CD34+CD38− cells associated with the increased SDF-1 secretion by Inc−CD34+CD38+ cells could result in a protective reaction of hematopoietic progenitors in response to a cellular stress situation. This hypothesis was consistent with results from Ponomaryov and coworkers pointing up a role for SDF-1 in alarm situations by protecting stem cells from DNA-damaging agents.19 Our results showing that exogenous SDF-1 exerted a survival effect on CD34+ cell survival and on colony formation derived from both sorted CD34+CD38−and CD34+CD38+ cells strongly supported the idea that SDF-1 acts on primitive and committed progenitor survival. In contrast, neutralization of intracellular SDF-1 increased annexin-V+ CD34+ cell number and decreased the viability and clonogenicity of sorted CD34+CD38+ cells. Autocrine/paracrine regulation requires both release of the ligand and expression of its cognate receptor. Our results demonstrating the coexpression of surface CXCR-4 and intracellular SDF-1, the secretion of the protein, and the neutralizing effect of anti–SDF-1 on sorted CD34+CD38+ cells strongly suggested that endogenous SDF-1 exerted a protective effect on CD34+ cell survival through an autocrine/paracrine loop. Majka et al45have recently reported that endogenous growth factors such as Tpo, Flt-3 ligand, and SF could participate in CD34+survival. Therefore, a possible synergy between SDF-1 and such growth factors could not be excluded.
Recent findings have indicated the involvement of PI3-K/Akt and MAP kinase pathways in cell survival and SDF-1 signaling.39-41,49 By using specific inhibitors, we demonstrated that exogenous SDF-1 displayed its antiapoptotic effect in CD34+ cells through activation of the PI3-K/Akt axis. This result is in agreement with the ability of SDF-1 to phosphorylate and activate PI3-K/Akt in CD34+ cells and in hematopoietic cell lines as demonstrated by Majka and coworkers, though in these studies the authors did not report any effect of SDF-1 on cell survival.40,50,51 Several hypotheses, including CD34+ cell source (BM versus PB) and SDF-1 doses (500 ng/mL versus 0.5 ng/mL), may account for such differences. Interestingly, we showed that specific inhibition of PI3-K/Akt by LY294002 affected the autonomous survival of CD34+ cells and that the antiapoptotic effect of endogenous SDF-1 did not go through a pathway different from the PI3-K/Akt axis. These results strongly suggested that endogenous SDF-1 participates in CD34+ autonomous cell survival through PI3-K/Akt signaling.52
In our experimental system, the MAP kinase/MEK pathway was required neither for autonomous CD34+ cell survival nor for the antiapoptotic effect of SDF-1. This may appear to contradict the findings of Lee and colleagues that SDF-1 was able to phosphorylate MAP kinases39; however, in their study, direct implication of such a pathway in CD34+ cell survival was not demonstrated. Moreover, conflicting data have been reported concerning the involvement of the MAP kinase/MEK pathway in adhesion protein activation and chemotaxis in response to SDF-1.50,51 53Taken together these studies illustrate the complexity of SDF-1–mediated signaling, depending on studied functions and experimental conditions.
Apoptosis and cell cycling are thought to be intimately linked processes involved in hematopoiesis homeostasis. When analyzed with a standard DNA histogram, up to 98% of PB CD34+ cells are in G0/G1 phases, whereas less than 85% of BM and cord blood CD34+ cells are quiescent.22,33,34This conventional cell cycle analysis allows definition of cells in G0/G1 and S+G2/M phases but does not discriminate G0 from G1phase.35 By using dual DNA/Ki67 staining we have demonstrated that exogenous SDF-1 was able to trigger resting G0 PB CD34+ cells into G1 and to make them progress through the cycle without executing a complete cell cycle. Such a triggering effect was confirmed by the overexpression of G0/G1-restricted D1 and E cyclins in response to SDF-1. To our knowledge, our study is the first reporting a role for a chemokine in the G0 to G1 transition in CD34+ hematopoietic progenitors. Very few studies have analyzed the role of growth factors in the control of early phase transitions. Among these factors, SF was reported to increase the percentage of CD34+ cells in S+G2/M without recruiting cells from G0.33 We show that Tpo and SF preferentially stimulate the transition from G1 to S+G2/M and, when combined with SDF-1, allow G0cells to progress to the late phases of the cell cycle. These findings suggest that SDF-1 acts as a priming factor by recruiting quiescent cells from G0 into cycle and by rendering them responsive to further growth factor stimulation.
As an attempt to explore the possible involvement of endogenous SDF-1 in CD34+ cell cycle regulation, we investigated whether its expression was related to cell cycle status. We showed that SDF-1 was expressed and secreted by sorted cycling cells but not by G0 resting cells and that its secretion was increased under apoptosis conditions. Because most of cycling cells expressed antigenic markers of CD34+CD38+ committed cells (data not shown), it could be suggested that the cycling and CD34+CD38+ cell populations (that produce SDF-1) overlap. We and others showed that CXCR-4 was expressed in both cycling and resting CD34+ cells,54 with a higher expression on sorted G1+S+G2/M cells. Under apoptosis-inducing conditions, CXCR-4 was up-regulated on both sorted cycling and resting cells. Addition of anti–SDF-1 maintained the percentage of G0 CD34+ cells at its steady-state value and inhibited their progression into cycle, suggesting that endogenous SDF-1 was able to trigger CD34+cells into cycle. Together these results suggested that CD34+ cells are able to control their own cycling and survival status via endogenous factors such as SDF-1.
In conclusion, our present study demonstrates a role for the SDF-1 chemokine in survival and cycling control of CD34+progenitors and provides evidence for an autocrine/paracrine mechanism. We propose that SDF-1 could play a part in hematopoiesis homeostasis by participating in the autonomous survival of circulating progenitors. SDF-1 may also occur in alarm situations by protecting them in response to cell damage. Preliminary results showing that treatment of lethally irradiated mice with SDF-1 significantly increased survival from 0% in control untreated mice to 30% in their SDF-1–treated counterparts, 30 days after irradiation, argued for an in vivo SDF-1 survival effect. By acting on stem cell trafficking, progenitor cell survival and cycling, SDF-1 chemokine could be of major interest in cellular therapy settings after myeloablative aggression.
We are indebted to Dr Loı̈c Niel for continuously supplying blood samples. We also thank Peggy Sanatine for her technical skill in flow cytometry.
Supported by grants from the DRET (Direction des Recherches Etudes et Techniques, Ministère de la Défense, no. 957) and the ANRB (Association Nouvelles Recherches Biomédicales).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marie-Caroline Le Bousse-Kerdilès, INSERM U268, Institut André Lwoff, Hôpital Paul Brousse, 14, Ave Paul Vaillant Couturier, 94800 Villejuif, France; e-mail:lebousse@ujf.inserm.fr.

![Fig. 1. Cell sorting strategy and purity of sorted fractions. / Stained cells were sorted according to CD34 and CD38 expression (A) or DNA/RNA staining (B) by using a Coulter Elite flow cytometer. For cell sorting, immunomagnetic-purified CD34+ cells were gated on a first R1 region by selecting lymphomononuclear cells and excluding dead cells (FSC/SSC dot plot). A second region R2 corresponding to CD34+ cells was drawn on a second dot plot (CD34/SSC) gated on R1. (A) The CD34+CD38−fraction was arbitrarily defined according to the CD38 expression level as the 10% of CD34+ cells with the lowest CD38 labeling. Two gates corresponding to CD34+CD38− and to CD34+CD38+ fractions were defined (Ai and Aii, respectively). Five percent of cells at the limits between these populations were excluded from the sort. (B) CD34+ cells were also sorted according to simultaneous DNA/RNA staining with Hoechst 33342 and pyronin Y. This cell cycle fractionation allowed isolation of viable [G0] CD34+ cells from [G1+S+G2/M] cells. Cells residing in G0 exhibit a low RNA content and appear at the bottom of the Hoechst/pyronin Y dot plot identified by a low pyronin Y and a low Hoechst staining (Bi). Cells in G1 show a brighter pyronin Y signal associated with a low Hoechst staining and cells in S+G2/M display both high Hoechst and pyronin Y staining. Two gates were defined corresponding to [G0] CD34+cells and to [G1+S+G2/M] CD34+ cells (Bi and Bii, respectively). The purity of the CD34+CD38−, CD34+CD38+, [G0] CD34+, and [G1+S+G2/M] CD34+ sorted fractions was more than 99% as established by the flow cytometry analysis following sorting. A representative analysis of each sorted fraction cell purity was shown on histograms of CD38 and pyronin Y fluorescences (Ai, Aii and Bi, Bii).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/4/10.1182_blood.v99.4.1117/6/m_h80422156001.jpeg?Expires=1769093350&Signature=Yt-tOGVcaHFCC25pyroa44CTrn7ojCTl6xaBBlB9OshpdJmEuIds6rFLasqxq3HQr8JvPZI7kgKZNwEdeNLV0PwDJ2jVSAWTz7SBUEeOuMcexbn5w9EdVmGAivUDqb4DrheBc0eru4CdDg3CzSnuSbYCJ2RXg~a12axb8~eNQAU7RtSoe4mVYoZr0HhraNk0J-UJVBhz1ZxElOc~RkR1dPchHZ2t03Rpo~voWFt5xsIHkBo09Cv5hiEjoTyUFAmuJVbbPP9I6MIP94rsu4y3lZYe-hzznT~XKozu3DOpdquoVH1EZJlIejMbLerGX8fYfSqYPla3T4aSky3DihqkrA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. SDF-1 counteracts apoptosis-associated nuclear DNA degradation in PB Inc+CD34+ cells. / PB CD34+ cells purified immediately after density gradient separation (Inc−) or after incubation on a plastic support (Inc+) were incubated (1 × 105 cells/mL) under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]). Freshly isolated cells (A) or cells harvested after a 4-day incubation (B) were processed for apoptosis assessment by “sub-G1 peak” detection as indicated in “Materials and methods.” The biologic effect of SDF-1 on apoptosis was evaluated by adding rhSDF-1α at various concentrations (0.01, 0.05, 0.1, 0.5 ng/mL) to the culture medium. The percentages of sub-G1apoptotic cells shown for each histogram are for one experiment representative of the 3 performed. The logarithm fluorescence of PI is expressed in channel numbers. Maximal effect of SDF-1 was obtained for 0.5 ng/mL in Inc− cells and for 0.05 ng/mL in Inc+ cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/4/10.1182_blood.v99.4.1117/6/m_h80422156002.jpeg?Expires=1769093350&Signature=CUAs99Ej85Gl66jgRRtgOgE42QfHYP-Ox0nUp8PkIicggBPbH1i4PLdrk2LN28gycZFMCo9FtOigfgnhDWkJK1yLyaXm-hPiR5TG0PHWs0nUpjK9xoWIEQ2U73~4HmfhGTv-Oe5QJ~HpIqp-zEMkE7sKQYdlXI7Jq5goP1DkvK5HJBYWYF14IsnblihbqLyf8tK8iF1GzkdrUvxviTOilEYVOtD320cw~K9C8bt5GGHLR8gjxcaVjgkFPDzIWr6dOQFTR9WhU2tXp6QHSUqlOXRcpKR-Iflx1nZjEMGL64qMPOdeEyLAONJ7A98lZ4at83Iky7w5lFF-SzPj2nzvEA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. SDF-1 decreases the expression of apoptosis-associated cell membrane proteins in PB CD34+ cells. / PB CD34+ cells purified immediately after density gradient separation (Inc−) or after incubation on a plastic support (Inc+) were incubated (1 × 105 cells/mL) under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]). Freshly isolated cells (A) or cells harvested after a 24-hour incubation program (B) were processed for apoptosis assessment by using the annexin-V apoptosis detection kit as indicated in “Materials and methods.” The biologic effect of SDF-1 on apoptosis was evaluated by adding rhSDF-1α at various concentrations (0.01, 0.05, 0.1, 0.5 ng/mL) to the culture medium. The percentages of annexin-V+PI− apoptotic cells and annexin-V+PI+ necrotic cells shown for each histogram are for one experiment representative of the 3 performed. Maximal effect of SDF-1 was obtained for 0.5 ng/mL in Inc− cells and for 0.05 ng/mL in Inc+ cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/4/10.1182_blood.v99.4.1117/6/m_h80422156003.jpeg?Expires=1769093350&Signature=4FgBLfl9mPwCfmxIFpJa3-H6gZmmM6vtbBFo3QNYga4huMTV8Rf0xZ6C3zQlJD2PLVzutIWKId2yi4wUzwhk1VfBQ8zU26eDKT42VG5mB59IFsyHoUdwsX1vk1iXHsS~Qr~CphQ55jc4XUHBxvPSAcwrPZhDQkscFzyJiGtfaadK2ss5SmyKWoXsUX3CksruozA5groZvfrJEFfVjrpwDKGHWzsMgBqNBCfFCm~72WbF7GFL1md8irw7JVZD4P9XLwBs4iDDAycw~t~BgxR-BgCmqXMapiHhjZXHKQ8yMaqFS~Ai2pdH1BihI9hTZksu1-y54IvMwnFi6TWv8QPP2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. SDF-1 decreases the expression of APO2.7 apoptosis-associated mitochondrial protein in PB Inc+CD34+ cells. / PB CD34+ cells freshly purified after incubation on a plastic support (Inc+) were incubated (1 × 105 cells/mL) under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]). Cells harvested after a 6-hour incubation program were processed for apoptosis assessment by using the mitochondrial APO2.7 antigen detection assay as indicated in “Materials and methods.” The biologic effect of SDF-1 on apoptosis was evaluated by adding 0.5 ng/mL rhSDF-1α to the culture medium. Biologic specificity of SDF-1 was demonstrated by using an anti–SDF-1 antibody (5 ng/mL) or its isotype control (5 ng/mL). A histogram from a typical donor is presented. The MFI (AU) of cells positive for APO2.7 is shown for each condition.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/4/10.1182_blood.v99.4.1117/6/m_h80422156004.jpeg?Expires=1769093350&Signature=NlO2GCL98oGjnBXQNgXUExHzX6Ma6UuX69nasJwM5jupvAfLxzqyStCGMn2ozI2b7idfVTQbuoAHKqURo-wiU5t9ZPDcmVGIc4u0czCii7F3ZlGCxkQz49y9vRWDA9c2b2Wq7x9fnfA4ksJz9oujDL-ZXyVSghv7Iu95YF7asw-WeX-vBssWpvfbvGb5Jjf172EGPh86F-RVLXGvcrr6lq1ywLbKjfgmnmc2~9F93u~Dp2goCiwWnqve9sj-keSN7161nBRF3W2nPj8RJ-wZ3lFaJJece5PdkD-1f2k7mWrjKjOyT2L7cI6eUIZW9buwkcOCQ~abmiV~0zf8g6t6-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. SDF-1 modulates apoptosis-regulatory gene products in PB Inc+CD34+ cells. / PB CD34+ cells freshly purified after incubation on a plastic support (Inc+) were incubated (1 × 105 cells/mL) under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]). Freshly isolated cells or cells harvested after a 72-hour incubation program were processed for intracellular detection of (A) antiapoptotic regulatory proteins (Bcl-2 and Bcl-xL) and (B) proapoptotic regulatory proteins (Bad and Bax) as indicated in “Materials and methods.” Modulation of Bcl-2 homolog protein expressions was evaluated in the absence or presence of SDF-1 (0.05 ng/mL). Data from at least 3 different donors with similar results were analyzed. Histograms from a typical donor are presented. The percentage or MFI (AU) or both of positive cells for Bcl-2, Bcl-xL, Bad, and Bax are shown within the histograms. The left-hand histogram represents the negative control (IgG).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/4/10.1182_blood.v99.4.1117/6/m_h80422156005.jpeg?Expires=1769093350&Signature=jdfFB7CI8kA7T36pV~j0nbZRphl7hzdiwIvaPK19eiX9NH2VHc8ogFM3WAlXtzBeUQ3dq0MaBIX2vkilxUHZeSfUqrJay6hS6vBtEuyl3fZOgLwfRJasmouXNYmAdvz3cKT4b9q69jJ4y3GcPi9rC4bSP6W0csvjfK-kmoFxIfByeip1kztLnEw3fGH7UWQp-3MrXe1NhOrw5XTxPAE4SLr7zIIXMPb33ARJONDZ10DliuvVvDkGHsG0jyN0NR39W9wkSQKAZiNVoKdJsO2zC59dNcAuy5lFtGpheVQ07AgRxWRmDE83yhXGzluHK~bF9kCqcolgpHhi~1CMCA~hnA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
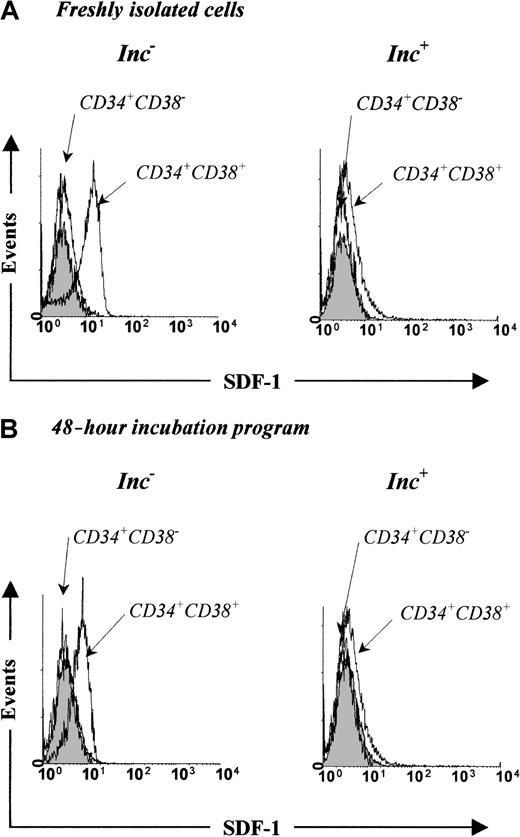
![Fig. 7. Endogenous SDF-1 improves PB Inc−CD34+ autonomous cell survival. / (A) PB CD34+ cells purified immediately after density gradient separation (Inc−) or (B) after incubation on a plastic support (Inc+) were incubated (1 × 105 cells/mL) for 48 hours under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]). The role of endogenous SDF-1 was evaluated by adding an anti–SDF-1 neutralizing antibody (10 ng/mL) to the culture. Freshly isolated cells and cells harvested after a 48-hour incubation were processed for apoptosis assessment by using the annexin-V apoptosis detection kit as indicated in “Materials and methods.” The percentages of annexin-V+ PI− apoptotic cells and annexin-V+ PI+ necrotic cells shown for each histogram are for one experiment representative of the 3 performed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/4/10.1182_blood.v99.4.1117/6/m_h80422156007.jpeg?Expires=1769093350&Signature=jSBaQ2aJyebgrro9nwfZex4yOaAP9diLuxMv5mvnLMwYkaG2j9jXzrdbgtJMhp0Rub6AWDYnTns3yNBN60oanUyon~n5UYjkSOVJmdoYniBJvxklEBERxRVR~MxDR2kr06roVZuaBLLWepRa5k-AJqOfjVp01xius2ZwiA1kBu3JvqoSTql8GC2TRLGu6fsf78pYbNroA88qR0ir2dtmkEo8zo5E9IIBuBzJWIdKCOJCq1RplDhWt5BYMX0M9PmhwvXMP6N1IO7ShKzDKdiT5DVdUOAZYYzOI7OeU27ggNoAGHhkrLHwemzR1mLdtYZLfVIeRL6hKzvAFFT-4XKwng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. SDF-1 protects PB CD34+ cells from apoptosis through the PI3-K pathway. / PB CD34+ cells purified immediately after density gradient separation (Inc−, ░) or after incubation on a plastic support (Inc+, ■) were incubated (1 × 105cells/mL) for 24 hours under apoptosis-inducing culture conditions (serum- and cytokine-free medium [IMDM]) in presence or absence of SDF-1 (0.05 and 0.5 ng/mL) and processed for apoptosis assessment by using the annexin-V apoptosis detection kit as indicated in “Materials and methods.” The role of endogenous SDF-1 was evaluated by adding an anti–SDF-1 neutralizing antibody (10 ng/mL) to the culture. Involvement of PI3-K/AKT and MAP kinase pathways was evaluated by using LY294002- and PD98059-specific inhibitors at concentrations varying from 10 to 100 μM. Data from 3 to 5 independent experiments are presented as mean percentage of annexin-V+ cells ± SD. The antiapoptotic effect of exogenous SDF-1 is shown in conditions ➁/➀. The role of PI3-K in the autonomous survival of CD34+ cells is shown in conditions ③/➁. Requirement of PI3-K axis for the antiapoptotic effect of exogenous SDF-1 is shown in conditions ➀/④. The antiapoptotic effect of endogenous SDF-1 produced by Inc−cells is shown in conditions ➁/⑤. Requirement of PI3-K axis for the antiapoptotic effect of endogenous SDF-1 is shown in conditions ⑥/③. No requirement of MAP kinase pathway in the autonomous survival of CD34+ cells and in the antiapoptotic effect of SDF-1 is shown in conditions ⑦/➁, and ⑧/⑨, ➀/⑤, respectively.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/4/10.1182_blood.v99.4.1117/6/m_h80422156008.jpeg?Expires=1769093350&Signature=MU7dwJvkD3aX8iOZjhGCrnc0sUNQPtMeCGQKlidgUvR~IYahx9o3FbgyuzGPthKkyqxVdArBA~x0o3A9rn5Nh1e3lJxup1zPTyxUCwkNGhDRt7VlsJORWNeaTA5L3O-3tFCrWuFNMwlryvQBQQH7ChO1iF-YRYBlUen5Nl7-qi3NALyxKKO3fyGjo38C0NBZ7WicL754h~cbNdstr~YIRoiyb~ATgJJHFB8H6lygnTO5LZBpvtejrOgA7B6wp1FVer~KEWunDUF1PPKvxEAL7Ljye7FuC2Q7kbsr5jKWiqxJcglvisV6an8UVu-rXtPLoI~wLJCwSUjmXiFpVmY5HA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
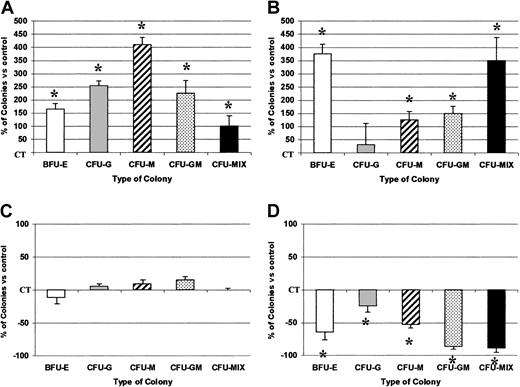
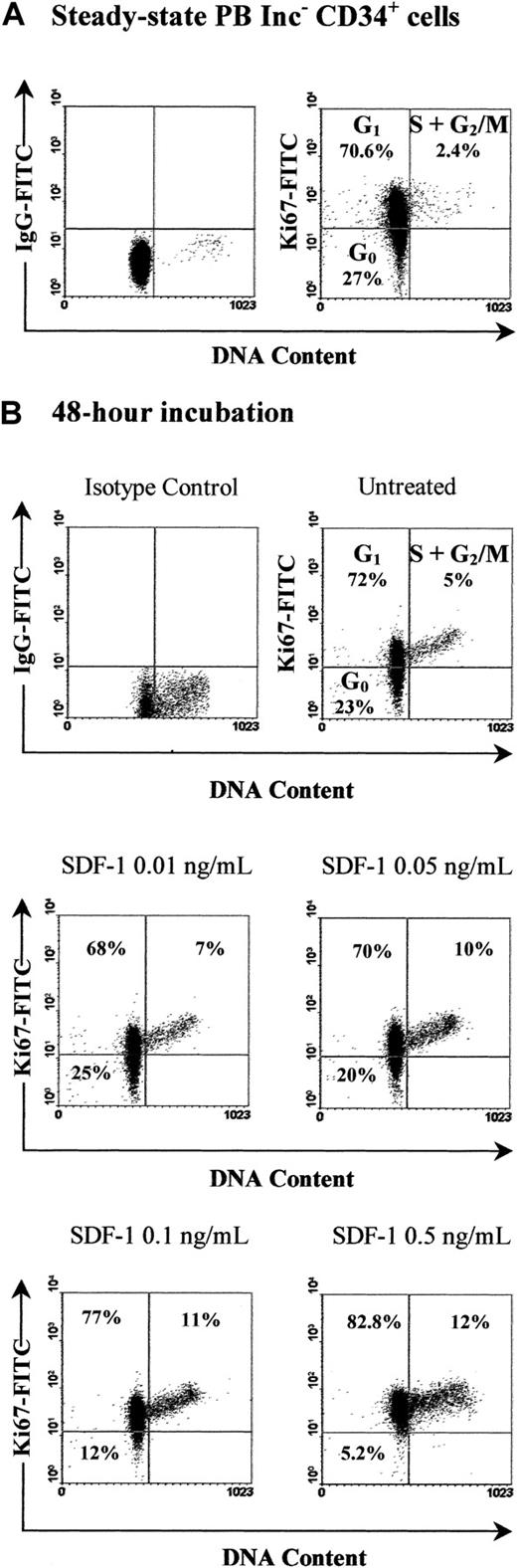
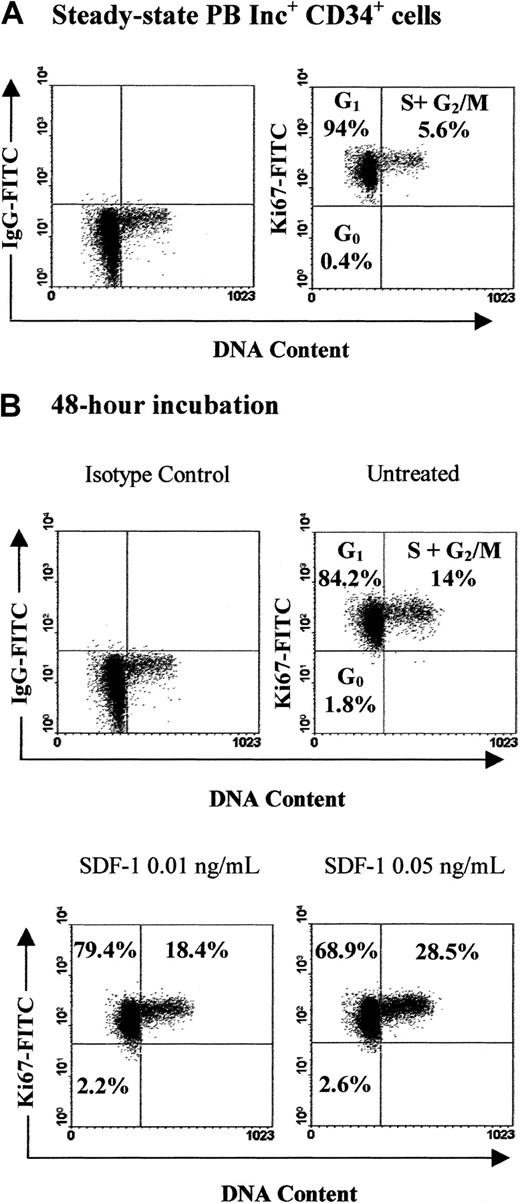

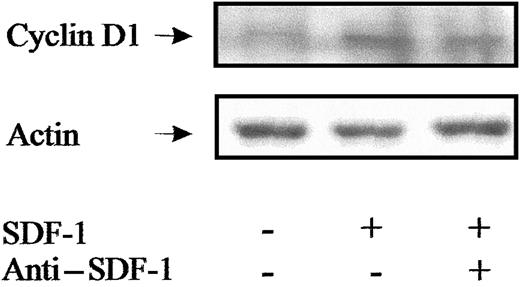
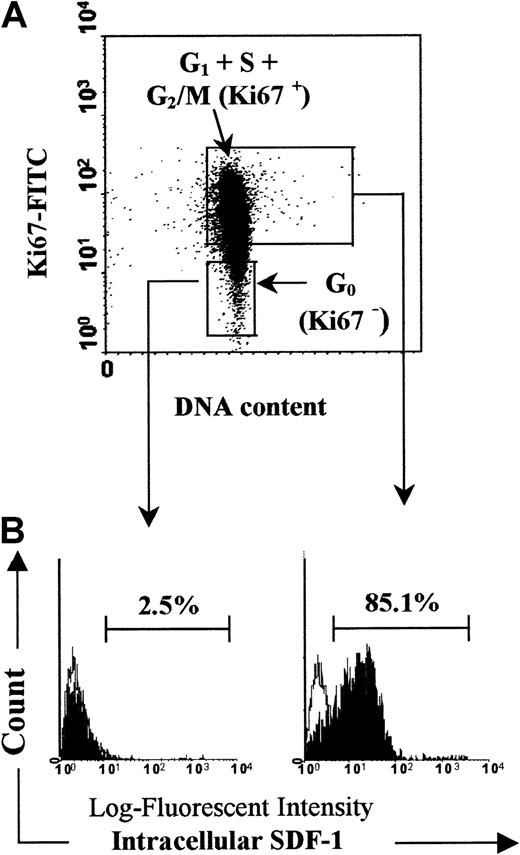
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal