Abstract
Mycosis fungoides is a low-grade cutanous T-cell lymphoma (CTCL) of unknown etiology. In advanced stages of CTCL, a shift in cytokine profile from TH1 to TH2 is observed, which coincides with eosinophilia, high levels of immunoglobulin E, and increased susceptibility to bacterial infections. It is, however, unknown why TH2 cytokines predominate in advanced CTCL, and the cellular source of these cytokines also remains to be identified. In several leukemias and lymphomas, constitutively activated signal transducer and activator of transcription (Stat) signaling pathways have been detected. In a previous study, constitutive activation of Stat3 was found in tumor cells isolated from affected skin and blood from CTCL patients. Here, it is shown that CTCL tumor cell lines, but not nonmalignant cell lines, spontaneously produce interleukin-5 (IL-5), IL-6, and IL-13. Transfection of tumor cells with dominant-negative Stat3 almost completely blocks IL-5 production and strongly inhibits IL-13 production, whereas IL-6 production is unaffected. Thus, the data show that malignant CTCL cells themselves might contribute to the change in cytokine pattern accompanying progression of CTCL. In conclusion, constitutively activated Stat3 is found to mediate a spontaneous IL-5 production and regulate IL-13 production in CTCL cell lines, pointing toward a new role of Stat3 in malignant transformation.
Introduction
Cutanous T-cell lymphomas (CTCLs) are a heterogeneous group of lymphomas, of which mycosis fungoides (MF) and the leukemic variant Sézary syndrome (SS) are the most common (reviewed in Samuelson,1 Diamandidou et al2). Although viral transformation with human T-cell lymphotropic virus type 1 has been found in some cases of CTCL,3 it does not seem to be the agent causing MF.4 Thus, the primary etiology of MF remains to be found. In the initial phase, which can endure for several years, MF is found as flat erythromatous skin lesions, resembling nonmalignant psoriasis or eczema. In later stages, tumor cells spread to other sites of the body with a fatal outcome. In SS, the leukemic form of MF, tumor T cells are present both in the skin and in the blood.1
Previously, several groups have investigated cytokine production in skin lesions or peripheral blood from CTCL patients to establish whether a unique cytokine profile could be associated with the disease. Although the results of these studies have not been entirely concordant, the general concept is that a shift in cytokine profile from TH1 to TH2 accompanies disease progression. In a report by Vowels et al,5 expression of TH2 cytokine messenger RNA (mRNA) was found to correlate with disease progression. In a study by Lee et al,6 an inability of T cells to produce interferon γ (IFN-γ) (a classical TH1 cytokine) was found to accompany CTCL progression. In contrast, other groups have not been able to find any clear polarization in cytokine production.7,8 These studies have, however, been performed with mixed cell populations from either skin lesions, blood, or nonmalignant tumor–infiltrating T cells and not on purified malignant T-cell populations. Thus, the mixture of cells may have blurred the picture and has made it difficult to determine the cellular source of the specific cytokines. Only very recently, a malignant CTCL clone was examined and characterized as TH3-like owing to its lack of IFN-γ production in combination with production of transforming growth factor β1.9
In CTCL patients, as well as many other lymphoma patients, concomitant eosinophilia, elevated levels of immunoglobulin (Ig)–E and IgA, and decreased cell-mediated immunity are often observed.10,11These complications could be caused by a shift in the cytokine pattern from a TH1-like to a TH2-like profile that accompanies progression of CTCL. In this regard, interleukin-5 (IL-5) is the predominant regulator of eosinophilia12 and has been shown to play an important role in the development of eosinophilia in Hodgkin disease.13 Likewise, cytokines such as IL-4, IL-10, and IL-13 stimulate antibody production and hence favor humoral immune responses at the expense of cellular immune responses that are needed to eliminate the tumor.12
Signal transducer and activator of transcription (Stat) proteins compose a family of transcription factors activated in response to most cytokines and growth factors. Upon receptor ligation, Stat proteins are activated by tyrosine phosphorylation mediated by receptor-associated Jak kinases. Following tyrosine phosphorylation, Stats dimerize and translocate to the nucleus, where they act directly as activators of transcription (reviewed in Imada and Leonard14). It was previously found that a member of the Stat family, Stat3, is constitutively activated in tumor cells from MF and SS15-17 and mediates the expression of constitutive suppressors of cytokine signaling–3.18 Recently, Stat3 was found to function as an oncogene,19 but the mechanism underlying its oncogenic effect is not yet clear.
Here, we have investigated cytokine production in tumor cell lines established from affected skin and blood from patients with CTCL. We show that malignant T cells produce TH2 cytokines such as IL-5 and IL-13. Furthermore, we demonstrate that IL-5 (and to some extent IL-13) production in MF tumor cells is mediated by constitutively activated Stat3.
Materials and methods
Antibodies and other reagents
Tyrphostin Ag490 was from Alexis (Laufelfigen, Switzerland); Jak3 inhibitor II was from Calbiochem (San Diego, CA); and free pyrophosphate (PP1) was from Biomol Research Laboratories (Plymouth Meeting, PA). Hemaglutinin (HA) monoclonal antibody (mAb) was from BAbCO (Richmond, CA); phospho-specific Stat3 (tyr705) mAb was from nanoTools (Denzlingen, Germany); phospho-specific Stat6 (tyr641) antibody was from New England Biolabs (Beverly, MA); and Stat3 (C-20) and Stat6 (S-20) antibodies were from Santa Cruz Biotechnology (CA). All antibodies and standards used for enzyme-linked immunosorbent assay (ELISA) were from Pharmingen/Becton Dickinson (San Diego, CA) except for rIL-13, which was from Biosource (Camarillo, CA). IFN-α-2b was from Schering-Plough (Kenilworth, NJ) (introna), and IL-4 was from Leinco (Ballwin, MO). Double-stranded Stat-binding sequences used for affinity purification were from the IgE promotor biotin-5′-TGCCTTAGTCAACTTCCCAAGAACAGAATCAAAAGGGAACTTCCAA-3′,20or from the c-fos promotor hSIE 5′-biotin-GTCGACATTTCCCGTAAATCGTCGA-3′.21
Cells
MF3675 and MF2000 are an early and a long-term culture, respectively, of a malignant cell line established from a plaque biopsy specimen from a patient with MF.22 A combination approach that included phenotyping, HLA-typing, and karyotyping verified the tumor origin of the cell line.23 MF2000 shares the same clonal chromosome aberrations as MF3675, and both cell lines display the same T-cell receptor rearrangement, Vβ13. Whereas the growth of MF2000 has become cytokine independent, growth of MF3675 is IL-2 dependent. MF1885 is a nonmalignant T-cell line established from peripheral blood from the same patient.23 SeAx is a tumor cell line established from peripheral blood from a patient with SS.24 MF2000 was cultured in RPMI 1640 (Gibco, Rockville, MD) supplemented with 10% fetal calf serum, 2 mM L-glutamine, 100 μg/mL penicillin, and 100 μg/mL streptomycin. MF3675 and SeAx cells were grown in RPMI 1640 supplemented with 10% pooled human serum, 1000 U/mL proleukin IL-2 (Chiron, Emeryville, CA), 2 mM L-glutamine, 100 μg/mL penicillin, and 100 μg/mL streptomycin. MF1885 was grown in the same medium as MF3675 and SeAx, execpt that 10 ng/mL IL-4 was added.
Transfection of MF tumor cells
Expression vectors pCAGGSneo-HA-Stat3 wild type (WT) or pCAGGSneo-HA-Stat3 dominant negative (D) have been described previously.25 In Stat3D, E434 and E435 have been replaced with alanines, rendering Stat3D unable to bind DNA. Stable transfections of MF2000 cells were performed by electroporation (240 V) using 25 μg DNA. Transfected cells were selected in medium containing 1.5 mg/mL Geneticin (G-418 sulfate) (Gibco), and expression of “exogeneous” Stat3 was examined by Western blotting with HA antibody.
RNAse protection assay
RNA extraction was performed by means of the QIA shredder and RNeasy Mini Kits from Qiagen (Valencia, CA) as described.26 The purity and size distribution of total RNA were determined by agarose gel electrophoresis. RNase protection assay was performed by means of the ribonuclease protection assay kit and the in vitro transcription kit from Pharmingen according to the manufacturer's protocol.
Enzyme-linked immunosorbent assay
Before the ELISA, cells were washed and incubated overnight in fresh medium at 1 × 106 cells per milliliter. The cytokine production was analyzed by sandwich ELISA with the use of the manufacturer's protocol (Pharmingen/BectonDickinson).
Oligonucleotide affinity purification of Stat proteins and Western blotting
Cells were lysed in ice-cold lysis buffer (1% NP-40; 20 mM Tris, pH 8.0; 137 mM NaCl; 5 mM MgCl2; 10% glycerol; and the following inhibitors: 5 mM EDTA, 1 mM Na3VO4, 10 μg/mL aprotinin, 4 μM iodoacetamide, 1 mM phenylmethyl sulfonyl fluoride). For oligonucleotide affinity purification, 150 pmol double-stranded biotinylated oligo was added to the cell lysate and incubated 2 hours. Stat/oligonucleotide complexes were recovered by means of streptavidin-conjugated beads. Purified proteins or total lysates were boiled in reducing sodium dodecyl sulfate sample buffer and analyzed by gel electrophoresis followed by electrophoretic transfer to a nitrocellulose membrane. The membrane was blocked in 3% skim milk and 1% bovine serum albumin in phosphate-buffered saline (PBS) and incubated with primary antibody in blocking buffer followed by washing in PBS and incubation with peroxidase-conjugated secondary antibody. Blots were evaluated by means of enhanced chemiluminescence, stripped, and reprobed as described (Amersham, Buckinghamshire, United Kingdom).27
Results
CTCL tumor cells produce TH2 cytokines
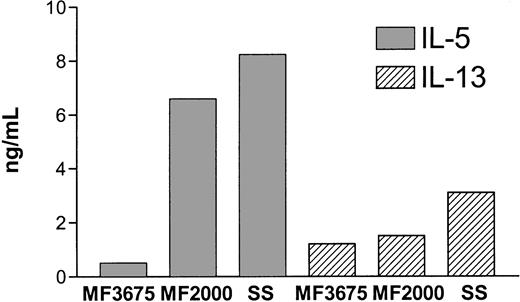
To evaluate cytokine expression in CTCL, we first examined production of classical TH1 (IFN-γ) and TH2 (IL-4, IL-5, and IL-13) cytokines in MF tumor T cells and nonmalignant T cells isolated from the same patient. MF2000 is a continuous tumor cell line, established from a plaque biopsy from a patient with classical MF, that has all the malignant characteristics of MF.22 As shown in Table 1, MF2000 did not produce any IFN-γ or IL-4, whereas high amounts of both IL-5 and IL-13 were detected. In contrast, the nonmalignant cell line, MF1885, produced neither IFN-γ, IL-4, nor IL-5 and produced only barely detectable amounts of IL-13. To further clarify whether production of the TH2 cytokines IL-5 and IL-13 is a general phenomenon in tumor cells from CTCL, an early culture of MF tumor cells (MF3675) as well as an SS (SeAx) tumor cell line were examined. As shown in Figure 1, all the tumor cell lines produced IL-5 and IL-13, although MF3675 did so to a lesser extent than MF2000. In a further ELISA screening for IL-2, IL-6, IL-7, IL-12, IL-14, IL-15, and tumor necrosis factor α production, only IL-6 was detected in MF2000 supernatants (data not shown).
Cytokine synthesis in nonmalignant (MF1885) or mycosis fungoides tumor cells (MF2000) measured by enzyme-linked immunosorbent assay (ng/mL)
| Cell type . | IFN-γ . | IL-4 . | IL-5 . | IL-13 . |
|---|---|---|---|---|
| MF1885 | < 0.03 | < 0.07 | < 0.03 | 0.7 |
| MF2000 | < 0.02 | < 0.02 | 14.3 | 6.7 |
| Cell type . | IFN-γ . | IL-4 . | IL-5 . | IL-13 . |
|---|---|---|---|---|
| MF1885 | < 0.03 | < 0.07 | < 0.03 | 0.7 |
| MF2000 | < 0.02 | < 0.02 | 14.3 | 6.7 |
Cells were washed and incubated overnight in fresh medium at 1 × 106 cells per milliliter before analysis. The data are from 2 independent experiments.
IFN indicates interferon; IL, interleukin.
Production of IL-5 and IL-13 by CTCL tumor cell lines.
MF or SeAx tumor cells were washed and incubated overnight in fresh medium at 1 × 106 cells per milliliter before IL-5 and IL-13 production was determined by ELISA.
Production of IL-5 and IL-13 by CTCL tumor cell lines.
MF or SeAx tumor cells were washed and incubated overnight in fresh medium at 1 × 106 cells per milliliter before IL-5 and IL-13 production was determined by ELISA.
Treatment with a Jak3 inhibitor inhibits IL-5 and IL-13 production in CTCL tumor cells
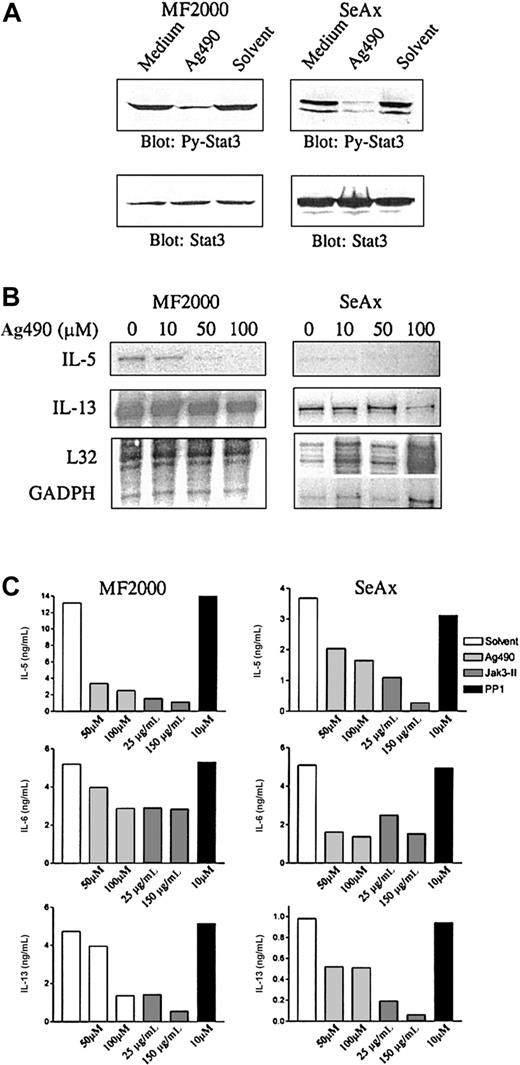
It was previously shown that SS and MF tumor cells express constitutively activated Stat3.15,16 Here, we addressed whether the constitutive Stat3 activation is involved in (dys)regulation of cytokine production in CTCL cells. As shown previously,16,28 and confirmed here, treatment of SS and MF tumor cells with tyrphostin Ag490, an inhibitor of Jak kinases, inhibits constitutive Stat3 activation (Figure2A). By means of RNAse protection assay, the effect of Ag490 incubation on cytokine mRNA was examined (Figure2B). Interestingly, expression of both IL-5 and IL-13 mRNA was strongly inhibited in both cell lines following treatment with Ag490. In contrast, mRNA for the housekeeping genes L32 and GADPH was not affected, indicating that it is a specific rather than a toxic effect of Ag490. To see whether the effect of Ag490 detected at the mRNA level was also evident at the protein level, ELISA assays for IL-5, IL-6, and IL-13 were performed. Because Jak3 is constitutively activated and/or associated with Stat3 in CTCL,15 17 a more selective inhibitor of Jak3 (Jak3-II) was also included in this experiment. As shown in Figure 2C, both IL-5 and IL-13 production were strongly inhibited by both Jak kinase inhibitors, in particular by the more selective Jak3-II inhibitor, which inhibited cytokine production by approximately 90%. In contrast, IL-6 production was not as strongly inhibited. PP1, an inhibitor of src kinases, did not have any effect on the production of any of the cytokines. Although Jak inhibitors might also inhibit Jak targets other than activated Stat3, these data suggest that constitutive active Stat3 might be involved in the disordered cytokine production in CTCL tumor cell lines.
Effect of Jak3 inhibitors on IL-5 and IL-13 production in MF2000 and SeAx tumor cells.
Treatment with Jak3 inhibitors inhibits IL-5 and IL-13 production in MF2000 and SeAx tumor cells. (A) Ag490 inhibits constitutive Stat3 activation. MF or SeAx cells were incubated for 16 hours in the indicated concentrations of Ag490. Cells were then lysed and analyzed by Western blotting with phosphotyrosine-Stat3 mAb (top) followed by reprobing with total Stat3 antibody (bottom). (B) Ag490 inhibits mRNA for IL-5 and IL-13. Cells were treated with Ag490 as mentioned above before RNA was isolated and analyzed by RNAse protection assay. L32 and GADPH are housekeeping genes. (C) Jak inhibitors inhibit cytokine production. Cells were treated with Ag490, Jak3-II (Jak inhibitors), or PP1 (src kinase inhibitor) for 16 hours. Cytokine production was then measured by ELISA.
Effect of Jak3 inhibitors on IL-5 and IL-13 production in MF2000 and SeAx tumor cells.
Treatment with Jak3 inhibitors inhibits IL-5 and IL-13 production in MF2000 and SeAx tumor cells. (A) Ag490 inhibits constitutive Stat3 activation. MF or SeAx cells were incubated for 16 hours in the indicated concentrations of Ag490. Cells were then lysed and analyzed by Western blotting with phosphotyrosine-Stat3 mAb (top) followed by reprobing with total Stat3 antibody (bottom). (B) Ag490 inhibits mRNA for IL-5 and IL-13. Cells were treated with Ag490 as mentioned above before RNA was isolated and analyzed by RNAse protection assay. L32 and GADPH are housekeeping genes. (C) Jak inhibitors inhibit cytokine production. Cells were treated with Ag490, Jak3-II (Jak inhibitors), or PP1 (src kinase inhibitor) for 16 hours. Cytokine production was then measured by ELISA.
Transfection of MF tumor cells with dominant-negative Stat3
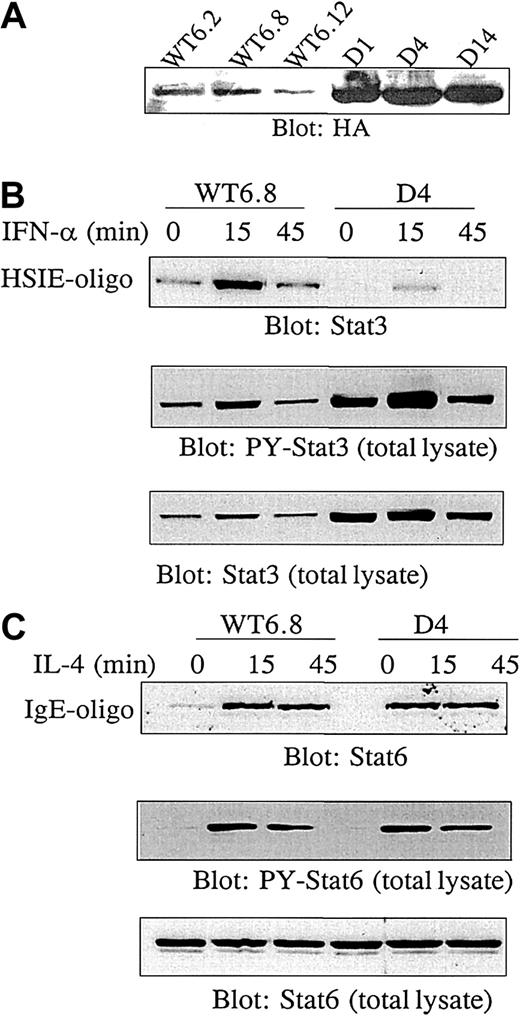
To investigate the role of constitutive Stat3 activation in cytokine production in a more direct way, we stably transfected MF tumor cells with either WT or dominant-negative (D) HA-tagged Stat3. Stat3D contains mutations at positions important for DNA binding.25 The expression level of transfected Stat3 was determined by blotting with antibody against the HA tag (Figure3A). In the following experiments, 3 Stat3WT (WT6.2, WT6.8, WT6.12) and 3 Stat3D (D1, D4, D14) transfectants were used. It was a consistent finding that Stat3D transfectants had a higher expression level of exogenous Stat3 than WT transfectants. To examine the dominant-negative effect of Stat3D, transfectants were stimulated with IFN-α, which is a strong inducer of Stat3 activation. Hereafter, binding to hSIE, an oligo representing a Stat-binding sequence from the c-fos promotor, was analyzed by affinity purification of proteins binding to the oligo. As shown in Figure 3B (upper panel), there was a constitutive background level of Stat3 binding to DNA in unstimulated Stat3WT cells, analogous to our previous findings.15 Upon IFN-α stimulation for 15 minutes, DNA binding was further increased and declined after 45 minutes to background level. In contrast, constitutive DNA binding of Stat3 in Stat3D cells was strongly reduced and was only marginally induced by IFN-α. Aliquots of the lysates used to study DNA binding were blotted with antibody against tyrosine-phosphorylated Stat3 (PY-Stat3) (Figure 3B, middle panel) to demonstrate that tyrosine phosphorylation of Stat3 was not inhibited. Reblotting the membrane with Stat3 antibody confirmed that the blocked DNA binding was not due to unequal amounts of protein in the samples (Figure 3B, lower panel). To ensure that other Stat signaling pathways were not affected, transfected cells were stimulated with IL-4 in order to examine Stat6 activation. As shown in Figure 3C (upper panel), DNA binding of Stat6 was equally well induced in Stat3WT and Stat3D cells. Likewise, blotting of total lysates demonstrated identical induction of Stat6 tyrosine phosphorylation (middle panel) and equal amounts of Stat6 in the samples (bottom panel). Thus, transfection of MF cells with Stat3D selectively inhibits constitutive active Stat3 in a dominant-negative manner.
Transfection of MF2000 with dominant-negative Stat3.
Transfection of MF2000 with dominant-negative Stat3 specifically inhibits Stat3 activity. MF tumor cells were stably transfected with HA-tagged Stat3 wild type (Stat3WT) or a dominant-negative Stat3 mutant (Stat3D). (A) Expression of exogenous Stat3. Transfectants were lysed and analyzed for HA expression by Western blotting. (B) Dominant-negative Stat3 inhibits DNA binding of Stat3. MF cells transfected with Stat3WT or Stat3D were stimulated with IFN-α for the indicated periods of time followed by lysis of the cells. Stat3 capable of binding to DNA was affinity purified by means of biotinylated hSIE oligos and identified by Western blotting with Stat3 antibody (top panel). Aliquots of the lysates were analyzed for tyrosine phosphorylation of Stat3 (middle panel) followed by reblotting of total Stat3 (lower panel). (C) Dominant-negative Stat3 does not affect Stat6 activation. MF cells transfected with Stat3WT or Stat3D were stimulated with IL-4, followed by lysis of the cells. Stat6 capable of binding to DNA was affinity purified by means of biotinylated IgE promotor oligos and identified by Western blotting with Stat6 antibody (top panel). Aliquots of the lysates were analyzed for tyrosine phosphorylation of Stat6 (middle panel) followed by reblotting of total Stat6 (lower panel). The experiments shown in panels B and C were repeated with WT6.2, WT6.12, D1, and D14 with similar results.
Transfection of MF2000 with dominant-negative Stat3.
Transfection of MF2000 with dominant-negative Stat3 specifically inhibits Stat3 activity. MF tumor cells were stably transfected with HA-tagged Stat3 wild type (Stat3WT) or a dominant-negative Stat3 mutant (Stat3D). (A) Expression of exogenous Stat3. Transfectants were lysed and analyzed for HA expression by Western blotting. (B) Dominant-negative Stat3 inhibits DNA binding of Stat3. MF cells transfected with Stat3WT or Stat3D were stimulated with IFN-α for the indicated periods of time followed by lysis of the cells. Stat3 capable of binding to DNA was affinity purified by means of biotinylated hSIE oligos and identified by Western blotting with Stat3 antibody (top panel). Aliquots of the lysates were analyzed for tyrosine phosphorylation of Stat3 (middle panel) followed by reblotting of total Stat3 (lower panel). (C) Dominant-negative Stat3 does not affect Stat6 activation. MF cells transfected with Stat3WT or Stat3D were stimulated with IL-4, followed by lysis of the cells. Stat6 capable of binding to DNA was affinity purified by means of biotinylated IgE promotor oligos and identified by Western blotting with Stat6 antibody (top panel). Aliquots of the lysates were analyzed for tyrosine phosphorylation of Stat6 (middle panel) followed by reblotting of total Stat6 (lower panel). The experiments shown in panels B and C were repeated with WT6.2, WT6.12, D1, and D14 with similar results.
IL-5 production in MF tumor cells is mediated through constitutively activated Stat3
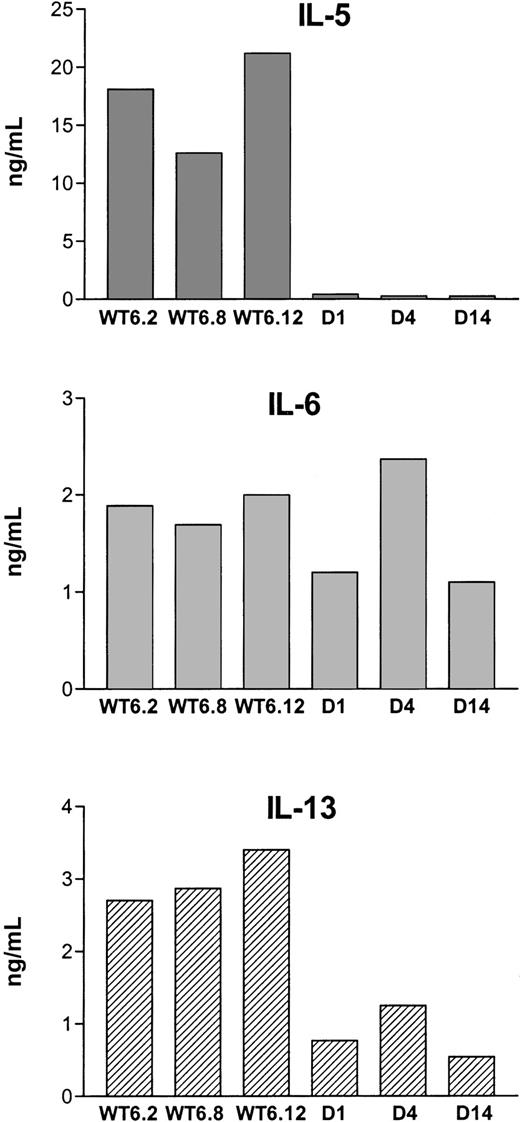
Production of IL-5, IL-6, and IL-13 was examined in 3 Stat3WT lines (WT6.2, WT6.8, WT6.12) and 3 Stat3D lines (D1, D4, D14). As shown in Figure 4, the spontaneous production of IL-5 was totally blocked in all Stat3D transfectants, demonstrating that constitutively active Stat3 plays a major role in IL-5 production in MF tumor cells. In contrast, there was no difference in IL-6 production between Stat3WT and Stat3D cells. As regards IL-13 production, Stat3 seemed to be partially involved, since cytokine levels decreased to about 30% to 40% in Stat3D transfectants compared with Stat3WT. A comparison of the transfectant studies in which Stat3 was specifically inhibited (Figure 4) with experiments using Jak inhibitors (Figure 2B-C) makes it evident that blocking Jak kinase activity affects targets other than Stat3, since Jak inhibition influenced IL-6 production whereas specific inhibition of Stat3 did not.
Mediation of IL-5 production in MF tumor cells.
IL-5 production in MF tumor cells is mediated through constitutively activated Stat3. Cytokine production in MF cells transfected with Stat3WT or Stat3D was measured by ELISA. The data are representative of 3 independent experiments.
Mediation of IL-5 production in MF tumor cells.
IL-5 production in MF tumor cells is mediated through constitutively activated Stat3. Cytokine production in MF cells transfected with Stat3WT or Stat3D was measured by ELISA. The data are representative of 3 independent experiments.
Discussion
In the present study, we provide the first evidence that CTCL tumor cell lines, but not an autologous nonmalignant T-cell line, spontaneously produce high amounts of IL-5, IL-6, and IL-13.
Production of TH2-like cytokines, such as IL-5 and IL-13, might promote disease progression, since TH2 cytokines have a negative influence on antitumor immune responses. Moreover, secretion of IL-5 and IL-13 could be involved in the complications that often follow CTCL such as eosinophilia, erythroderma, and elevated titers of IgE, since it is well known that IL-5 is one of the main controlling cytokines for eosinophilia and IL-13 induces IgE synthesis in B cells. Catovsky et al11 found that eosinophil counts fell during remission and rose during relapse in patients with T-lymphoblastic lymphomas, supporting the idea that eosinophilia could be induced by cytokines produced by the malignant cells.
Experiments using inhibitors of Jak kinases suggested that constitutively activated Stat3 might be involved in the production of IL-5 and IL-13, as inhibition of Stat3 was followed by decreased production of these cytokines. However, this indirect way of inhibiting Stat3 might also affect other Jak3 targets. Therefore, in order to address the question in a more direct way, we investigated cytokine production in MF tumor cells stably transfected with dominant-negative Stat3. These studies provided strong evidence that constitutive Stat3 activation plays a major role in inducing IL-5 production in MF tumor cells and is involved in regulation of IL-13 production. In contrast, constitutive activation of Stat3 was found to be not involved in IL-6 production. The importance of Stat3 in IL-5 production might also be reflected in the finding that MF3675 has a lower production of IL-5 than MF2000, since MF3675 cells have a lower level of constitutive Stat3 activation than MF2000 (Figure 1 and data not shown). Whether Stat3 induces IL-5 transcription directly or via a secondary mechanism is not clear at the moment. Although IL-5 synthesis is known to be regulated at the transcriptional level, only limited information about the promotor sequence is available. However, some important regulatory elements have been reported (ie, Cle0, REI, II, III).29 30We searched these promotor sequences for Stat3-binding sites, but failed to identify any sequences that could actually bind Stat3 in experiments using MF tumor cells. Although we cannot exclude the possibility that a Stat3-binding motif could be present in the IL-5 promotor, these findings might suggest that Stat3 mediates IL-5 production via an indirect mechanism. Blocking experiments with cytokine and cytokine receptor antibodies have indicated that IL-5 production is not induced by IL-6 or IL-13 (or vice versa; data not shown). Likewise, the constitutive activation of Stat3 is not induced via an autocrine loop involving these cytokines (data not shown).
As mentioned above, IL-5 has been associated with eosinophilia and the clinical syndrome erythroderma. In this respect, it should be noted that all cell lines used in this study were obtained from patients suffering from severe erythoderma. It has been proposed that in Hodgkin disease IL-5 is a key mediator of eosinophilia,13and studies are now in progress to elucidate whether Stat3 is also involved in IL-5 production in Hodgkin disease and other lymphomas.
In conclusion, constitutively activated Stat3 mediates a spontaneous IL-5 production and regulates IL-13 production in CTCL cell lines, pointing toward a new role of Stat3 in malignant transformation. Thus, Stat3 might be a highly relevant target for therapeutical intervention in CTCL.
Supported in part by The Weimann Foundation (M.N.), The Foundation of 17/12-1981 (J.G.), The Danish Medical Research Council, The Danish Biotechnological Center for Cellular Communication, The Carlsberg Foundation, The Novo Nordic Foundation, The Danish Medical Associations Research Foundation (Lægeforeningens Forskningsfond), The Danish Cancer Research Foundation (Dansk Kræftforsknings fond), and The Danish Cancer Society (Kræftens Bekæmpelse).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Niels Ødum, Institute of Medical Microbiology and Immunology, University of Copenhagen, Panum 22.5, Blegdamsvej 3, 2200 Copenhagen N, Denmark; e-mail: n.odum@immi.ku.dk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal