Abstract
Inflammatory processes are associated with the rapid migration of dendritic cells (DCs) to regional lymph nodes and depletion of these potent antigen-presenting cells (APCs) from the inflamed tissue. This study examined whether sites of cutaneous inflammation can be repopulated with DCs from a pool of immature DCs circulating in the blood. In adoptive transfer experiments with ex vivo–generated radioactively labeled primary bone marrow–derived DCs injected into mice challenged by an allergic contact dermatitis reaction, immature DCs were actively recruited from the blood to sites of cutaneous inflammation, whereas mature DCs were not. Immature, but not mature, DCs were able to adhere specifically to immobilized recombinant E- and P-selectin under static as well as under flow conditions. P-selectin–dependent adhesion of immature DCs correlates with their higher level of expression of the carbohydrate epitope cutaneous lymphocyte-associated antigen (CLA) and is blocked by a novel inhibitory antibody against mouse P-selectin glycoprotein ligand 1 (PSGL-1). Surprisingly, however, emigration of immature DCs into inflamed skin is retained in the presence of this anti–PSGL-1 antibody and is also normal when immature DCs are generated from fucosyltransferase (Fuc-T) Fuc-TVII–deficient mice. By contrast, emigration of wild-type immature DCs is reduced by adhesion-blocking anti–E- and P-selectin antibodies, and immature DCs generated ex vivo from Fuc-TVII/Fuc-TIV double-deficient mice emigrate poorly. Thus, fucosylated ligands of the endothelial selectins, determined in part by Fuc-TIV, and independent of PSGL-1, are required for extravasation of DCs into sites of cutaneous inflammation.
Introduction
Dendritic cells (DCs) are bone marrow–derived leukocytes that are specialized in antigen capture, processing, and presentation to T lymphocytes and are essential for the initiation and modulation of antigen-specific immune responses.1 DC progenitors as well as more mature DCs are present in small numbers in the blood.2 They seed nonlymphoid tissues and are primarily localized within epithelia, such as Langerhans cells in the epidermis.3 On activation these cells undergo phenotypic changes that allow them to migrate from their site of residence to the T-cell areas of regional lymph nodes.4 The factors that mediate trafficking from the periphery to lymphoid organs are well defined.5-10 However, not much is known about the immigration of DC precursors to their tissue of residence and only a little information is available about immigration and turnover of DCs in inflamed tissues. Because pathogens, allergens, or contact with CD40L-expressing cells all lead to activation and emigration of resident DCs toward regional lymph nodes,11 the site of inflammation is rapidly depleted of resident antigen-presenting cells (APCs). Thus, for the maintenance of the antigen-specific immune response, it appears necessary that nonresident APCs be actively recruited to inflamed tissue. The role of the small population of blood DCs is not clear, but it is possible that this cell type forms a “task force” of potent APCs that can rapidly relocate to sites of inflammation.12 This would result in enhanced local antigen presentation to infiltrating effector T cells and sustained priming of naive T cells after subsequent migration to regional lymph nodes.2
Selectins and their ligands play a major role for extravasation of leukocytes from blood into inflamed tissue.13,14 They are essential for tethering and rolling of leukocytes on vascular endothelium, the initial steps of the adhesion cascade.15The best analyzed selectin ligand on leukocytes is P-selectin glycoprotein ligand 1 (PSGL-1),16-18 which is essential for T-cell as well as for neutrophil emigration.19-22 For selectin-binding, PSGL-1 and all other known selectin counterreceptors need to be modified by the minimal ligand structure for all 3 selectins, the tetrasaccharide sialyl Lewis X (sLex) (NeuAcα2,3Galβ1,4 [Fucα1,3]-GlcNAc). The biosynthesis of sLex requires the sequential action of a number of glycosyltransferases of which the final reaction is mediated by α1,3-fucosyltransferases (Fuc-T). Two of them, Fuc-TIV and Fuc-TVII, have been implicated in the generation of selectin ligands.23 The lack of Fuc-TVII severely reduces extravasation of lymphocytes as well as neutrophils,24whereas the lack of Fuc-TIV has only minor effects on leukocyte endothelial interactions.25
In humans, it was demonstrated that a DC progenitor population bears a skin homing molecule known as cutaneous lymphocyte-associated antigen (CLA) on its surface, defined by the monoclonal antibody (mAb) HECA452.26 This epitope is a carbohydrate modification whose precise structure has not yet been determined definitively, but ample evidence suggests that it resembles or is a derivative of sLex. The mAb HECA452 was shown to block lymphocyte binding to E-selectin,27 28 suggesting a role of this epitope as a ligand for E-selectin.
Cutaneous lymphocyte-associated antigen was reported to be prominently if not exclusively expressed on the sialomucin PSGL-1 on activated human T cells.29 Although CLA is not well defined in the mouse, and the HECA452 antibody stains murine skin homing lymphocytes less reliably than human ones, antigen-specific activation of a mouse CD8+ T-cell clone also induced expression of CLA, and PSGL-1 was identified as the major glycoprotein carrier of this carbohydrate modification on these cells.30 In subsequent studies, CLA was found to be expressed on human DCs, which prompted studies to investigate the ability of DCs to interact with the endothelial selectins. Using intravital microscopy it could indeed be demonstrated that human DCs were able to roll in postcapillary venules in the noninflamed mouse skin and these interactions could be inhibited with antibodies against E- and P-selectin.31Preliminary, nonquantitative data also suggested that immature DCs, differentiated from mouse bone marrow cells, could be recruited into inflamed mouse skin,31 although neither the potential adhesion mechanisms that mediate this recruitment nor the capacity of these cells to bind to the endothelial selectins have been investigated so far.
In the present study, we have performed a detailed and quantitative analysis of the migration of immature mouse DCs into inflamed mouse skin. We found that immature, but not mature, DCs are recruited in significant numbers into the inflamed tissue. Moreover, we show that immature bone marrow–derived mouse DCs transiently express CLA and a selectin-binding glycoform of PSGL-1, bind to P- and E-selectin, and lose their selectin-binding capacity as well as their ability to emigrate into inflamed skin on full maturation. Emigration of immature DCs was dependent on E- and P-selectin and was reduced if DCs were generated from Fuc-TVII/Fuc-TIV double-deficient mice. Surprisingly, however, emigration was not reduced if DCs were preincubated with an adhesion-blocking antibody against PSGL-1 or if DCs were generated from mice deficient in Fuc-TVII. Our results suggest that immature DCs require fucosylated ligands of the endothelial selectins for emigration into inflamed skin and that these ligands are independent of PSGL-1 and are in part determined by Fuc-TIV.
Materials and methods
Mice
Antibodies and selectin-IgG chimeras
To monitor the DC phenotype the following mAbs were used for flow cytometry: B7-2 (clone G1-1), CD11b/Mac1 (clone M1/70), CD11c (clone HL3), CD44 (clone IM7) (all from Pharmingen, San Diego, CA) and I-Ab,d,q, I-Ed,k (clone M5/114), intercellular adhesion molecule 1 (ICAM-1; clone YN1/1.7.4), very late activation antigen 4 (VLA-4; clone PS/2), as hybridoma supernatants, were purchased from the American Type Culture Collection (ATCC, Rockville, MD). As secondary antibodies a DTAF-labeled and affinity-purified F(ab′)2 fragment goat-antirat IgG + IgM (H + L) (Dianova, Hamburg, Germany) or a fluorescein isothiocyanate (FITC)–conjugated antihamster IgG cocktail (Pharmingen) in case of CD11c (clone HL3) were used. As secondary antibody in Western blot a peroxidase-labeled and affinity-purified F(ab′)2 fragment goat-antirat IgG + IgM (H + L) (Dianova) was used.
Two novel mAbs against mouse PSGL-1 were generated by immunizing Lewis rats with a PSGL-1–human IgG1 chimeric protein. This recombinant protein was constructed by fusing a complementary DNA (cDNA) fragment coding for the extracellular part of mouse PSGL-1 (base pairs 1-918) with a cDNA fragment coding for the hinge region and domains C2 and C3 of human IgG1.33 The cDNA construct was cloned into the pcDNA-3 vector (Invitrogen, Groningen, The Netherlands) and transfected into COS-7 cells; the fusion protein was purified by affinity isolation with protein A Sepharose (Pharmacia, Uppsala, Sweden). Lymphocytes isolated from the spleen and lymph nodes of immunized rats were fused with the mouse myeloma SP2/0. Hybridoma supernatants were tested in enzyme-linked immunosorbent assays (ELISAs) for binding to the fusion protein with human IgG1 as negative control. Two antibodies were obtained that bound to the fusion protein as well as to PSGL-1 on mouse myeloid cells: 4RA10 (rat-IgG1) and 4RB12 (rat-IgG2a).
The following mAbs were purified from protein-free hybridoma supernatants (Nanotools, Teningen, Germany): 2PH1 against mouse PSGL-1,19 RB40.34 against mouse P-selectin,344RA10 and 4RB12 against mouse PSGL-1 (described here). Purified rat-IgM mAb HECA452 was a kind gift from Dr Louis Picker and mAb UZ4 (rat-IgM) against E-selectin had been kindly provided by Dr Rupert Hallmann. Negative control antibodies were mAb S7 (rat-IgG) against CD43 and mAb R4-22 (rat-IgM, κ isotype) (Pharmingen). The P-selectin–IgG and E-selectin–IgG chimeras were produced as described before.20 Human IgG1 was purchased from Sigma (St Louis, MO).
For sorting with magnetic cell-sorting (MACS) beads (Miltenyi Biotec, Bergisch Gladbach, Germany) the following additional mAbs were used: The mAb Tib164 (anti-CD45R/B220, clone 14.8) was obtained from ATCC. The Fc-block rat-antimouse CD16/CD32 mAb (clone 2.4G2) and the mAbs CD11b/Mac1 (clone M1/70), Gr1 (clone RB6-8C5), CD4 (clone GK1.5), CD8 (clone 53-6.7), and CD24/HSA (clone 30-F1) were obtained from Pharmingen.
Cells
For the generation of DCs, published methods were used,35 36 with slight modifications. Mouse bone marrow was collected from tibias and femurs of female mice, and passed through a 70-μm nylon mesh after hypotonic lysis of erythrocytes for 3 minutes at room temperature. Cells were resuspended in complete medium (RPMI 1640 containing 5% heat-inactivated fetal calf serum [FCS], 50 μM β-mercaptoethanol, 2 mM l-glutamine, 0.1 mM nonessential amino acids, and 20 μg/mL gentamicin; all from PAA, Linz, Austria), and seeded into 90-mm tissue culture dishes (Becton Dickinson, Heidelberg, Germany) for 2 hours at 37°C and 10% CO2. Nonadherent cells were carefully washed from dishes and collected and adherent cells were discarded. In some experiments lineage-positive (Lin+) cells were depleted by MACS after incubation with mAbs against CD45R/B220, CD11b/Mac1, CD3, GR1, I-Ab,d,q, I-Ed,k (all rat-IgG) and goat-antirat IgG microbeads (Miltenyi Biotec) according to the manufacturer's instructions. The lineage-negative (Lin−) cell fraction was separated in an automated Magnetic Cell Sorter (Miltenyi Biotec). Cells were cultured in 80-cm2 bottles (Nunclone, Wiesbaden, Germany) in the presence of 150 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) (both from conditioned cell culture supernatants). Cultures were grown for 5 days (immature DCs) or 9 to 11 days (mature DCs) with complete change of medium every second day. In some experiments, mature DCs were incubated for the final 24 hours of culture with 0.1 μg/mL lipopolysaccharide (LPS) from Escherichia coli (Sigma).
The T cells were obtained from the axillary and inguinal lymph nodes of mice sensitized 7 and 6 days before with 2% (wt/vol) oxazolone (4-ethoxymethyl-2-phenyl-2-oxazolin-5-one; Sigma) freshly dissolved in olive oil/acetone (1:4); 50 μL was applied to the shaved abdomen and 10 μL solution to each footpad of the mice. Lymph nodes were collected and pressed through a metal sieve with cold phosphate-buffered saline (PBS; 1% [vol/vol] FCS) to generate single-cell suspensions. T cells were resuspended in RPMI 1640 containing 5% FCS and subjected to a sterile nylon wool column equilibrated in the same medium. After incubation at 10% CO2 for 45 minutes, nonadherent cells were collected by washing the column with 40 mL warm media. In a second purification step, this fraction was incubated with an antibody cocktail including mAbs against CD45R/B220, CD11b/Mac1, CD16/CD32 (Fc-block), Gr1, and CD24/HSA for negative depletion. In case of T cells from Fuc-TVII−/− and wild-type control strains the mAb MEL14 against L-selectin was included to also remove naive T cells. After washing and incubation with goat-antirat IgG microbeads (Miltenyi Biotec), the antibody-negative cell fraction was separated with an automated Magnetic Cell Sorter (Miltenyi Biotec). Purity was controlled by flow cytometry analysis. More than 98% of cells were CD4+ or CD8+.
The mouse neutrophilic progenitor 32Dcl3 and the Chinese hamster ovary (CHO) cell line CHO DUKX B1 and the mouse CD8+ cytotoxic T-cell clone 4G3 were cultivated as previously described.30,37,38 The immature DC cell line XS52 was cultured in the presence of GM-CSF and conditioned media from NS47 cells as described previously.39
Flow cytometry
Flow cytometry was done as described.40
Immunoprecipitation and Western blot
Static adhesion assay
Static adhesion assays were done in 96-well flat-bottom plates (Maxisorp, Nunc, Wiesbaden, Germany) coated with 5 μg/mL selectin-IgG chimera or human IgG1 in Hanks balanced salt solution (HBSS) overnight at 4°C. Nonspecific binding sites were blocked by incubating with Dulbecco modified Eagle medium (DMEM) containing 10% (vol/vol) FCS for 3 hours.41 To minimize unspecific binding, cells (107 cells/mL) were preincubated with 1 μg CD16/CD32 (Fc-block, Pharmingen) in HBSS per 106 cells. For inhibition studies, purified mAbs were added to the cells prior to the assay at 10 μg/mL and incubated on ice for 10 minutes. To allow adhesion, 5 × 105 cells were added per well and incubated for 20 minutes at 4°C under mild rotation (80 rpm). Plates were kept on ice and washed 4 times with cold HBSS. To check for calcium dependence of the interaction, wells were washed with PBS containing 5 mM EDTA. Adherent cells were fixed with CellFIX in PBS (Becton Dickinson) and numbers were evaluated by computer-aided image analysis with the NIH Image 1.55 software as described.20
Cell attachment assay under flow
Assays were essentially performed as described.31 42 Glass coverslips were coated overnight at room temperature with 0.75 μg/mL selectin-IgG chimera or human IgG1 in HBSS as negative control and subsequently blocked with 5% (vol/vol) bovine serum albumin (BSA; grade V; Sigma) in HBSS for 3 hours at room temperature in a moist chamber. Cells were used at a density of 5 × 106 cells in 6 mL DMEM containing 10% (vol/vol) FCS and 0.04% (vol/vol) azide. To avoid nonspecific activation of rolling cells via Fc-receptor binding to coated selectin-IgG fusion proteins, cells were preincubated with 1 μg anti-CD16/CD32 (Fc-block, Pharmingen) per 106 cells. Adhesion-blocking antibodies were added at 10 μg/mL final concentration. Immediately before the assay, cells were removed from ice and perfused at room temperature through a rectangular transparent laminar flow perfusion chamber containing the protein-coated cover slip. Evaluation started 60 seconds after starting the pump. The number of rolling cells was counted for 10 areas of 0.125 mm2 each.
In vivo contact hypersensitivity model
The procedure was performed as described,21 36 with slight modifications. Mice were sensitized twice 7 and 6 days before injection of DCs with 2% (wt/vol) oxazolone (Sigma) in olive oil/acetone (1:4). At 24 hours (in some experiments 6 or 2 hours) before injection of cells mice were challenged at the right ear with 10 μL 0.5% (wt/vol) oxazolone. The untreated left ear served as a control. From this point on mice were caged individually to prevent allergen contamination of the contralateral ear. In some experiments hapten challenge was repeated 4 hours before injection.
Cells were counted and labeled at a density of 107 cells/mL for 1 hour with 51Cr, 100 μCi/107 (3.7 MBq/107) cells (Amersham, Braunschweig, Germany) at 37°C in RPMI 1640, 20% (vol/vol) FCS. After labeling, cells were washed 3 times in PBS to remove nonincorporated radioactivity. DCs (5 × 106), 5 × 106 32Dcl3 cells, or 107 T cells were incubated in 200 μL PBS with 250 μg/mL antibodies against selectin ligands or control antigen, incubated on ice for 15 minutes, and injected intravenously together with the antibodies. Antibodies against selectins (400 μg/mouse) were injected intravenously directly before injection of radiolabeled cells. In control experiments, cells were fixed with 2% (wt/vol) formaldehyde for 10 minutes and washed with PBS prior to injection. Immediately before injection, cells were resuspended to break up clusters. Six hours after injection mice were killed and organs removed. In some experiments, a topical steroid (0.1% momethasone-17-[2-furoat] Ecural cream, Essex, Munich, Germany) was applied to the eczematous ear lesions 6 and 24 hours after injection of cells, and organs were removed after 48 hours. Ears, lungs, liver, and spleen were counted in a γ counter for 2 minutes per probe (LKB Systems, Freiburg, Germany). The percentage of radioactivity in each ear relative to the sum counted in all organs was calculated.
Results
Characterization of DCs differentiated from mouse bone marrow cells
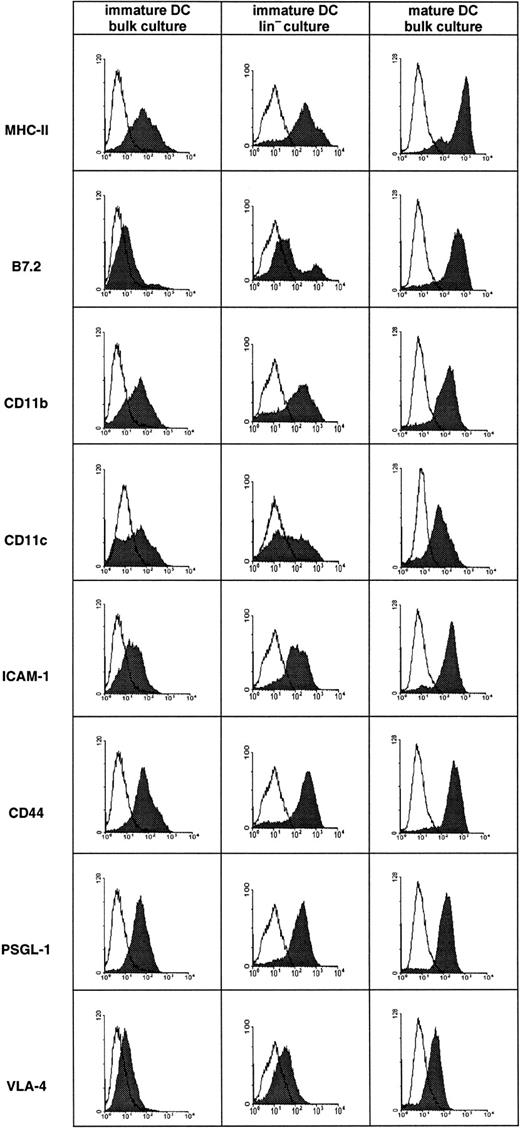
To obtain mouse DCs, bone marrow cells were cultured in the presence of GM-CSF and IL-4.35 36 Typically, DCs are characterized by expression of a combination of surface molecules such as major histocompatibility complex class II (MHC-II), B7-2, CD11c, and ICAM-1 that are strongly up-regulated during maturation and that are generally low but not negative on immature DCs. In cultures growing for 5 days we generally observed most cells as DCs of immature morphology and surface phenotype (Figure 1), whereas in cultures growing for 9 to 11 days with LPS added for the last 24 hours, almost all cells uniformly exhibited a mature morphology and surface phenotype (Figure 1). The broader distribution of several markers that we observed on immature DCs at day 5 indicates that the differentiation status of the DC population was still heterogeneous at this time. VLA-4 expression was often found to be stronger than in the particular experiment shown here, especially on mature DCs. In some cultures, Lin+ cells were depleted from bone marrow cells before starting the culture. After 5 days, DCs in these cultures exhibited an almost identical pattern of surface markers as did cells from the nondepleted cultures (Figure 1). All cultures tested were consistently negative for lineage markers such as CD19, CD56, Gr1, and CD3, indicating that the cultures were devoid of significant contaminations by other cell types. Similar results were obtained with cultures from Balb/c as well as from 129Sv mice (not shown).
Surface phenotype of in vitro–derived mouse DCs.
DCs were derived from bone marrow of Balb/c mice that had been depleted (Lin− culture) or not depleted (bulk culture) of Lin+ cells. Cells cultured for 5 days in the presence of GM-CSF and IL-4 gave rise to immature DCs, whereas cells cultured for 9 days in identical medium and with LPS added for the last 24 hours gave rise to mature DCs. Flow cytometry was performed as described in “Materials and methods.” The mature and immature DCs depicted here originated from aliquots of the same bone marrow preparation.
Surface phenotype of in vitro–derived mouse DCs.
DCs were derived from bone marrow of Balb/c mice that had been depleted (Lin− culture) or not depleted (bulk culture) of Lin+ cells. Cells cultured for 5 days in the presence of GM-CSF and IL-4 gave rise to immature DCs, whereas cells cultured for 9 days in identical medium and with LPS added for the last 24 hours gave rise to mature DCs. Flow cytometry was performed as described in “Materials and methods.” The mature and immature DCs depicted here originated from aliquots of the same bone marrow preparation.
Migration of DCs into inflamed skin in the allergic contact dermatitis model
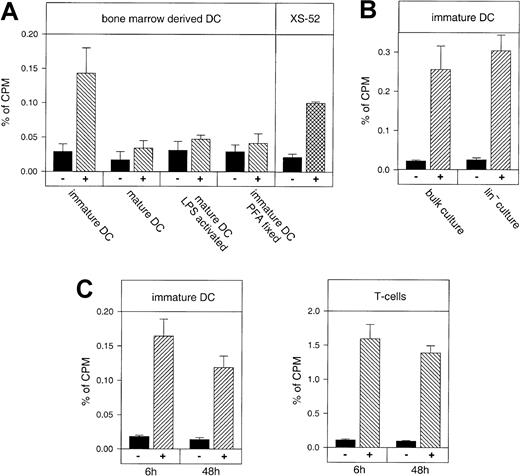
We investigated the capacity of the mouse DCs of different maturation stages to migrate into inflamed skin and compared their homing ability with that of other cell types. Recipient mice were sensitized, challenged with oxazolone, and 1 day later, radioactively labeled DCs were injected into the tail veins. As shown in Figure2, immature DCs cultured for 5 days in the presence of GM-CSF and IL-4 accumulated in the inflamed ear 5 to 12 times better than in the noninflamed ear. This migration was dependent on the immature differentiation state of these cells, because fully mature DCs cultured for 11 days did not specifically accumulate in the inflamed ear. Likewise, mature DCs that had been cultured in the presence of LPS during the last 24 hours of culture did not migrate into inflamed ear skin (Figure 2A). To further control whether the accumulation of DCs required the active participation of these cells we injected paraformaldehyde (PFA)–fixed immature DCs. Again, no specific accumulation of these cells in the inflamed ear was observed (Figure 2A).
In vivo migration of mature and immature DCs into skin during a contact hypersensitivity reaction in comparison to sensitized T cells.
Balb/c bone marrow–derived DCs, freshly isolated T cells from oxazolone-sensitized Balb/c donors, or the mouse DC line XS52 were labeled with 51Cr and injected into the tail vein of sensitized and challenged Balb/c recipients. After 6 hours mice were killed and the accumulation of radioactivity in the oxazolone-challenged inflamed ear (hatched bars, +) was measured by γ counting and compared to the vehicle-treated control ear (black bars, −). The y-axis (percent of cpm) refers to radioactivity in the ear with respect to radioactivity of all harvested organs set as 100%. (A) Immature DCs cultured for 5 days, mature DCs cultured for 11 days without LPS (mature DC) or with LPS for the last 24 hours (mature DC, LPS activated), immature DCs fixed in PFA (immature DC, PFA fixed), and the immature DC line XS52 were compared. The means and SDs for each value were calculated from 3 independent animals. Immigration of DCs was statistically significant for immature DCs and XS52, respectively (P < .001), but not for mature DCs. (B) Bulk cultures of immature DCs were not depleted for Lin+ cells, whereas Lin− cultures were immature DCs generated from bone marrow cells that had been depleted for CD3+, B220+, Gr1+, and MHC-II+ cells at the beginning of the culture period. Lin− DCs and undepleted DCs were cultured for 5 days and injected intravenously as above. (C) Accumulation of immature DCs (left panel) or T cells (right panel) 6 hours and 48 hours after tail vein injection. For the 48-hour time points, ears of recipient mice were treated twice at 6 and at 24 hours after cell injection with a steroid cream to resolve inflammation due to the allergic contact dermatitis. The values represent groups of at least 3 mice each.
In vivo migration of mature and immature DCs into skin during a contact hypersensitivity reaction in comparison to sensitized T cells.
Balb/c bone marrow–derived DCs, freshly isolated T cells from oxazolone-sensitized Balb/c donors, or the mouse DC line XS52 were labeled with 51Cr and injected into the tail vein of sensitized and challenged Balb/c recipients. After 6 hours mice were killed and the accumulation of radioactivity in the oxazolone-challenged inflamed ear (hatched bars, +) was measured by γ counting and compared to the vehicle-treated control ear (black bars, −). The y-axis (percent of cpm) refers to radioactivity in the ear with respect to radioactivity of all harvested organs set as 100%. (A) Immature DCs cultured for 5 days, mature DCs cultured for 11 days without LPS (mature DC) or with LPS for the last 24 hours (mature DC, LPS activated), immature DCs fixed in PFA (immature DC, PFA fixed), and the immature DC line XS52 were compared. The means and SDs for each value were calculated from 3 independent animals. Immigration of DCs was statistically significant for immature DCs and XS52, respectively (P < .001), but not for mature DCs. (B) Bulk cultures of immature DCs were not depleted for Lin+ cells, whereas Lin− cultures were immature DCs generated from bone marrow cells that had been depleted for CD3+, B220+, Gr1+, and MHC-II+ cells at the beginning of the culture period. Lin− DCs and undepleted DCs were cultured for 5 days and injected intravenously as above. (C) Accumulation of immature DCs (left panel) or T cells (right panel) 6 hours and 48 hours after tail vein injection. For the 48-hour time points, ears of recipient mice were treated twice at 6 and at 24 hours after cell injection with a steroid cream to resolve inflammation due to the allergic contact dermatitis. The values represent groups of at least 3 mice each.
Because we had used primary cultures as the source for DCs, we were concerned that contaminating non-DCs could have contributed to the selective accumulation of radioactivity at the site of inflammation. To exclude this possibility, we generated DC cultures that had been vigorously depleted for Lin+ cells. As shown in Figure 2B, these Lin− DCs accumulated in the inflamed tissue with the same efficiency as DCs from bulk cultures (Figure 2B). We conclude that the radioactivity that accumulated in the inflamed ear was indeed due to the migration of DCs. In agreement with this, we found that an immature DC line derived from neonatal mouse skin (XS52)39also homed into the inflamed ear, although slightly less efficiently than the primary differentiated cells (Figure 2A).
To exclude the possibility that the radioactivity measured in the inflamed ear would originate from cells being simply trapped in the dilated microvasculature rather than having emigrated into the surrounding tissue, we topically applied a steroid cream (mometasone-17-[2-furoate]) to the eczematous ears 6 and 24 hours after injection of cells to down-regulate local inflammation and vasodilation. In addition, radioactivity in the ear was analyzed 48 hours after injecting the cells, to exclude transient trapping of labeled cells within the vasculature. At this time point, steroid-treated ears were no longer visibly inflamed and ear swelling had returned to baseline levels of control-treated ears. We found that the accumulated radioactivity measured after 48 hours was in a similar range as after 6 hours (Figure 2C, left panel), indicating that cells had entered the inflamed tissue and had not just been trapped in the dilated vasculature of the ear. Radioactively labeled T cells isolated from the lymph nodes of oxazolone-sensitized mice also accumulated in the inflamed ear skin and were found in comparable numbers at 6 hours and at 48 hours after injection (Figure 2C, right panel).
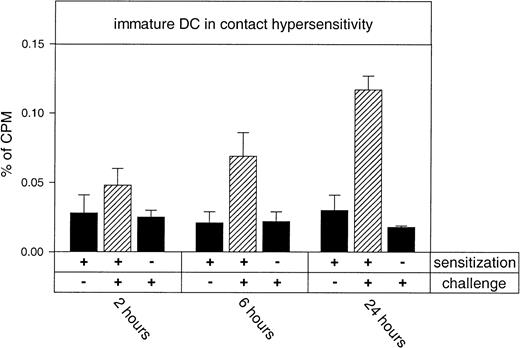
We next investigated whether the emigration of DCs preceded the allergen-induced skin inflammation, which would suggest a functional relevance of these cells in elicitation of acute allergic contact dermatitis. We therefore analyzed the efficiency of DC accumulation in the inflamed ear at different time points of the inflammation. As shown in Figure 3, immature DCs homed with increasing efficiency into the inflamed skin when cells were injected at 2, 6, and 24 hours after challenging the ear with oxazolone. In addition, this experiment demonstrates that DCs only home to hapten-challenged ears of sensitized, but not of naive, mice. Thus, the efficiency of DC emigration into inflamed skin was largely proportional to the severity of inflammation, although DCs were observed to extravasate into allergen-challenged skin as early as 2 hours after hapten application.
Time course of immigration of immature DCs into hapten-challenged ears in an evolving allergic contact hypersensitivity reaction.
51Cr-labeled DCs from untreated donor Balb/c mice were injected into the tail vein of differentially treated recipients of the same strain. After 6 hours mice were killed and the accumulation of radioactivity in organs was measured in a γ counter. Y-axis (percent of cpm) refers to radioactivity in the ear with respect to the radioactivity of all harvested organs set as 100%. Recipient mice were sensitized on the shaved abdomen (+) or left untreated (−) as indicated. Seven days later, one ear was challenged with hapten (+) 2, 6, or 24 hours before injection of DCs. The untreated ears from sensitized mice served as negative controls (−) and are shown in the first column of each set. The hatched bars represent measured radioactivity in sensitized and challenged ears. Specific homing to hapten-challenged ears was statistically significant at 6 and 24 hours (P < .001).
Time course of immigration of immature DCs into hapten-challenged ears in an evolving allergic contact hypersensitivity reaction.
51Cr-labeled DCs from untreated donor Balb/c mice were injected into the tail vein of differentially treated recipients of the same strain. After 6 hours mice were killed and the accumulation of radioactivity in organs was measured in a γ counter. Y-axis (percent of cpm) refers to radioactivity in the ear with respect to the radioactivity of all harvested organs set as 100%. Recipient mice were sensitized on the shaved abdomen (+) or left untreated (−) as indicated. Seven days later, one ear was challenged with hapten (+) 2, 6, or 24 hours before injection of DCs. The untreated ears from sensitized mice served as negative controls (−) and are shown in the first column of each set. The hatched bars represent measured radioactivity in sensitized and challenged ears. Specific homing to hapten-challenged ears was statistically significant at 6 and 24 hours (P < .001).
Immature, but not mature, mouse DCs bind to P- and E-selectin
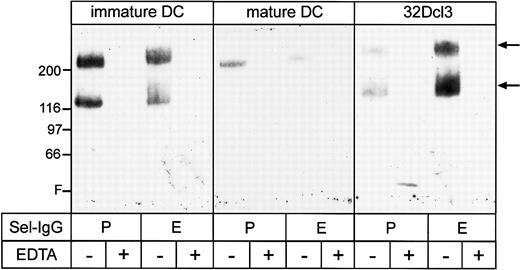
E- and P-selectin are known to be essential for the emigration of T cells from blood into inflamed skin.20,43 In addition, human DCs bind to E- and P-selectin in vitro and rolling of these cells in mouse venules is dependent on E- and P-selectin.31Therefore, we first investigated whether immature mouse DCs express ligands for E- and P-selectin. As analyzed by flow cytometry, immature as well as mature DCs expressed PSGL-1 (Figure 1). Whether PSGL-1 on these cells was able to bind to P- or E-selectin (or both) was analyzed in affinity isolation experiments with P- and E-selectin–IgG. As shown in Figure 4, bands at 230 and 130 kd, affinity isolated from immature DCs as well as from 32Dcl3 cells, reacted specifically with the mAb 4RA10 against PSGL-1. As often seen with PSGL-1, not all 230-kd dimers were completely reduced to the 130-kd monomer on the gels. The fact that the E-selectin–binding form of PSGL-1 was slightly larger than the P-selectin–binding form has also been described for a mouse T- cell clone.30 Mature DCs only allowed precipitation of very small amounts of the dimeric form of PSGl-1. Thus, the glycoform of PSGL-1, which can efficiently bind to E- and P-selectin, is preferentially expressed on immature and not on mature DCs. In agreement with this the carbohydrate epitope CLA (resembling sLeX) was clearly expressed on immature mouse DCs, whereas it was strongly reduced on mature DCs (Figure5). Although CLA is also found on human DCs,31 our finding is surprising because CLA has usually not been described on mouse primary leukocytes.
Affinity isolation of PSGL-1 from 32Dcl3 cells and DCs with selectin-IgG fusion proteins.
Total cell lysates of immature and mature DCs from Balb/c mice and 32Dcl3 cells were subjected to immunoprecipitations with selectin-IgG fusion proteins bound to protein A Sepharose in the presence (+) or absence (−) of EDTA as indicated. Precipitated proteins were immunoblotted with the novel mAb 4RA10 against mouse PSGL-1 (Figure 6). Molecular weight markers (kd) are indicated on the left, and the front (F) of the gel is marked.
Affinity isolation of PSGL-1 from 32Dcl3 cells and DCs with selectin-IgG fusion proteins.
Total cell lysates of immature and mature DCs from Balb/c mice and 32Dcl3 cells were subjected to immunoprecipitations with selectin-IgG fusion proteins bound to protein A Sepharose in the presence (+) or absence (−) of EDTA as indicated. Precipitated proteins were immunoblotted with the novel mAb 4RA10 against mouse PSGL-1 (Figure 6). Molecular weight markers (kd) are indicated on the left, and the front (F) of the gel is marked.
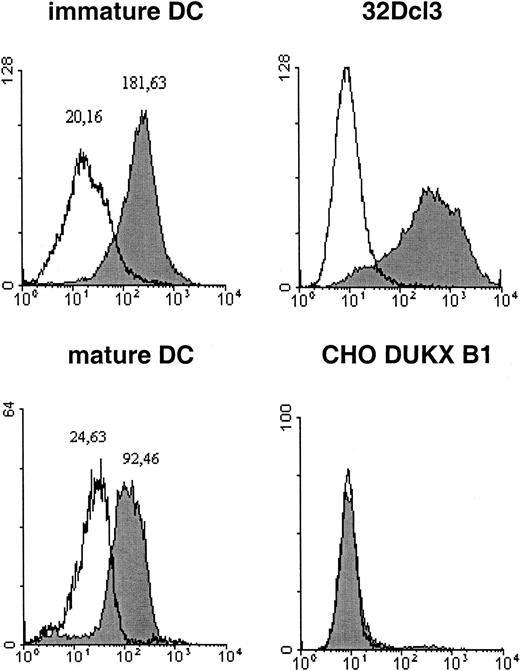
CLA expression on the surface of DCs.
Immature and mature DCs from cultures of aliquots from the same bone marrow preparation were incubated with identical amounts of mAb HECA452 and subjected to flow cytometry. The cell lines 32Dcl3 and CHO DUKX B1 served as positive and negative controls, respectively. The open region represents the IgM isotype control and the filled region the HECA452 signal. Flow cytometry was performed as described in “Materials and methods.” The calculated peak maxima are indicated as numbers above the peaks.
CLA expression on the surface of DCs.
Immature and mature DCs from cultures of aliquots from the same bone marrow preparation were incubated with identical amounts of mAb HECA452 and subjected to flow cytometry. The cell lines 32Dcl3 and CHO DUKX B1 served as positive and negative controls, respectively. The open region represents the IgM isotype control and the filled region the HECA452 signal. Flow cytometry was performed as described in “Materials and methods.” The calculated peak maxima are indicated as numbers above the peaks.
The expression of CLA and the selectin-binding form of PSGL-1 selectively on immature DCs prompted us to test whether these cells can bind to the endothelial selectins and whether PSGL-1 would be involved. Although one adhesion-blocking mAb against mouse PSGL-1 was already available (2PH1), we included the novel antibody 4RA10 in this analysis because the antibody blocks PSGL-1–mediated binding to P-selectin more efficiently (see below). In addition, we raised the nonblocking anti–PSGL-1 antibody 4RB12. As shown in Figure6A both antibodies as well as mAb 2PH1 immunoprecipitated PSGL-1 from surface biotinylated 32Dcl3 cells. Analyzing the epitopes of the 2 new antibodies on PSGL-1 revealed that the 4RA10 epitope, but not the 4RB12 epitope, was sensitive to treatment of 32Dcl3 cells with O-sialoglycoprotease (not shown). This suggests that the 4RB12 epitope is located more proximal to the transmembrane region of PSGL-1 than the 4RA10 epitope.
Comparison of mAbs 2PH1, 4RA10, and 4RB12 against PSGL-1.
(A) The total cell lysate from surface biotinylated 32Dcl3 cells was subjected to immunoprecipitations with the mAb 2PH1, 4RA10, and 4RB12 against mouse PSGL-1 (α-PSGL-1) as indicated. The mAb 2PH1 described by Borges et al19 served as control. The first lane (Co) is the negative control with a mAb against CD44. Precipitated antigens were blotted and the filter was probed with horseradish peroxidase–conjugated streptavidin. The 230/130-kd double band of PSGL-1 is indicated by arrows. Molecular weight markers (kd) are indicated on the left, and the front (F) of the gel is marked. (B) Inhibition of P-selectin–IgG binding to the mouse T-cell clone 4G3, as analyzed by flow cytometry. Cells were incubated either with P-selectin–IgG (P-Sel-IgG) or as negative control with human IgG (hIgG) and binding was detected with a fluorescence-labeled secondary antibody. To test the inhibitory activity of the anti–PSGL-1 antibodies, cells were first incubated with the anti–PSGL-1 mAb 4RA10, 2PH1, or 4RB12 (as indicated) and subsequently incubated with P-selectin–IgG fusion proteins (P-Sel-IgG). Binding of P-selectin–IgG was detected with a fluorescence-labeled secondary antibody and staining was determined by FACS analysis. For positive controls, P-selectin–IgG was analyzed without preincubation with an anti–PSGL-1 mAb; for negative controls, cells were incubated with human IgG (hIgG) and the secondary reagent.
Comparison of mAbs 2PH1, 4RA10, and 4RB12 against PSGL-1.
(A) The total cell lysate from surface biotinylated 32Dcl3 cells was subjected to immunoprecipitations with the mAb 2PH1, 4RA10, and 4RB12 against mouse PSGL-1 (α-PSGL-1) as indicated. The mAb 2PH1 described by Borges et al19 served as control. The first lane (Co) is the negative control with a mAb against CD44. Precipitated antigens were blotted and the filter was probed with horseradish peroxidase–conjugated streptavidin. The 230/130-kd double band of PSGL-1 is indicated by arrows. Molecular weight markers (kd) are indicated on the left, and the front (F) of the gel is marked. (B) Inhibition of P-selectin–IgG binding to the mouse T-cell clone 4G3, as analyzed by flow cytometry. Cells were incubated either with P-selectin–IgG (P-Sel-IgG) or as negative control with human IgG (hIgG) and binding was detected with a fluorescence-labeled secondary antibody. To test the inhibitory activity of the anti–PSGL-1 antibodies, cells were first incubated with the anti–PSGL-1 mAb 4RA10, 2PH1, or 4RB12 (as indicated) and subsequently incubated with P-selectin–IgG fusion proteins (P-Sel-IgG). Binding of P-selectin–IgG was detected with a fluorescence-labeled secondary antibody and staining was determined by FACS analysis. For positive controls, P-selectin–IgG was analyzed without preincubation with an anti–PSGL-1 mAb; for negative controls, cells were incubated with human IgG (hIgG) and the secondary reagent.
In agreement with this, we found that mAb 4RA10 recognizes the 19–amino acid peptide covering the N-terminus of the mature form of PSGL-1 (amino acids 42-60), whereas mAb 4RB12 did not recognize this epitope (not shown). The binding of P-selectin–IgG to the mouse T-cell clone 4G3 as analyzed by flow cytometry could be blocked by 99% (reduction of mean fluorescence intensity) with mAb 4RA10, whereas 2PH1 only reduced the binding of P-selectin–IgG by 80% and no reduction was observed with 4RB12 (Figure 6B).
We next performed static and dynamic adhesion assays with immature and mature DCs. As shown in Figure 7, immature, but not mature, DCs bound to immobilized P- and E-selectin–IgG under static conditions. The number of cells specifically bound was comparable to 32Dcl3 cells. Binding of DC to P-selectin–IgG was completely blocked by mAb 4RA10. As a negative control, mAb PS/2 did not block any cell binding to the selectins. We conclude that immature, but not mature, DCs are able to interact with each of the 2 endothelial selectins and that PSGL-1 is involved in this binding. To exclude that the binding of immature DCs to selectins was due to contaminating non-DC cell populations present in the 5-day cultures, the cultures were depleted for Lin+ cells by magnetic bead cell sorting. As shown in Figure8, removal of potential contaminants resulted in largely unaffected efficiency of cell binding in the cell adhesion assays.
Binding of dendritic cells to P- and E-selectin in a static adhesion assay.
Immature and mature DCs from Balb/c mice were allowed to bind to immobilized P-selectin–IgG (A) or E-selectin–IgG (B) in 96-well microtiter plates. The 32Dcl3 cells served as a positive control. Numbers on the y-axis refer to the number of bound cells/mm2 remaining in the wells after washing. For antibody-blocking studies, cells were preincubated either with 10 μg/mL 4RA10 (α-PSGL-1) or 10 μg/mL PS/2 against VLA-4 (control mAb). Human IgG refers to wells coated with plain human IgG1 as negative control.
Binding of dendritic cells to P- and E-selectin in a static adhesion assay.
Immature and mature DCs from Balb/c mice were allowed to bind to immobilized P-selectin–IgG (A) or E-selectin–IgG (B) in 96-well microtiter plates. The 32Dcl3 cells served as a positive control. Numbers on the y-axis refer to the number of bound cells/mm2 remaining in the wells after washing. For antibody-blocking studies, cells were preincubated either with 10 μg/mL 4RA10 (α-PSGL-1) or 10 μg/mL PS/2 against VLA-4 (control mAb). Human IgG refers to wells coated with plain human IgG1 as negative control.
Binding of lineage-depleted and nondepleted immature DCs to P- and E-selectin under static conditions.
DCs were derived from bone marrow of Balb/c mice that had been depleted (Lin− culture) or not depleted (bulk culture) of Lin+ cells. Cells cultured for 5 days in the presence of GM-CSF and IL-4 gave rise to immature DCs. Cells were allowed to bind to immobilized P-selectin–IgG (A) or E-selectin–IgG (B) in 96-well microtiter plates (as indicated). Numbers on the y-axis refer to the number of bound cells/mm2 remaining in the wells after washing. For antibody-blocking studies, cells were preincubated either with 10 μg/mL 4RA10 (α-PSGL-1) or 10 μg/mL PS/2 against VLA-4 (control mAb). Human IgG refers to wells coated with plain human IgG1 as negative control.
Binding of lineage-depleted and nondepleted immature DCs to P- and E-selectin under static conditions.
DCs were derived from bone marrow of Balb/c mice that had been depleted (Lin− culture) or not depleted (bulk culture) of Lin+ cells. Cells cultured for 5 days in the presence of GM-CSF and IL-4 gave rise to immature DCs. Cells were allowed to bind to immobilized P-selectin–IgG (A) or E-selectin–IgG (B) in 96-well microtiter plates (as indicated). Numbers on the y-axis refer to the number of bound cells/mm2 remaining in the wells after washing. For antibody-blocking studies, cells were preincubated either with 10 μg/mL 4RA10 (α-PSGL-1) or 10 μg/mL PS/2 against VLA-4 (control mAb). Human IgG refers to wells coated with plain human IgG1 as negative control.
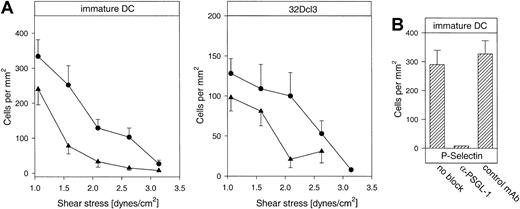
We also performed dynamic adhesion assays under flow, using a laminar flow chamber system with the selectin-IgG fusion proteins immobilized on glass coverslips.44 45 We found that immature DCs could interact with each of the 2 selectins under shear stress in the physiologic range (Figure 9A). The number of interacting cells decreased with increasing shear stress and was comparable to the number of interacting 32Dcl3 cells. Rolling of DCs was qualitatively similar to other cell types such as neutrophils. Again, the interaction with P-selectin was completely inhibited by the mAb 4RA10 (Figure 9B), demonstrating that also under shear stress PSGL-1 was the predominant P-selectin ligand on immature DCs. Similar to the results in the static adhesion assays, mature DCs did not interact with the immobilized selectin fusion proteins under flow conditions (not shown).
Rolling of immature DCs to P- and E-selectin in an adhesion assay under hydrodynamic flow.
(A) Immature DCs from Balb/c mice (left panel) and 32Dcl3 cells (right panel) were allowed to interact under flow with P-selectin–IgG (●) or E-selectin–IgG (▴) immobilized on glass coverslips that were inserted into a planar flow chamber with a variable flow rate of 1.0 to 3.2 dynes/cm2. (B) At a constant flow rate of 1 dyne/cm2, cells were either used directly (no block) or preincubated with 10 μg/mL of the blocking antibody 4RA10 against mouse PSGL-1 (α-PSGL-1) or 10 μg/mL of the mAb PS/2 against VLA-4 (control mAb). The depicted experiments represent 1 of 3 independent experiments with similar results.
Rolling of immature DCs to P- and E-selectin in an adhesion assay under hydrodynamic flow.
(A) Immature DCs from Balb/c mice (left panel) and 32Dcl3 cells (right panel) were allowed to interact under flow with P-selectin–IgG (●) or E-selectin–IgG (▴) immobilized on glass coverslips that were inserted into a planar flow chamber with a variable flow rate of 1.0 to 3.2 dynes/cm2. (B) At a constant flow rate of 1 dyne/cm2, cells were either used directly (no block) or preincubated with 10 μg/mL of the blocking antibody 4RA10 against mouse PSGL-1 (α-PSGL-1) or 10 μg/mL of the mAb PS/2 against VLA-4 (control mAb). The depicted experiments represent 1 of 3 independent experiments with similar results.
Blocking of the endothelial selectins or lack of fucosylated selectin ligands, but not selective blocking of PSGL-1 or lack of Fuc-TVII, reduces migration of DCs into inflamed skin
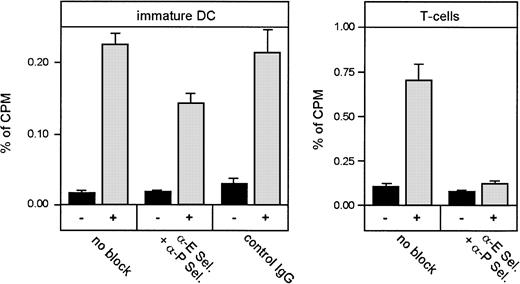
Because immature, but not mature, DCs clearly and specifically interacted with the endothelial selectins under physiologic shear conditions, we expected that these adhesion mechanisms would also be important for the migration of these cells into inflamed skin in the allergic contact dermatitis model presented above. Therefore, we tried to inhibit the migration of radioactively labeled, immature DCs into the inflamed skin of the ear by intravenous injection of a mixture of the adhesion-blocking antibodies UZ4 and RB40.34 against mouse E- and P-selectin, respectively. These mAbs have been shown to completely block the entry of Th-1 cells into inflamed skin in vivo.43 In control experiments we used in vivo–activated T cells isolated from lymph nodes of inflamed tissues that we labeled radioactively and injected into mice similarly to the immature DCs. As expected, we observed almost complete blockage of T-cell migration into the inflamed ear with antibodies against E- and P-selectin (Figure10). However, homing of immature DCs was only partially inhibited, suggesting that the endothelial selectins are clearly involved in this process, but are less essential than for T cells (Figure 10).
Blockade of selectin-mediated adhesion strongly affects homing of T cells, but only weakly inhibits homing of DCs, to inflamed skin.
Freshly isolated T cells from lymph nodes of oxazolone-sensitized donor mice and immature DCs were labeled with 51Cr and injected into the tail vein of sensitized and challenged recipient Balb/c mice. Some groups of mice were coinjected with antibodies against P-selectin (RB40.34, 400 μg/mouse) and E-selectin (UZ4, 400 μg/mouse) immediately before injecting the cells. After 6 hours, mice were killed and radioactivity that accumulated in ears, liver, lung, and spleen was measured in a γ counter. Radioactivity measured in noninflamed control ears (▪, −) or inflamed oxazolone-challenged ears (░, +) was calculated as percent of total radioactivity measured in all harvested organs (percent of cpm). Each value represents groups of 4 mice. Inhibition of homing due to blockade of selectins was statistically significant (DCs, P = .001; T cells,P < .0001).
Blockade of selectin-mediated adhesion strongly affects homing of T cells, but only weakly inhibits homing of DCs, to inflamed skin.
Freshly isolated T cells from lymph nodes of oxazolone-sensitized donor mice and immature DCs were labeled with 51Cr and injected into the tail vein of sensitized and challenged recipient Balb/c mice. Some groups of mice were coinjected with antibodies against P-selectin (RB40.34, 400 μg/mouse) and E-selectin (UZ4, 400 μg/mouse) immediately before injecting the cells. After 6 hours, mice were killed and radioactivity that accumulated in ears, liver, lung, and spleen was measured in a γ counter. Radioactivity measured in noninflamed control ears (▪, −) or inflamed oxazolone-challenged ears (░, +) was calculated as percent of total radioactivity measured in all harvested organs (percent of cpm). Each value represents groups of 4 mice. Inhibition of homing due to blockade of selectins was statistically significant (DCs, P = .001; T cells,P < .0001).
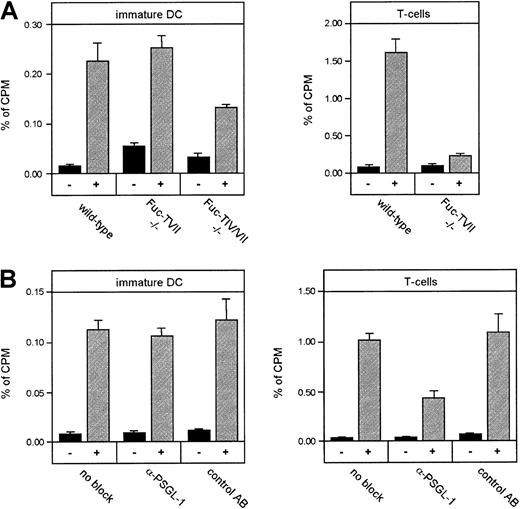
Next we tested the requirement of selectin ligands on DCs for their entry into inflamed tissue in the same experimental system. Because Fuc-TIV and Fuc-TVII are essential for the generation of ligands for each of the 3 selectins,24 25 we generated T cells and immature DCs from bone marrow cells of mice that were either deficient for the FUCTVII gene or for both, the genes ofFUCTIV and FUCTVII. Differentiation of DCs from bone marrow of these mice was largely normal, although in Fuc-TIV/VII double-deficient mice, fewer DCs could be generated and the cells had a slightly less differentiated surface phenotype. T cells isolated from lymph nodes of hapten-sensitized Fuc-TVII−/− donor mice showed almost complete inability to migrate into hapten-challenged skin when compared to T cells from wild-type donors (Figure 11A). Surprisingly, DCs from Fuc-TVII−/− mice were not impaired in their migration into inflamed ears. Interestingly, however, DCs generated from Fuc-TIV/VII double-deficient mice showed partial inhibition of their ability to home into inflamed skin (Figure 11A), a reduction that was in a similar range as the effect caused by simultaneously blocking both endothelial selectins. Thus, fucosylated ligands are involved in DC homing into inflamed skin, but in contrast to neutrophils and T cells, Fuc-TVII is not the major fucosyltransferase that is responsible for the generation of such ligands.
Fucosylated selectin ligands, but not PSGL-1 alone, partially contribute to homing of DCs to inflamed skin.
(A) Immature DCs from Fuc-TVII–deficient mice (Fuc-TVII−/−), Fuc-TIV/VII double-deficient mice (Fuc-TIV/VII−/−), and wild-type control mice, as well as T cells from same strains were compared for their ability to home into the inflamed ear. Inhibition of homing in Fuc-TVII−/−mice was statistically significant for T cells (P < .001), but not for DCs. Inhibition of homing in Fuc-TIV/VII−/− mice was statistically significant for DCs (P = .048). (B) Immature DCs or T cells from Balb/c mice were incubated without antibody (no block) or with 50 μg 4RA10 per mouse (α-PSGL-1), or of the non–adhesion-blocking mAb 4RB12 against PSGL-1 (control mAb) prior to injection. Otherwise, the experimental protocol was identical to that described in Figure 10. Inhibition of homing due to blockade of PSGL-1 was statistically significant for T cells (P < .01), but not for DCs.
Fucosylated selectin ligands, but not PSGL-1 alone, partially contribute to homing of DCs to inflamed skin.
(A) Immature DCs from Fuc-TVII–deficient mice (Fuc-TVII−/−), Fuc-TIV/VII double-deficient mice (Fuc-TIV/VII−/−), and wild-type control mice, as well as T cells from same strains were compared for their ability to home into the inflamed ear. Inhibition of homing in Fuc-TVII−/−mice was statistically significant for T cells (P < .001), but not for DCs. Inhibition of homing in Fuc-TIV/VII−/− mice was statistically significant for DCs (P = .048). (B) Immature DCs or T cells from Balb/c mice were incubated without antibody (no block) or with 50 μg 4RA10 per mouse (α-PSGL-1), or of the non–adhesion-blocking mAb 4RB12 against PSGL-1 (control mAb) prior to injection. Otherwise, the experimental protocol was identical to that described in Figure 10. Inhibition of homing due to blockade of PSGL-1 was statistically significant for T cells (P < .01), but not for DCs.
In agreement with this finding, although surprising in light of the in vitro adhesion results (Figures 7 and 8), we found that preincubation of DCs with the adhesion-blocking mAb 4RA10 against mouse PSGL-1 failed to inhibit the migration of immature DCs into the inflamed ear, whereas it profoundly affected homing of T cells in the same experimental setting (Figure 11B). Increasing the amount of antibody 3-fold gave similar results (not shown). The nonblocking mAb 4RB12 against PSGL-1 had no effect on T cell migration, verifying the specificity of the blocking effect of 4RA10 on T-cell homing.
Thus, the endothelial selectins and their ligands are involved in the homing of T cells as well as DCs into inflamed skin, but they vary in their importance for both processes. The endothelial selectins are only partially responsible for DC extravasation. In addition, the selectin ligands that are involved seem to be different from the ones on T cells. PSGL-1, although clearly a major ligand for P-selectin in in vitro adhesion assays, is not essential for DC extravasation in vivo. In agreement with this, Fuc-TVII, which plays a major role in generation of E- and P-selectin ligands in T cells and neutrophils and which is the essential enzyme for the formation of the selectin-binding glycoform of PSGL-1 in neutrophils,46 is dispensable for DC homing into inflamed skin. Instead, the selectin ligands on DCs involved in this process depend on the expression of Fuc-TIV, a minor component in the generation of selectin ligands on T cells and neutrophils.
Discussion
In this study, we have shown that immature DCs can be recruited into inflamed areas of murine skin. Because isolation of DCs from the blood is not feasible in the mouse, we used a well-established culture system35,36 to generate DCs of different stages of maturity for our study. By analyzing the migration of radioactively labeled DCs into sites of allergic contact dermatitis, we could quantitatively compare the homing efficiency of DCs with that of activated T cells. Although recruitment of DCs was less efficient than that of activated T cells, several controls demonstrated that the accumulation of DCs was specific. First, about 10 times more DCs were recruited to the inflamed than to the noninflamed ear. Second, only immature, but not mature DCs (resulting from the same DC cultures) homed to inflamed skin. Third, PFA-fixed immature DCs did not accumulate in the inflamed ear, demonstrating that this process required the active participation of the recruited cells. Fourth, accumulated radioactivity persisted for at least 48 hours and was not affected by steroid-induced resolution of cutaneous inflammation, arguing against the possibility that the DCs were simply trapped within inflamed blood vessels without actually transmigrating into tissue. In addition, transmigration of calcein-labeled DCs from blood into inflamed skin was demonstrated by confocal microscopy in a previous study.31 Fifth, by flow cytometry, all DC cultures were virtually devoid of T cells, B cells, and granulocytes, suggesting that contaminating non-DCs were not responsible for the selective accumulation of radioactively labeled cells at the inflamed tissue site. To further exclude this possibility, we depleted the bone marrow cultures of differentiated T cells, B cells, granulocytes, and macrophages. These Lin− DC cultures showed the same homing capacity to inflamed skin. Sixth, an immature DC cell line, XS52, also exhibited selective homing capacity (Figure 2). Thus, these results demonstrate that immature DC can be actively recruited into inflamed skin during allergic contact dermatitis.
In earlier studies, we found that human DCs can bind to E- and P-selectin.31 Moreover, by intravital microscopy, we observed that human DCs roll in postcapillary venules of mouse ears.31 This prompted us to now analyze whether this is also the case for mouse DCs and whether binding was dependent on the differentiation state of these cells. We now demonstrate binding of immature, but not of mature, DCs to immobilized recombinant forms of the selectins in vitro under static assay conditions and under physiologic conditions of flow. This confirms that differentiation of mouse bone marrow cells in the presence of GM-CSF and IL-4 leads to the induction of E- and P-selectin ligands on the differentiating DCs. Expression of these ligands, but not of PSGL-1, is transient and vanishes when cells reach the mature phenotype.
It has been shown previously that human DCs can roll along the surface of mouse blood vessels and that this rolling depends on E- and P-selectin.31 However, it was not analyzed whether this interaction was indeed responsible for the extravasation of DCs. Here we show that the combined administration of adhesion-blocking antibodies against E- and P-selectin inhibited the entry of immature mouse DCs into inflamed skin, although inhibition was only partial. The same batches of antibodies were able to completely abolish the recruitment of hapten-specific T cells into inflamed skin, which rules out that the amount or quality of the antibodies might not have been sufficient to block the function of the endothelial selectins. We conclude that the endothelial selectins are indeed involved in the migration of immature mouse DCs into inflamed skin, but that additional mechanisms enable these cells to extravasate independently of the endothelial selectins. One obvious alternative candidate would be the integrin VLA-4 that is expressed on these cells (Figure 1) and that has been reported to also support leukocyte extravasation47 and rolling.48 Furthermore, it is conceivable that DCs, due to their large size, might already be in sufficiently tight contact with the blood vessel wall when they exit from the capillary system so that they are less dependent on selectin-mediated rolling to overcome the endothelial barrier. Yet another possibility is that DCs make use of adhesion mechanisms that are not found on other leukocytes. Recently, a novel lectin called DC-SIGN was identified on human DCs that mediates the binding of these cells to ICAM-2 and -3 on T cells and that is essential for DC-induced T-cell proliferation.49
Thus, novel adhesion mechanisms could exist on DCs, which might assist or even dominate some of the known ones. In this context it is interesting that we recently found that monocytes and DCs are still able to extravasate into tissues in CD18 (β2integrin)–deficient mice, whereas the extravasation capacity of T cells and neutrophils was severely impaired (S.G., manuscript in preparation).
Further differences between the molecular mechanisms involved in the extravasation of DCs on the one hand and T cells on the other hand were found on the level of the selectin ligands. Blocking of PSGL-1 with antibodies did not inhibit extravasation of DCs although it blocked extravasation of T cells almost completely. This was especially surprising because our in vitro studies showed that PSGL-1 was the major P-selectin ligand on immature DCs in static as well as in adhesion assays under flow (1 dyne/cm2). A possible explanation for this apparent discrepancy would be that, as stated above, additional adhesion mechanisms or the large size of immature DCs might enable them to be in sufficiently tight contact with the blood vessel wall when they exit from the capillary system that they are less dependent on a highly effective capturing molecule such as PSGL-1 and that other normally less effective selectin ligand molecules might compensate for a lack of PSGL-1. In agreement with this interpretation, we found that DC generated from Fuc-TVII−/− bone marrow cells extravasated at completely normal levels, although lack of this enzyme in T cells and neutrophils usually leads to dramatic inhibition of the extravasation of T cells and neutrophils in various inflammation and homing models.24,25,46 We found recently that Fuc-TVII is the major fucosyltransferase responsible for the generation of the adhesion mediating glycoform of PSGL-1 in mouse neutrophils.46 Thus, the lack of necessity of PSGL-1 is in agreement with the lack of necessity of Fuc-TVII for DC extravasation. In preliminary studies, DCs from Fuc-TIV single-deficient mice also showed no abnormalities in their capacity to emigrate into inflamed skin (data not shown). Only if the immature DCs were also deficient in the second fucosyltransferase in leukocytes, Fuc-TIV, was extravasation inhibited in a similar range as we had observed when E- and P-selectin were simultaneously blocked. Again, this is in contrast to what has been found for T cells and neutrophils where the additional loss of Fuc-TIV causes only minor additional inhibitory effects on extravasation when compared with the single deficiency of Fuc-TVII. Thus, indeed, the selectin ligands required for the extravasation of DCs differ from those on T cells and neutrophils. Fuc-TIV is clearly relevant for the generation of selectin ligands involved in DC extravasation, whereas it plays only a minor role for T cells and neutrophils.
The generation of carbohydrate epitopes on mouse DCs related to selectin functions seems to differ from such epitopes on other mouse leukocytes in yet another aspect. The carbohydrate epitope CLA is clearly transiently induced on the surface of DC during the course of differentiation. This is remarkable because neither primary isolated leukocytes from mouse bone marrow nor any major lineage population of leukocytes from mouse peripheral blood express the carbohydrate epitope CLA, which is known to be expressed on E-selectin–binding human leukocytes. The only other mouse leukocytes known to express CLA on their surface are the myeloid cell line 32Dcl3 (this report) and the mouse CD8+ T-cell clone 4G3 on antigen-specific activation.30 The structure of this carbohydrate epitope is not known in all detail; however, it is likely that it is a derivative of the tetrasaccharide sLex. It is generally assumed that mouse leukocytes express carbohydrate epitopes that somehow resemble sLex and bind to the selectins, but that are derived in a way that prevents recognition by the known anti-sLex antibodies.14 Our results suggest that enzymes that affect antibody recognition of sLex-like structures on these cells seem to be regulated during the course of DC differentiation in the mouse.
We demonstrate here that immature, but not mature, DCs are actively recruited from blood to inflamed tissues, which might constitute a possible mechanism to provide potent APCs for presentation of antigen to effector T cells within inflamed tissues. This recruitment of APCs to inflamed tissue would allow for a sustained inflammatory response in the case of persisting antigenic challenge. Moreover, this mechanism would ensure a continuous flow of antigen-loaded DCs to regional lymph nodes, resulting in prolonged priming of naive T cells during chronic inflammation. Although much less efficient, the time course of DC recruitment is comparable to that of T cells,43 starting as early as 2 hours after hapten application, which is before dermatitis becomes visible. It will be interesting to test whether recruitment of APCs is not only involved in the maintenance but also in the initiation of the inflammatory tissue response. In addition it will be important to further elucidate the molecular mechanisms that allow immature DCs to enter sites of inflammation.
We thank Dr Louis Picker for donating the mAb HECA452 and Dr Rupert Hallmann for the mAB UZ4.
Supported in part by grants to D.V. (SFB 293/A1) and S.G. (SFB293/B1, Gr1022/3-3, Gr1022/7-1) from the Deutsche Forschungsgemeinschaft. J.B.L. is an Investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dietmar Vestweber, Institute of Cell Biology, ZMBE, and Stephan Grabbe, Department of Dermatology, University of Münster, Von-Esmarch Str 56, D-48149 Münster, Germany; e-mail: vestweb@uni-muenster.de and grabbe@uni-muenster.de.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal