Abstract
Previously, it was reported that homocysteine (Hcy) specifically inhibits the growth of endothelial cells (ECs), suppresses Ras/mitogen-activated protein (MAP) signaling, and arrests cell growth at the G1/S transition of the cell cycle. The present study investigated the molecular mechanisms underlying this cell-cycle effect. Results showed that clinically relevant concentrations (50 μM) of Hcy significantly inhibited the expression of cyclin A messenger RNA (mRNA) in ECs in a dose- and time-dependent manner. G1/S-associated molecules that might account for this block were not changed, because Hcy did not affect mRNA and protein expression of cyclin D1 and cyclin E. Cyclin D1- and E-associated kinase activities were unchanged. In contrast, cyclin A–associated kinase activity and CDK2 kinase activity were markedly suppressed. Nuclear run-on assay demonstrated that Hcy decreased the transcription rate of the cyclin A gene but had no effect on the half-life of cyclin A mRNA. In transient transfection experiments, Hcy significantly inhibited cyclin A promoter activity in endothelial cells, but not in vascular smooth muscle cells. Finally, adenovirus-transduced cyclin A expression restored EC growth inhibition and overcame the S phase block imposed by Hcy. Taken together, these findings indicate that cyclin A is a critical functional target of Hcy-mediated EC growth inhibition.
Introduction
Epidemiologic and case control studies have consistently indicated that moderate and mild elevations of plasma homocysteine (Hcy), an intermediate metabolite of methionine, are an important and independent risk factor for arteriosclerosis and venous thrombosis in the general population.1-6 The level of risk for cardiovascular disease attributable to hyperhomocysteinemia is equivalent to that associated with smoking or hypercholesterolemia, and elevated Hcy levels synergistically increase the risk associated with smoking and hypertension.4 Recently, studies in animal models have revealed that hyperhomocysteinemia enhances atherosclerotic lesion formation and neointimal hyperplasia following arterial injury.7,8 Although experimental evidence has demonstrated that Hcy causes endothelial dysfunction,9-13thrombosis activation,14-19 and vascular smooth muscle cell proliferation,20-23 the fundamental biochemical mechanisms underlying these phenomena are not well understood.
In vitro studies found that some biologic effects of Hcy can be mimicked by hydrogen peroxide or other sulfhydryl-containing agents, are inhibited by catalase, and require oxygen.9,24 These results have provided support to the proposal that increased oxidation mediated through the sulfhydryl group of Hcy is a major mechanism responsible for Hcy-induced vascular pathogenesis.25-27However, these effects typically require relatively high concentrations of Hcy, above the levels that confer increased cardiovascular risk. In addition, another intermediary metabolite of methionine, cysteine (Cys), has similarities in chemical structure and redox properties with Hcy, but does not constitute a risk factor for cardiovascular disease, suggesting that these characteristics alone are not sufficient to explain the deleterious effects of Hcy.
Because the increased risk of cardiovascular disease associated with hyperhomocysteinemia occurs at mild and moderate elevations of Hcy, we have sought to identify biologic effects on vascular cells that occur at clinically observable concentrations of Hcy, and that are not produced by Cys. Along these lines, we previously found that endothelial cell (EC) growth is inhibited by Hcy at concentrations between 10 to 50 μM, with cell-cycle arrest occurring at the G1/S transition. This effect was cell-type specific and was not mimicked by Cys.28 We also found an accumulation of S-adenosylhomocysteine (SAH) in ECs that is associated with a decrease in Ras protein methylation and inhibition of the Ras/mitogen-activated protein (MAP) signaling pathway. Our findings are consistent with a hypomethylation mechanism, in which elevated levels of Hcy lead to increased SAH, which in combination with adenosine, a nucleic acid metabolic product, act to inhibit cellular methylation. Clinical evidence in support of a mechanism involving SAH and decreased methylation is available; elevated Hcy levels have been linked with increased SAH in adult women,29 and increased SAH has been reported in hyperhomocysteinemic uremic patients in association with a significant reduction in red cell membrane protein methylation levels.30-32
To understand better the mechanism of EC growth inhibition by Hcy, we characterized the effects of Hcy on the expression and activity of molecules controlling cell-cycle progression. We now report that clinically relevant concentrations of Hcy (25-50 μM) selectively inhibited cyclin A gene expression in EC. This effect on cyclin A expression resulted from decreased transcription of the cyclin A gene, which occurred in a cell-type specific manner. The inhibition of cyclin A gene expression correlated with its growth inhibiting and G1/S blocking effects. In addition, we found that overexpression of cyclin A was sufficient to overcome the G1/S phase block caused by Hcy. These results indicate that down-regulation of cyclin A expression in ECs is an important consequence of exposure to Hcy, and point to signaling mechanisms that specifically converge on the cyclin A promoter as targets for Hcy.
Materials and methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) (Clonetics, Walkersville, MD) and rat aortic smooth muscle cells (RASMCs) were cultured as previously described.28 Cells from passages 6 to 8 were used in the experiments. Adenosine (25 μM, Sigma, St Louis, MO) and 10 μM erythro-9-(2-hydroxy-3-nonyl)-adenine (Sigma, RBI), an adenosine deaminase inhibitor to stabilize adenosine, were added in all the experiments as control medium. The addition of adenosine and erythro-9-(2-hydroxy-3-nonyl)-adenine served to enhance the conversion of Hcy to SAH.
[3H]-thymidine incorporation
Northern blot analysis
Total RNA was obtained from cultured cells and 10 μg RNA from each sample was analyzed as described.23 28 Radioactivity was measured on a PhosphorImager running the ImageQuant software (Molecular Dynamics, Sunnyvale, CA). The filters were rehybridized with different probes and finally with an oligonucleotide probe complementary to 18S ribosomal RNA to correct for loading differences.
Western blot analysis
Whole-cell extracts were prepared from HUVECs and 50 μg protein from each sample was analyzed with antibodies (all from Santa Cruz Biotechnology, Santa Cruz, CA) against cyclin A (SC 751), cyclin D1 (SC 246), cyclin E (SC 481), or CDK2 (SC 163), respectively. Protein extraction and probing procedures were as described.28
Immunoprecipitation and histone H1/retinoblastoma protein kinase assay
The HUVECs were harvested for histone H1 or retinoblastoma (RB) protein kinase assay as described.23 Total protein (50 μg) from each sample was immunoprecipitated with a 1:100 dilution of the same antibodies used for Western blot. The immunoprecipitated complexes were reacted with γ-32P]-adenosine triphosphate (ATP; 6000 cpm/pmol; 100 mM) and desired substrate, histone H1 (Sigma; 0.5 mg/mL) or glutathione-S-transferase (GST)–RB protein (Santa Cruz, SC 4112) for 20 minutes at 30°C. Kinase activity was detected by autoradiography and PhosphorImaging after electrophoresis on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels.
Nuclear run-on analysis
The HUVECs were lysed and nuclei were isolated as described.33 The 32P-labeled RNA probes were prepared and hybridized at 40°C for 4 days with identical dot blots prepared by immobilizing denatured cyclin A and β-actin complementary DNA (cDNA; 1 μg) on nitrocellulose filters as described.23 The filters were then autoradiographed with x-ray film for 3 days.
Transient transfection and luciferase assay
The HUVECs or RASMCs were plated in 6-well culture plates (3 × 105/well), grown to 70% to 80% confluence, and transfected with lipofectin (Gibco BRL, Gaithersburg, MD).34 PGL2 plasmids (Promega, Madison, WI) containing SV40 early promoter or cyclin A promoter (−266/+205)35,36 and the luciferase reporter gene were used. Lipofectin (10 μL for HUVECs or 15 μL for RASMCs) diluted with 90 μL Opti minimum essential medium (MEM) was mixed with 2 μg plasmid DNA diluted in 100 μL Opti MEM, and incubated for 1 hour at room temperature. The lipofectin/DNA mix was overlaid onto cells freshly washed with 3 mL M199 medium. The transfected cells were then incubated for 6 hours and changed to fresh culture medium for another 10 to 12 hours for recovery. The cells were changed to fresh control medium and exposed to 50 μM dl-Hcy for 24 hours. To correct for variability in transfection efficiency, we cotransfected 0.5 μg plasmid cytomegalovirus (pCMV)–βGAL expressing β-gal (β-gal) in all experiments. The luciferase and β-gal activity were performed as described35 36 and the ratio of luciferase activity to β-gal activity in each transfection served as a measure of normalized luciferase activity. Comparisons were made by applying a factorial ANOVA followed by the Student ttest.
Construction of recombinant adenovirus and adenovirus infection
Replication-defective adenovirus expressing β-gal in pACCMVplpa vector and plasmid DNAs of this vector were obtained from Dr Perry Nisen (Abbott Laboratories, North Chicago, IL).37,38A human cyclin A cDNA (gift from Dr James Roberts at Fred Hutchinson Cancer Research Center, Seattle, WA) was inserted into the pACCMVplpa vector. The resulting plasmid was cotransfected with the pJM17 plasmid into 293 cells (adenovirus E1a-transformed human embryonic kidney cells) by calcium phosphate/DNA coprecipitation. Homologous recombination between the 2 plasmids resulted in a recombinant replication-defective virus containing the human cyclin A gene. Virus from cytopathic 293 cells were harvested after 48 hours of virus infection, collected by 3 freeze-thaw cycles followed by centrifugation, and purified by gradient cesium chloride (62%, 36%, and 51%) centrifugation at 35 000g for 2 hours. Viral titers were determined by plaque assay on 293 cells as described.39 HUVECs at 50% confluence were infected with purified adenovirus at indicated multiplicity of infection (MOI) (plaque-forming unit [PFU]/cell). Infected HUVECs were treated with or without Hcy after 24 hours of virus infection. Western blotting (with anticyclin A antibody) was carried out to examine ectopic gene expression.
β-Gal staining
Treated cells were washed with phosphate-buffered saline (PBS) plus 2 mM MgCl2, fixed with 0.2% glutaraldehyde for 5 minutes, and stained with 1 mg/mL X-gal for 10 minutes after 2 additional washes.
Flow cytometric analysis
The HUVECs were infected with indicated adenovirus for 24 hours and then exposed to 50 μM dl-Hcy for an additional 24 hours. The cells were harvested, stained with propidium iodide (50 μg/mL), and subjected to fluorescence-activated cell sorting analysis of DNA content on an EPICS XL-MCL Flow Cytometer (Beckman Coulter, Miami, FL) as described.28 Cell-cycle distribution was analyzed using a Multicycle software (Phoenix Flow Systems, San Diego, CA).
Results
Hcy inhibits DNA synthesis in human ECs and this inhibition is reversible
We have previously demonstrated that pathophysiologically relevant concentrations of Hcy specifically inhibit growth and Ras/MAP signaling, and cause cell-cycle arrest near the G1/S transition in ECs.28 In the present study, we investigated the molecular mechanisms underlying this cell-cycle effect. To evaluate possible cellular damage caused by Hcy, we first determined if this growth inhibition is reversible. HUVECs were grown to subconfluency and treated with 50 μM of dl-Hcy for 24 hours. Then, 50 μM Hcy, which is similar to the plasma level observed in moderate hyperhomocysteinemia, resulted in a 75% decrease in [3H]-thymidine incorporation (Figure1). After 24 hours, Hcy was washed out with PBS and HUVECs were cultured in control medium for an additional 48 hours. DNA synthesis increased to 30% and 100% of control after 24 hours and 48 hours of recovery, respectively (Figure 1). Thus, the inhibitory effect of Hcy on DNA synthesis in HUVECs appears to be reversible.
The inhibitory effect of Hcy on DNA synthesis is reversible in ECs.
HUVECs were exposed to dl-Hcy for 24 hours, then washed with PBS, and cultured with fresh control medium for the time indicated. [3H]-thymidine incorporation was measured at indicated times. The value of no Hcy treatment control for each time point was set as 100%, and [3H]-thymidine incorporation was divided by the control value. Values represent the mean ± SD of 3 separate experiments (n = 9). *P < .01 versus 0 hour sample in control medium after Hcy treatment.
The inhibitory effect of Hcy on DNA synthesis is reversible in ECs.
HUVECs were exposed to dl-Hcy for 24 hours, then washed with PBS, and cultured with fresh control medium for the time indicated. [3H]-thymidine incorporation was measured at indicated times. The value of no Hcy treatment control for each time point was set as 100%, and [3H]-thymidine incorporation was divided by the control value. Values represent the mean ± SD of 3 separate experiments (n = 9). *P < .01 versus 0 hour sample in control medium after Hcy treatment.
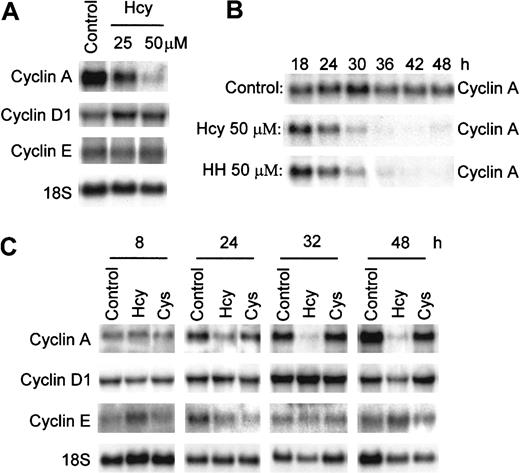
Hcy selectively reduces cyclin A mRNA in ECs
We have reported that Hcy arrests the cell cycle at G1/S transition in ECs.28 Cyclins D, E, and A are key cyclins regulating the G1 and G1/S transition of the cell cycle. To understand how Hcy affects regulation of the cell cycle, we examined the mRNA expression of cyclins D1, E, and A in HUVECs in response to Hcy. RNA was harvested from HUVECs treated with 25 or 50 μM of Hcy for 30 hours and analyzed by Northern blotting (Figure 2A). The cyclin A message decreased by 20% with 25 μM Hcy and by 85% with 50 μM, indicating a dose-sensitive response, whereas cyclin D1 and E mRNA levels were not affected (Figure 2A). Cyclins D2 and D3 are expressed at very low levels relative to cyclin D1 and are not major D cyclins in HUVECs (data not shown). To characterize the time course of this effect, we then treated HUVECs with 50 μM Hcy or Cys for 8, 24, 32, and 48 hours and measured the mRNA expression of cyclins A, D1, and E. Cys was used as a sulfhydryl amino acid control. The reduction in cyclin A message started as early as 24 hours, with cyclin A mRNA decreased to 12% of control at 32 hours and 5% of control at 48 hours after Hcy exposure (Figure 2C). Cyclin D1 and cyclin E messages remained unchanged, whereas Cys did not affect mRNA expression of cyclin A and had only minor effects on cyclin D1 and cyclin E mRNA levels (Figure 2C). Therefore, Hcy selectively decreases cyclin A mRNA levels in a dose- and time-dependent manner in ECs. Because most of the homocysteine in the blood is in the oxidized form, such as homocystine (HH), we also examined the effects of HH on cyclin A mRNA expression. HH, at 50 μM, markedly inhibited cyclin A mRNA expression in an identical time-dependent fashion as Hcy in ECs after 18, 24, 30, 36, 42, and 48 hours of exposure (Figure 2B), indicating that the Hcy-mediated reduction in cyclin A expression is pathophysiologically relevant and is independent of the free sulfhydryl group.
Suppression of cyclin A, but not cyclin D1 and cyclin E mRNA expression by Hcy.
HUVECs were exposed to 25 μM or 50 μM dl-Hcy for 30 hours (A), to 50 μM dl-Hcy or l-HH (B), and to 50 μM dl-Hcy or l-Cys (C) for the indicated times. Total cellular RNA (10 μg) was used for Northern blot analysis. The RNA blot was hybridized to human cyclin A, D1, and E probes successively, and then to an 18S oligonucleotide probe (n = 3).
Suppression of cyclin A, but not cyclin D1 and cyclin E mRNA expression by Hcy.
HUVECs were exposed to 25 μM or 50 μM dl-Hcy for 30 hours (A), to 50 μM dl-Hcy or l-HH (B), and to 50 μM dl-Hcy or l-Cys (C) for the indicated times. Total cellular RNA (10 μg) was used for Northern blot analysis. The RNA blot was hybridized to human cyclin A, D1, and E probes successively, and then to an 18S oligonucleotide probe (n = 3).
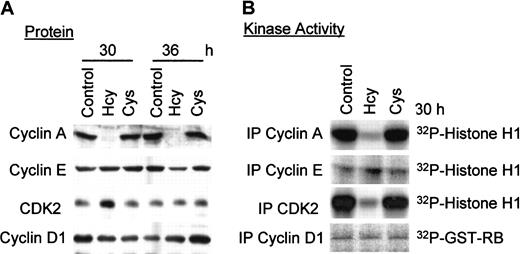
Hcy decreases cyclin A protein expression and cyclin A–associated kinase activity in ECs
Cyclin A protein regulates cell-cycle progression during S phase in association with protein kinase CDK2. To assess whether Hcy-induced decreases in cyclin A mRNA levels were reflected in decreases in protein levels and associated kinase activity, we performed Western blot analysis and kinase activity assays. Protein lysates from HUVECs were divided, with half subjected to immunoprecipitation with the indicated antibodies for kinase activity assay, and the second half used for Western blot analysis. Cyclin A protein expression was barely detectable at 30 or 36 hours after 50 μM Hcy addition (Figure3); this was accompanied by similar reductions in cyclin A–associated kinase activity. In comparison, Cys had no effect on cyclin A protein expression and its associated kinase activity, suggesting once again that this is a free sulfhydryl group–independent effect. CDK2 protein expression was not altered by Hcy, but its net activity was greatly reduced, consistent with the decrease in expression of its positive regulator, cyclin A. Cyclin D1 and cyclin E protein expression and associated kinase activities were unchanged (Figure 3), consistent with the mRNA results in Figure 2. Both cyclins E and A regulate CDK2 activity during G1 and at the G1/S transition, respectively; the lack of change in cyclin E levels suggests that the decrease in CDK2 activity in response to Hcy is most likely due to cyclin A inhibition. Thus, in ECs, Hcy selectively inhibits cyclin A gene expression at the mRNA and protein levels and decreases its associated kinase activity.
Effect of Hcy on the protein expression and kinase activity of G1/S cyclins and CDK2.
HUVECs were exposed to 50 μg dl-Hcy or l-Cys for the indicated times. Proteins (50 μg) were analyzed by Western blotting with antibodies against cyclins A, D1, E, or CDK2 respectively (A), and by immunoprecipitation with the same antibody for associated kinase or kinase activity (B). Histone H1 (200 μg/mL) was used as phosphorylation substrate for cyclin A, E, or CDK2. GST-RB (40 μg/mL) was used as phosphorylation substrate for cyclin D1.
Effect of Hcy on the protein expression and kinase activity of G1/S cyclins and CDK2.
HUVECs were exposed to 50 μg dl-Hcy or l-Cys for the indicated times. Proteins (50 μg) were analyzed by Western blotting with antibodies against cyclins A, D1, E, or CDK2 respectively (A), and by immunoprecipitation with the same antibody for associated kinase or kinase activity (B). Histone H1 (200 μg/mL) was used as phosphorylation substrate for cyclin A, E, or CDK2. GST-RB (40 μg/mL) was used as phosphorylation substrate for cyclin D1.
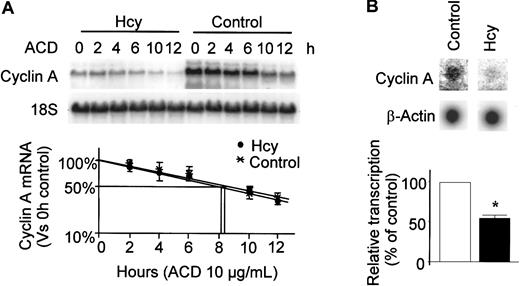
Hcy decreases the transcription of the cyclin A gene and does not change mRNA stability
The selective decrease in cyclin A expression suggests that Hcy specifically affects the production or degradation of cyclin A mRNA. To determine which mechanism is involved, we first measured the half-life of cyclin A mRNA in the presence and absence of Hcy. After 24 hours (t = 0) of Hcy (50 μM) treatment, actinomycin D was added to stop transcription. RNA was extracted at different time points and Northern blot analysis was performed to measure the rate of mRNA degradation (Figure 4A). Consistent with our previous findings in Figure 2, cyclin A mRNA levels were substantially reduced by this concentration of Hcy. In control cells, the calculated half-life for cyclin A mRNA was approximately 8.1 hours, whereas in cells treated with Hcy, the half-life was 8.3 hours. Thus, the decreases in cyclin A mRNA levels by Hcy was not mediated by a decrease in the stability of cyclin A mRNA.
Effects of Hcy on half-life of cyclin A mRNA and gene transcription.
(A) mRNA half-life; HUVECs were exposed to dl-Hcy for 24 hours followed by administration of actinomycin D (ACD; 10 μg/mL; t = 0) to stop transcription. Total cellular RNA was extracted at indicated times and used for Northern analysis. The corrected signal density was plotted as a percentage of 0 hour value against time in log scale. The values represent the means ± SD from 3 independent experiments (n = 3). (B) Nuclear run-on experiment; HUVECs were exposed to dl-Hcy for 30 hours. Equal amounts of32P-labeled, in vitro–transcribed RNA probes from each group were hybridized to 1 μg denatured cyclin A and β-actin cDNA that had been immobilized on the nitrocellulose filters. The values represent the means ± SD from 3 independent experiments (n = 3). *P < .01 versus control.
Effects of Hcy on half-life of cyclin A mRNA and gene transcription.
(A) mRNA half-life; HUVECs were exposed to dl-Hcy for 24 hours followed by administration of actinomycin D (ACD; 10 μg/mL; t = 0) to stop transcription. Total cellular RNA was extracted at indicated times and used for Northern analysis. The corrected signal density was plotted as a percentage of 0 hour value against time in log scale. The values represent the means ± SD from 3 independent experiments (n = 3). (B) Nuclear run-on experiment; HUVECs were exposed to dl-Hcy for 30 hours. Equal amounts of32P-labeled, in vitro–transcribed RNA probes from each group were hybridized to 1 μg denatured cyclin A and β-actin cDNA that had been immobilized on the nitrocellulose filters. The values represent the means ± SD from 3 independent experiments (n = 3). *P < .01 versus control.
We then evaluated the rate of cyclin A gene transcription by nuclear run-on assay. Hcy had no effect on β-actin transcription, but decreased the rate of cyclin A gene transcription by about 50% (Figure 4B). Taken together, these data indicate that Hcy-induced decreases of cyclin A mRNA levels result from a decrease in cyclin A gene transcription.
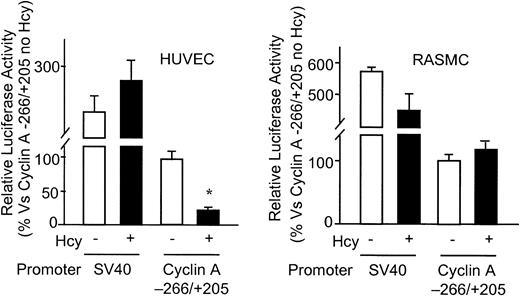
Hcy inhibits cyclin A promoter activity specifically in ECs
Further evidence for Hcy-induced cyclin A transcriptional inhibition was obtained by promoter-reporter gene transient transfection experiments. Hcy (50 μM) significantly inhibited cyclin A promoter activity, but had no effect on that of the control SV40 promoter (PGL2-control) in ECs (Figure5A). This finding is consistent with our earlier experiments demonstrating that Hcy decreases the transcription rate of the cyclin A gene.
Effect of Hcy on cyclin A promoter activity in vascular cells.
HUVECs and RASMCs were transfected with 2 μg PGL2 plasmids containing SV40 early promoter or cyclin A promoter (−266/+205) by lipofectin transfection. Starting 24 hours after transfection, cells were treated with or without 50 μM dl-Hcy, and harvested 24 hours later. The corrected luciferase activity for each time point was divided by that of control (cyclin A −266/+205 plasmid in cells not treated with Hcy) and is presented as relative luciferase activity. Values represent the mean ± SD from 2 separate experiments (n = 6). *P < .01 versus control.
Effect of Hcy on cyclin A promoter activity in vascular cells.
HUVECs and RASMCs were transfected with 2 μg PGL2 plasmids containing SV40 early promoter or cyclin A promoter (−266/+205) by lipofectin transfection. Starting 24 hours after transfection, cells were treated with or without 50 μM dl-Hcy, and harvested 24 hours later. The corrected luciferase activity for each time point was divided by that of control (cyclin A −266/+205 plasmid in cells not treated with Hcy) and is presented as relative luciferase activity. Values represent the mean ± SD from 2 separate experiments (n = 6). *P < .01 versus control.
We previously found that clinically relevant concentrations of Hcy inhibit EC growth, but not that of other vascular cell types.28 In this study, we were interested in determining whether the inhibitory effect of Hcy on the cyclin A promoter is also cell-type specific. As shown in Figure 5B, activity of the cyclin A promoter transfected into RASMCs was not affected by Hcy. This result suggests that regulation of cyclin A transcription in ECs may be an important mechanism by which Hcy is able to selectively inhibit EC growth.
Adenovirus-transduced cyclin A expression restores DNA synthesis and cell-cycle progression in Hcy-treated HUVECs
To test the possibility that decreased cyclin A transcription was responsible for EC growth arrest, we used a heterologous promoter not inhibited by Hcy treatment to drive cyclin A transcription and then assessed the ability of ECs to enter S phase and synthesize DNA. HUVECs were transduced with the cyclin A adenovirus (Ad cyclin A) for 24 hours, and then treated with 50 μM of Hcy for an additional 24 hours. As shown in Figure 6A, compared to cells infected with a control adenovirus vector (Ad vector), Ad cyclin A yielded a robust, dose-dependent increase in cyclin A protein expression in HUVECs. Hcy did not affect this expression. As expected, endogenous cyclin A protein expression was suppressed in cells without adenovirus infection, and is comparable with that in cells infected with Ad vector. Thus, the control adenovirus vector does not appear to interfere with endogenous cyclin A gene expression in ECs.
The effect of adenovirus-transduced cyclin A gene on DNA synthesis in Hcy-treated HUVECs.
HUVECs were infected with control adenovirus (Ad vector), adenovirus expressing cyclin A (Ad cyclin A) or adenovirus expressing β-gal (Ad β-gal) at the indicated MOI for 24 hours before being challenged with Hcy. Cells were then treated with or without 50 μM ofdl-Hcy in control medium for another 24 hours. (A) Western analysis; total protein (50 μg) from each sample was analyzed with antibodies against cyclin A. (B) Staining for β-gal; cells were infected with Ad β-gal at the indicated MOI and stained for β-gal. Photograph was taken with × 100 magnification. (C) Thymidine uptake; cells treated for 24 hours were metabolically labeled with 1 μCi (0.037 MBq)/mL [3H]-thymidine during the last 3 hours. [3H]-thymidine incorporation was measured in a liquid scintillation counter. ■, control; ▪, Hcy, 24 h. Values represent mean ± SD from 3 separate experiments from 3 wells (n = 9).
The effect of adenovirus-transduced cyclin A gene on DNA synthesis in Hcy-treated HUVECs.
HUVECs were infected with control adenovirus (Ad vector), adenovirus expressing cyclin A (Ad cyclin A) or adenovirus expressing β-gal (Ad β-gal) at the indicated MOI for 24 hours before being challenged with Hcy. Cells were then treated with or without 50 μM ofdl-Hcy in control medium for another 24 hours. (A) Western analysis; total protein (50 μg) from each sample was analyzed with antibodies against cyclin A. (B) Staining for β-gal; cells were infected with Ad β-gal at the indicated MOI and stained for β-gal. Photograph was taken with × 100 magnification. (C) Thymidine uptake; cells treated for 24 hours were metabolically labeled with 1 μCi (0.037 MBq)/mL [3H]-thymidine during the last 3 hours. [3H]-thymidine incorporation was measured in a liquid scintillation counter. ■, control; ▪, Hcy, 24 h. Values represent mean ± SD from 3 separate experiments from 3 wells (n = 9).
To test whether this forced expression of cyclin A could rescue cell-cycle progression and DNA synthesis, we measured [3H]-thymidine incorporation in Ad cyclin A–infected HUVECs and compared this level with uninfected and Ad vector–infected cells. Cells infected with high concentrations of control virus (Ad vector) showed moderate decreases in [3H]-thymidine incorporation (decreases of 20% and 32% at 200 and 500 MOI, respectively), possibly reflecting nonspecific toxicities at these viral titers; at lower MOI, there was no evidence of toxicity. Hcy treatment inhibited [3H]-thymidine incorporation by approximately 80% to 90% in these cells, and this inhibition was not affected by addition of control adenovirus at MOI of 10 to 500 (Figure6C). In contrast, Ad cyclin A infection reversed the Hcy-induced suppression of [3H]-thymidine incorporation, because DNA synthesis recovered to 57% of that of the uninfected control cells at 100 MOI, 68% at 200 MOI, and 84% at 500 MOI (Figure 6C). Transduction efficiency, assessed with adenovirus expressing β-gal (Ad β-gal), was approximately 80% at 48 hours after infection with 100 MOI (Figure6B).
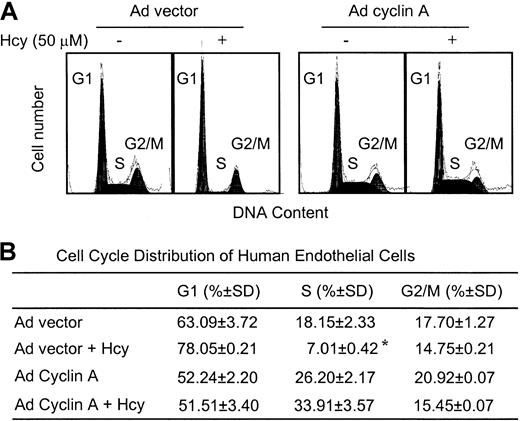
Finally, we used flow cytometric analysis to examine the effect of Ad cyclin A on cell-cycle progression. As shown in Figure7, in ECs infected with the Ad vector, the S phase population decreased in response to Hcy from 18.15% ± 2.33% to 7.01% ± 0.42%, consistent with our previous observation in cells not exposed to adenovirus.28Infection with Ad cyclin A resulted in a decrease in the fraction of cells in G1, accompanied by an increase in the S and G2/M fractions. Furthermore, with overexpression of cyclin A, the ECs became resistant to Hcy-mediated G1/S arrest. These results indicate that exogenous expression of cyclin A is sufficient to overcome the G1/S block imposed by Hcy, and suggest that down-regulation of cyclin A is essential for the growth inhibitory effects of Hcy on ECs.
Flow cytometric analysis of ECs after adenovirus cyclin A infection in the presence of Hcy.
(A) HUVECs were infected with adenovirus vector (Ad vector) or adenovirus expressing cyclin A (Ad cyclin A) at 100 MOI, and exposed to 50 μM dl-Hcy for 24 hours. The cells were harvested and subjected to FACS analysis of DNA content. (B) Cell-cycle distribution of HUVECs. Cell-cycle distribution was analyzed using a Multicycle software. The values represent the means ± SD from 5 independent experiments (n = 10). *P < .05 versus control.
Flow cytometric analysis of ECs after adenovirus cyclin A infection in the presence of Hcy.
(A) HUVECs were infected with adenovirus vector (Ad vector) or adenovirus expressing cyclin A (Ad cyclin A) at 100 MOI, and exposed to 50 μM dl-Hcy for 24 hours. The cells were harvested and subjected to FACS analysis of DNA content. (B) Cell-cycle distribution of HUVECs. Cell-cycle distribution was analyzed using a Multicycle software. The values represent the means ± SD from 5 independent experiments (n = 10). *P < .05 versus control.
Discussion
Endothelial dysfunction is broadly implicated in conditions that increase the risk of atherosclerosis, such as hypercholesterolemia, diabetes, and smoking. Although previous work on Hcy has focused on its prothrombotic effects on ECs, we have identified and characterized an Hcy-mediated effect on EC growth that occurs at clinically relevant concentrations.
The goal of this study was to identify the specific molecular targets of Hcy that lead to EC growth inhibition and cell-cycle arrest. We previously observed that Hcy specifically arrests EC at the G1/S transition of the cell cycle, and therefore focused our studies on the effect of Hcy on G1 and S phase regulators. We found that expression and activity of the G1cyclins, D1 and E, were not affected by Hcy exposure. In contrast, cyclin A expression and its associated kinase activity were markedly decreased, consistent with cell-cycle arrest occurring in very late G1 or at the G1/S transition, when cyclin A activity is required for cell-cycle progression.40 41 Our mechanistic studies indicated that the decrease in cyclin A expression by Hcy was due to inhibition of cyclin A transcription. Adenovirus-transduced expression of cyclin A was sufficient to overcome the Hcy-mediated growth inhibition and G1/S cell-cycle block.
The reversibility of EC growth inhibition after withdrawal of Hcy and the rescue of cell-cycle progression by forced expression of cyclin A argue against nonspecific toxic effects at these Hcy concentrations and suggest that discrete cell-cycle control mechanisms similar to checkpoints may be activated by exposure to Hcy. Checkpoints have been proposed as mechanisms by which cells halt cell-cycle progression at precise stages during stress to provide time for repair.42-44 Cell-cycle checkpoint studies have led to the identification of a number of therapeutic anticancer targets.45-47 Understanding the molecular basis for the checkpoint activated by Hcy is thus of considerable interest because it may lead to identification of therapeutic targets for Hcy-related and other vasculoproliferative diseases.48
Expression of cyclin A is essential for normal cell-cycle progression.23,35,49,50 In ECs, regulation of cyclin A gene transcription has been specifically implicated in the phenomenon of contact inhibition.35 We found that Hcy decreased cyclin A promoter activity in ECs, but not in aortic smooth muscle cells, whose growth is not inhibited by Hcy.28Thus, the mechanism leading to inhibition of the cyclin A promoter and growth arrest of ECs appears to be vascular cell-type specific. In previous work, we found that Hcy treatment decreased cellular methylation and inhibited RAS/MAP kinase signaling in ECs.28 In the human cyclin A promoter, an activating transcription factor-1/cyclic AMP-responsive element-binding protein (ATF/CREB)–binding site (bases −73 to −80) and E2F (+30 to +40) are important positive regulators of activity.51,52 Regulation of ATF and CREB activity depends in turn on phosphorylation status.53 The CREB transcription factor is activated by a broad range of extracellular stimuli, including many growth factors, by phosphorylation at Ser133.54 Although several kinases appear to be capable of phosphorylating CREB at this position, some of these candidates are downstream targets of RAS/MAP signaling pathways, including RSK1-3, MSK1, and MAPKAP-K2/3.54 E2F transcription factors are required for RAS-induced gene activation.55 RAS/RB/E2F signaling is a major regulation of cell proliferation and has been linked with cyclin A transactivation.56-61 Future studies will investigate such intermediates that may link Hcy-mediated inhibition of RAS/MAP signaling to inhibition of cyclin A gene transcription and of EC growth.
Our finding that Hcy, at clinically relevant concentrations, inhibits EC growth by blocking cyclin A transcription has important implications for understanding the mechanisms of Hcy-induced vascular pathogenesis. The endothelium plays a pivotal role in neointima formation after injury. Increased endothelial recovery, or re-endothelialization, correlates with diminished neointimal hyperplasia.62Inhibition of EC growth by Hcy may therefore contribute to accelerated neointima formation and postangioplasty restenosis. Indeed, it was recently reported that diet-induced hyperhomocysteinemia exacerbates neointima formation after denuding injury, and that this effect may be mediated through Hcy-induced attenuation of re-endothelialization.7,63 Growing evidence demonstrates that Hcy impairs the normal antithrombotic property of the endothelium.64-66 The ability of Hcy to induce cell-cycle arrest in ECs may further function to promote the thrombotic potential of the vessel wall by limiting the extent of EC regrowth at sites of EC injury. Furthermore, Hcy inhibition of EC growth may be a mechanism for impaired angiogenesis in hyperhomocysteinemia. Nagai et al67 have found that Hcy inhibits angiogenesis in vitro and in vivo by preventing the proliferation and migration of ECs. Identification of the molecular mechanism of EC growth inhibition by Hcy may permit specific targeting of the cell cycle in the pharmacologic and genetic therapy of Hcy-induced vascular vasculoproliferative disease.
We thank Dr Mu-En Lee for his support for some experiments initiated in his laboratory, Drs Michael A Gimbrone, Stephen J Elledge, and Zhou SongYang for helpful discussions and critical review of this manuscript, and Dr Terry L. Timme for assistance with FACS analysis.
Supported by National Institutes of Health grant HL-62467 and HL- 59976 (A.I.S.), and HL-59976 (W.D.); by American Heart Association Texas Affiliate Grant 0160041y (H.W.); and by American Health Assistance Foundation grant H2001-010 (H.W.). W.D. is an Established Investigator of the American Heart Association. H.W. is an awardee of the Junior Faculty Scholar Award from the American Society of Hematology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hong Wang, VA Medical Center, Baylor College of Medicine, 2002 Holcombe Ave 109-129, Houston, TX, 77030; e-mail:hongw@bcm.tmc.edu.

![Fig. 1. The inhibitory effect of Hcy on DNA synthesis is reversible in ECs. / HUVECs were exposed to dl-Hcy for 24 hours, then washed with PBS, and cultured with fresh control medium for the time indicated. [3H]-thymidine incorporation was measured at indicated times. The value of no Hcy treatment control for each time point was set as 100%, and [3H]-thymidine incorporation was divided by the control value. Values represent the mean ± SD of 3 separate experiments (n = 9). *P < .01 versus 0 hour sample in control medium after Hcy treatment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/3/10.1182_blood.v99.3.939/4/m_h80322043001.jpeg?Expires=1765948569&Signature=lmmXU-HVnKY7DmvLDORYeXaUNaM1n1V-ic7y9HDd24vNHQuaWmRWqfECuWpgSfeAdiyYeIgj-mM7h9IjLbDERPJNo1KtzD2Bb4oqlK9lWH3NFg5uiLiD-Y~uwnpHpyYahqzbzCa4CqU~PabIk4pZTBUa32LWjULlbQL10UpKDrfCNG6loULv2vXB~0Nd03UgHtFZfx31EvVw25T30SQ0x7ZXhTk9Ea0qZgBQoT3cOM54tjWIW~yuapTiuegbKuDjwaNyusjPUfubAOR7xxbe4cacZe1OWqMF6pZQJHhnYVJ5q2nL80af7Bd2qFOH7lfjviLQ0wXKuATEZ-ezxCMJFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. The effect of adenovirus-transduced cyclin A gene on DNA synthesis in Hcy-treated HUVECs. / HUVECs were infected with control adenovirus (Ad vector), adenovirus expressing cyclin A (Ad cyclin A) or adenovirus expressing β-gal (Ad β-gal) at the indicated MOI for 24 hours before being challenged with Hcy. Cells were then treated with or without 50 μM ofdl-Hcy in control medium for another 24 hours. (A) Western analysis; total protein (50 μg) from each sample was analyzed with antibodies against cyclin A. (B) Staining for β-gal; cells were infected with Ad β-gal at the indicated MOI and stained for β-gal. Photograph was taken with × 100 magnification. (C) Thymidine uptake; cells treated for 24 hours were metabolically labeled with 1 μCi (0.037 MBq)/mL [3H]-thymidine during the last 3 hours. [3H]-thymidine incorporation was measured in a liquid scintillation counter. ■, control; ▪, Hcy, 24 h. Values represent mean ± SD from 3 separate experiments from 3 wells (n = 9).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/3/10.1182_blood.v99.3.939/4/m_h80322043006.jpeg?Expires=1765948569&Signature=A9Ke1DE3SqA7OJTQmc2tZi-zIWiCotCuGFbsquh7WtMFWtoJPftqTDlW143veMpHAAyTKQhj5B9oY8p1G64ZyS6hB90OJN1-slGyjoyh5f~ghfqBv6z5~IEn~0ufp8gAEzyjQDVIAbwjzT1EjboZnhln0MBEpJtNZ77xfj1fXEv3ueiEz6bhu8qCLik90MAb2Heyv-oyXPNUWv~PdhjerQuTCXHQHEpI8Fov-2~5jZkggh1L4jCc2wS9Or0hvmkP0UJgapp7WwhuC7TaRuZbmwIvfplsK4~AfENpa5Z0oGSEQiBW5jbVkRX7eEOuF-it0FB5JnpkPEIZjhw2DaP7Vw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal