Abstract
A culture of human blood outgrowth endothelial cells (BOECs) was established from a sample of peripheral blood and was transfected using a nonviral plasmid carrying complementary DNA for modified human coagulation factor VIII (B domain deleted and replaced with green fluorescence protein). BOECs were then chemically selected, expanded, cryopreserved, and re-expanded in culture. Stably transfected BOECs were administered intravenously daily for 3 days to NOD/SCID mice at 4 cell dose levels (from 5 × 104 to 40 × 104 cells per injection). In 156 days of observation, mice showed levels of human FVIII that increased with cell dose and time. Mice in all cell dose groups achieved therapeutic levels (more than 10 ng/mL) of human FVIII, and mice in the 3 highest dose groups acquired levels that were normal (100-200 ng/mL) or even above the normal range (highest observed value, 1174 ng/mL). These levels indicate that the BOECs expanded in vivo after administration. When the mice were killed, it was found that BOEC accumulated only in bone marrow and spleen and that these cells retained endothelial phenotype and transgene expression. Cell doses used here would make scale-up to humans feasible. Thus, the use of engineered autologous BOECs, which here resulted in sustained and therapeutic levels of FVIII, may comprise an effective therapeutic strategy for use in gene therapy for hemophilia A.
Introduction
Hemophilia A is an inherited X-linked disorder caused by a deficiency of coagulation factor VIII (FVIII).1 This defect results in a severe bleeding phenotype leading to death, significant illness, and enormous use of health system resources. The available standard therapy, intravenous infusion of FVIII, is expensive. Aside from certain specific situations such as preoperative factor coverage, FVIII therapy is usually used to treat acute bleeding rather than to provide prophylaxis. Furthermore, FVIII has a relatively short half-life of approximately 12 hours, mandating repeated and prolonged administration. In addition, historical disasters have occurred, such as the emergence of human immunodeficiency virus in this patient population because of viral contamination of commercial factor products.
Gene therapy for hemophilia A would be of tremendous benefit,2 and many investigators have actively pursued this goal. An attractive aspect of FVIII therapy is that only small amounts of FVIII are required to shift hemophilia phenotype from severe to mild. For example, patients with FVIII levels greater than 5% of normal (ie, greater than 5-10 ng/mL) have a mild phenotype; patients whose levels are lower than 1% have a severe phenotype. Unfortunately, numerous impediments hamper achieving a sustained, therapeutically useful elevation in circulating FVIII through a gene therapy strategy. The FVIII gene is large and suppresses its own expression. This problem has been ameliorated by deleting the large, centrally located FVIII B domain, which is not necessary for function.3-6 Various vector systems have been designed for expression of FVIII in a gene therapy setting,7-12 and encouraging results have been observed in mice subjected to intravenous administration of recombinant adenoviral,13,14 rAAV,11lentiviral,12 and retroviral vectors.15 Each of these approaches has entailed the potential disadvantage of systemic exposure to viral vectors. Notably, viral vectors carry potential risks, including untoward inflammatory reactions, germline transmission, and carcinogenesis.
The current work is based on the premise that, regardless of which expression vector ultimately proves to be optimal for the production of FVIII, a novel delivery vehicle for that vector might be useful. We propose that blood outgrowth endothelial cells (BOECs) can comprise such a vehicle. Monolayer cultures of BOECs can be reproducibly established from human peripheral blood buffy coat mononuclear cells.16 Outgrowth BOECs have typical endothelial cobblestone morphology, take up acetylated low-density lipoprotein (LDL), contain Weibel-Palade bodies, and express multiple endothelial cell markers: CD34, von Willebrand factor (VWF), P1H12, vascular-endothelial (VE)–cadherin, flk-1, CD36, thrombomodulin, and platelet/endothelial cell adhesion molecule (PECAM).16 BOECs can be expanded from 20 cells at the start of culture to 1019 cells by 65 days.16 Thus, a virtually unlimited number of BOECs can be available, offering a potential advantage for gene transfer protocols that require chemical selection, because it may overcome the endothelial cell's relative intolerance of growth at low density (that typically results from chemical selection).
Materials and methods
BOEC culture
We obtained 100 mL venous blood from a healthy human volunteer donor and prepared buffy coat mononuclear cells from diluted blood using Histopaque-1077 (Sigma Chemical, St Louis, MO), as previously described.16 After suspending these cells in EBM-2/EGM-2 culture medium (Clonetics, San Diego, CA), we plated them into a culture well coated with collagen I (Sigma Chemical). After 24 hours, we changed the medium and removed nonadherent cells and debris. Thereafter, we changed culture medium daily until first passage, corresponding to expansion to approximately 3 × 104cells, and we changed it every other day thereafter. After BOECs had expanded to approximately 107 cells, we lifted them with trypsin (Gibco BRL, Grand Island, NY) and plated them onto a 10-cm culture dish coated with 6 μg/cm2 type 1 collagen and 5 mL 1% gelatin containing 50 μg/mL fibronectin (Sigma Chemical). They were then used for gene transfer.
Construction of chimeric eGFP-FVIII expression vector
We used a mammalian expression plasmid containing cDNA encoding a human B–domainless FVIII molecule, designated HSQ/ReNeo,6 as described previously.17 HSQ contains the FVIII domain sequence A1-A2-ap-A3-C1-C218 and a 14-amino acid linker segment between the A2 domain and the ap segment. The linker corresponds to the first 5 and last 9 amino acids of the human FVIII B domain (SerPheSerGlnAsn and ProProValLeuLysArgHisGlnArg, respectively). PEGFP-N1, a plasmid encoding enhanced green fluorescence protein (eGFP), a 31-kd red-shifted variant (λex 488 nm, λem 505 nm) of wild-type GFP, was purchased from Clontech (Palo Alto, CA). We then constructed cDNA encoding an active eGFP-FVIII fusion protein by splicing-by-overlap (SOE) mutagenesis19using HSQ/ReNeo and PEGFP-N1 as templates in the polymerase chain reaction (PCR). The resultant plasmid, eGFP-FVIII/ReNeo, contains eGFP inserted between SerPheSerGlnAsn and ProProValLeuLysArgHisGlnArg in the linker segment of HSQ. To test for the functional integrity of eGFP-FVIII in vitro, we stably expressed eGFP-FVIII/ReNeo in a baby hamster kidney-derived cell line as described previously20and purified it in a single step by SP-Sepharose chromatography. Specific coagulant activity of eGFP-FVIII, measured by one-stage FVIII assay,21 was indistinguishable from the parent HSQ protein (7000 U/A280). Fluorescence emission maximum of eGFP-FVIII was 512 nm.
We digested plasmid HSQ-eGFP/ReNeo with XhoI andNotI to yield a 5.4-kb fragment containing the cDNA for the chimeric eGFP-FVIII protein. We then ligated this fragment into the Neo-containing plasmid pcDNA3.1(−) (Invitrogen, Carlsbad, CA) that had been predigested with XhoI and NotI. The resultant plasmid is referred to as pcF8G.
Gene transfer to BOECs
We exposed BOECs (at 75% confluence in a 10-cm dish prepared as above) to 25 μg Fugene6 and 10 μg plasmid pcF8G in a culture medium composed of MCDB-131 base medium-supplemented 20% heat-inactivated male human serum, 0.246 mg/mL dibutyl cyclic adenosine monophosphate, 0.04 mg/mL heparin, 1% PenStrepFungizone (Gibco Invitrogen, Carlsbad, CA), 1.5 mg/mL L-glutamine, 1 μg/mL hydrocortisone, and 10 μg/mL endothelial cell growth factors (ECGF) (Clonetics).22Cells were exposed in this manner for 72 hours at 37°C in a humidified environment with 5% CO2. We added fresh medium at 24 hours without removing the old medium. Thereafter, we cultured the transfected BOECs in EBM-2/EGM-2 medium (Clonetics) containing 50 μg/mL G418 (Gibco-BRL) for 10 days. They were cultured in this medium without G418 for 45 days, after which G418 was again added (at 50 μg/mL) for 14 days.
Supernates from 5 × 105 transfected BOECs were tested after 72 hours and after final G418 selection for content of human FVIII. We obtained several positive clones, as evidenced by enzyme-linked immunosorbent assay (ELISA) for human FVIII on culture supernatants (see below) and flow cytometry detection of the chimeric eGFP-FVIII protein (not shown). We chose one BOEC clone, pcF8G-6, producing 22 ng/mL FVIII into 72-hour–conditioned culture medium as the stably transfected BOEC (tBOEC) for use in this study.
For one type of control BOEC, we used vector pLEIN (Clontech), which is the retroviral vector pLXIN with an eGFP sequence inserted in the multiple cloning site. PA317 cells were used in the packaging. Particles of replication-incompetent virus were used to transduce human BOECs; 1 × 105 BOECs, prepared and seeded as above, were exposed at 75% confluence to virus-containing medium in combination with 4 μg/mL polybrene. Fresh medium was added at 24 hours. After this, cells were selected as described above.
Characterization of tBOECs
To demonstrate the presence of the transgene, we isolated genomic DNA from wild-type BOEC (wtBOEC) and tBOEC (clone pcF8G-6) using DNAeasy tissue kit (Qiagen, Valencia, CA). Five hundred nanograms genomic DNA from wtBOEC or tBOEC or 50 pg plasmid pcF8G (positive control) was subjected to PCR using specific primers. The sense primer (5′-GTC TCC ACC CCA TTG-3′) was located in the cytomegalovirus (CMV) promoter region of the pcF8G expression vector; the antisense primer (5′-TGG CAC TCT AGG AGG-3′) was located in the A domain of the human FVIII cDNA in the expression vector. The expected size of the PCR product was 415 base pair (bp). Thermocycling parameters were 31 cycles of incubation at 94°C (45 seconds), 55°C (30 seconds), and 72°C (45 seconds), followed by a final 10-minute extension at 72°C. PCR products were visualized by electrophoresis on ethidium bromide–stained 0.8% agarose gel. The gel was subjected to Southern blot analysis using a 415-bp digoxigenin-labeled PCR probe spanning the junction between sequences for CMV promoter and human FVIII.
To estimate the number of transgene copies per cell, we took 0.5 pg to 100 pg pcF8G plasmid DNA and 3 μg tBOEC genomic DNA and subjected them to dot blot analysis using the DIG system29; 415 bp digoxigenin-labeled PCR product was used as the probe. The transgene copy number in tBOEC was estimated by comparison of the dot intensities on the dot blot, compared to standards, where 1 pg pcF8G plasmid DNA equals 1 copy of the transgene per cell in 600 ng tBOEC genomic DNA.
Phenotype of tBOEC was determined as previously described16for wtBOEC based on morphology, uptake of acetylated LDL, expression of the transgene human FVIII, expression of endothelial markers (VE-cadherin, thrombomodulin, VWF, flk-1, vascular cell adhesion molecule 1 [VCAM-1], PECAM-1, CD34, P1H12, and CD36), and expression of makers of monocytes (CD14), hematopoietic cells (CD45), and putative endothelial progenitors (AC133). Each fluorescence activated cell sorting (FACS) analysis used unlabeled cells and cells with isotype control antibody as controls. Because these 2 controls were always identical in these studies, presentation of the results illustrates the unlabeled cell control only.
ELISA for human FVIII and eGFP
We measured the level of human FVIII in BOEC supernates or in murine platelet-poor plasma using an ELISA kit that detects human, but not murine, FVIII (American Diagnostica, Greenwich, CT). We verified the lack of cross-reactivity by examining dilutions of mouse plasma (with or without added human FVIII). The presence of any mouse plasma added a nonspecific background signal, but this did not increase as the amount of mouse plasma increased from 10% to 100%. Each animal's own prestudy background signal was subtracted from each subsequent measurement. In individual mice monitored sequentially day to day, the level of background signal varied by ± 5 ng/mL. All measurements of FVIII were made in duplicate. For this ELISA, the average coefficient of variation of duplicate measurements was 49.5% for FVIII levels lower than 30 ng/mL, 17.6% for those between 30 and 100 ng/mL, and 3.3% for FVIII levels greater than 100 ng/mL.
To detect the eGFP component of the eGFP-FVIII chimeric gene product in murine plasma, we similarly used an ELISA with rabbit anti-GFP antibody (Clontech). The average coefficient of variation of replicate measurements in this ELISA was 8.9%.
Cell administration and animal monitoring
The NOD/SCID mice used in these studies weighed 35 g. Although they exhibit a greatly truncated lifespan, they have a severe immunodeficiency so there could be no immunologic reactivity against the human BOEC and human FVIII used for the experiment.23 24 We suspended BOEC in 500 μL mouse saline (330 mOsm/L) and injected them by tail vein once a day for 3 consecutive days. Mice received either 5, 10, 20, or 40 × 104 BOECs per injection (ie, total dose from 4.3 × 106 to 3.4 × 107 cells per kg). Control mice received no cells (injection of saline only), or they received unmanipulated wtBOEC or BOEC transduced to express eGFP only (gfpBOEC) at the dose level of 20 × 104 cells per injection. We obtained blood from the retro-orbital plexus at multiple time points after BOEC administration for ELISA measurement of human FVIII, as above.
We performed histologic examination of available (deceased or killed) animals to localize tBOECs. We sampled multiple tissues (heart, lung, liver, spleen, kidney, bone marrow, abdominal lymph nodes, and gut wall) and probed fixed sections with a goat antibody to human β2-microglobulin, which did not cross-react with murine tissues (Accurate Chemical and Scientific, Westbury, NY). After blocking endogenous peroxidase activity and nonspecific biotin–avidin binding (with H2O2 and Avidin/Biotin Blocking Kit [Vector Laboratories, Burlingame, CA], respectively), we used a biotin–SP-conjugated donkey anti–goat immunoglobulin secondary antibody (Jackson ImmunoResearch, West Grove, PA) and the R.T.U. Vectastain Elite ABC reagent and peroxidase substrate DAB (both from Vector Laboratories) to visualize human BOECs in murine tissues. To assess nonspecific background staining, we omitted the primary antibody, and we used tissues from untreated mice as negative controls.
To identify the phenotype of tBOEC detected in murine marrows, we took frozen sections of marrow at day 156 and fixed them with 4% paraformaldehyde. BOECs were characterized by double staining for β2-microglobulin and for either VE-cadherin or FVIII. To detect the combination of β2-microglobulin and FVIII, we used a goat antibody to human β2-microglobulin (Accurate Chemical and Scientific) and a sheep antibody to human FVIII (Accurate Chemical and Scientific); secondary antibodies were an alkaline phosphatase-conjugated antigoat antibody and a peroxidase-conjugated antisheep antibody; substrates were Fast-Red (Sigma Chemical) and DAB (Vector Laboratories). To detect β2-microglobulin and VE-cadherin, we used a rabbit antibody to human β2-microglobulin (Accurate Chemical and Scientific) and a goat antibody to human VE-cadherin (Santa Cruz Biotechnology, CA); secondary antibodies were an alkaline phosphatase–conjugated antigoat antibody and a peroxidase-conjugated antirabbit antibody; substrates were as above. Marrow from untreated NOD-SCID mice was used as a negative control; human BOEC served as a positive control. In similar fashion, we evaluated murine marrow sections for staining using a monoclonal antibody to human CD45 (Serotech, Oxford, United Kingdom).
To determine the percentage of nucleated cells in murine marrow that were human BOECs, we evaluated marrow sections stained for human β2-microglobulin as above. For this we used 5 of the study animals (Table 2 and Figures 2, 3) that were killed at 156 days and 2 that were killed at 28 days. We also evaluated one animal killed at 9 days that had been given tBOEC but was not included as part of the FVIII level study because a baseline FVIII level was unavailable. In animals at 28 and 156 days, we counted more than 1200 nucleated cells in 100 high-power fields to determine percentages of tBOEC. In the mouse examined at 9 days, we counted 5000 nucleated cells to obtain the result.
Results
We established a culture of BOECs from a healthy human volunteer and expanded them approximately 106-fold to obtain approximately 107 cells. After confirming that these BOECs had the endothelial markers we previously observed for BOECs,16 we transfected them using a nonviral plasmid vector (pcF8G) containing a cDNA encoding a modified form of human FVIII. In this cDNA, the unnecessary B domain was replaced by sequence encoding eGFP. We selected a clone of stably transfected BOECs (clone pcF8G-6) and expanded them another 106-fold before use in the current experiments. Thus, this clone of tBOEC was analyzed and used after the cells had undergone an overall 3.4 × 1012-fold expansion since establishment of the initial BOEC culture.
In these tBOECs, we demonstrated the presence of the transgene by PCR and Southern blot analysis by probing for the junction between the CMV promoter and the A domain of FVIII. tBOECs were positive, and wtBOECs were negative (data not shown). We also obtained an estimate of transgene copy number of 1.5 per cell from dot blot intensities (data not shown). This clone of cells produced 22 ng/mL FVIII into culture medium (supernate from 5 × 105 cells) in 72 hours (Table1). Phenotypic characterization of the tBOECs (Figure 1) showed that they were somewhat more elongated than the starting wtBOECs, they took up acetylated LDL, and they retained their endothelial markers (VE-cadherin, thrombomodulin, von Willebrand factor, flk-1, PECAM, CD34, P1H12, and CD36). They were negative for activation antigen VCAM-1 but expressed it when deliberately stimulated in vitro (data not shown). In addition, they were negative for monocyte marker CD14 (Figure 1) and negative for hematopoietic marker CD45 and endothelial progenitor marker AC133 (data not shown). Thus, the tBOECs retained the general phenotype of wtBOECs (ie, that of differentiated, quiescent, microvascular endothelial cells).
FVIIII secretion from cells in culture
| Endothelial cell type . | Culture supernate FVIII (ng/mL) . |
|---|---|
| HUVEC | 1 ± 3 |
| MVEC | 0 ± 0 |
| wtBOEC | 5 ± 4 |
| gfpBOEC | 4 ± 8 |
| BOEC transient transfection | 104 ± 1 |
| tBOEC (clone pcF8G-6) | 22 ± 5 |
| Endothelial cell type . | Culture supernate FVIII (ng/mL) . |
|---|---|
| HUVEC | 1 ± 3 |
| MVEC | 0 ± 0 |
| wtBOEC | 5 ± 4 |
| gfpBOEC | 4 ± 8 |
| BOEC transient transfection | 104 ± 1 |
| tBOEC (clone pcF8G-6) | 22 ± 5 |
FVIII secretion levels are indicated for supernate from 5 × 105 cultured BOECs after 72 hours (n = 4 each). Levels for transiently and stably transfected BOECs were significantly increased over those of background culture medium (P < .001), whereas those for the untransfected BOECs were not (though wtBOECs approached significance at P = .055).
Characterization of tBOECs.
tBOECs were characterized before they were administered to mice. At this time, they had expanded approximately 106-fold since gene transfer and approximately 1012-fold overall since the establishment of initial BOEC culture from blood. Panels illustrate morphology and uptake of acetylated LDL, negativity for monocyte marker CD14, positivity for the transgene product FVIII, and positivity for multiple endothelial cell markers. The illustrated control peak for unlabeled cells was identical to the control peak for isotype control antibody in all cases.
Characterization of tBOECs.
tBOECs were characterized before they were administered to mice. At this time, they had expanded approximately 106-fold since gene transfer and approximately 1012-fold overall since the establishment of initial BOEC culture from blood. Panels illustrate morphology and uptake of acetylated LDL, negativity for monocyte marker CD14, positivity for the transgene product FVIII, and positivity for multiple endothelial cell markers. The illustrated control peak for unlabeled cells was identical to the control peak for isotype control antibody in all cases.
We gave BOECs by tail vein to NOD-SCID mice, each of which received 5, 10, 20, or 40 × 104 eGFP-FVIII tBOECs each day for 3 consecutive days. Our 3 types of control animals received 3 injections of saline vehicle, 20 × 104 unmanipulated wtBOEC, or 20 × 104 BOECs transduced with retroviral vector pLEIN to express eGFP only (gfpBOEC). We then obtained retro-orbital plexus blood from each experimental animal at 9, 28, 47, 97, and 156 days for an ELISA that detected human, but not murine, FVIII. Each measurement was reported after subtraction of the baseline, background signal obtained from blood of the same mouse 3 days before BOEC infusion.
After the administration of tBOEC, levels of human FVIII increased with cell dose and elapsed time (Table 2, Figure 2). All mice that received eGFP-FVIII tBOECs, even those given the lowest cell dose, achieved a therapeutic level of FVIII (greater than 10 ng/mL). Mice in the 3 highest dose groups achieved levels that are normal (100-200 ng/mL) or even substantially higher than normal (highest observed level was 1174 ng/mL at 156 days). Even with this small number of experimental animals, FVIII levels reached statistically significant levels compared to baseline for mice in the highest cell-dose treatment groups (analysis by analysis of variance; Table 2). In mice at 156 days, our evaluation by separate ELISAs for human FVIII and for eGFP showed a strong positive correlation between these 2 independent measures for presence of expressed gene product in murine plasma (r = 0.903; n = 10; P < .001).
Plasma levels of human FVIII in NOD/SCID mice
| Treatment group . | Plasma level of human FVIII in ng/mL (nM) . | ||||
|---|---|---|---|---|---|
| Day 9 . | Day 28 . | Day 47 . | Day 97 . | Day 156 . | |
| No. cells given | −3 (2) | −4 (2) | −2 (1) | 0 (1) | |
| 20 × 104wtBOEC | 1 (2) | 9 (2) | 11 (2) | 17 (2) | 9 (2) |
| 20 × 104gfpBOEC | 1 ± 4 (3) | 7 ± 5 (3) | 12 ± 7 (3) | 26 ± 4 (3) | 7 ± 4 (3) |
| 5 × 104 tBOEC | 9 (2) | 14 (2) | 45 (2) | 53 (2) | 58 (2) |
| 10 × 104 tBOEC | 9 ± 3 (3) | 19 ± 6 (3) | 57 (1) | 49 (1) | 145 (1) |
| 20 × 104 tBOEC | 16 ± 11 (4) | 39 ± 24 (4) | 114 ± 65 (4) | 146 ± 96 (4)† | 581 ± 517 (3)* |
| 40 × 104 tBOEC | 35 ± 26 (3) | 368 ± 50 (3)* | 534 ± 363 (3)* | 246 (2)‡ | 346 (1) |
| Treatment group . | Plasma level of human FVIII in ng/mL (nM) . | ||||
|---|---|---|---|---|---|
| Day 9 . | Day 28 . | Day 47 . | Day 97 . | Day 156 . | |
| No. cells given | −3 (2) | −4 (2) | −2 (1) | 0 (1) | |
| 20 × 104wtBOEC | 1 (2) | 9 (2) | 11 (2) | 17 (2) | 9 (2) |
| 20 × 104gfpBOEC | 1 ± 4 (3) | 7 ± 5 (3) | 12 ± 7 (3) | 26 ± 4 (3) | 7 ± 4 (3) |
| 5 × 104 tBOEC | 9 (2) | 14 (2) | 45 (2) | 53 (2) | 58 (2) |
| 10 × 104 tBOEC | 9 ± 3 (3) | 19 ± 6 (3) | 57 (1) | 49 (1) | 145 (1) |
| 20 × 104 tBOEC | 16 ± 11 (4) | 39 ± 24 (4) | 114 ± 65 (4) | 146 ± 96 (4)† | 581 ± 517 (3)* |
| 40 × 104 tBOEC | 35 ± 26 (3) | 368 ± 50 (3)* | 534 ± 363 (3)* | 246 (2)‡ | 346 (1) |
Data are expressed as mean (number of mice contributing). SD is shown if n > 2. Cell dose is indicated as the number of cells per injection; 3 injections were administered.
P values are compared to the baseline measurement for the whole group.
BOEC prefixes are as follows: wt indicates unmanipupated BOECs; gfp, BOECs transduced to express GFP only; and t, BOECs stably transfected to express eGFP-FVIII.
P < .001.
P < .05.
P < .01.
Levels of human FVIII in NOD/SCID mouse plasma.
Blood was sampled on days 9, 28, 47, 97, and 156 after the administration of BOECs, and human FVIII was measured by ELISA. Results are shown for each mouse in the 4 groups that received eGFP-FVIII tBOECs. Cell dose is indicated per injection.
Levels of human FVIII in NOD/SCID mouse plasma.
Blood was sampled on days 9, 28, 47, 97, and 156 after the administration of BOECs, and human FVIII was measured by ELISA. Results are shown for each mouse in the 4 groups that received eGFP-FVIII tBOECs. Cell dose is indicated per injection.
Although control animals given saline only showed no appearance of human FVIII, animals given control BOEC (either wtBOEC or gfpBOEC) developed detectable amounts of human FVIII. Levels in these control animals were low compared with those generated by eGFP-FVIII tBOEC, but they did eventually become transiently therapeutic themselves. This occurrence is consistent with our observation in preliminary experiments that unmanipulated BOECs may produce small amounts of FVIII in culture (Table 1). This possibility will have to be confirmed by separate study of a larger number of animals given control BOECs.
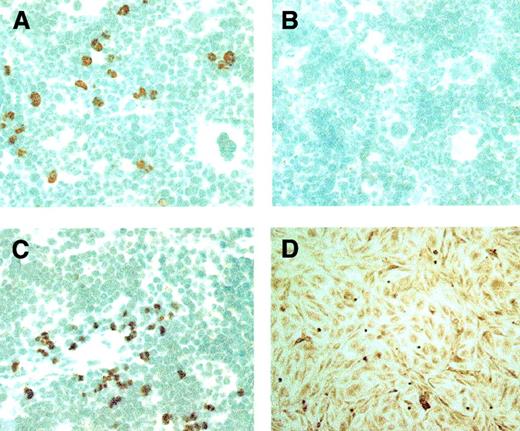
Histologic examination of mice after tBOEC administration revealed detectable accumulations of BOEC only in bone marrow and in spleen (Figure 3). Otherwise, we rarely observed a single cell in other locations. As assessed by double staining, 100% of the human tBOECs detected in murine marrow at 156 days were also positive for human VE-cadherin, indicating preservation of a differentiated endothelial phenotype. Similarly, virtually all (more than 97%) tBOECs were positive for human FVIII, indicating the preservation of transgene product production up to that time point. Conversely, the tBOECs detected in murine marrow were uniformly negative for hematopoietic marker CD45.
Transfected BOECs detected in mouse marrow and spleen.
tBOECs are identified in murine tissues by peroxidase staining using an antibody to human β2-microglobulin. (A, C) Human tBOECs in murine marrow and spleen, respectively, at 156 days after administration. (B) Negative control—marrow from a NOD-SCID mouse that did not receive BOEC. (D) Positive control—monolayer culture of BOEC. Original magnification, × 600.
Transfected BOECs detected in mouse marrow and spleen.
tBOECs are identified in murine tissues by peroxidase staining using an antibody to human β2-microglobulin. (A, C) Human tBOECs in murine marrow and spleen, respectively, at 156 days after administration. (B) Negative control—marrow from a NOD-SCID mouse that did not receive BOEC. (D) Positive control—monolayer culture of BOEC. Original magnification, × 600.
We were able to analyze several mice to determine the percentages of nucleated cells in the marrow or spleen that were composed of human BOECs. The limited data available (Table3) allowed a direct comparison only for the 20 × 104 cell dose group at day 9 versus day 156, but they seem to confirm a substantial increase in tBOEC cell numbers in marrow over time after the initial administration (see “Discussion”).
Enumeration of human tBOECs in murine marrow and spleen
| Cell dose group . | N . | Time (d) . | tBOECs (% nucleated cells) . | |
|---|---|---|---|---|
| Marrow . | Spleen . | |||
| 10 × 104 | 2 | 28 | 0.7, 1.0 | 1.3, 1.6 |
| 20 × 104 | 1 | 9 | 0.02 | NA |
| 2 | 156 | 3.0, 5.2 | 2.4, 1.6 | |
| 40 × 104 | 1 | 156 | 4.8 | 3.6 |
| Cell dose group . | N . | Time (d) . | tBOECs (% nucleated cells) . | |
|---|---|---|---|---|
| Marrow . | Spleen . | |||
| 10 × 104 | 2 | 28 | 0.7, 1.0 | 1.3, 1.6 |
| 20 × 104 | 1 | 9 | 0.02 | NA |
| 2 | 156 | 3.0, 5.2 | 2.4, 1.6 | |
| 40 × 104 | 1 | 156 | 4.8 | 3.6 |
Five animals used for this enumeration were from the formal FVIII level study (Table 2, Figure 2) (2 killed at 28 days and 3 killed at 156 days). We also included an animal killed at 9 days after tBOEC administration; this animal was not included in the formal FVIII study because no baseline FVIII was available for it.
NA indicates not available.
During this study, 4 mice died of unknown causes (1 of 2 no-cell control mice and 3 of 17 mice that received some form of BOEC). This degree of animal loss was within the limits of expected mortality for these NOD/SCID mice that were older than 3 months at the start of this study, and it paralleled mortality in the parent colony from which these animals were derived. Careful observation of multiple random sections prepared from liver, heart, lung, spleen, and kidney of 6 animals (1 at 9 days, 2 at 28 days, and 3 at 156 days) showed no evidence of thrombosis, vascular occlusion, or infarction. Thus, even animals with the very highest FVIII levels (approximately 1000 ng/mL) had no evidence of thrombosis.
Discussion
We have shown that the administration of engineered human BOECs can be used to attain sustained (up to 156 days, the longest time point examined) and therapeutic (even normal or supranormal) levels of FVIII in immunodeficient mice. This experiment thus demonstrates the feasibility of using BOECs as a delivery system for the administration of FVIII expression vectors, and we have done so using a nonviral plasmid vector. This therapeutic strategy would use autologous cells that obviate concerns about immunologic incompatibility or pathogenic viral infection. Growth characteristics of BOECs in culture allow for chemical selection of engineered cells in vitro and, thus, avert the need for systemic exposure to transducing agents in vivo. The specific plasmid construct used is not necessarily the preferable choice for this purpose. After further development of vector science in general, and as it applies to the expression of FVIII in particular, better expression systems than the one used here likely will be identified. An attraction of our strategy is that any viral or nonviral expression system presumably could be applied using the BOEC delivery system.
Several aspects of our results deserve specific comment. The fact that human FVIII levels in murine plasma increased over time suggests that BOECs expanded more than 100-fold in vivo after their administration. To arrive at this, we used the conservative assumptions that the half-life of human FVIII in our mice was the same as previously reported for human FVIII in immunodeficient mice (1 hour)25and that all administered BOECs remained viable. The latter assumption is almost certainly incorrect, which means that the degree of expansion is even greater. The alternative explanation—that BOECs produce more FVIII in vivo than in vitro—cannot be ruled out, but it seems less plausible. In fact, cell counts performed on available marrow sections are consistent with there having been a substantial expansion of tBOECs over time in vivo. For example, the animals in the 20 × 104 cell dose group were given a total of only 6 × 105 cells, yet at 156 days approximately 5% of marrow cells were composed of tBOECs. If we take 100 × 106 as the total number of nucleated marrow cells in the long bones of the adult mouse, 5% tBOEC represents 5 × 106 cells, an order of magnitude greater than the total number originally given. Further studies are needed to obtain more precise quantitative data on tBOEC expansion in vivo.
Detailed histologic examination revealed accumulations of tBOEC only in spleen and marrow. This suggests a seeding specificity consistent with a homing mechanism involving specific adhesion receptors, analogous to that of hematopoietic stem cells, though this, too, will have to be defined by further studies. It is important to note that analysis of the tBOECs found in murine marrow revealed that the endothelial phenotype was preserved; they were positive for VE-cadherin and negative for CD45. Most important, the tBOECs in murine marrow were still virtually all positive for the transgene product even at 156 days, consistent with the persistence of human FVIII in murine plasma observed here.
Sustained transgene expression, specificity for seeding marrow and spleen, and proliferation of tBOECs in vivo may all be unique aspects of BOEC biologic function. This could account for the current result being particularly encouraging, compared to other attempts to use ex vivo gene transfer and subsequent administration of autologous cells for hemophilia therapy. Chuah et al26 used the retroviral expression of FVIII in bone marrow stromal cells given to mice and found only low-level and transient FVIII expression. Recently, Roth et al27 gave engineered autologous fibroblasts to humans with severe hemophilia A. Although results were disappointing, this was only a phase 1 trial, and the usefulness of the approach remains to be seen. It is encouraging that Qiu et al28 had earlier reported better success using that same strategy for hemophilia B. Whether engineered autologous BOECs truly present a unique system with an advantageous biologic function for gene therapy can only be answered by continued studies.
Nevertheless the results presented here predict that this strategy can, indeed, be scaled up to support preclinical trials in dogs and clinical trials in humans. For example, the second highest cell dose used here, 6 × 105 cells (ie, 3 doses of 20 × 104cells) is equivalent to 4 × 108 BOECs given to an adult dog on a weight-equivalent basis. An additional 4-fold scale up to humans would require 1.6 × 109 cells. This is not a trivial number of cells, but this degree of cell expansion is certainly achievable for human BOECs, which easily expand to 1019cells.16 There are at least 2 reasons to assume that efficacy of the approach in humans would be even better than observed here. First, the half-life of human FVIII in the human patient should be substantially longer than in the mouse,25 and this should allow an order of magnitude fewer cells per kilogram to be used. In addition, because FVIII levels apparently rise with time, it may be that clinical efficacy could be achieved using the lower range of cell doses. Therefore, we believe this approach should be considered for the treatment of hemophilia A. Similarly, it could be a useful therapy for other disorders in which the generation of a gene product into the bloodstream would be beneficial. Hemophilia B is but one example.
We thank Ann Kerimo for technical assistance.
Supported by National Institutes of Health grants HL30160 and HL62931 (R.P.H.) and HL40921 (P.L.).
P.L. has a financial interest in a company that has licensed the technology reported here. P.L. and R.P.H. receive research support from this company.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert P. Hebbel, Dept of Medicine, University of Minnesota Medical School, Mayo Mail Code 480, 420 Delaware St SE, Minneapolis, MN 55455; e-mail hebbe001@tc.umn.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal