Abstract
Among patients with von Willebrand disease (VWD) who are unresponsive to desmopressin therapy, replacement with plasma-derived concentrates is the treatment of choice. Because prospective studies are lacking, such treatment has been largely empirical. A multicenter, prospective study has been conducted in 81 patients with VWD (15 patients with type 1, 34 with type 2, and 32 with type 3 disease) to investigate the efficacy of a high-purity factor VIII/von Willebrand factor (FVIII/VWF) concentrate for treatment of bleeding and surgical prophylaxis. Two preparations of the concentrate—one virally inactivated with solvent detergent, the other with an additional heat-treatment step—were evaluated. Pharmacokinetic parameters were similar for both preparations. Using pre-established dosages based on the results of pharmacokinetic studies, 53 patients were administered either preparation for the treatment of 87 bleeding episodes, and 39 patients were treated prophylactically for 71 surgical or invasive procedures. Sixty-five (74.7%) and 10 (11.5%) of the bleeding episodes were controlled with 1 or 2 infusions, respectively. Patients with severe type 3 VWD typically required more infusions and higher doses, at shorter time intervals, than did patients with generally milder types 1 and 2. Among patients undergoing surgical procedures, blood loss was lower than that predicted prospectively, and losses exceeding the predicted value did not correlate with the postinfusion skin bleeding time. In conclusion, the concentrate effectively stopped active bleeding and provided adequate hemostasis for surgical or invasive procedures, even in the absence of bleeding time correction.
Introduction
Von Willebrand disease (VWD) is the most common hereditary bleeding disorder, affecting both males and females with an estimated prevalence of 1% in the population worldwide.1,2 VWD results from a quantitative or a qualitative abnormality of von Willebrand factor (VWF), a large multimeric adhesive glycoprotein contained in plasma, platelets, and endothelial cells. As a critical component of hemostasis, VWF initiates platelet adhesion at sites of vascular injury, transports coagulation factor VIII (FVIII) to sites where it can participate in the formation of fibrin clots, and protects FVIII from in vivo proteolysis. Patients with VWD usually manifest a dual hemostatic defect characterized by a prolonged skin bleeding time and low plasma levels of FVIII coagulant activity (FVIII:C).3
VWD is heterogeneous.3 Seventy percent to 80% of patients have type 1 VWD, in which there is a decrease in the quantity of VWF but no functional abnormality. It is usually characterized by relatively mild symptoms; spontaneous soft-tissue bleeding is rare, excessive postoperative bleeding is unpredictable, and mucosal tract bleeding, typically epistaxis and menorrhagia, is usually not severe. Approximately 20% of patients have type 2 VWD, which has qualitative deficiencies of VWF and is functionally abnormal in its interactions with platelets. Bleeding in mucosal tracts is frequent in patients with type 2 VWD, whereas soft-tissue bleeding is rare because FVIII:C levels are usually higher than they are in type 1. Approximately 1% to 3% of patients have type 3 VWD, which has virtually a complete absence of VWF and very low levels of FVIII:C. Patients with type 3 disease have more severe clinical symptoms, such as hemarthroses, muscle hematomas, and consistent postoperative and postpartum bleeding.
Mild forms of VWD can be treated with desmopressin acetate (1-desamino-8-D-arginine vasopressin), which acts efficaciously to prevent or stop bleeding by releasing endogenous VWF and FVIII from storage sites.4 Patients with more severe forms of VWD, however, do not respond to desmopressin or may have contraindications to its use.5 In addition, tachyphylaxis may develop in those initially responsive if repeated infusions are given to maintain hemostasis (eg, in the postpartum period or after surgery).6 Thus, in 20% to 30% of patients, the replacement of VWF and FVIII with plasma-derived concentrates is the mainstay of treatment.7
Cryoprecipitate, derived from fresh-frozen plasma thawed at 4°C, is no longer considered the optimal replacement because of the risk for transfusion-associated viral infection and the inconvenience of storage and preparation for use. Virally attenuated plasma-derived FVIII/VWF concentrates, originally developed for the treatment of hemophilia A, are used in desmopressin-unresponsive patients.8-12 A prospective study of replacement therapy in VWD has not been previously conducted. Therapy has been largely empirical, not tailored to different VWD types, and it has been based primarily on FVIII for dosing recommendations.13 Therefore, the main objective of this prospective study was to assess the efficacy of a FVIII/VWF concentrate in patients known not to respond adequately to desmopressin or to have a contraindication to this compound; dosage was based on the content of VWF, the protein missing or dysfunctional in VWD.
Patients and methods
Patients
We conducted a multicenter, prospective study of eligible patients recruited at 27 hemophilia centers in the United States and Europe between 1993 and 1998. Patients of both sexes were eligible for the study if they had hereditary VWD, were 7 years of age or older, and met one of the following criteria: were known not to respond adequately to desmopressin, had a contraindication to this compound, or required supplemental treatment in the past. Inadequate response to desmopressin was defined as little or no increase in plasma FVIII:C level, VWF ristocetin cofactor activity (VWF:RCof) level and no shortening in the skin bleeding time. Patients recruited to participate in the early pharmacokinetic study and in the crossover pharmacokinetic study were not experiencing bleeding episodes at the time of the study. Patients enrolled in the subsequent phases of the study required treatment for non–life-threatening bleeding episodes, and those enrolled in the surgical prophylaxis portion of the study were scheduled for various types of surgical or invasive diagnostic procedures judged to require bleeding prophylaxis. Eighty-seven patients were enrolled in the various phases of the study, but 6 never underwent treatment. Patients were classified by VWD subtype according to the criteria of Sadler.14 The study protocol and the informed consent form were approved by the ethics committees of the participating institutions.
FVIII/VWF concentrates
Alphanate Solvent Detergent (A-SD) (Alpha Therapeutic, Los Angeles, CA) is prepared from pooled human plasma by cryoprecipitation and further purification by using heparin-coupled, cross-linked agarose with affinity for the heparin-binding domain of VWF. The preparation is treated with a mixture of tri(n-butyl) phosphate (solvent) and polysorbate 80 (detergent) to reduce the risk for transmission of infectious viruses. Each vial was labeled with the FVIII:C and the VWF:RCof potency, expressed in international units as defined by the World Health Organization.
Alphanate Solvent Detergent/Heat Treated (A-SD/HT) (Alpha Therapeutic) is prepared similarly to A-SD but with an added step of heat treatment at 80°C for 72 hours to provide an additional safeguard against non–lipid-enveloped viruses resistant to solvent detergent alone. Results of in vitro inactivation studies documenting the effect of the heat treatment step on reduction of surrogate non–lipid-enveloped viruses are contained in the package insert for the product.
Study design
Our study was originally designed to assess the efficacy of A-SD in the treatment of bleeding episodes and for bleeding prophylaxis for elective surgery or invasive procedures. Dosages used for treatment of bleeding episodes and for bleeding prophylaxis were based on the results of pharmacokinetic studies conducted early during the study (data not shown). When A-SD/HT became available, an open-label, randomized, crossover study was conducted in 12 patients with type 3 VWD from 8 different kindreds (4 sibling pairs) at 4 centers (1 in Italy and 3 in the United States) to compare the pharmacokinetics of the 2 preparations. The protocol was amended by substituting A-SD/HT for A-SD for the treatment of bleeding episodes and for bleeding prophylaxis. Doses and treatment schedules used for the treatment of bleeding episodes and for bleeding prophylaxis were identical for the 2 preparations. Patients were eligible to participate in more than one phase of the study.
Crossover pharmacokinetic study.
Twelve patients with type 3 VWD were administered, in random order, one infusion of either A-SD or A-SD/HT at a dose of 60 VWF:RCof IU/kg, followed in 7 or more days by an identical infusion of the other preparation (median, 109 days; range, 7-186 days). This dosage was chosen because, on the basis of data available from the literature13 and of pharmacokinetic studies conducted early during this study (data not shown), it was expected that plasma levels would be attained in excess of 100 IU/dL (100% of normal). For each infusion, blood samples for platelet count, plasma levels of VWF:RCof, FVIII:C, and VWF:Ag, and determination of VWF multimeric distribution at a central laboratory (Special Coagulation Laboratory, Pathology Specialty Services, Miami, FL) were obtained at baseline and at 0.25, 1, 3, 6, 12, 24, and 48 hours after infusion. Platelet-poor plasma was obtained by centrifugation of venous blood collected in 0.129 M trisodium citrate. Plasma samples were snap-frozen and stored at −70°C before testing or shipping in dry ice. Skin bleeding times were determined at baseline and at 1, 6, and 24 hours after infusion.
Treatment of bleeding episodes.
Patients were infused with A-SD or A-SD/HT, 40 VWF:RCof IU/kg (50 VWF:RCof IU/kg in pediatric patients) for the treatment of bleeding episodes. The initial dose was chosen on the basis of the results of pharmacokinetic studies, and the goal was to raise patients' VWF levels to 100 IU/dL (100% of normal) regardless of initial VWF levels. The dose used in pediatric patients was 50 VWF:RCof IU/kg to account for an average 20% greater plasma volume, on a per kilogram basis, in persons younger than 18.15 Additional doses, if required, were not standardized, and dosing intervals were based on the clinical judgment of each investigator. No patients treated under this part of the study were excluded from analysis.
Prophylaxis for elective surgery or invasive procedures.
An infusion of A-SD (or A-SD/HT), 60 VWF:RCof IU/kg (75 VWF:RCof IU/kg in pediatric patients) was given before surgical or invasive procedures. This dosage was intended to achieve a plasma level in excess of 100%. A blood sample was obtained at 15 minutes after the first infusion for a FVIII:C level and at 1 hour after infusion to determine levels of VWF:RCof, FVIII:C, VWF:Ag, VWF multimeric distribution, and platelet count. Bleeding time was determined before and 30 minutes after infusion. Quantity of treatment to be given after the preoperative infusion was not standardized. Additional concentrate was administered at doses and intervals based on the investigators' determination of the need for further treatment, which was in turn based on type and severity of the procedure and on the results of laboratory measurements. The use of cryoprecipitate, alternate factor VIII/VWF concentrate, and platelet transfusion was not allowed. Treatment with the antifibrinolytic agent tranexamic acid was allowed during oral surgery and in presence of mucosal tract bleeding because this adjuvant drug is the standard of care for patients with coagulopathies. Platelet transfusion was allowed for patients with type 2B VWD because thrombocytopenia is sometimes associated with this subtype. Before surgery, the surgeons were asked to provide an estimation of expected blood loss for a healthy person of the same sex and of similar height and age as the patient. For each patient the estimated intra-operative blood loss was obtained from the operative report or as noted elsewhere in the patient's record. No patients treated under this part of the study were excluded from analysis.
Laboratory tests
Skin bleeding times were performed on the forearm by making 2 incisions with Simplate devices (Organon Teknika, Durham, NC) (in 17 centers) or other template devices (in 3 centers). If incisions were bleeding at 30 minutes, the test was stopped, and bleeding time was recorded as longer than 30 minutes. Bleeding time was considered corrected if it was equal to or shorter than the laboratory upper limit of normal (from 7 to 9.5 minutes, depending on the center and the device used) and partially corrected if it was shortened by more than 30% from the patient's baseline value.16
One-stage FVIII:C assays were performed at each participating site using various sources of FVIII-deficient substrate plasmas and reference plasmas. VWF:RCof was measured by aggregometric methods using lyophilized platelets from Bio/Data (Horsham, PA) and ristocetin (ABP, Marlton, NJ) at a concentration of 1.0 mg/mL. VWF:Ag was measured by enzyme immunoassay (Diagnostica Stago, Parsippany, NJ) in most clinical centers. A few centers used the Laurell electroimmunoassay, which is known to be sensitive to multimer distribution and tends to overestimate VWF:Ag levels when large multimers are lacking, as they are in type 2 VWD. We chose to combine the results of the 2 assays because VWF:Ag values are thought to be of little relevance in the management of patients with type 2 VWD. Calibrator plasma for all assays is traceable to the Third International Standard for FVIII and VWF. All values were expressed as percentage normal relative to the calibrator plasma. Multimeric analysis of VWF in concentrates was carried out in the Miami Central Laboratory by sodium dodecyl sulfate (SDS) 1.2% or 1.7% agarose gel electrophoresis, and bands were visualized by autoradiography using a F(ab')2 rabbit anti–human VWF antibody (Diagnostica Stago).17
Pharmacokinetic analysis
In vivo decay curves of plasma VWF:RCof, FVIII:C, and VWF:Ag were analyzed by noncompartmental pharmacokinetic methods.18 19 In vivo incremental recovery values were calculated from the increase of VWF:RCof, FVIII:C, and VWF:Ag above baseline 15 minutes after the completion of infusion divided by the amount of the component administered intravenously and expressed in IU/kg. Pharmacokinetic parameters for A-SD and A-SD/HT were compared by a repeated-measures mixed model using treatment, sequence, and period effect as factors.
Results
Fifteen lots of A-SD and 7 lots of A-SD/HT were used in the crossover pharmacokinetic, bleeding episode treatment, and bleeding prophylaxis portions of the study. The median FVIII:C to VWF:RCof ratio of the 2 products was 1.6, with a range from 1.0 to 2.6. Both preparations, electrophoretically run at an agarose concentration of 1.7%, lacked a fraction of the high–molecular-weight VWF multimers present in normal plasma, as also seen from the lower calculated percentage of such multimers (Figure 1, Table 1).
SDS agarose electrophoresis VWF multimer analysis of paired lots of A-SD and A-SD/HT.
Gels were prepared in 1.7% agarose.
SDS agarose electrophoresis VWF multimer analysis of paired lots of A-SD and A-SD/HT.
Gels were prepared in 1.7% agarose.
VWF multimer analyses
| Samples . | Low–molecular-weight multimers, % . | High–molecular-weight multimers, % . |
|---|---|---|
| Normal plasma (control) | 46 | 54 |
| Lot AP3034A | 54 | 46 |
| Lot AP3034A + heat treatment | 60 | 40 |
| Lot AP3020 | 61 | 39 |
| Lot AP3020 + heat treatment | 65 | 36 |
| Lot AP3018A | 63 | 37 |
| Lot AP3018A + heat treatment | 63 | 37 |
| Samples . | Low–molecular-weight multimers, % . | High–molecular-weight multimers, % . |
|---|---|---|
| Normal plasma (control) | 46 | 54 |
| Lot AP3034A | 54 | 46 |
| Lot AP3034A + heat treatment | 60 | 40 |
| Lot AP3020 | 61 | 39 |
| Lot AP3020 + heat treatment | 65 | 36 |
| Lot AP3018A | 63 | 37 |
| Lot AP3018A + heat treatment | 63 | 37 |
High–molecular-weight multimers were determined from densitometric scans and were defined as the area under the 5 molecular peaks from the high–molecular-weight end of the scan as described by Cumming et al.20
Eighty-one patients overall (50 female, 31 male; 72 white, 6 black, 3 Hispanic) were treated in one or more phases of the study. Fifteen patients had severe type 1 VWD, 29 had type 2A, 5 had type 2B, and 32 had type 3. Forty-five patients participated in only one phase of the study, including 24 who participated only in the early or crossover pharmacokinetic studies, one patient who participated only in the study on the treatment of bleeding episode, and 20 patients who participated only in the surgical prophylaxis study. Thirty-six patients were enrolled in multiple phases of the study. Overall, 14 patients (3 with type 1, 7 with type 2A, 4 with type 3 VWD) were treated for bleeding episodes, and 39 patients (6 with type 1, 17 with type 2A, 2 with type 2B, 14 with type 3 VWD) were administered prophylaxis for surgical or invasive procedures.
The median age of all 81 patients was 34 (range, 7-75) years. Eighteen patients were younger than 18. Baseline bleeding times and plasma levels of VWF:RCof, FVIII:C, and VWF:Ag for each VWD subtype are summarized in Table 2. Patients with type 3 disease had lower FVIII:C levels than did patients with other types, and VWF:RCof and VWF:Ag levels were below the limits of detection. In some type 1 patients, baseline values of VWF:RCof were normal; these results were likely to be attributed to variations or recent treatments in patients who historically met criteria for type 1 VWD. Median bleeding times were prolonged in all 4 VWD subtypes and were longest in patients with type 3 VWD.
Baseline laboratory values by subtype of von Willebrand disease
| VWD subtype . | Measurement . | |||
|---|---|---|---|---|
| VWF:Rcof, % median (range) . | FVIII:C, % median (range) . | VWF:Ag, % median (range) . | Bleeding time, min median (range) . | |
| Type 1 | 20 | 47 | 20 | 20 |
| (n = 15) | (6-80) | (14-145) | (0-68) | (3-30) |
| Type 2A | 19 | 56 | 49 | 26 |
| (n = 29) | (7-52) | (2-175) | (< 10-271) | (6-46) |
| Type 2B | 40 | 60 | 57 | > 30 |
| (n = 5) | (16-63) | (33-121) | (40-180) | (6-> 30) |
| Type 3 | < 10 | 6.0 | < 1 | > 30 |
| (n = 32) | (< 1-< 10) | (2-46) | (< 1-< 10) | (15-> 30) |
| Normal range | 56-150 | 54-158 | 52-160 | < 8-9.5 |
| VWD subtype . | Measurement . | |||
|---|---|---|---|---|
| VWF:Rcof, % median (range) . | FVIII:C, % median (range) . | VWF:Ag, % median (range) . | Bleeding time, min median (range) . | |
| Type 1 | 20 | 47 | 20 | 20 |
| (n = 15) | (6-80) | (14-145) | (0-68) | (3-30) |
| Type 2A | 19 | 56 | 49 | 26 |
| (n = 29) | (7-52) | (2-175) | (< 10-271) | (6-46) |
| Type 2B | 40 | 60 | 57 | > 30 |
| (n = 5) | (16-63) | (33-121) | (40-180) | (6-> 30) |
| Type 3 | < 10 | 6.0 | < 1 | > 30 |
| (n = 32) | (< 1-< 10) | (2-46) | (< 1-< 10) | (15-> 30) |
| Normal range | 56-150 | 54-158 | 52-160 | < 8-9.5 |
Value ranges of some measurements, particularly for patients with type 1 and type 2 VWD, include some values that are within the normal range for that measurement. They are likely to be explained by variation in results for patients who historically met criteria for VWD or by previous treatments not reported by the patient.
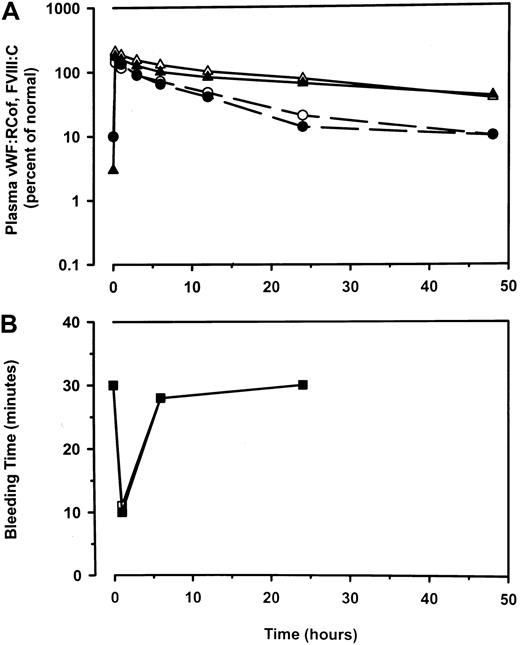
Crossover pharmacokinetic study
Eight lots of concentrate were used in the crossover pharmacokinetic study (median FVIII:C to VWF:RCof ratio, 1.6; range, 1.0-2.6). Median plasma levels of VWF:RCof, FVIII:C, and bleeding times before and after infusion of A-SD and A-SD/HT (60 VWF:RCof IU/kg) in 11 of 12 patients with type 3 VWD are shown in Figure2. Data from one patient were excluded from analysis because, during the second infusion (A-SD), she was found to have in vivo recovery of FVIII:C, VWF:Ag, and VWF:RCof of approximately 50% that observed after the first infusion (A-SD/HT). Inhibitor testing on the second preinfusion sample revealed a weak inhibitor to FVIII:C of 0.5 Bethesda units (normal, less than 0.4). Although no inhibitors were found in her plasma on enrollment, on retrospective questioning the patient recalled having been told by other physicians that she had a weak inhibitor to FVIII/:C.
Median values of plasma levels.
VWF:RCof (circles, dash-dot lines) and FVIII:C (triangles, solid lines) (A) and of bleeding times (squares, short dash lines) (B) before and after administration of A-SD (open symbols) and A-SD/HT (closed symbols), 60 IU/kg VWF:RCof, in 11 patients with type 3 VWD. Before treatment, median plasma levels of VWF:RCof, FVIII:C, and VWF:Ag were very low or were below detection, and bleeding times were longer than 30 minutes. Plasma levels shown at baseline reflect the lower limits of detection.
Median values of plasma levels.
VWF:RCof (circles, dash-dot lines) and FVIII:C (triangles, solid lines) (A) and of bleeding times (squares, short dash lines) (B) before and after administration of A-SD (open symbols) and A-SD/HT (closed symbols), 60 IU/kg VWF:RCof, in 11 patients with type 3 VWD. Before treatment, median plasma levels of VWF:RCof, FVIII:C, and VWF:Ag were very low or were below detection, and bleeding times were longer than 30 minutes. Plasma levels shown at baseline reflect the lower limits of detection.
Plasma levels at 15 minutes after infusion were similar after infusions of A-SD and of A-SD/HT. With each preparation there was a disproportionate increase of FVIII:C level relative to VWF measurements, especially relative to VWF:RCof, in the late postinfusion times. Median bleeding time at baseline was longer than 30 minutes; at 1 hour after infusion, it was shortened to 10.5 minutes (range, 4.5-20.8 minutes). Bleeding time was partially corrected in 14 infusions and completely corrected in 8 infusions (7 partial corrections and 4 complete corrections for both A-SD and A-SD/HT). The patient excluded from analysis had partial correction with A-SD/HT and complete correction with A-SD. VWF multimers, undetectable before the infusion, appeared in the plasma of all patients but lacked the high–molecular-weight fraction the infused concentrate had. A representative pattern of multimer distribution on 1.2% agarose gel electrophoresis is shown in Figure 3.
SDS agarose electrophoresis VWF plasma multimer analysis before and after infusion of A-SD/HT (60 IU/kg VWF:RCof) in a representative patient with type 3 VWD.
Gels were prepared in 1.2% agarose. Lane 1, normal pooled plasma; lane 2, before infusion; lane 3, 15 minutes after infusion; lanes 4 through 9, 1, 3, 6, 12, 24, and 48 hours after infusion. Increased size in circulating VWF multimers was observed for more than 24 hours after infusion.
SDS agarose electrophoresis VWF plasma multimer analysis before and after infusion of A-SD/HT (60 IU/kg VWF:RCof) in a representative patient with type 3 VWD.
Gels were prepared in 1.2% agarose. Lane 1, normal pooled plasma; lane 2, before infusion; lane 3, 15 minutes after infusion; lanes 4 through 9, 1, 3, 6, 12, 24, and 48 hours after infusion. Increased size in circulating VWF multimers was observed for more than 24 hours after infusion.
Mean in vivo half-lives of FVIII:C, VWF:Ag, and VWF:RCof for A-SD and A-SD/HT were 20.9 versus 23.8 hours, 12.4 versus 12.9 hours, and 7.1 versus 6.5 hours, respectively. Mean in vivo incremental recoveries for FVIII:C and VWF:RCof were 2.0 versus 2.1% per IU/kg and 2.5 versus 2.9% per IU/kg, respectively. These and other pharmacokinetic parameters for A-SD and A-SD/HT were not statistically different (Table3). Moreover, the effects of each product on the bleeding time and the pattern of VWF multimers were similar. This overall equivalence led us to combine the clinical results obtained with each preparation.
Summary of pharmacokinetic analysis in 11 patients with type 3 von Willebrand disease
| Parameter . | Plasma VWF:RCof . | Plasma FVIII:C . | Plasma VWF:Ag . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD . | Mean ± SD . | Mean ± SD . | |||||||
| A-SD . | A-SD/HT . | P . | A-SD . | A-SD/HT . | P . | A-SD . | A-SD/HT . | P . | |
| AUCINF | 1295 ± 568 | 1403 ± 918 | .82 | 4995 ± 1843 | 5054 ± 1600 | .95 | 3972 ± 988 | 4369 ± 1442 | .42 |
| CL | 0.05 ± 0.025 | 0.06 ± 0.028 | .84 | 0.01 ± 0.005 | 0.01 ± 0.004 | .78 | 0.02 ± 0.004 | 0.02 ± 0.005 | .63 |
| Vss | 0.50 ± 0.139 | 0.51 ± 0.269 | .92 | 0.38 ± 0.095 | 0.43 ± 0.115 | .32 | 0.28 ± 0.052 | 0.27 ± 0.086 | .97 |
| T1/2 | 7.1 ± 2.89 | 6.5 ± 2.41 | .65 | 20.9 ± 6.68 | 23.8 ± 6.15 | .23 | 12.4 ± 2.13 | 12.9 ± 2.06 | .60 |
| Cmax | 152 ± 44.4 | 180 ± 85.8 | .37 | 192 ± 40.9 | 175 ± 56.3 | .35 | 272 ± 67.2 | 281 ± 96.5 | .90 |
| Tmax | 0.39 ± 0.30 | 0.32 ± 0.23 | .30 | 0.3 ± 0.2 | 0.4 ± 0.3 | .30 | 0.32 ± 0.23 | 0.32 ± 0.23 | .90 |
| Incremental in vivo recovery | 2.5 ± 0.7 | 2.9 ± 1.3 | .32 | 2.04 ± 0.38 | 2.11 ± 0.44 | .58 | ND | ND | — |
| Parameter . | Plasma VWF:RCof . | Plasma FVIII:C . | Plasma VWF:Ag . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD . | Mean ± SD . | Mean ± SD . | |||||||
| A-SD . | A-SD/HT . | P . | A-SD . | A-SD/HT . | P . | A-SD . | A-SD/HT . | P . | |
| AUCINF | 1295 ± 568 | 1403 ± 918 | .82 | 4995 ± 1843 | 5054 ± 1600 | .95 | 3972 ± 988 | 4369 ± 1442 | .42 |
| CL | 0.05 ± 0.025 | 0.06 ± 0.028 | .84 | 0.01 ± 0.005 | 0.01 ± 0.004 | .78 | 0.02 ± 0.004 | 0.02 ± 0.005 | .63 |
| Vss | 0.50 ± 0.139 | 0.51 ± 0.269 | .92 | 0.38 ± 0.095 | 0.43 ± 0.115 | .32 | 0.28 ± 0.052 | 0.27 ± 0.086 | .97 |
| T1/2 | 7.1 ± 2.89 | 6.5 ± 2.41 | .65 | 20.9 ± 6.68 | 23.8 ± 6.15 | .23 | 12.4 ± 2.13 | 12.9 ± 2.06 | .60 |
| Cmax | 152 ± 44.4 | 180 ± 85.8 | .37 | 192 ± 40.9 | 175 ± 56.3 | .35 | 272 ± 67.2 | 281 ± 96.5 | .90 |
| Tmax | 0.39 ± 0.30 | 0.32 ± 0.23 | .30 | 0.3 ± 0.2 | 0.4 ± 0.3 | .30 | 0.32 ± 0.23 | 0.32 ± 0.23 | .90 |
| Incremental in vivo recovery | 2.5 ± 0.7 | 2.9 ± 1.3 | .32 | 2.04 ± 0.38 | 2.11 ± 0.44 | .58 | ND | ND | — |
AUCINF indicates area under the plasma concentration curve from zero to infinity (% h); CL, clearance (U/kg)/(% h); Vss, volume at steady state (μm/kg per percentage); T1/2, half-life (h); Cmax, maximum concentration (%); Tmax, time to maximum concentration (h); incremental in vivo recovery, % IU/kg; ND, not done.
Patients with type 3 VWD were administered, in random order, 1 infusion of either A-SD or A-SD/HT at a dose of 60 IU/kg WF:RCof, followed 7 or more days later by an identical infusion of the other preparation.P values are from a repeated-measures mixed model with treatment, sequence, and period effects comparing A-SD and A-SD/HT, based on the actual data (no transformations).
Treatment of bleeding episodes
Fourteen patients were given 135 infusions of either preparation for 87 bleeding episodes. Doses administered for the treatment of bleeding episodes for each VWD type and for treatment intervals are summarized in Table 4. All the bleeding episodes were ultimately controlled by concentrate infusion. On average, however, patients with type 3 VWD required more infusions (3.0 vs 1.0) to stop bleeding, and at shorter time intervals (median, 16 vs 24 hours) than did patients with type 2A VWD. Median initial treatment dosage was 40 VWF:RCof IU/Kg for patients with type 2A and type 3 VWD. Median dosage for subsequent infusions, chosen by each investigator, was higher in patients with type 3 than in patients with type 2A VWD (60 vs 40 VWF:RCof IU/Kg), probably a reflection that bleeding was more severe and less promptly controlled in type 3. Only one patient with type 1 VWD required a second infusion, at a dosage of 55 VWF:RCof IU/Kg. An oral antifibrinolytic agent was administered during 8 (9.2%) bleeding episodes. The most common bleeding sites were the gastrointestinal tract (72.4%), nasopharynx (14.9%), musculoskeletal system (9.2%), and genitourinary tract (3.4%). Patients with type 2A VWD bled predominantly from mucosal tracts, whereas patients with type 3 VWD also had musculoskeletal bleeding episodes (hemarthrosis, muscle hematomas). Table 5 shows that musculoskeletal and genitourinary bleeding required, on average, more treatments per bleeding episode than did gastrointestinal and nasopharyngeal bleeding (median, 3.5 and 3.0 vs 1.0) and higher median overall dosages (55 and 60 vs 40 VWF:RCof IU/Kg).
Doses used to treat bleeding episodes by subtype of von Willebrand disease
| . | VWD subtype . | ||
|---|---|---|---|
| Type 1 . | Type 2A . | Type 3 . | |
| No. patients | 3 | 7 | 4 |
| No. bleeding episodes | 4 | 67 | 16 |
| Total no. infusions | 5 | 86 | 44 |
| Median no. infusions per bleeding episode (range) | 1.0 (1-2) | 1.0 (1-7) | 3.0 (1-6) |
| Median VWF:RCof dosage per infusion, IU/kg | |||
| 1 infusion (range) | 45 (33-56) | 40 (14-50) | 40 (14-79) |
| 2 or more infusions (range) | 55 (55-55) | 40 (14-50) | 60 (19-79) |
| All infusions (range) | 50 (33-56) | 40 (14-50) | 60 (14-79) |
| Median treatment interval, h (range) | 24 (24-24) | 24 (20-71) | 16 (7-69) |
| . | VWD subtype . | ||
|---|---|---|---|
| Type 1 . | Type 2A . | Type 3 . | |
| No. patients | 3 | 7 | 4 |
| No. bleeding episodes | 4 | 67 | 16 |
| Total no. infusions | 5 | 86 | 44 |
| Median no. infusions per bleeding episode (range) | 1.0 (1-2) | 1.0 (1-7) | 3.0 (1-6) |
| Median VWF:RCof dosage per infusion, IU/kg | |||
| 1 infusion (range) | 45 (33-56) | 40 (14-50) | 40 (14-79) |
| 2 or more infusions (range) | 55 (55-55) | 40 (14-50) | 60 (19-79) |
| All infusions (range) | 50 (33-56) | 40 (14-50) | 60 (14-79) |
| Median treatment interval, h (range) | 24 (24-24) | 24 (20-71) | 16 (7-69) |
Median treatment interval was measured from preceding infusion, across all infusions.
Relation between dosage and bleeding site
| . | Bleeding site . | |||
|---|---|---|---|---|
| Gastrointestinal . | Nasopharynx . | Musculoskeletal . | Gynecologic . | |
| No. bleeding episodes | 63 | 13 | 8 | 3 |
| Total no. infusions administered | 86 | 14 | 26 | 9 |
| Median no. infusions per bleeding episode (range) | 1.0 (1-7) | 1.0 (1-2) | 3.5 (1-5) | 3.0 (2-4) |
| Median VWF:Rcof dosage per infusion, IU/kg (range) | 40 (14-60) | 40 (14-56) | 55 (19-79) | 60 (38-60) |
| . | Bleeding site . | |||
|---|---|---|---|---|
| Gastrointestinal . | Nasopharynx . | Musculoskeletal . | Gynecologic . | |
| No. bleeding episodes | 63 | 13 | 8 | 3 |
| Total no. infusions administered | 86 | 14 | 26 | 9 |
| Median no. infusions per bleeding episode (range) | 1.0 (1-7) | 1.0 (1-2) | 3.5 (1-5) | 3.0 (2-4) |
| Median VWF:Rcof dosage per infusion, IU/kg (range) | 40 (14-60) | 40 (14-56) | 55 (19-79) | 60 (38-60) |
Prophylaxis of elective surgery and invasive procedures
Thirty-nine patients (6 with type 1, 17 with type 2A, 2 with type 2B, 14 with type 3 VWD) were administered either product for prophylaxis of bleeding in 71 surgical or invasive diagnostic procedures (Table 6). This included 10 gastrointestinal endoscopic procedures. Biopsy samples were required in only 3 of the 10. The other 7 procedures have been included in this analysis because the potential for direct mucosal trauma during the procedure was judged to require bleeding prophylaxis. An antifibrinolytic agent was administered during 8 (11.3%) surgical procedures, mainly in the oral cavity. The median number of infusions per surgical procedure was 3.0, the dosages for the first and subsequent infusions were 60 IU/kg and 40 IU/kg VWF:RCof, respectively, and the median treatment interval across all infusions was 24 hours (Table 6). Overall, in 63 surgical procedures to correct prolonged bleeding time, the 30-minute postinfusion test showed that it was fully corrected in 25 (39.7%) patients, partially corrected in 25 (39.7%) patients, demonstrated no correction in 12 (19.0%) patients, and was not done in one patient.
Prophylaxis during surgery or invasive procedures for 39 patients
| Description of procedure . | No. procedures . |
|---|---|
| Dental extractions (1 to 10 teeth) | 16 |
| Dental scaling/root planing | 6 |
| Gastroscopy/colonoscopy (3 with biopsy samples) | 10 |
| Invasive vascular procedures | 8 |
| Laparoscopic surgery | 5 |
| Abdominal laparotomic surgery | 4 |
| Orthopedic surgery | 9 |
| Ear, nose, and throat surgery | 2 |
| Inguinal hernial repairs | 2 |
| Pulmonary procedures (percutaneous lung aspiration, thoracentesis) | 2 |
| Urologic procedures | 2 |
| Dermatologic procedures (superficial tumor removal) | 2 |
| Miscellaneous procedures (hemorrhoidectomy, enteroclysis, cervical excision) | 3 |
| Total | 716-150 |
| Total no. infusions | 298 |
| Median no. infusions per procedure (range) | 3.0 (1-18) |
| Median WF:RCof dosage (IU/kg) | |
| 1 infusion (range) | 60 (20-76) |
| 2 or more infusions combined (range) | 40 (10-75) |
| Median treatment interval (range) | 24 (1.3-721) |
| Description of procedure . | No. procedures . |
|---|---|
| Dental extractions (1 to 10 teeth) | 16 |
| Dental scaling/root planing | 6 |
| Gastroscopy/colonoscopy (3 with biopsy samples) | 10 |
| Invasive vascular procedures | 8 |
| Laparoscopic surgery | 5 |
| Abdominal laparotomic surgery | 4 |
| Orthopedic surgery | 9 |
| Ear, nose, and throat surgery | 2 |
| Inguinal hernial repairs | 2 |
| Pulmonary procedures (percutaneous lung aspiration, thoracentesis) | 2 |
| Urologic procedures | 2 |
| Dermatologic procedures (superficial tumor removal) | 2 |
| Miscellaneous procedures (hemorrhoidectomy, enteroclysis, cervical excision) | 3 |
| Total | 716-150 |
| Total no. infusions | 298 |
| Median no. infusions per procedure (range) | 3.0 (1-18) |
| Median WF:RCof dosage (IU/kg) | |
| 1 infusion (range) | 60 (20-76) |
| 2 or more infusions combined (range) | 40 (10-75) |
| Median treatment interval (range) | 24 (1.3-721) |
Median treatment interval was measured from preceding infusion, across all infusions.
Intention to treat population. One patient had 2 orthopedic procedures canceled after concentrate administration.
Mean actual blood loss for the combined surgical procedures was less than the prospectively estimated value, 173.5 ± 231.8 mL (median, 50 mL; range, 0-1000 mL) versus 141.8 ± 341.9 mL (median, 25 mL; range, 0-2500 mL), respectively (P = .0001). Only 3 patients had bleeding that exceeded the amount predicted by more than 50 mL; the bleeding time 30 minutes after infusion was normal in 1 patient and only slightly lengthened in 2 patients. Mitigating factors were present in each of these 3 patients. In contrast, in each of the 9 patients in whom the postinfusion bleeding time was longer than 30 minutes and in all 22 additional patients in whom the postinfusion bleeding time was between 10 and 30 minutes, blood loss was less than predicted or was no more than 50 mL over that predicted. One patient with type 2B VWD and preoperative thrombocytopenia received 6 U platelets after surgery. No other patient required an alternative FVIII/VWF concentrate or platelet transfusions.
Safety
The proportion of patients with adverse events possibly, probably, or definitely related to treatment was similar for A-SD and A-SD/HT—9 of 66 (13.6%) and 5 of 36 (13.9%) patients, respectively. Most adverse events were mild. Two patients, both with type 3 VWD, had reduced in vivo recovery during the course of the study. Anti-VWF alloantibodies were not studied in one patient, and enzyme-linked immunosorbent assay for anti-VWF gave conflicting results in the other. One patient, a 10-year-old girl with type 1 VWD, contracted B19 parvovirus infection, characterized by the transient appearance of erythema multiforme, 36 days after an infusion of A-SD. Serologic test results for immunoglobulin G (IgG) and IgM anti–B-19, negative before treatment, were positive on day 35 after the infusion.
Two patients had thrombotic complications. A 30-year-old man with type 2A VWD was given prophylaxis with A-SD for surgical resection of volvulus involving the sigmoid colon. Superficial thrombophlebitis developed in his left arm 12 hours after 4 infusions of A-SD, 27 IU/kg VWF:RCof, given over 48 hours; plasma levels of VWF:RCof, FVIII:C, and VWF:Ag were 223%, 116%, and 298%, respectively. The intravenous line placed in the affected arm was changed, and the patient continued to receive infusions of A-SD without further complications. A 41-year-old man with type 3 VWD received prophylaxis with A-SD for surgical hemorrhoidectomy, with doses initially starting at 60 IU/kg VWF:RCof once daily on days 1 (surgical procedure) and 2, then de-escalating to 40 to 50 IU/kg VWF:RCof once a day on days 3, 5, 7, 9, 10, and 12. On day 13 (18 hours after the 8th infusion of A-SD on day 12), deep vein thrombosis developed in his right leg. Postinfusion plasma levels of VWF:RCof, FVIII:C, and VWF:Ag after the infusion of A-SD on day 12 were 191%, 248%, and 221%, respectively. The patient, who also had human immunodeficiency virus, had undergone arthrodesis to the right knee 25 years earlier, and it was impossible to mobilize him fully early after the surgical procedure. He was treated with heparin and oral anticoagulants and continued to receive treatment with A-SD during his hospital stay; subsequently, a permanent vena cava filter was applied to avoid further thromboembolic complications.
Discussion
A review of the literature discloses only case reports and small retrospective series of patients with VWD treated with plasma concentrates. The current study is the only large prospective study conducted of treatment and prophylaxis. It was designed to assess the efficacy of a FVIII/VWF concentrate for the control of bleeding and for bleeding prophylaxis in patients undergoing surgery or invasive procedures in whom desmopressin was either ineffective or contraindicated.
Early pharmacokinetic studies of A-SD and a crossover pharmacokinetic study of A-SD and A-SD/HT carried out in patients with type 3 VWD with unmeasurable plasma VWF levels and very prolonged bleeding times provided the basis for the concentrate regimens used in the clinical efficacy study. Overall mean in vivo incremental recoveries for VWF:RCof (2.7 IU/dL per IU/kg) and for FVIII:C (2.1 IU/dL per IU/kg) were similar to values reported for these and other FVIII/VWF concentrates.8-12 The mean half-life of FVIII:C in patients with VWD, approximately 24 hours, was substantially longer than the half-life seen in patients with hemophilia A, approximately 12 hours. This is because of the endogenous synthesis of FVIII in VWD after the administration of VWF.21 The half-life of VWF:RCof, approximately 7 hours, was substantially shorter than the half-life of VWF:Ag, approximately 13 hours, similar to values reported for another FVIII/VWF concentrate.12 Postinfusion shortening of bleeding time was transient and generally lasted less than 6 hours. Like other FVIII/VWF concentrates, both preparations lack a fraction of high–molecular-weight multimers.22 A multimeric pattern reproducing the defective multimeric pattern of the concentrates appeared in plasma after infusion, and the effect persisted for longer than 24 hours. These observations confirm the previously described dissociation between the bleeding time and the VWF multimeric integrity.22 23
Regimens used in this study for the treatment of bleeding episodes (initial dose, 40 IU/kg VWF:RCof) and for prophylaxis of bleeding at surgery (initial dose, 60 IU/kg VWF:RCof) were chosen with the arbitrary goal to achieve plasma levels in excess of 100 IU/dL (100% of normal) irrespective of the baseline level. They were highly effective—three fourths of the bleeding episodes required only one dose of concentrate to be controlled, and no treated patient required alternative therapy for control of hemorrhage. Patients with type 3 VWD needed a larger number of infusions per bleeding episode than patients with type 1 or 2 VWD; doses were higher and intervals between infusions were shorter. These differences likely reflect more severe disease in these patients. Numbers of infusions needed to control bleeding were greater for musculoskeletal and genitourinary bleeding than for gastrointestinal and nasopharynx bleeding. These clinical observations provide information and guidelines not previously available for treating patients with severe VWD. However, a limit of the study design was that only the first dose of the concentrate was standardized; the approach to subsequent doses was left to the choice of the individual physician. It cannot be excluded, therefore, that the results do not distinguish true need for more therapy from physician perception that more therapy was needed for patients with ostensibly more severe disease.
It is unclear whether the correction of the skin bleeding time is necessary for performing surgical procedures in patients with moderate and severe VWD. In this study, bleeding that exceeded the amount predicted prospectively did not correlate with whether there was correction of bleeding time. Excess bleeding did not occur during the 9 surgical procedures in which there was no correction of bleeding time after treatment, and no patient required alternative therapy for the control of hemorrhage. It is likely that the successful outcome of surgical procedures, imperfect correction of bleeding time notwithstanding, is related to having attained normal postoperative FVIII:C levels in all patients after concentrate infusion. As early as in 1963, Biggs and Matthews23demonstrated that normal levels of FVIII:C are the main determinants of surgical hemostasis in VWD, regardless of whether the bleeding time is corrected.
Treatment with concentrates was generally well tolerated. Two patients had reduced in vivo recovery, suggestive of alloantibody formation, though this was not documented in either of them. It has been estimated, based on 2 relatively large surveys, that alloantibodies to VWF develop in 7.5% and 9.5% of patients with type 3 VWD.24 One patient in the current study contracted B19 parvovirus infection after infusion of the solvent/detergent-treated preparation. B19 is not lipid-enveloped and is, therefore, resistant to inactivation by solvent detergent.25 The virus is also highly thermoresistant, so heat treatment does not afford additional protection.25 B19 infection is a well-known risk for all currently manufactured plasma coagulation products,25 but it has not been reported to be associated with serious clinical consequences when transmitted by factor concentrates.25 Venous thromboembolic complications developed in 2 patients, though it was mild in 1 and involved an arm vein phlebitis in the site of infusion of A-SD. In the second patient, a 41-year-old man, the development of deep vein thrombosis after surgery perhaps resulted from his previous orthopedic operation that led to poor mobilization in the postoperative period. There is also a possible contributing role for the high postoperative levels of factor VIII, known to be a risk factor for venous thromboembolism.26
In conclusion, for the first time, patients with VWD were treated with the same prospectively defined regimens for bleeding episodes and prophylaxis for surgical or invasive procedures, and dosages were based on the VWF rather than on the FVIII content of the preparations. This is also the first study to analyze the efficacy of the dosages used to treat patients with various subtypes of VWD by bleeding site, to analyze treatment intervals, and to provide evidence that surgery for VWD patients can be safely undertaken even if the bleeding time does not correct. Therefore, patients with VWD who do not respond to desmopressin can now be treated effectively using this concentrate, obviating the need to use cryoprecipitate. At the moment, it should not be extrapolated from this study that other available FVIII/VWF concentrates would behave in the same manner. Two prospective studies with similar designs are being conducted in Europe and the United States, and they should provide information in the near future about the use of other FVIII/VWF concentrates.
We thank the staff of the Pathology Specialty Services, Special Coagulation Laboratory, Miami, FL; Patrick Gallagher, Cheryl Graul, Lisa Potter, Roger Davies, and Sarah Broadhurst for monitoring the study; Bob Warnock and Keith Gregg for statistical analyses; Henry Eran for supplying the gel electrophoresis of A-SD and A-SD/HT; and Mary Darahdgian for support and excellent administrative assistance. Even though the database of this study was kept by Alpha Therapeutics, all the authors had full access to the data at any time during the study.
Participants in the Alphanate Study Group are as follows: Angelo Bianchi Bonomi Hemophilia and Thrombosis Centre, University of Milan, Italy; Pier M. Mannucci, Filippo Tradati, Augusto Federici, Illinois Masonic Medical Center, Chicago; Juan Chediak, Betty Maxey, University of Miami, FL; John J. Byrnes, Marlies Ledford, East Tennessee Comprehensive Hemophilia Center, Knoxville; Wahid T. Hanna, Cherys Zimmerman, George Washington University Medical Center, Washington, DC; Craig M. Kessler, Carolyn Francis, University of Kentucky Medical Center, Lexington; Ewa J. Marciniak, Susan Peterson, University of California at Davis, Sacramento; Jerry S. Powell, Janet Harrison, Brigham & Women's Hospital, Boston, MA; Bruce M. Ewenstein, Carol Sweeney, Children's Hospital Medical Center of Akron, OH; Carl E. Krill, Jr, Elizabeth Miller, University of Colorado Health Sciences Center, Denver; Sally P. Stabler, Sheryl Giambartolomei, St Michael's Medical Center, Newark, NJ; Alice J. Cohen, Bebet Navia, University of Louisville, KY; Benjamin Djulbegovic, Mary Marasa, The Milton S. Hershey Medical Center, Hershey, PA; M. Elaine Eyster, Sara Neagley, Children's Hospital, Los Angeles, CA; Jonathan Goldsmith, Robert Miller, Oxford Radcliffe Hospital, United Kingdom; David M. Keeling, Laura Oyesiku, Children's Hospital, Columbus, OH; Frederick B. Ruymann, Charmaine Biega, Children's Hospital, Oakland, CA; Joseph E. Addiego, Jr, Beth Chase, Arkansas Children's Hospital, Little Rock; David L. Becton, Berlinda McAdory, The Medical Center of Central Massachusetts, Worcester; Doreen B. Brettler, Patricia Forand, University of Missouri, Columbia; Nasrollah Hakami, Priscilla Lanigan, Manchester Royal Infirmary, United Kingdom; Charles R. M. Hay, Janet Goldstone, Long Island Jewish Medical Center, New Hyde Park, NY; Richard A. Lipton, Christine Pece, Children's National Medical Center, Washington, DC;Naomi L. C. Luban, Susan Shannan, The Royal Free Hospital, London, United Kingdom; John Pasi, Christine A. Lee, Ioana Nitu, University of Oklahoma Children's Hospital of Oklahoma, Oklahoma City; Charles L. Sexauer, Sara Hawk, Children's Mercy Hospital, Kansas City, MO; Brian M. Wicklund, Ann Mehrhof, Alpha Therapeutic Corporation, Los Angeles, CA; Anastassios Retzios, Barbara Kapelan, Patrick Gallagher, and Richard S. Schwartz (Consultant), Alpha Therapeutic Corporation, Los Angeles, CA.
Supported by a grant from Alpha Therapeutic Corporation, Los Angeles, CA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Pier M. Mannucci, University of Milan, Via Pace 9, 20122 Milan, Italy.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal