LFA-1 (CD11a/CD18, αLβ2) is an integrin expressed in a tissue-specific fashion and is important in inflammatory and immune responses. Promoter analysis has identified transcription factors that may be involved in CD11a expression, but the mechanisms contributing to its tissue-specific expression are incompletely characterized. In this report we have asked if DNA methylation and/or chromatin structure could contribute to tissue-specific CD11a expression. Bisulfite sequencing was used to compare methylation patterns in the promoter and 5′ flanking regions of the ITGAL gene, encoding CD11a, in normal human T cells, which express LFA-1, and fibroblasts, which do not. The region was found to be heavily methylated in fibroblasts but not T cells, and methylation correlated with an inactive chromatin configuration as analyzed by deoxyribonuclease 1 sensitivity. Patch methylation of the promoter region revealed that promoter activity was methylation-sensitive but that methylation of the 5′ flanking regions more than 500 base pairs 5′ to the transcription start site could also suppress promoter function. Treating fibroblasts with a DNA methylation inhibitor decreased ITGAL promoter methylation and increased CD11a messenger RNA. The results thus indicate that methylation and chromatin structure may contribute to the tissue-specific expression of CD11a.
Introduction
The integrin LFA-1 (CD11a/CD18, αLβ2) is a cell surface heterodimer expressed on leukocytes and mediates essential adhesive interactions by binding members of the intercellular adhesion molecule family.1 The importance of LFA-1 in inflammatory conditions is evidenced by the leukocyte adhesion deficiency syndrome, in which LFA-1 deficiency results in a lack of an inflammatory response and increased susceptibility to infectious diseases.2LFA-1 is also important in adhesive interactions between T cells and other cells of the immune system, including macrophages, dendritic cells, and B lymphocytes, and is essential for recruitment into sites of inflammation, antigen-specific T-cell activation, alloreactive responses, cytotoxic T-cell responses, natural killer responses, and B-cell help.3ITGAL, the gene encoding CD11a, is located on chromosome 16p11.2, near genes encoding other members of the integrin family, including CD11b and CD11c.4
The regulation of CD11a expression is complex and on T cells is affected by the state of activation as well as differentiation and aging.5,6 Deletional analysis has revealed that the first 40 base pairs (bp) 5′ to the major transcription start site are essential for promoter function, and sequence analysis shows that the promoter contains binding sites for Sp1 and PU.1, located in the first 120 bp 5′ to the start site.7,8 A 1.7-kilobase (kb) fragment containing the ITGAL promoter and 5′ flanking region has been shown to be sufficient to direct leukocyte-specific expression of the ITGAL promoter in transgenic mice, indicating that sequences directing tissue specificity of expression are located in this region.9 Similarly, transfection of reporter constructs containing this region into T cells, Hela, and K562 cells reveals that the promoter is preferentially expressed in T cells, although lower levels of expression are detectable in the other cells as well.8 The reason for expression of the reporter construct in nonmyeloid cells, while the native gene is not, is not known.
Recent evidence has persuasively shown that transcriptional suppression also involves the related mechanisms of promoter methylation and chromatin condensation.10 Methylation patterns and chromatin structure are typically established during differentiation and serve to prevent expression of genes not necessary for the function of a given cell type.11 It is therefore possible thatITGAL promoter function is suppressed in nonexpressing cells by methylation and/or changes in chromatin structure. In this report we have asked if alterations in methylation patterns and chromatin structure could contribute to ITGAL regulation. We compared methylation patterns and chromatin structure in the ITGALpromoter and flanking regions in 2 cell types discordant inITGAL expression: T cells and fibroblasts. The effects of regional methylation on ITGAL promoter function were tested by patch methylation. The studies indicate that both methylation and differences in chromatin structure may contribute to suppressITGAL expression in fibroblasts and other nonmyeloid cells.
Materials and methods
Cells and cell lines
Dermal fibroblasts from a healthy donor were donated by Dr Samir Hanash, and synovial fibroblasts from a patient with osteoarthritis were provided by Dr C. William Castor. T cells were isolated from the peripheral venous blood of healthy donors by density gradient centrifugation followed by e-rosetting, and monocytes were enriched by adherance, as described.12 T-cell purity was checked by staining with anti-CD3–fluorescein isothiocyanate and analysis by flow cytometry as described13 and was typically more than 93% CD3+. Monocyte enrichment was tested by staining with anti-CD14–fluorescein isothiocyanate and was typically about 35%. Where indicated the cells were stimulated with phytohemagglutinin (PHA) using previously published protocols.13 The CD4+ and CD8+subsets were isolated from purified T cells by magnetic cell sorting using CD4 and CD8 microbeads and protocols provided by the manufacturer (Miltenyi Biotec, Auburn, CA). Purity was checked by flow cytometry and was typically more than 94% CD4+ or CD8+. Jurkat cells were cultured as previously described.14Where indicated fibroblasts were treated with 1 μM 5-azacytidine (5-azaC) (Sigma, St Louis, MO) for 3 days.
Real-time RT-PCR
Real-time semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) was performed using a LightCycler (Roche, Indianapolis, IN) and previously published protocols.15 A series of 5 dilutions of 1 RNA sample were included to generate a standard curve, and this was used to obtain relative concentrations of the transcript of interest in each of the RNA samples. Relative concentrations were then determined by the second derivative method using the LightCycler computer software. Amplification of β-actin and L32 was performed to confirm that equal amounts of total RNA were added for each sample and that the RNA was intact and equally amplifiable among all samples.
Primers included the following: CD11a primers: forward: 5′-AAATGGAAGGACCCTGATGCTC-3′; backward: 5′-TGTAGCGGATGATGTCTTTGGC-3′; β-actin primers: forward: 5′-GCACCACACCTTCTACAATGAGC-3′; backward: 5′-GGATAGCACAGCCTGGATAGCAAC-3′; and L32 primers: forward: 5′-GGCATTGACAACAGGGTTCGTAG-3′; backward: 5′-GATGGCTTTGCGGTTCTTGG-3′.
Bisulfite sequencing
One to 5 μg purified T-cell DNA was treated with sodium bisulfite,16 and then the 2.3 kb-CD11a promoter fragment7 was amplified in 5 overlapping fragments. The fragments were cloned into PBS+ (Stratagene, La Jolla CA), and 5 independent clones were sequenced by the University of Michigan Sequencing Core for each of the amplified fragments.
DNase1 sensitivity
Deoxyribonuclease 1 (DNase1) sensitivity was performed using a modification of procedures described by others.17,18 A total of 2 × 107 cells was suspended in 1.2 mL harvest buffer (10 mM HEPES [pH 8.0], 50 mM KCl, 5 mM MgCl2, 3 mM CaCl2, 1 mM dithiothreitol, 0.1% Nonidet P-40, and 8% glycerol), and then the cells were disrupted with a Dounce homogenizer. Then, 280 μL aliquots were incubated with 0, 40, 80, or 160 U/mL DNase1 (Worthington, Lakewood, NJ) at room temperature for 3 minutes, and then the reaction was stopped by the addition of 300 μL 20 mM ethyleneglycotetraacetic acid/1% SDS. Then 5.8 μL DNase-free ribonuclease A (10 mg/mL) was added and the mixture incubated at 37°C for 2 hours, and then 11.6 μL proteinase K (10 mg/mL) was added and the mixture incubated at 55°C overnight. The DNA was then isolated, digested with SacI, fractionated by agarose gel electrophoresis, transferred to nylon filters, and hybridized with a32P-labeled fragment spanning bp 1060 to 1264 (relative to the transcription start site) of the ITGAL gene amplified by PCR, all using previously published protocols.19 The labeled fragments were then visualized using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Patch methylation
A 1.9-kb XhoI fragment containing the humanITGAL promoter and 5′ flanking region (bp −1818 to +79 of the published fragment, kindly provided by Dr Dennis Hickstein)7 was cloned into the luciferase-containing reporter vector pGL3-Basic (Promega, Madison, WI). An NdeI site was engineered into the fragment at bp −382 using the QuikChange site-directed mutagenesis kit (Stratagene) and intact function was confirmed by transfection into Jurkat cells. The regions from the beginning of the fragment to the NdeI site and from theNdeI site to the end of the fragment were excised, methylated with SssI and S-adenosylmethionine (both from New England Biolabs, Beverly, MA) using instructions provided by the manufacturer, and then ligated back into the reporter construct and purified by gel electrophoresis. Completeness of methylation was tested by digestion with the methylation-sensitive restriction endonucleaseAciI (New England Biolabs). Controls included a mock-methylated construct prepared by omitting theSssI.
Site-directed mutagenesis
Mutation of deoxycytosine (dC) to deoxythyamidine (dT) residues in the ITGAL promoter was performed using the QuikChange site-directed mutagenesis kit (Stratagene) and protocols provided by the manufacturer.
Transient transfection
Plasmid DNA was introduced into Jurkat cells by electroporation using a modification of previously described protocols.20,21 Twenty-four hours later the cells were washed twice, suspended in 400 μL reporter lysis buffer (Promega), and lysed by freeze/thaw. Insoluble material was removed by centrifugation and luciferase assays were performed using 100 μL as described.19 Similarly, 20 μL was used for β-galactosidase determinations, performed using the Galacto-Light system as per the manufacturer's protocol (Tropix, Bedford, MA).
Results
ITGAL promoter methylation in T cells and fibroblasts
ITGAL promoter methylation patterns were compared in T lymphocytes as a representative expressing cell type and in fibroblasts as a representative nonexpressing cell type. Real-time RT-PCR was used to first confirm that CD11a is expressed in T cells but not in fibroblasts. Relative to fibroblasts, T cells expressed about 80-fold higher levels of CD11a messenger RNA (mRNA) (CD11a/β-actin ratio 15.8 vs 0.2, T vs fibroblast in arbitrary units), consistent with other reports.22
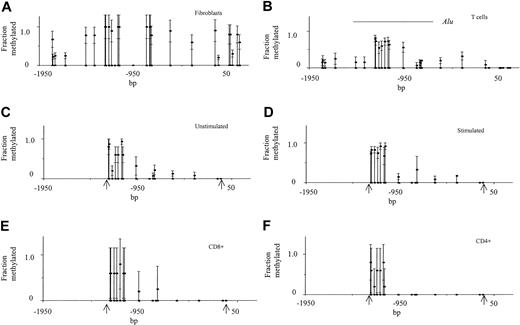
Bisulfite sequencing was then used to determine the methylation pattern of the ITGAL promoter and flanking regions in fibroblasts and T cells. Figure 1 shows theITGAL promoter numbered relative to the transcription start site.7 There are 22 potentially methylatable CpG dimers between the beginning of the fragment and the transcription start site, and there are 5 following. For reference, the transcriptionally relevant PU.1 and Sp1 sites are also shown.7 DNA was isolated from synovial fibroblast and dermal fibroblast lines, treated with bisulfite, and then the ITGAL promoter and 5′ flanking region were amplified in overlapping fragments, cloned into theEcoRI/XbaI sites of the PBS+ vector, and 5 fragments from each amplification sequenced. Figure2A shows the methylation status of each CpG dimer averaged over the 10 fragments from both fibroblast types. Nearly all CpG pairs are relatively heavily methylated (> 50%) in the DNA from both lines. Figure 2B shows the methylation pattern from the same region in T lymphocytes isolated from 4 to 6 healthy donors. The transcribed region was completely demethylated in all fragments from the 4 healthy subjects examined, while most of the sequence 5′ to the transcription start site was partially methylated in all controls. Of note is the region containing Alu elements, identified by the bar, which was more heavily methylated in all subjects, consistent with previous reports that repetitive DNA sequences are usually heavily methylated.23
ITGAL promoter structure.
The ITGAL promoter is shown, numbered relative to the transcription start site. Deoxycytosine residues in CG pairs are represented by the filled ovals, the transcription start site by an arrow, and the PU.1 and Sp1 binding sites by lines.
ITGAL promoter structure.
The ITGAL promoter is shown, numbered relative to the transcription start site. Deoxycytosine residues in CG pairs are represented by the filled ovals, the transcription start site by an arrow, and the PU.1 and Sp1 binding sites by lines.
The ITGAL promoter is methylated in fibroblasts but not T cells.
(A) DNA was isolated from 2 fibroblast cell lines, treated with bisulfite, and the ITGAL promoter amplified in 5 overlapping regions. For each amplified region, 5 fragments were cloned and sequenced. The filled circles on the x-axis represent each potentially methylatable dC residue, and the filled circles with error bars represent the average methylation (mean ± SEM) for each site of the 5 sequenced fragments from both fibroblast lines. (B) T-cell DNA was similarly isolated, treated with bisulfite, amplified, and sequenced. The region from −1261 to −68 represents the average methylation (mean ± SEM) of 5 fragments from each of 6 donors, while the remainder of the sequence represents the average methylation of 5 fragments from each of 4 healthy donors. The horizontal line indicates the region containing Alu elements. (C) DNA was isolated from the T cells of 3 healthy donors, and then bisulfite sequencing of 5 fragments from each donor was performed as above for the region from bp −1261 to −68 (identified by the arrows). Results are presented as in panel A. (D) T cells from the same donors shown in panel C were stimulated with PHA and DNA similarly isolated, treated with bisulfite, and the region from bp −1261 to −68 (identified by the arrows) sequenced. Results again represent the mean ± SEM of 5 determinations from each of the 3 donors. (E) DNA was isolated from CD8+ T cells and bisulfite sequencing was performed on the region from bp −1261 to −68 (identified by the arrows) as in panel A. Results represent the mean ± SD of 5 determinations for each dC residue. (F) DNA was isolated from CD4+ T cells and analyzed as in panel E. Results again represent the mean ± SD of 5 determinations for each dC residue.
The ITGAL promoter is methylated in fibroblasts but not T cells.
(A) DNA was isolated from 2 fibroblast cell lines, treated with bisulfite, and the ITGAL promoter amplified in 5 overlapping regions. For each amplified region, 5 fragments were cloned and sequenced. The filled circles on the x-axis represent each potentially methylatable dC residue, and the filled circles with error bars represent the average methylation (mean ± SEM) for each site of the 5 sequenced fragments from both fibroblast lines. (B) T-cell DNA was similarly isolated, treated with bisulfite, amplified, and sequenced. The region from −1261 to −68 represents the average methylation (mean ± SEM) of 5 fragments from each of 6 donors, while the remainder of the sequence represents the average methylation of 5 fragments from each of 4 healthy donors. The horizontal line indicates the region containing Alu elements. (C) DNA was isolated from the T cells of 3 healthy donors, and then bisulfite sequencing of 5 fragments from each donor was performed as above for the region from bp −1261 to −68 (identified by the arrows). Results are presented as in panel A. (D) T cells from the same donors shown in panel C were stimulated with PHA and DNA similarly isolated, treated with bisulfite, and the region from bp −1261 to −68 (identified by the arrows) sequenced. Results again represent the mean ± SEM of 5 determinations from each of the 3 donors. (E) DNA was isolated from CD8+ T cells and bisulfite sequencing was performed on the region from bp −1261 to −68 (identified by the arrows) as in panel A. Results represent the mean ± SD of 5 determinations for each dC residue. (F) DNA was isolated from CD4+ T cells and analyzed as in panel E. Results again represent the mean ± SD of 5 determinations for each dC residue.
Hypomethylation of the region closest to the transcription start site was confirmed in other LFA-1–expressing cells. The region spanning the CpG pairs at bp −346, −122, and −108 was amplified from T-depleted (e-rosette–negative) peripheral blood mononuclear cells. This population contains B lymphocytes, monocytes, and natural killer cells and typically contains less than 5% T cells by flow cytometry. Analysis of 5 fragments revealed that 2 of the 5 fragments were methylated at −346, but none of the 5 were methylated at −122 or −108, correlating with LFA-1 expression3 and identical to T cells (Figure 2B). Similarly, analysis of this region in adherent cells, enriched for monocytes, showed that none of the dC residues at these 3 sites were methylated in the 5 fragments studied. In contrast, fibroblasts demonstrated 60% methylation at bp −346, 90% at −122, and 20% at −108.
Because fibroblasts are proliferating cells, while the T cells were not stimulated it was possible that the differences in methylation were due to the activation status of the cells. To test this possibility, CD11a promoter methylation patterns were compared in unstimulated and PHA-stimulated T cells from 3 donors, examining all CpG pairs from the 5′ end of the Alu sequence (bp −1261) to the transcription start site (Figure 2C,D). No significant differences were observed. Methylation patterns were also compared in purified CD4+and CD8+ T-cell subsets isolated from a healthy donor (Figure 2E,F). Again, no significant differences were observed.
Chromatin structure and CD11a expression
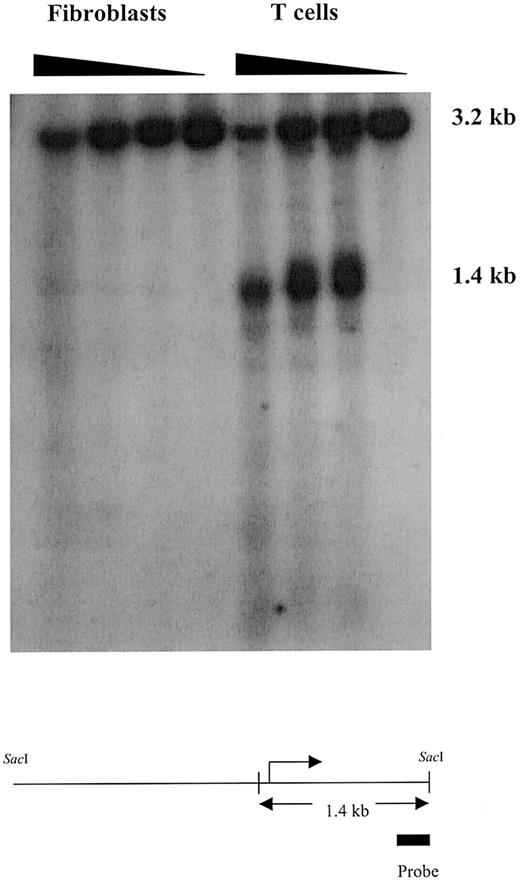
Methylation changes have been implicated in directing alterations of chromatin structure.10 Therefore, chromatin structure around the ITGAL gene was compared in T cells and fibroblasts, using DNase1 sensitivity to detect changes in the availability of DNA to digestion by this enzyme. Cellular homogenates of normal human fibroblasts and T cells were incubated with increasing amounts of DNase1, and then the DNA was isolated, digested withSacI, and fractionated by agarose gel electrophoresis. The digests were transferred to nylon filters and hybridized with a probe from the coding sequence (bp 1060-1264). Figure3 shows that SacI digestion gives an approximate 3.2-kb fragment spanning the sequence shown in Figure 1 and extending into the coding region. Digestion with low concentrations of DNase1 causes the appearance of a prominent 1.4 kb band in T cells but not fibroblasts, while the highest concentration tested appears to cause some nonspecific digestion in both cell types. Because the probe is complementary to sequences at the 3′ end of theSacI fragment, the DNase1 susceptible site is located near bp −132, just 5′ to the transcription start site and close to the CpG pairs at −108 and −122 (Figure 3, lower panel). Similar results were seen in a confirming experiment (not shown). This is consistent with the differences in the methylation patterns between the 2 cell types.
Chromatin structure is condensed around theITGAL locus in fibroblasts but not T cells.
Freshly isolated T cells and 2 × 107 fibroblasts were homogenized in phosphate-buffered saline and then treated with 0, 40, 80, or 160 U/mL DNase1. DNA was then isolated, digested withSacI, fractionated by agarose gel electrophoresis, transferred to nylon filters, hybridized with a 32P-labeled complementary DNA probe amplified from bp 1060 to 1264 of theITGAL gene, and developed using a PhosphorImager. Fragment size is shown in the right column, and the figure at the bottom shows the 3.2-kb SacI fragment, the relative size and location of the probe, and the approximate location of the 1.4 kb fragment. The broken arrow represents the transcription start site.
Chromatin structure is condensed around theITGAL locus in fibroblasts but not T cells.
Freshly isolated T cells and 2 × 107 fibroblasts were homogenized in phosphate-buffered saline and then treated with 0, 40, 80, or 160 U/mL DNase1. DNA was then isolated, digested withSacI, fractionated by agarose gel electrophoresis, transferred to nylon filters, hybridized with a 32P-labeled complementary DNA probe amplified from bp 1060 to 1264 of theITGAL gene, and developed using a PhosphorImager. Fragment size is shown in the right column, and the figure at the bottom shows the 3.2-kb SacI fragment, the relative size and location of the probe, and the approximate location of the 1.4 kb fragment. The broken arrow represents the transcription start site.
Methylation of the CD11a promoter suppresses function
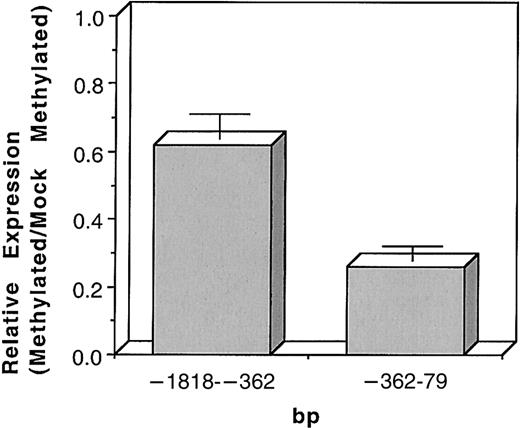
We previously reported that methylation of the entireITGAL promoter suppresses expression.19 To determine the relative importance of methylation of the promoter region versus the 5′ flanking region, an NdeI site was engineered into the ITGAL promoter at bp −382. This was necessary because the region lacks other unique restriction sites. To test if the mutation affected promoter function, the modified fragment was cloned into pGL3 and transfected into Jurkat cells. The mutation did not significantly affect promoter function (not shown). The regions from bp −1818 to −382 and from −382 to +79 were then excised, methylated in vitro, ligated back into pGL3, and transfected into Jurkat cells. Figure 4 shows that methylation of the region from the beginning of the promoter (−1818) fragment to −382 partially suppresses promoter activity relative to mock-methylated controls, with the expression of the methylated construct being about 60% that of the control (n = 5,P < .0001). Methylation of the region containing the active promoter (−362 to +79) suppressed to a greater degree, with expression of the methylated construct being only about 25% of the control (n = 4, P < .0001). Because the CpG residues at −102 and −68 are close to the PU.1 and Sp1 binding sites, we tested whether mutating the dC residues to dT at these sites would alter promoter function. Using the same expression system, no effect was seen on promoter function in 2 independent experiments (luciferase/β-galactosidase 0.282 vs 0.269 and 0.170 vs 0.184, wild type vs mutant) as reported by others.24
Methylation suppresses ITGAL promoter function.
The regions from −1818 to −362 and −362 to +79 were excised, methylated with SssI in vitro, ligated back into a luciferase reporter construct, and transfected into Jurkat cells, using cotransfection with β-galactosidase as a control. Controls consisted of similar preparations but without the addition of SssI. The results are presented as the ratio of luciferase/β-galactosidase expression in arbitrary units standardized to the mock-methylated controls and represent the mean ± SEM of 5 experiments for the region from −1818 to −362 and 4 experiments for the region from −362 to +79.
Methylation suppresses ITGAL promoter function.
The regions from −1818 to −362 and −362 to +79 were excised, methylated with SssI in vitro, ligated back into a luciferase reporter construct, and transfected into Jurkat cells, using cotransfection with β-galactosidase as a control. Controls consisted of similar preparations but without the addition of SssI. The results are presented as the ratio of luciferase/β-galactosidase expression in arbitrary units standardized to the mock-methylated controls and represent the mean ± SEM of 5 experiments for the region from −1818 to −362 and 4 experiments for the region from −362 to +79.
Effect of 5-azaC on fibroblast CD11a expression
Because these results suggest that DNA methylation may play a role in suppressing CD11a expression in fibroblasts, we asked if inhibiting DNA methylation would increase fibroblast CD11a expression. Cultured fibroblasts were treated with 5-azaC for 3 days, and then CD11a, L32, and β-actin transcripts were quantitated by real-time RT-PCR in treated and untreated cells. The 5-azaC caused an approximate 2-fold increase in CD11a mRNA (0.078 vs 0.143, untreated and treated, respectively, relative to total RNA in arbitrary units) while β-actin transcripts decreased about 60% and L32 decreased about 20% in the treated cells relative to controls, suggesting that methylation was contributing to CD11a regulation in fibroblasts. We have previously reported that 5-azaC also increases CD11a, but not β-actin, mRNA in T cells.25 The effect of 5-azaC on ITGAL promoter methylation was confirmed using bisulfite sequencing to compare methylation of the dC residues at bp −346, −122, and −108 in the active portion of the ITGAL promoter, shown to be important in the patch methylation experiments. Five fragments spanning this region were analyzed as before. The overall methylation of these 3 loci decreased from 53.3% methylated to 33.3% following 5-azaC treatment, in agreement the increase in CD11a mRNA. Specifically, methylation at −108 decreased from 80% to 20%, at −122 from 80% to 40%, while methylation at −346 increased from 0% to 40%.
Discussion
In this report we demonstrate that the ITGAL promoter and flanking regions are extensively methylated in fibroblasts, which do not express CD11a, but are largely demethylated in T lymphocytes, which express this gene. The mechanisms directing the methylation of specific sequences are largely unknown. However, it has been reported that Sp1 sites can protect adjacent regions from methylation.26 The ITGAL promoter contains an Sp1 site but is extensively methylated in fibroblasts. This is likely due to a requirement for multiple Sp1 sites to protect a region from methylation.27 Notably, the ITGAL promoter methylation pattern observed in normal T cells differs significantly from that of Jurkat cells, a transformed human T-cell line.19 However, others have reported that DNA methylation is frequently abnormal in transformed lines,27 and the present results confirm that methylation patterns observed in transformed lines do not necessarily reflect those of normal cells.
The patch methylation studies suggest that methylation may suppressITGAL promoter function by more than one mechanism. Methylation of cytosine residues in the CpG dimers closest to the transcription start site suppressed promoter function almost completely. Methylation of transcription factor recognition sequences can prevent the binding of some factors such as AP-2, ATF/CREB, and c-myc.28-30 In addition, methylcytosine binding proteins can recognize and bind the residues, preventing the binding of the transcription factors. This mechanism appears to prevent Sp1 from interacting with its recognition sequence,31 which may be relevant to the suppression of ITGAL expression in these studies. Our observation that mutating 2 of the CpG pairs near the transcription start site did not affect ITGAL expression is consistent with the interpretation that methylcytosine binding proteins interacting with these methylated bases contribute to ITGALsuppression. We also found that methylation of bases located more than 500 bp 5′ to the transcription start site suppressed promoter function, albeit to a lesser degree. This is most likely mediated by effects on chromatin structure. Others have reported that methylcytosine binding proteins such as MeCP2, which contain a transcription repressor domain, can suppress promoter function from a distance.32 This protein interacts with Sin3A, which in turn binds a chromatin inactivation complex containing histone deacetylases, which promote chromatin condensation into an inactive configuration.33The interpretation of patch methylation studies may be limited by 2 considerations. First, the methylation achieved in vitro withSssI may not necessarily reflect methylation patterns in vivo, because SssI gave essentially complete methylation as measured by AciI digestion. However, this concern is mitigated by our observation that most of the CpG sites in the fibroblast ITGAL promoter are 75% to 100% methylated, and so it seems likely that the patch methylation studies give a good approximation of the in vivo conditions, and the conclusion that the promoter is methylation-sensitive seems reasonable. The second is that the transfected construct may not represent endogenous chromatin structure. While the construct may not reflect total chromatin structure, studies by Kass et al report that regional methylation of constructs has effects that resemble those observed in intact chromatin.34
The DNase1 studies also support the contention that chromatin inactivation contributes to ITGAL suppression in the fibroblasts. Digestion of T-cell DNA with DNase1 demonstrated that the region just 5′ to the ITGAL transcription start site was relatively sensitive to degradation while the corresponding region in fibroblasts was resistant. This suggests that this region has a structure making the DNA more accessible to the enzyme in T cells, consistent with active chromatin.
The mechanisms establishing methylation patterns are unknown. Two enzymes with de novo methyltransferase activity, Dnmt3a and Dnmt3b, have recently been identified.35 These enzymes are required for normal development, and it is reasonable to propose that during development they methylate this region in cells not destined to express LFA-1. However, the mechanisms directing these enzymes to theITGAL promoter in fibroblasts remain to be identified.
Inhibiting DNA methylation in fibroblasts increased CD11a mRNA. This effect was observed on CD11a but not β-actin or L32 mRNA, indicating specificity. While 5-azaC is also a DNA synthesis inhibitor, we have previously reported that hydroxyurea, another DNA synthesis inhibitor, does not increase CD11a expression.25 Because the increase induced with 5-azaC correlated with promoter demethylation and patch methylation studies indicate that the promoter is methylation sensitive, it is reasonable to propose that the increase is due to promoter demethylation. However, the increase was not to the levels seen in T cells. The partial response could be due to at least 2 factors. First, methylation was only partially inhibited, suggesting that only a fraction of the cells may be expressing the transcripts. Second, fibroblasts may lack other factors necessary for fullITGAL expression. Nonetheless, the results presented here support the concept that DNA methylation and chromatin structure contribute to the suppression of ITGAL in fibroblasts and likely other nonmyeloid cells.
The authors thank Ms Janet Stevens for her expert secretarial assistance. Drs S. Hanash, C. W. Castor, and D. Hickstein are thanked for their generous contribution of essential materials.
Supported by Public Health Service grants AG014783, AR42525, and AI42753 and a Merit grant from the Department of Veterans Affairs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bruce Richardson, 5310 Cancer Center and Geriatrics Center Bldg, Ann Arbor MI 48109-0940; e-mail:brichard@umich.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal