Stromal cell–derived factor 1 (SDF-1/CXCL12) is a multifunctional cytokine. We previously reported that myelopoiesis was enhanced in SDF-1α transgenic mice, probably due in part to SDF-1α enhancement of myeloid progenitor cell (MPC) survival. To understand signaling pathways involved in this activity, we studied the effects on factor-dependent cell line MO7e cells incubated with SDF-1α alone or in combination with other cytokines. SDF-1α induced transient activation of extracellular stress–regulated kinase (ERK1/2), ribosomal S6 kinase (p90RSK) and Akt, molecules implicated in cell survival. Moreover, ERK1/2, p90RSK, and Akt were synergistically activated by SDF-1α in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF), Steel factor (SLF), or thrombopoietin (TPO). Similar effects were seen after pretreatment of MO7e cells with SDF-1α followed by stimulation with the other cytokines, suggesting a priming effect of SDF-1α. Nuclear factor-κB (NF-κB) did not appear to be involved in SDF-1α actions, alone or in combination with other cytokines. These intracellular effects were consistent with enhanced myeloid progenitor cell survival by SDF-1α after delayed addition of growth factors. SDF-1α alone supported survival of highly purified human cord blood CD34+++ cells, less purified human cord blood, and MO7e cells; this effect was synergistically enhanced when SDF-1α was combined with low amounts of other survival-promoting cytokines (GM-CSF, SLF, TPO, and FL). SDF-1 may contribute to maintenance of MPCs in bone marrow by enhancing cell survival alone and in combination with other cytokines.
Introduction
Blood cell production is maintained by a balance between growth and death, processes regulated in part by cytokine-cell interactions. Hematopoietically relevant cytokines have been identified and their implications in proliferation and survival studied.1-4
Chemokines are part of a family of cytokines having chemotactic activities; they mediate effects by binding 7 transmembrane domain receptors associated with heterotrimeric Gi proteins.5-8The human chemokine system includes more than 50 chemokines and 18 chemokine receptors.7-9 Many chemokines bind more than one receptor, and receptors generally bind more than one chemokine. However, stromal cell–derived factor 1 (SDF-1; CXCL12) is a CXC chemokine apparently interacting with only one receptor, CXCR4.10-13 This single chemokine–single receptor is supported by the nearly identical phenotypes of SDF-1−/−and CXCR4−/− mice including impaired hematopoiesis.10,11 SDF-1 protects against human immunodeficiency virus (HIV) infection by competing with gp120 for binding to CXCR4 and by down-regulation of the receptor.14
Studies have implicated SDF-1 in the migration of myeloid progenitor cells (MPCs) and stem cells.5-7,15-18 SDF-1 is constitutively expressed in specific lymphoid or nonlymphoid tissues, in contrast to inflammatory chemokines expressed in inflamed tissues.9 SDF-1 is also involved in differentiation, proliferation, and survival of various cellular systems. SDF-1 was originally cloned from a stromal cell line as a pre-B cell growth–stimulating factor.19 SDF-1 acts with thrombopoietin (TPO) to enhance development of megakaryocytic progenitor cells.20 Stromal cells or cytokines in synergy with SDF-1 support survival of human leukemic B-cell precursors.21 Blood-derived nurselike cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through SDF-1.22 We reported that myelopoiesis is enhanced in SDF-1α transgenic mice, and SDF-1α modulates myelopoiesis by regulating progenitor cell survival and inhibitory effects of myelosuppressive chemokines.23
A number of intracellular pathways have been implicated in cell survival. Phosphoinositol 3-kinase (PI3K)/protein kinase B (PKB)(Akt) and mitogen-activated protein kinase (MAPK)/p90 ribosomal S6 kinase (RSK) pathways are 2 major survival pathways induced by cytokines.24,25 After activation of specific growth factor receptors, PI3K is recruited to the inner surface of the plasma membrane and generates phosphoinositol-3,4,5-triphosphate (PIP3).26,27 The PI3K-generated phospholipids act by multiple mechanisms that cooperate to regulate Akt activity.24 Akt is phosphorylated and activated by phospholipid-dependent kinase 1 (PDK1). Targets of Akt include BAD, caspase-9, forkhead family transcription factors, and the nuclear factor-κB (NF-κB) regulator inhibitor of kB (IκB) kinase (IKK).24 Akt also phosphorylates cyclic adenosine monophosphate (cAMP) responsive element binding protein (CREB), which increases binding of CREB to CREB-binding protein and enhances CREB-mediated transcription.28 These Akt activities modulate proapoptotic or antiapoptotic proteins through transcriptional or posttranscriptional modes.24 RSKs are mediators of ERK signal transduction.29 The RSKs are serine/threonine kinases with 2 functional kinase domains activated in sequential manner by a series of phosphorylation.29 RSK activity is regulated by ERK as well as PDK1.30,31 RSK phosphorylates a number of substrates including c-Fos, NFκB/IκBα, BAD, CREB, histone H3, and Myt1 in different cell situations, which direct transcriptional activation of immediate early genes and promote cell survival.25 Studies on the PI3K/Akt and ERK/RSK pathways offer some insight into the potential for cross-talk in survival/antiapoptotic mechanisms.7
Because cells in vivo are likely subjected to multiple cytokines that can influence growth and survival, it is probable that the combined actions of several cytokines will be of physiologic relevance. Here we demonstrate that SDF-1α in synergy with other cytokines has the capacity to enhance survival of primary MPCs and the factor-dependent myeloid cell line MO7e subjected to delayed addition of growth factors; this is associated with synergistic stimulation in MO7e cells of intracellular signaling pathways implicated in mediating survival/antiapoptotic effects.
Materials and methods
Cells and cell culture
MO7e cells were cultured in RPMI 1640 medium with 20% fetal bovine serum (FBS; Hyclone, Logan, UT), 100 U/mL penicillin, 100 μg/mL streptomycin, and 10 ng/mL human granulocyte-macrophage colony-stimulating factor (GM-CSF). This cell line and its characteristics have been described elsewhere.3,32 33Before stimulation with cytokines, cells were factor starved for 16 to 18 hours in RPMI 1640 supplemented with 0.5% bovine serum albumin (BSA). For intracellular signaling studies, factor-starved cells were incubated with SDF-1α alone or in combination with GM-CSF, Steel factor (SLF; stem cell factor), or TPO. When inhibitors were used, cells were preincubated for 1 hour at 37°C before factor stimulation.
Heparinized human marrow cells were collected from healthy volunteers with informed consent. Heparinized human cord blood was collected from healthy, full-term neonates according to institutional guidelines. Mononuclear cells were separated by density gradient centrifugation on Ficoll Hypaque (1.077 g/mL; Pharmacia, Piscataway, NJ). CD34+ cells were positively selected by MACS CD34+ isolation kit (Miltenyi Biotec, Auburn, CA; purity was more than 90% in each experiment). More highly purified CD34+++ cells (containing the top 20% highest CD34-expressing cells; ≥ 98% pure CD34+) were isolated by FACS.34
Cytokines and antibodies
Recombinant human (rhu) GM-CSF and rhu Flt3 ligand (FL) were provided by Immunex (Seattle, WA). Rhu TPO was from Genentech (Emeryville, CA). Rhu erythropoietin (Epo) was purchased from Amgen (Thousand Oaks, CA). Rhu SLF, rhu SDF-1α, rhu macrophage inflammatory protein 1α (MIP-1α), and rhu regulated on activation, normal T cell–expressed and secreted (RANTES) were purchased from R & D Systems (Minneapolis, MN). PC-5–conjugated APO2.7 monoclonal antibody (mAb) was obtained from Immunotech (Marseille, France). Fluorescein isothiocyanate (FITC)–conjugated mAb to c-kit and purified mAb to c-mpl were purchased from Becton Dickinson (Bedford, MA) and Genzyme (Cambridge, MA), respectively. Phycoerythrin (PE)–conjugated mAb to CXCR4 was from BD Pharmingen (San Diego, CA). Antibodies to ERK1/2, phospho-ERK1/2 (Thr202/Tyr204), phospho-Elk-1 (Ser383), phospho-p90RSK (Ser381), Akt, phospho-Akt (Ser473) were from Cell Signaling Technology (Beverly, MA). Anti-p90RSK (Rsk-1) was from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell lysate preparation
MO7e cells were washed twice with phosphate-buffered saline (PBS). The cell pellet was resuspended in lysis buffer (20 mM Tris-HCl, pH 8.0, 137 mM NaCl, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 0.15 U/mL aprotonin, 10 mM EDTA, 10 μg/mL leupeptin, 100 mM NaF, 2 mM Na3VO4, and 1% NP-40) and incubated for 30 minutes on ice. Insoluble fractions were removed by centrifugation at 14 000g for 10 minutes, and the supernatants were frozen at −20°C. Protein concentration of the lysate was measured by bicinchoninic acid (BCA) protein assay reagent (Pierce, Rockford, IL).
Western blotting
Equal amounts of protein were loaded on sodium dodecyl sulfate-polyacrylamide gel, subjected to electrophoresis (SDS-PAGE), and electrotransferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA). Membranes were blocked in Tris-buffered saline containing 0.05% Tween 20 and 2% BSA for 1 hour at room temperature, and incubated with appropriate primary antibody for 1 to 2 hours. Immunoreactive proteins were detected by horseradish peroxidase–conjugated secondary antibody and an enhanced chemiluminescence reagent (Amersham Pharmacia Biotech, Piscataway, NJ). To reprobe with another primary antibody, membranes were incubated in striping buffer (62.5 mM Tris-HCl, pH 6.7, 100 mM 2-mercaptoethanol, and 2% SDS) for 30 minutes at 50°C, washed, and then used for further study.
Immunoprecipitation and MAPK activity assay
Cell lysates were immunoprecipitated with anti–phospho-ERK1/2 mAb. Immunoprecipitates were washed twice with lysis buffer and then twice with kinase buffer (25 mM Tris, pH 7.5, 5 mM β-glycerolphosphate, 2 mM dithiothreitol [DTT], 0.1 mM Na3VO4, 10 mM MgCl2). Immunoprecipitates were resuspended in 50 μL kinase buffer containing 200 μM adenosine triphosphate (ATP) and 2 μg GST-Elk-1 fusion protein, and then incubated at 30°C for 30 minutes. Reactions were terminated by adding SDS-PAGE sample buffer, and boiled samples were separated by 10% SDS-PAGE. Phosphorylated GST–Elk-1 fusion protein was visualized by immunoblotting with anti–phospho-Elk-1 antibody.
Immunofluorescent staining
Factor-starved MO7e cells were incubated with SDF-1α alone or with other cytokines, fixed using PBS containing 4% paraformaldehyde for 10 minutes, and permeabilized with PBS containing 0.1% Triton X-100 for 10 minutes. To investigate the cellular localization of NF-κB, samples were treated with a mAb against human NF-κB p65 (Santa Cruz Biotechnology, 1:100) for 1.5 hours. After extensive washing in PBS, samples were further incubated with FITC-conjugated donkey antimouse IgG (Jackson Immunotech Laboratory, 3:400) for 1 hour. Nuclei were stained by 5 μg/mL Hoechst no. 33258 (Sigma, St Louis, MO). After extensive washing, samples were examined by laser scanning confocal microscopy (Carl Zeiss, Thornwood, NY).
Primary hematopoietic progenitor cell assays
Magnetic bead–separated human cord blood CD34+(103 cells/mL), FACS-sorted CD34+++ (100-150 cells/mL), or MO7e cells (103 cells/mL) were plated without or with single or multiple cytokines at time 0 hour. For human cord blood progenitor cells plated in 0.9% methylcellulose culture medium with 30% FBS, the combination of rhu GM-CSF (10 ng/ml), rhu interleukin 3 (IL-3; 10 ng/mL), and rhu SLF (50 ng/mL) or rhu Epo (1 U/mL) plus rhu FL (100 ng/mL) was used as a maximally potent combination of cytokines for stimulation of colonies. Plates were incubated at 5% CO2 and 5% O2 in a humidified atmosphere and scored for progenitor cell–derived colonies 14 days after addition of the maximally stimulatory growth factors. These assays have been described in detail elsewhere.35
Apoptosis assay after growth factor withdrawal
Factor-starved MO7e or CD34+ marrow cells were incubated in serum-free media with either 100 ng/mL SDF-1α, 10 ng/mL SLF, or the combination of these 2 cytokines. After 4 days, cells were stained with PC-5–conjugated APO2.7 mAb and analyzed by flow cytometry (EPICS XL, Coulter, Miami, FL). The antigen defined by this antibody (7A6 antigen) is a 38-kd protein localized to the membrane of mitochondria and is involved in the molecular cascade of apoptosis.36,37 The expression of 7A6 antigen is preferentially detected on apoptotic cells, but not on the normal cell surface or digitonin-permeabilized cells. Expression of 7A6 antigen represents an early event of apoptosis.36
Statistical analysis
Colony results are expressed as mean ± SD from triplicate plates for each experiment. Statistical significance was determined using Student t test.
Results
SDF-1α triggers activation of ERK, p90RSK, and Akt
We reported that myelopoiesis was enhanced in SDF-1α transgenic mice, and SDF-1α modulated myelopoiesis by regulating progenitor cell survival and the inhibitory effects of myelosuppressive chemokines.23 To understand possible molecular mechanisms involved in this survival-enhancing activity of SDF-1α, we used the factor-dependent cell line MO7e,3,32,33 which expresses the SDF-1 receptor, CXCR4. First, we incubated the cells with SDF-1α alone at an optimal concentration (100 ng/mL) and checked for activation of signaling molecules by Western blotting using phosphospecific antibodies. As shown in Figure1A, SDF-1α induced activation of ERK1/2 as reported in other cells.38-40 Additionally, p90RSK, a crucial downstream effector molecule of ERK, was activated. Here, we used antibody recognizing phosphorylated p90RSK(Ser 381), which represents its activation by ERK.29 Because activation of Akt correlates with its phosphorylation at residues Thr308 and Ser473,24 we used antibody recognizing phosphorylated Akt(Ser473) to check if Akt, a downstream molecule of PI3K, was activated by SDF-1α. SDF-1α also activated Akt, consistent with results in other cells.38-41
Activation of Akt, ERK1/2, and p90RSK in MO7e cells after stimulation with SDF-1α.
(A) MO7e cells were incubated with 100 ng/mL SDF-1α for the indicated time periods. (B) MO7e cells were preincubated in the presence of dimethyl sulfoxide (DM) vehicle control or LY 294002 (LY, 30 μM), wortmannin (W, 100 nM), PD 98059 (PD, 25 μM), or rapamycin (R [also called sirolimus], 10 nM) for 1 hour, and stimulated with 100 ng/mL SDF-1α for 5 minutes. Cell lysates were analyzed by Western blotting with phosphospecific antibodies to Akt (Ser473), ERK1/2 (Thr202/Tyr204), or p90RSK (Ser381). Amounts of p90RSK (A) or Akt (B) are shown on the bottom panel of each as a loading control. This experiment was performed 3 times with similar results.
Activation of Akt, ERK1/2, and p90RSK in MO7e cells after stimulation with SDF-1α.
(A) MO7e cells were incubated with 100 ng/mL SDF-1α for the indicated time periods. (B) MO7e cells were preincubated in the presence of dimethyl sulfoxide (DM) vehicle control or LY 294002 (LY, 30 μM), wortmannin (W, 100 nM), PD 98059 (PD, 25 μM), or rapamycin (R [also called sirolimus], 10 nM) for 1 hour, and stimulated with 100 ng/mL SDF-1α for 5 minutes. Cell lysates were analyzed by Western blotting with phosphospecific antibodies to Akt (Ser473), ERK1/2 (Thr202/Tyr204), or p90RSK (Ser381). Amounts of p90RSK (A) or Akt (B) are shown on the bottom panel of each as a loading control. This experiment was performed 3 times with similar results.
We used pathway-specific inhibitors to determine if activation of ERK was sensitive to MEK1 inhibitor PD98059 (50 μM) and Akt activation was sensitive to PI3K inhibitors, LY 294002 (30 μM) or wortmannin (100 nM; Figure 1B). Wortmannin reduced ERK activation, but another PI3K inhibitor LY 294002 did not. Considering the inhibitory effects of wortmannin on MAPK activation,42 and the greater specificity of LY 294002 for PI3K, which acts on the ATP binding site of this enzyme,43 we consider it reasonable that PI3K contributes little if anything to the MEK1/ERK activation induced by SDF-1α.
SDF-1α in combination with other cytokines synergistically activates MAPK/p90RSK and Akt
To determine if SDF-1α has synergistic activity in combination with other cytokines, we stimulated MO7e with SDF-1α alone, other cytokines alone, or SDF-1α plus other cytokines, and checked for activation of ERK, p90RSK, and Akt. Here, we used optimal concentrations of cytokines such as GM-CSF (10 ng/mL), SLF (50 ng/mL), or TPO (50 ng/mL).
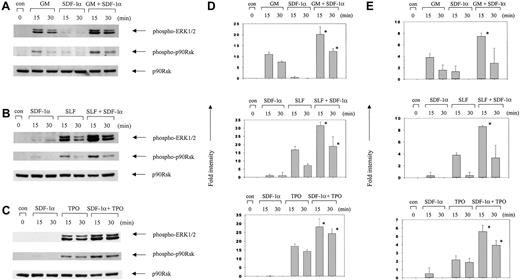
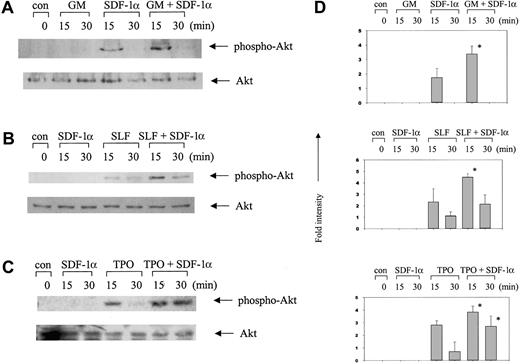
We found that GM-CSF alone, SLF alone, and TPO alone activated ERK and the combined stimulation with SDF-1α plus either of these other cytokines triggered a synergistic activation of ERK (Figure2). Because stimulation of ERK activation by SDF-1α showed much faster kinetics of signaling than those by the other cytokines, we could not detect much activation by 15 or 30 minutes after stimulation with SDF-1α. This is consistent with previous observations that signaling pathways from the G-protein coupled CXCR4 receptor are activated faster than those from growth factor receptors.44-46 Activation of p90RSK, a downstream molecule of ERK, showed similar patterns of response to SDF-1α alone or in combination with GM-CSF, SLF, or TPO (Figure 2). GM-CSF alone, SLF alone, or TPO alone induced activation of Akt in MO7e cells, and SDF-1α in combination with these cytokines induced synergistic activation of Akt (Figure 3).
Synergistic activation of ERK1/2 and p90RSK by SDF-1α in combination with other cytokines.
MO7e cells were incubated for the indicated time periods with SDF-1α (100 ng/mL), GM-CSF (10 ng/mL; GM), SLF (50 ng/mL), or TPO (50 ng/mL) each alone, or with the combination of SDF-1α plus one of these cytokines. Cell lysates were analyzed by Western blotting with phosphospecific antibodies to ERK1/2 (Thr202/Tyr204) or p90RSK (Ser381). The amount of p90RSK is shown as a loading control in the bottom panels. (A) SDF-1α plus GM-CSF. (B) SDF-1α plus SLF. (C) SDF-1α plus TPO. (D,E) Columns represent relative band intensities of phospho-ERK (D) and phospho-RSK (E) ± SD from 3 experiments. *P < .05 (greater than additive).
Synergistic activation of ERK1/2 and p90RSK by SDF-1α in combination with other cytokines.
MO7e cells were incubated for the indicated time periods with SDF-1α (100 ng/mL), GM-CSF (10 ng/mL; GM), SLF (50 ng/mL), or TPO (50 ng/mL) each alone, or with the combination of SDF-1α plus one of these cytokines. Cell lysates were analyzed by Western blotting with phosphospecific antibodies to ERK1/2 (Thr202/Tyr204) or p90RSK (Ser381). The amount of p90RSK is shown as a loading control in the bottom panels. (A) SDF-1α plus GM-CSF. (B) SDF-1α plus SLF. (C) SDF-1α plus TPO. (D,E) Columns represent relative band intensities of phospho-ERK (D) and phospho-RSK (E) ± SD from 3 experiments. *P < .05 (greater than additive).
Synergistic activation of Akt in MO7e cells by SDF-1α in combination with other cytokines.
Cell lysates were analyzed by Western blotting with phosphospecific antibodies to Akt (Ser473). The amount of total Akt is shown as a loading control in the bottom panels. (A) SDF-1α plus GM-CSF. (B) SDF-1α plus SLF. (C) SDF-1α plus TPO. (D) Columns represent relative band intensities of phospho-Akt ± SD from 3 experiments. *P < .05 (greater than additive).
Synergistic activation of Akt in MO7e cells by SDF-1α in combination with other cytokines.
Cell lysates were analyzed by Western blotting with phosphospecific antibodies to Akt (Ser473). The amount of total Akt is shown as a loading control in the bottom panels. (A) SDF-1α plus GM-CSF. (B) SDF-1α plus SLF. (C) SDF-1α plus TPO. (D) Columns represent relative band intensities of phospho-Akt ± SD from 3 experiments. *P < .05 (greater than additive).
To confirm the synergism of SDF-1α with these other cytokines, we evaluated signaling events using different concentrations of SDF-1α and the other cytokines. The concentrations of cytokines used were 100 or 10 ng/mL SDF-1α; 10, 1, or 0.1 ng/mL GM-CSF; 50, 10, or 1 ng/mL SLF; and 10 or 1 ng/mL TPO. The results in Figure4 demonstrate clear synergistic activation of ERK. As shown later, the lower concentrations of cytokines do not alone enhance survival of primary MPCs or MO7e colony-forming cells. However, low concentrations of SDF-1α with low concentrations of GM-CSF, SLF, or TPO do enhance the survival of these cells.
Synergistic activation of ERK1/2 by SDF-1α in combination with nonproliferative concentrations of other cytokines.
MO7e cells were stimulated for 15 minutes with SDF-1α alone, another cytokine alone, or SDF-1α plus another cytokine at the indicated concentrations. Cell lysates were analyzed by Western blotting using phosphospecific antibodies to ERK1/2 (Thr202/Tyr204). The membrane was reblotted with antibody recognizing p90RSK to show equal loading. (A) SDF-1α plus GM-CSF. (B) SDF-1α plus SLF. (C) SDF-1α plus TPO. (D) Columns represent relative band intensities of phospho-ERK ± SD from 3 experiments with similar results. *P < .05 (greater than additive).
Synergistic activation of ERK1/2 by SDF-1α in combination with nonproliferative concentrations of other cytokines.
MO7e cells were stimulated for 15 minutes with SDF-1α alone, another cytokine alone, or SDF-1α plus another cytokine at the indicated concentrations. Cell lysates were analyzed by Western blotting using phosphospecific antibodies to ERK1/2 (Thr202/Tyr204). The membrane was reblotted with antibody recognizing p90RSK to show equal loading. (A) SDF-1α plus GM-CSF. (B) SDF-1α plus SLF. (C) SDF-1α plus TPO. (D) Columns represent relative band intensities of phospho-ERK ± SD from 3 experiments with similar results. *P < .05 (greater than additive).
To determine if SDF-1α influenced surface expression of other cytokine receptors, we checked the level of the c-kit (SLF receptor) and c-mpl (TPO receptor) gene products by flow cytometry analysis after treatment of cells with SDF-1α (100 ng/mL) for 30 and 60 minutes. SDF-1α did not change the expression levels of c-kit or c-mpl as assessed by mean fluorescence intensity (data not shown). To exclude the possibility that one of the cytokines may have affected the surface expression level of CXCR4, we treated MO7e cells with GM-CSF for 1 hour and 24 hours and checked the expression level of surface CXCR4 by flow cytometry. We did not see any change of CXCR4 expression level induced by GM-CSF as assessed by mean fluorescence intensity (data not shown).
Priming with SDF-1α induces enhancement of survival signaling
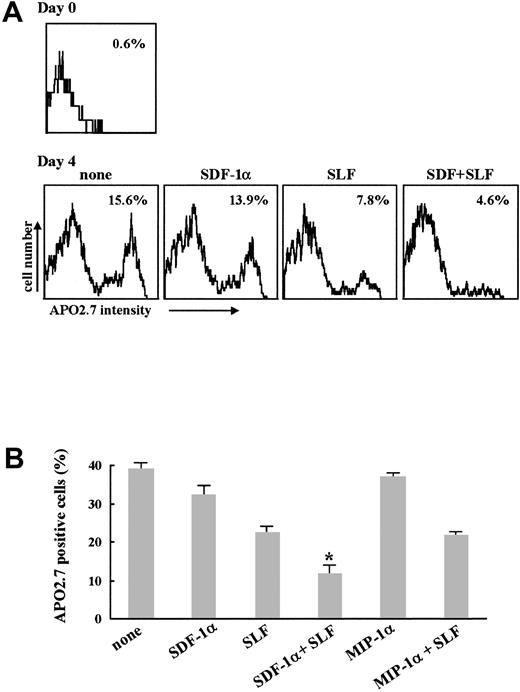
To determine if the other cytokines had to be given simultaneously with SDF-1α to detect synergistic enhancement in intracellular signaling, we pretreated MO7e cells for 30 minutes with SDF-1α and then added one of the other cytokines prior to analysis of effects on ERK activation. We confirmed activation of ERK by using an in vitro kinase assay with ERK1/2 immunoprecipitates (Figure5A). Because SDF-1α–induced ERK activation kinetics are very fast (Figure 1A), we did not detect ERK activation by SDF-1α alone at this time point. SLF alone induced ERK activation, and SLF given 30 minutes after SDF-1α induced clear synergism of ERK activation (Figure 5Ai). We detected the same pattern of synergistic activation of ERK when GM-CSF was added to the cells 30 minutes after SDF-1α (Figure 5Aii). To check if this effect was unique to SDF-1α, we pretreated MO7e cells with other chemokines (MIP-1α or RANTES) instead of SDF-1α, and monitored for their possible effects on ERK activity. Neither of these 2 chemokines enhanced the ERK activation induced by subsequent addition of SLF (Figure 5Aii). Pretreatment with SDF-1α induced synergistic activation of ERK even when SLF was added 1 hour after SDF-1α when ERK activation by SDF-1α alone could not be detected (Figure 5Aiii). Enhanced activation of ERK was sensitive to pretreatment of the cells with the MEK1 inhibitor PD98059 (Figure 5Aiv). To see if Akt was also synergistically activated after pretreatment with SDF-1α, we checked phosphorylation levels of Akt. As shown in Figure 5B, Akt activation was significantly enhanced by SLF after pretreatment with SDF-1α, but not after pretreatment with MIP-1α or RANTES. MO7e cells do express the receptors for MIP-1α and RANTES, CCR1 and CCR3 (data not shown). Pretreatment of cells with SDF-1α for 30 minutes and subsequent treatment with GM-CSF or TPO also resulted in synergistic enhancement of ERK and Akt activation (data not shown). Therefore, we conclude that SDF-1α activates ERK as well as Akt in synergy with other cytokines and that SDF-1α may act as a priming agent to sensitize the cells to the actions of these other cytokines.
Enhanced activation of MAPK and Akt signaling by pretreatment of MO7e cells with SDF-1α.
Factor-starved MO7e cells were preincubated with or without 100 ng/mL of either SDF-1α, MIP-1α, or RANTES for 30 minutes or the indicated periods of time (Aiii), followed by treatment with 10 ng/mL SLF or 1 ng/mL GM-CSF for 5 minutes. In some experiments, cells were pretreated with PD98059 for 1 hour before SLF treatment (Aiv). (A) ERK immunoprecipitates obtained from total cell lysate using anti–phospho-ERK mAb were subjected to MAPK activity assay as described in “Materials and methods.” Recombinant active MAPK (20 ng) was included as a positive control (Ai). These results are representative of 3 independent experiments. (B) Phosphorylation levels of Akt were determined by Western blotting with phosphospecific antibodies to Akt (Ser473). The amount of total Akt is shown as a loading control in the bottom panels of B. This is a representative of 3 separate experiments with similar results.
Enhanced activation of MAPK and Akt signaling by pretreatment of MO7e cells with SDF-1α.
Factor-starved MO7e cells were preincubated with or without 100 ng/mL of either SDF-1α, MIP-1α, or RANTES for 30 minutes or the indicated periods of time (Aiii), followed by treatment with 10 ng/mL SLF or 1 ng/mL GM-CSF for 5 minutes. In some experiments, cells were pretreated with PD98059 for 1 hour before SLF treatment (Aiv). (A) ERK immunoprecipitates obtained from total cell lysate using anti–phospho-ERK mAb were subjected to MAPK activity assay as described in “Materials and methods.” Recombinant active MAPK (20 ng) was included as a positive control (Ai). These results are representative of 3 independent experiments. (B) Phosphorylation levels of Akt were determined by Western blotting with phosphospecific antibodies to Akt (Ser473). The amount of total Akt is shown as a loading control in the bottom panels of B. This is a representative of 3 separate experiments with similar results.
SDF-1α alone or SDF-1α in combination with other cytokines does not trigger nuclear localization of NF-κB in MO7e cells
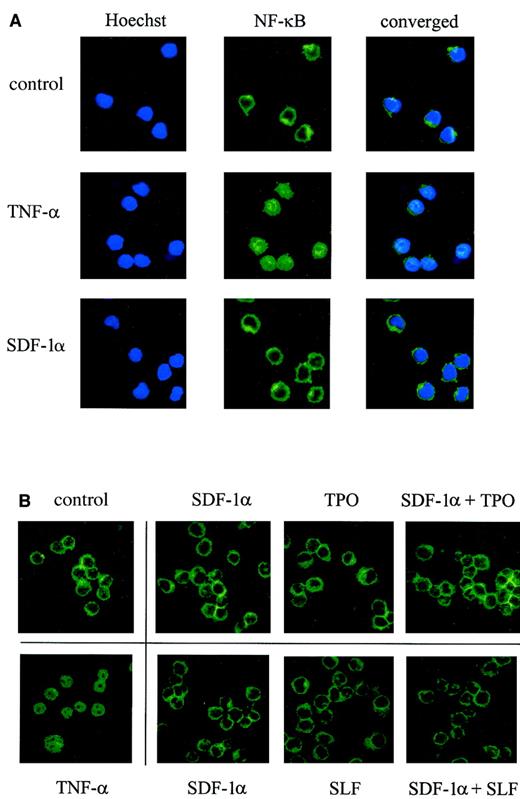
Previously, SDF-1 was reported to activate NF-κB in other cells.38,47 Activated NF-κB is known to translocate into the nucleus.48 To examine the localization of NF-κB, we made use of immunofluorescence staining with antibody against human NF-κB p65 and confocal microscopy. As seen in Figure6A, tumor necrosis factor-α (TNF-α; 10 ng/mL) induced very clear translocation of NF-κB into the nucleus within 15 minutes, but we did not detect any significant change induced by SDF-1α alone, GM-CSF alone, or SDF-1α plus GM-CSF in MO7e cells. SLF alone, TPO alone, or SDF-1α in combination with SLF or TPO did not cause the translocation of NF-κB to the nucleus either (Figure6B). The same results were obtained up to 2 hours after stimulation. Additionally, we checked NF-κB DNA binding activity by gel shift assay using NF-κB consensus binding site, and the results obtained from 30 minutes and 1 hour time points after stimulation were in agreement with confocal microscopy data; no activation of NF-κB was noticed (data not shown). Therefore, it is likely that NF-κB activation is not involved in the survival-enhancing activity of MO7e cells induced by SDF-1α alone or in synergy with these other cytokines.
No effect of SDF-1α or other cytokines on translocation of NF-κB.
Factor-starved MO7e cells were stimulated with SDF-1α alone, other cytokines alone, or combinations of cytokines for 15 minutes, and the cellular localization of NF-κB was determined by confocal microscopy after staining with a mAb against human NF-κB p65. Nuclei were stained by Hoechst no. 33258. Cells treated with TNF-α (15 minutes) are shown as a positive control for NF-κB nuclear translocation. Magnification, × 100.
No effect of SDF-1α or other cytokines on translocation of NF-κB.
Factor-starved MO7e cells were stimulated with SDF-1α alone, other cytokines alone, or combinations of cytokines for 15 minutes, and the cellular localization of NF-κB was determined by confocal microscopy after staining with a mAb against human NF-κB p65. Nuclei were stained by Hoechst no. 33258. Cells treated with TNF-α (15 minutes) are shown as a positive control for NF-κB nuclear translocation. Magnification, × 100.
SDF-1α synergistically enhances survival in vitro of primary myeloid progenitor and MO7e cells in the context of delayed growth factor addition with low concentrations of other cytokines
Because we had detected synergistic activation of intracellular signaling pathways known to be associated with cell survival and antiapoptotic effects after treatment of MO7e cells with SDF-1α plus other cytokines, we checked the survival-enhancing activities of these cytokines on primary myeloid progenitor cells as well as on MO7e cells. To assess survival-enhancing effects of SDF-1α and other cytokines, we performed colony-forming assays after delayed addition of growth factors. In brief, we incubated the cells with and without the test cytokines at time 0 and then added maximally potent combinations of growth factors used to stimulate colony formation to the plates at 24 and 48 hours later.
We previously determined that SDF-1α at 1 to 1000 ng/mL did not stimulate colony or cluster formation of primary granulocyte-macrophage colony forming units (CFU-GMs), erythroid burst-forming units (BFU-Es) or granulocyte-erythrocyte-macrophage-megakaryocyte colony forming units (CFU-GEMMs) from human or murine bone marrow, human cord blood, or MO7e cells. Moreover, SDF-1α at these concentrations did not enhance colony formation stimulated by Epo, GM-CSF, or IL-3 each alone or in the absence or presence of SLF or FL (data not shown).
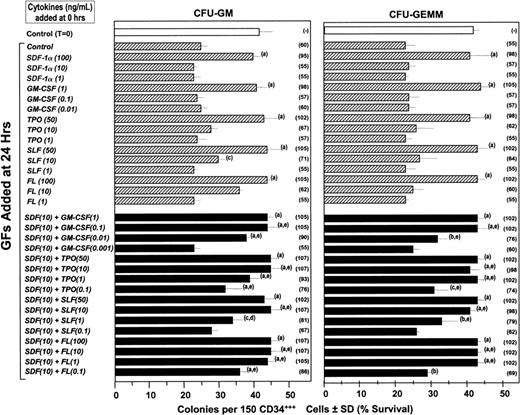
A number of cytokines have been shown to enhance the survival of hematopoietic progenitor cells in vitro that have been subjected to withdrawal and subsequent delayed addition of growth factors. These survival enhancing cytokines include but are not limited to IL-1,49 IL-6,50 FL,51SLF,51 and TPO.2 Moreover, the combinations of IL-1 plus IL-650 or FL and SLF51synergized in this effect when used at concentrations below which each acted alone to enhance survival of myeloid progenitors. Based on our above data on intracellular effects, we hypothesized that SDF-1 would synergize with other cytokines to enhance survival of primary myeloid progenitor cells in human cord blood and of MO7e cells subjected to delayed addition of growth factors that would stimulate these cells to form colonies in semisolid medium. One hundred and fifty FACS-sorted CD34+++ human cord blood cells were plated in methylcellulose culture medium with varying concentrations of SDF-1α, GM-CSF, TPO, SLF, or FL alone, or the combination of SDF-1α plus varying concentrations of GM-CSF, TPO, SLF, or FL, prior to addition of maximally stimulating concentrations of Epo (1 U/mL) plus GM-CSF (10 ng/mL) with IL-3 (10 ng/mL) and SLF (50 ng/mL) at 24 hours compared to at time 0. As shown in Figure 7 at high, but not lower concentrations of each cytokine alone, survival enhancement of CFU-GMs and CFU-GEMMs was noticed. We did not evaluate BFU-Es in these studies because we previously showed that the addition of SLF to cultures with Epo plus colony-stimulating factors will stimulate CFU-GM and CFU-GEMM but not BFU-E colonies.52Low concentrations of SDF-1α with low concentrations of either GM-CSF, TPO, SLF, or FL, that each alone did not enhance survival of CFU-GMs or CFU-GEMMs, synergized with each other to enhance the survival of these very highly enriched populations of progenitor cells in which more than 1 of 2 cells plated in the presence of optimal concentration of the combination of growth factors formed a CFU-GM or CFU-GEMM colony. Similar results were seen in one other experiment using CD34+++ cord blood cells, one experiment using bead-separated CD34+ cord blood cells (> 90% CD34+ cells with > 15% optimal cloning efficiency at 1000 cells/mL) and 2 experiments using Ficol-Hypaque separated low density cord blood cells plated at 2.5 × 104 cells/mL.
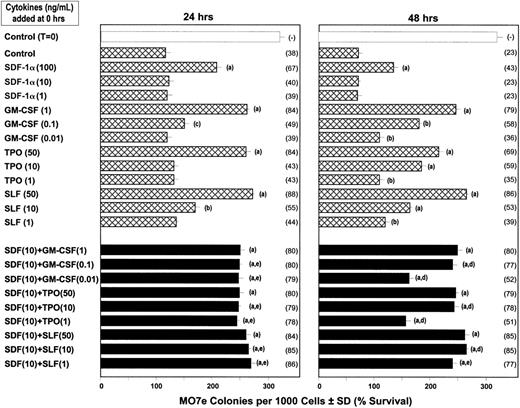
Influence of SDF-1α, GM-CSF, TPO, SLF, and FL, alone and in combination on the survival of CFU-GMs and CFU-GEMMs in FACS-sorted CD34+++ cord blood cells subjected to delayed addition of a combination of maximally stimulating growth factors.
Human CD34+++ cells were plated at time 0 in the absence and presence of various concentrations of SDF-1α, GM-CSF, TPO, SLF, FL, or SDF-1α plus either GM-CSF, TPO, SLF, or FL. The combination of rhu Epo (1 U/mL), rhu GM-CSF (10 ng/mL), rhu IL-3 (10 ng/mL), and rhu SLF (50 ng/mL), a maximally potent combination of cytokines to stimulate colony formation, was added to the plates at either time 0 or 24 hours and cultures were scored for CFU-GM and CFU-GEMM colonies 14 days after the addition of the maximally stimulating cytokines. Results are given as colonies ± SD (with the percent survival given in parentheses). (A) Significant increase in survival compared to control plates at the same time of delayed growth factor addition (P < .001). (B) Significant increase in survival compared to control plates at same time of delayed growth factor addition (P < .01). (C) Significant increase in survival compared to control plates at same time of delayed growth factor additions (P < .05). (D) Significantly greater survival than either cytokine alone and additive to slightly less than additive effects at the same time of delayed growth factor addition (P < .05). (E) Significantly greater survival than with either cytokine alone and greater than additive effect at the same time of delayed growth factor addition (P < .01). The addition of either SDF-1, GM-CSF, TPO, SLF, FL, or the combination of SDF-1 plus these cytokines along with the maximally stimulating combination of Epo, GM-CSF, IL-3, and SLF at time 0 had no significant effect (P > .05) compared to control medium added with the maximally stimulating combination of growth factors at time 0 (data not shown).
Influence of SDF-1α, GM-CSF, TPO, SLF, and FL, alone and in combination on the survival of CFU-GMs and CFU-GEMMs in FACS-sorted CD34+++ cord blood cells subjected to delayed addition of a combination of maximally stimulating growth factors.
Human CD34+++ cells were plated at time 0 in the absence and presence of various concentrations of SDF-1α, GM-CSF, TPO, SLF, FL, or SDF-1α plus either GM-CSF, TPO, SLF, or FL. The combination of rhu Epo (1 U/mL), rhu GM-CSF (10 ng/mL), rhu IL-3 (10 ng/mL), and rhu SLF (50 ng/mL), a maximally potent combination of cytokines to stimulate colony formation, was added to the plates at either time 0 or 24 hours and cultures were scored for CFU-GM and CFU-GEMM colonies 14 days after the addition of the maximally stimulating cytokines. Results are given as colonies ± SD (with the percent survival given in parentheses). (A) Significant increase in survival compared to control plates at the same time of delayed growth factor addition (P < .001). (B) Significant increase in survival compared to control plates at same time of delayed growth factor addition (P < .01). (C) Significant increase in survival compared to control plates at same time of delayed growth factor additions (P < .05). (D) Significantly greater survival than either cytokine alone and additive to slightly less than additive effects at the same time of delayed growth factor addition (P < .05). (E) Significantly greater survival than with either cytokine alone and greater than additive effect at the same time of delayed growth factor addition (P < .01). The addition of either SDF-1, GM-CSF, TPO, SLF, FL, or the combination of SDF-1 plus these cytokines along with the maximally stimulating combination of Epo, GM-CSF, IL-3, and SLF at time 0 had no significant effect (P > .05) compared to control medium added with the maximally stimulating combination of growth factors at time 0 (data not shown).
As shown in Figure 8, SDF-1α synergized with low concentrations of GM-CSF, TPO, and SLF to enhance survival of MO7e colony-forming cells subjected to 24- and 48-hour delayed growth factor addition. These results were representative of 2 additional studies with MO7e cells. Because MO7e cells do not express Flt3, the receptor for FL, we could not evaluate FL effects on these cells nor the signaling events triggered by SDF-1α plus FL or FL alone. Neither SDF-1α nor the low concentrations of the other cytokines used for cell survival stimulated colony formation by MO7e cells when used alone or together (data not shown).
Influence of SDF-1α, GM-CSF, TPO, and SLF on survival of MO7e colony-forming cells (CFCs) after growth factor withdrawal.
MO7e cells were plated at 103 cells/mL in agar culture medium at time 0 with control medium or the amounts of SDF-1α, GM-CSF, TPO, or SLF, or SDF-1α plus either GM-CSF, TPO, or SLF shown. The combination of GM-CSF (10 ng/mL) plus SLF (50 ng/mL), a maximal concentration of factors to stimulate MO7e colony formation, was added to these plates at either time 24 or 48 hours. Results are shown as colonies ± SD (with the percent survival compared to time 0 culture plated with control medium shown in parentheses). Statistical differences between groups are noted by the same letters as for the results in Figure 7. The addition of either SDF-1, GM-CSF, TPO, SLF, or SDF-1 plus these cytokines along with the maximally stimulating combination of GM-CSF plus SLF at time 0 had no significant effect (P > .05) compared to control medium added with the maximally stimulating combination of growth factors at time 0 (data not shown).
Influence of SDF-1α, GM-CSF, TPO, and SLF on survival of MO7e colony-forming cells (CFCs) after growth factor withdrawal.
MO7e cells were plated at 103 cells/mL in agar culture medium at time 0 with control medium or the amounts of SDF-1α, GM-CSF, TPO, or SLF, or SDF-1α plus either GM-CSF, TPO, or SLF shown. The combination of GM-CSF (10 ng/mL) plus SLF (50 ng/mL), a maximal concentration of factors to stimulate MO7e colony formation, was added to these plates at either time 24 or 48 hours. Results are shown as colonies ± SD (with the percent survival compared to time 0 culture plated with control medium shown in parentheses). Statistical differences between groups are noted by the same letters as for the results in Figure 7. The addition of either SDF-1, GM-CSF, TPO, SLF, or SDF-1 plus these cytokines along with the maximally stimulating combination of GM-CSF plus SLF at time 0 had no significant effect (P > .05) compared to control medium added with the maximally stimulating combination of growth factors at time 0 (data not shown).
We performed apoptosis assays to see if the enhanced survival read-outs of the colony-forming cell assays correlated with antiapoptotic activity. Here, we assessed apoptosis by measuring APO2.7+cells expressing the 7A6 antigen, which is involved in the molecular cascade of apoptosis.36 As shown in Figure9, SDF-1α alone slightly suppressed apoptosis in response to growth factor withdrawal in human CD34+ cells (Figure 9A) as well as in MO7e (Figure 9B) cells. SLF alone induced more apoptosis inhibition, and SDF-1α plus SLF significantly enhanced suppression of apoptosis of these cells compared to SDF-1α alone or SLF alone. When we treated MO7e cells with MIP-1α (Figure 9B) or RANTES (data not shown) instead of SDF-1α, there was no significant effect suggesting that the survival enhancing/antiapoptotic activity we noted is not a common property of chemokines.
Effect of SDF-1α on apoptosis induced by growth factor withdrawal.
Factor-starved CD34+ cells prepared from human bone marrow (A) or MO7e cells (B) were further incubated in serum-free media in the presence of either 100 ng/mL SDF-1α alone, 10 ng/mL SLF alone, or the combination of these 2 cytokines. After 4 days, cells were stained with PC-5–conjugated APO2.7 mAb and analyzed by flow cytometry. (A) Values in histogram represent percent of APO2.7+ cells. This is representative of 3 separate experiments. (B) Columns represent the average percent of APO2.7+ cells ± SD of 3 separate experiments. *P < .01 versus SLF alone.
Effect of SDF-1α on apoptosis induced by growth factor withdrawal.
Factor-starved CD34+ cells prepared from human bone marrow (A) or MO7e cells (B) were further incubated in serum-free media in the presence of either 100 ng/mL SDF-1α alone, 10 ng/mL SLF alone, or the combination of these 2 cytokines. After 4 days, cells were stained with PC-5–conjugated APO2.7 mAb and analyzed by flow cytometry. (A) Values in histogram represent percent of APO2.7+ cells. This is representative of 3 separate experiments. (B) Columns represent the average percent of APO2.7+ cells ± SD of 3 separate experiments. *P < .01 versus SLF alone.
Discussion
It is known that SDF-1 is an important chemokine in the bone marrow microenvironment. It has been implicated in migration of MPCs and stem cells through its chemotactic activity,5-7,15-18events consistent with abnormalities in the marrow of both SDF-1−/− and CXCR4−/−mice.10,11 We postulated that SDF-1 might have other activities in addition to that of chemotaxis. Because SDF-1−/− mice die perinatally, we made SDF-1α transgenic mice and found that these mice had enhanced myelopoiesis with a higher MPC cell cycling status and a greater absolute number of MPCs per femur and spleen compared to their littermate controls. This did not appear to be due to SDF-1α activities in inducing the proliferation of MPCs. Rather, we found that SDF-1α had survival-enhancing effects on MPCs in vitro.23 SDF-1α has previously been shown to induce,53,54 protect against apoptosis,55,56 or to have no effects on cell survival.57 These contradictory results might be due to differences in cell types or experimental conditions.
In this present study, we have additionally found that SDF-1α can synergize with other cytokines at very low concentrations in the survival enhancement of primary MPCs subjected to the delayed addition of growth factors. Our results revealed that SDF-1α enhanced survival of purified human CD34+++ and CD34+ cord blood CFU-GMs and CFU-GEMMs in synergy with other cytokines such as GM-CSF, FL, SLF, or TPO. We obtained similar results with the factor-dependent cell line MO7e. Strikingly, the effective concentrations of other cytokines used in combination with SDF-1α were as low as 1 ng/mL for FL, 0.01 ng/mL for GM-CSF, 1 ng/mL for SLF, and 1 ng/mL for TPO. Therefore, SDF-1α clearly had survival-enhancing activity that synergized with these other cytokines. Based on our own observations, SDF-1α did not change the expression level of other cytokine receptors such as c-kit and c-mpl and GM-CSF did not change the expression level of CXCR4 in MO7e cells; this latter observation is in agreement with a previous report using monocytes.58Therefore, we believe that the synergistic survival-enhancing activity of SDF-1α likely results from enhanced intracellular signaling rather than effects on cytokine receptor expression.
Because we observed the same results using primary human MPCs as we did using MO7e cells in the survival-enhancing activity of SDF-1α, we felt that biochemical data from the MO7e cell line could serve as a model system to understand some of the intracellular signaling mechanisms that might be involved in these effects. We checked activation of 2 survival-related signal transduction pathways, the PI3K/Akt and MAPK/RSK pathways, using phosphospecific antibodies against Akt/PKB, ERK1/2, or RSK in MO7e cells after stimulation with SDF-1α alone or SDF-1α in combination with other cytokines such as GM-CSF, SLF, and TPO. These 2 pathways were activated by stimulation with SDF-1α alone and inhibited by PI3K inhibitors and a MEK1 inhibitor, respectively. These results imply that PI3K and MAPK pathways activated by SDF-1α are independent of each other. Combined stimulation with SDF-1α plus another of the cytokines we used here activated these 2 pathways synergistically. These results were in agreement with biologic effects showing enhanced survival by SDF-1α alone and SDF-1α in synergy with other cytokines in primary cells as well as MO7e cells. Interestingly, when MO7e cells were stimulated with other cytokines after pretreatment with SDF-1α, synergistic activation of ERK and Akt was still evident, even 1 hour after pretreatment with SDF-1α. In contrast, when we checked ERK activation after stimulation with SDF-1α alone for 30 minutes, we could not detect significant activity. This suggests that SDF-1α and the other cytokines trigger signal transduction pathways in agreement with their unique biologic activities. Furthermore, SDF-1α may prime or sensitize cells to be more responsive to other cytokines for cell survival enhancement. We have previously noted that SDF-1α blocks suppression of murine MPC proliferation in vitro by CC chemokines MIP-1α, CKβ-11, TECK, and MCP-1, CXC chemokines IL-8 and PF4, and the C chemokine lymphotactin.23 MPCs from SDF-1α transgenic, but not littermate control, mice were insensitive to inhibition by these chemokines.23 We speculate that a priming effect of SDF-1α may somehow also contribute to these phenomena, at least, in part.
Nuclear factor-κB is a ubiquitous heterodimeric transcription factor, and IκB binds NF-κB and keeps it localized to the cytoplasm.48 Phosphorylation of IκB targets it for ubiquitination and proteosome-mediated degradation, which frees NF-κB from IκB, allowing NF-κB nuclear translocation and activation of target genes. It was previously reported that SDF-1α activated NF-κB in the CTS cell line38 and megakaryocytes,47 and information from the literature suggests that both the PI3K/Akt and MAPK/RSK pathways can activate NF-κB.24,25 Therefore, we checked NF-κB activation in MO7e cells after stimulation with SDF-1α alone or in combination with other cytokines. NF-κB activation and localization to nucleus was detected after stimulation with TNF-α, but not after stimulation with other cytokines such as GM-CSF, SLF, and TPO. This is in agreement with other reports that in MO7e cells TNF-α, but not GM-CSF and IL-3, can activate NF-κB.59 No change in localization of NF-κB was induced by SDF-1α alone or in combination with these other cytokines. Thus, NF-κB activation by SDF-1α may be cell type specific. There is a report describing differential effects of HIV type 1 through CD4 and CXCR4 receptors. Using Jurkat T cells it was shown that SDF-1 did not affect expression of cytokine and chemokine genes regulated by NF-κB.60 In astrocytes, SDF-1α was reported to activate NF-κB via TNF induction.61 Taken together, we believe that SDF-1α does not activate NF-κB directly or if it does it may be very weak in MO7e cells and that NF-κB may not be involved in the survival enhancing activities of SDF-1α in synergy with the other cytokines we evaluated. Whether this is true for primary MPCs remains to be determined.
The PI3K/Akt and MAPK pathways have also been reported to be involved in cytokine- or chemokine-induced migration of various cell types.38,62-65 SDF-1α activated both pathways in the hematopoietic cell line CTS60 and also in the progenitor cell line MO7e that we used in this study. Because our studies did not distinguish between signaling that might be involved in chemotaxis versus enhancement of MPC survival, the synergistic activation of the PI3K/Akt and MAPK pathways may contribute to chemotaxis also. It has been shown by us and others that SDF-1α in combination with SLF enhances chemotaxis of MO7e cells, CTS cells, and progenitor cells.16,66 Although activation of the ERK signaling pathway has been shown to promote cell motility, either by regulating gene expression64 or by directly activating myosin light chain kinase,65 this was not the case for SDF-1α–induced cell migration.67 Based on inhibitor studies, PI3K appears to be required for SDF-1α–mediated phosphorylation of focal adhesion proteins and the migration of both hematopoietic cell line CTS and primary marrow CD34+ cells, whereas MAPK ERK1/2 is not.67 The MAPK pathway has also been implicated in mitogen-stimulated proliferation,68 but SDF-1α alone or in combination with low concentrations of the other cytokines we used here did not induce cellular proliferation. Therefore, MAPK activation in our study may reflect the survival-enhancing activity of SDF-1α alone or SDF-1α in combination with the other cytokines in MO7e cells, and possibly in primary MPCs.
SDF-1 and its receptor CXCR4 have unique properties in terms of structure and function.9 SDF-1 and CXCR4 are highly conserved between mouse and human (99% and 90%, respectively), compared with other chemokines such as mitotic control protein 1 (MCP-1; 55%) or MIP-1α (75%) or other CXC chemokine receptors such as IL-8 receptor A (68%) or B (71%).69Among more than 50 identified chemokines, only a very small number of these chemokines, including SDF-1, is known to induce chemotaxis of primitive MPCs and stem cells. The function of SDF-1 is considered essential and nonredundant based on the embryonic lethal phenotypes of SDF-1 or CXCR4 knock-out mice.10,11 We could not detect any significant effect on survival and signaling in MO7 cells treated with MIP-1α or RANTES. Although we have not checked other chemokines for survival-enhancing effects, our studies suggest that the survival-enhancing activity of SDF-1 for MPCs is not a common property of all chemokines. A recent report that SDF-1 alone among many different chemokines induced significant and prolonged activation of ERK and Akt in T lymphocytes supports this possibility.40
Hematopoiesis involves a complex set of interactions that are dynamically and finely regulated by the environment and specifically by stromal cells.69 Stromal cells regulate hematopoiesis by binding directly to hematopoietic cells and also by providing numerous secreted cytokines.70-72 Therefore, the capability of SDF-1α in synergy with other cytokines to enhance survival of primary MPCs in vitro may be of physiologic relevance. We suggest from our studies another function of SDF-1, in addition to promoting migration to and retention in bone marrow of MPCs and stem cells.15,16 73 We believe that SDF-1 not only helps direct MPCs and stem cells to the marrow and keeps them there, but in the process also helps to enhance their survival while in the marrow, either alone or in combination with other cytokines.
We thank Ayako Hirota for her excellent technical assistance with the apoptosis studies.
Supported by Public Health Service grants RO1 HL67384, RO1 HL56416, and RO1 DK53674 to H.E.B. L.K. was supported by training grant T32 DK 07519 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hal E. Broxmeyer, Walther Oncology Center, Indiana University School of Medicine, Bldg R4, Rm 302, 1044 W Walnut St, Indianapolis, IN 46202; e-mail: hbroxmey@iupui.edu.

![Fig. 1. Activation of Akt, ERK1/2, and p90RSK in MO7e cells after stimulation with SDF-1α. / (A) MO7e cells were incubated with 100 ng/mL SDF-1α for the indicated time periods. (B) MO7e cells were preincubated in the presence of dimethyl sulfoxide (DM) vehicle control or LY 294002 (LY, 30 μM), wortmannin (W, 100 nM), PD 98059 (PD, 25 μM), or rapamycin (R [also called sirolimus], 10 nM) for 1 hour, and stimulated with 100 ng/mL SDF-1α for 5 minutes. Cell lysates were analyzed by Western blotting with phosphospecific antibodies to Akt (Ser473), ERK1/2 (Thr202/Tyr204), or p90RSK (Ser381). Amounts of p90RSK (A) or Akt (B) are shown on the bottom panel of each as a loading control. This experiment was performed 3 times with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/12/10.1182_blood.v99.12.4307/4/m_h81222709001.jpeg?Expires=1769086190&Signature=1yMUiJl3YM8~oggyoXD-irRadTT3g5-G7L910cK0bURUPhHpSe3Yu3Rc4hKZwbmwoVpw~HpQU1BUtWbotB9-UoYAyQBpo~vkt5Du1MI10zVUgxI~5qFFtvg4B7KBb282u4WvM-BCX5UuEwqf18OOpvN4ArHumh5b6EncpC2t8sz9jz4lBpdQ7DtTIKnkO8~zvG4C-JlZgDqSF81ReOX8CZdytAgOe470RW9~8KpxY9xRxHVJzQdTjvGKZrpVsxCIx9G9vq4J~AYFmy035gYeJLan5N2UjJeC9sjinlYOptUAnYQTjgKlYMF00BMlm0JCkxYIzDYfH~~a3CHdlpRGiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal