The lack of diagnostic certainty in some patients makes it difficult to distinguish between primary and secondary forms of thrombocytosis. To augment current diagnostic studies for thrombocytosis, we retrospectively evaluated clinical records and bone marrow trephine specimens of 183 patients with thrombocytosis—164 with essential thrombocythemia (ET), 19 with reactive thrombocytosis (RT)—for bone marrow angiogenesis, bone marrow megakaryocyte c-Mpl staining, and morphologic evidence of megakaryocyte proliferation. Angiogenesis was increased in patients with ET compared with healthy controls (P < .0001) and patients with RT (P = .006). In addition, an increase in angiogenesis was associated with certain disease features such as splenomegaly (P = .004) and reticulin fibrosis (P = .005). Decreased megakaryocyte c-Mpl staining was observed in a heterogeneous pattern in ET compared with healthy controls (P < .0001) and RT (P < .0001). Histologic stratifying criteria incorporating increased angiogenesis, decreased megakaryocyte c-Mpl expression, and marked megakaryocyte proliferation in the bone marrow was highly sensitive (97%) and specific (95%) for distinguishing ET from RT (P < .0001). However, with the current duration of follow-up available on the patients, none of the histologic features evaluated have yet demonstrated prognostic value for subsequent clinical course, vascular events, or survival.
Introduction
Essential thrombocythemia (ET) is a chronic myeloid stem cell disorder lacking a defining cytogenetic abnormality or an absolute diagnostic feature. Sustained thrombocytosis that is not attributable to known secondary causes is the primary feature of ET. However, unexplained thrombocytosis is not unique to ET. It may be present in various related myeloid disorders, including polycythemia vera, agnogenic myeloid metaplasia, and chronic myeloid leukemia. In the absence of a clear diagnostic standard, the diagnosis of ET has traditionally been made on the basis of criteria designed to exclude secondary causes of thrombocytosis and related primary marrow disorders. The Polycythemia Vera Study Group established the criteria for the diagnosis of ET as a sustained platelet count of more than 600 × 109/L, the exclusion of secondary causes of thrombocytosis (eg, iron deficiency), normal red cell mass (to exclude polycythemia vera), absence of significant myelofibrosis, and absence of t(9:22) (to exclude chronic myeloid leukemia).1 These diagnostic criteria, though clinically useful, have left many patients with thrombocytosis with some degree of diagnostic uncertainty.
The lack of absolute certainty in the diagnosis of ET and the occasional difficulty in distinguishing ET from reactive thrombocytosis (RT) have led to a variety of proposed clinical, laboratory, and morphologic methods of distinguishing ET from RT. Specifically, features from a detailed clinical history of unexpected vascular events (thrombosis or hemorrhage) or physical examination findings of myeloproliferation (eg, increased splenic volume2) can be helpful in distinguishing ET from RT. In addition, serologic evidence of increased levels of acute-phase reactants such as interleukin-6 (IL-6) and C-reactive protein3 have aided in distinguishing ET from RT. Spontaneous in vitro growth of erythroid or megakaryocyte colonies in the absence of required cytokines has been reported by several investigators to be of value in distinguishing ET from RT.4-6 However, the use of colony-forming assays for diagnostic purposes is limited by the lack of objective standards, and the method is technically demanding enough to limit the widespread availability of this assay. All the diagnostic features discussed above can aid in the diagnosis of ET, yet there remain patients with thrombocytosis for whom a firm diagnosis cannot be made. Accordingly, we explored additional objective disease features of ET to aid in the diagnosis and prognosis of this disorder.
A histologic feature of potential diagnostic relevance is increased bone marrow angiogenesis. Neo-angiogenesis is important for the growth and proliferation of many solid malignancies.7 Similarly, pathologic increases in bone marrow stromal microvessels have been demonstrated in a number of primary marrow disorders. In fact, various clonal and malignant hematologic disorders share the characteristic of increased angiogenesis, including lymphoproliferative disorders (multiple myeloma,8 chronic lymphocytic leukemia,9 acute lymphoblastic leukemia10) and myeloid disorders (acute myeloid leukemia,11myelodysplasia,12 and chronic myeloid leukemia12). Similarly, we previously reported on a marked increase in angiogenesis in myelofibrosis with myeloid metaplasia.13 The increase in angiogenesis is of prognostic significance and is potentially a useful therapeutic target in myeloma14,15 and myelofibrosis with myeloid metaplasia.3 16
Another histologic characteristic of interest in ET is the decreased expression of the thrombopoietin (TPO) receptor, c-Mpl, on the surfaces of megakaryocytes. The abnormal proliferation of platelets that characterizes ET has been associated with dysregulation of the TPO–c-Mpl axis. Specifically, recent studies have demonstrated inappropriately elevated levels of TPO with an associated decrease in surface expression of c-Mpl on platelets and megakaryocytes in the chronic myeloproliferative disorders—ET,17polycythemia vera,18 and agnogenic myeloid metaplasia.19 We have demonstrated that c-Mpl expression on megakaryocytes can be heterogeneous and that it is of diagnostic value in polycythemia vera.20
The definitive goal in the investigation of ET is to elucidate the molecular aberration or cellular regulatory dysfunction responsible for the disease. However, until an appropriate molecular marker is identified, we should make maximal use of the diagnostic information obtainable from our current histologic markers. We therefore chose to evaluate the independent diagnostic and prognostic values of increased marrow angiogenesis and aberrant c-Mpl expression in a retrospective histopathologic study involving a large cohort of patients with ET patients compared with appropriate healthy controls and patients with RT. In addition, we attempted to synthesize the diagnostic and prognostic values of the various individual marrow morphologic features (angiogenesis, c-Mpl expression, and megakaryocyte proliferation) into a single stratifying morphologic grade.
Patients and methods
Patients
After approval of the study by the institutional review board, we identified study patients by searching our institutional comprehensive database of medical diagnoses and procedures. We reviewed the medical records of all patients with ET who were seen at our institution from 1991 through 1998 for whom bone marrow biopsy specimens were available for analysis (n = 164). Patient selection was dictated by the availability of bone marrow sections. The study cohort included patients who were either newly diagnosed (n = 113, 68.9%) or further along in the course of their disease.
All study patients had histologic and clinical confirmation of the diagnosis of ET according to accepted criteria.1 All patients with a secondary cause of thrombocytosis or another chronic myeloid disorder were excluded. A group of patients from the same era with documented RT were included for comparison (n = 19). For these patients the cause of the thrombocytosis was clearly documented: inflammatory process, 9; lymphoma, 5; iron deficiency, 2; malignancy, 2; and liver disease, 1; corresponding bone marrow was available. In addition, we had for comparison normal bone marrow (n = 44 for CD34 and n = 22 for c-Mpl) and advanced chronic myeloproliferative disorder (CD34 only: postthrombocythemic myeloid metaplasia, n = 18; myelofibrosis with myeloid metaplasia, n = 96). Clinical and laboratory information at diagnosis, at the time of the bone marrow study, and during the subsequent clinical course were obtained in patients with ET and RT and in healthy controls and comparison patients. Specific attention was paid to details concerning vascular events (thrombosis or hemorrhage) and their outcomes (type of event and recurrences).
Evaluation of bone marrow histologic features
General.
Bone marrow biopsy slides were prepared from paraffin-embedded blocks. The marrows were stained with hematoxylin and eosin in standard fashion. All marrows were reviewed by one of the authors (S.-Y.Y. or C.A.H.) to ensure the accuracy of the diagnosis, whether ET or RT. Each marrow was uniformly evaluated for cellularity. In addition, the marrows were subsequently graded for the presence of reticulin fibrosis (grades 0-4), osteosclerosis (grades 0-2), and presence and degree of megakaryocyte proliferation (as judged by megakaryocyte number [1-4] and megakaryocyte clustering [0-2]).
Bone marrow angiogenesis: staining.
Bone marrow microvessels were visualized by immunohistochemical staining for the CD34 antigen with the use of a labeled streptavidin–biotin peroxidase method. We previously reported the value of CD34 as the antigen of choice for evaluating bone marrow angiogenesis in patients with myeloproliferative disorders.13 Other commonly used antigens for vessel staining (CD31 and factor VIII) are of little value in the myeloproliferative disorders because they also stain the large number of megakaryocytes usually seen in these disorders. Therefore, biopsy samples were prepared in the following manner. After deparaffinization, slides were steam pretreated in 0.01M EDTA buffer, pH 8, in a Black & Decker Handy Steamer Plus (Towson, MD) for 30 minutes. After rinsing in cool water, slides were immunostained by a Ventana ES automated stainer (Ventana Systems, Tucson, AZ) using buffers and detection reagents supplied by the manufacturer. The primary antibody (clone HPCA-1; Becton-Dickinson, San Jose, CA) was incubated with tissue sections for 24 minutes in a 1:50 dilution. The AEC (aminoethyl carbazole) detection kit (Ventana Systems) was used for antigen visualization; sections were counterstained with a light hematoxylin and coverslipped with Kaiseri glycerol jelly (Mayo Medical Laboratories, Rochester, MN).
Estimating angiogenesis.
Angiogenesis was assessed in each marrow trephine sample by 2 masked reviewers through visual microvessel grading, as we have previously described.13 Specifically, the study slides were visually scanned at × 100, × 200, and × 400 magnification and were semiquantitatively graded for extent of vessels by CD34 staining. Morphologic analysis was performed carefully to ensure vessel specificity of the CD34-stained stroma considered for analysis. Each specimen was graded on a semiquantitative scale: microvessel grade 1, scant vessels; grade 2, diffuse modest increase in vessels; grade 3, areas with marked increases in vessels; and grade 4, extensive marked increases in vessels (Figure 1). We previously compared 3 different methods to quantitate microvessel density and found that efforts to specifically count vessels are open to significant bias because of sample error and the inherent heterogeneity of vessel density in bone marrow trephine samples.
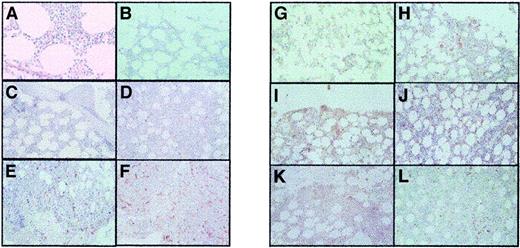
Bone marrow trephine specimens showing angiogenesis (CD34 staining) and megakaryocyte c-Mpl grading.
The panels show the following: (A) Microvessel grade 1 (MVG 1) (normal); (B) MVG 1 (RT); (C) MVG 1 (ET); (D) MVG 2 (ET); (E) MVG 3 (ET); (F) MVG 4 (ET); (G) c-Mpl 1 (normal); (H) c-Mpl 1 (RT); (I) c-Mpl 1 (ET); (J) c-Mpl 2 (ET); (K) c-Mpl 3 (ET); and (L) c-Mpl 4 (ET). All images were obtained using light microscopy at × 200 magnification.
Bone marrow trephine specimens showing angiogenesis (CD34 staining) and megakaryocyte c-Mpl grading.
The panels show the following: (A) Microvessel grade 1 (MVG 1) (normal); (B) MVG 1 (RT); (C) MVG 1 (ET); (D) MVG 2 (ET); (E) MVG 3 (ET); (F) MVG 4 (ET); (G) c-Mpl 1 (normal); (H) c-Mpl 1 (RT); (I) c-Mpl 1 (ET); (J) c-Mpl 2 (ET); (K) c-Mpl 3 (ET); and (L) c-Mpl 4 (ET). All images were obtained using light microscopy at × 200 magnification.
c-Mpl staining of megakaryocytes.
Immunohistochemical studies for megakaryocyte c-Mpl have been performed in our laboratory with reproducible success with commercially available antibodies20; therefore, we chose those same antibodies for this project and prepared the slides in the following manner. After deparaffinization, slides were steam pretreated in 0.01M EDTA buffer, pH 8, in a Black & Decker Handy Steamer Plus for 30 minutes. After rinsing in cool water, slides were immunostained by a Ventana ES automated stainer (Ventana Systems) using buffers and detection reagents supplied by the manufacturer. The primary antibody (Genzyme, catalog no. 3724; R&D Systems, Cambridge, MA) was incubated with tissue sections for 24 minutes in a 1:400 dilution. The AEC detection kit (Ventana Systems) was used for visualization of antigen. Sections were counterstained with a light hematoxylin and were coverslipped with Kaiseri glycerol jelly (Mayo Medical Laboratories).
Estimating c-Mpl staining of megakaryocytes. We previously noted that c-Mpl staining for megakaryocytes can be heterogeneous in patients with myeloproliferative disorders. We therefore tried to convey the heterogeneity seen in our study specimens by using a visual grading system. Specifically, the study slides were visually scanned at × 100, × 200, and × 400 magnification and were semiquantitatively graded for megakaryocyte staining with c-Mpl. Each specimen was graded on a semiquantitative scale: grade 1, strong staining in more than 80% of megakaryocytes; grade 2, strong staining in 50% to 80% of megakaryocytes; grade 3, strong staining in 20% to 50% of megakaryocytes; and grade 4, strong staining in less than 20% of megakaryocytes (Figure 1). All slides were reviewed by 2 masked reviewers, who were also blinded not only to the clinical information but also to the diagnosis of ET versus RT.
ET histologic score
We wanted to explore whether combining the information obtained from the various histologic findings in ET had diagnostic or prognostic relevance. We chose to assign an ET histologic score. Specifically, 1 point was assigned for marked megakaryocyte proliferation or clustering (on hematoxylin and eosin), marked increase in angiogenesis (microvessel grade 3 or higher, or marked decrease in megakaryocyte c-Mpl staining (grade 3 or higher). Thus, scores of 0 to 3 were possible.
Statistical analysis
Overall survival was defined as the interval from diagnosis to death or last contact. Survival from the date of bone marrow study was also analyzed. An event was defined as a death from any cause unless otherwise indicated. Kaplan-Meier methodology was used to estimate the distributions of survival and time to disease progression.21 The 2-tailed log-rank test was used to assess whether survival from diagnosis and survival from bone marrow study differed between categories. Associations between each histologic characteristic (microvessel grade, megakaryocyte c-Mpl staining grade, and ET histologic score) and pertinent clinical, histologic, and laboratory variables obtained at the time of the bone marrow study were also studied. Fisher exact test was used to assess the significance of the difference between the distributions of categorical data. The Wilcoxon rank-sum test or the Kruskal-Wallis test was used to assess whether continuous variables differed significantly between categories. Multivariate analysis was conducted with the Cox proportional hazards model.22 All data were analyzed by using SAS software (SAS, Cary, NC).
Results
One hundred eighty-three patients with thrombocytosis (164 with ET and 19 with RT) and 44 healthy controls with available bone marrow were studied. Three different immunohistochemical features (increased marrow microvessel density, decreased megakaryocyte c-Mpl expression, or a combination of these along with evidence of megakaryocyte proliferation) proved useful in distinguishing RT from ET (P < .0001 for all 3). Clinical and histologic data for the patients with thrombocytosis are summarized in Table1. The prognostic and diagnostic value of each type of immunohistochemical marrow analysis is reported below.
Characteristics of 183 patients with thrombocytosis
| . | Essential thrombocythemia . | Reactive thrombocytosis . |
|---|---|---|
| No. patients | 164 | 19 |
| Cause of thrombocytosis | ET (n = 164) | Inflammatory disorder (n = 8) |
| Lymphoma (n = 5) | ||
| Iron deficiency (n = 2) | ||
| Malignancy (n = 2) | ||
| Liver disease (n = 2) | ||
| Sex | ||
| Male | 63 (38.4%) | 10 (52.6%) |
| Female | 101 (61.6%) | 9 (47.4%) |
| Age at diagnosis, median (y) | 63.2 (range, 12-91.7) | NA |
| Age at bone marrow study, median (y) | 64.9 (range, 18-91.7) | 54.6 (range, 2.5-74.8) |
| Duration of follow-up from diagnosis, median (mo) | 71.6 (range, 0.03-403) | NA |
| Duration of follow-up from bone marrow study, median (mo) | 58.9 (range, 0-148) | 9 (1-98) |
| Interval, diagnosis to bone marrow study, median (mo) | 0 (range, 0-397) | NA |
| < 3 mos from diagnosis | 113 (68.9%) | NA |
| Status at last follow-up | ||
| Alive | 128 (78.0%) | 17 (89.5%) |
| Deceased | 36 (22.0%) | 2 (10.5%) |
| Cause of death | 5 ET related (3.0%) | NA |
| Chemotherapy naive at time of bone marrow study | 127 (77.4%) | NA |
| Laboratory values/examinations at diagnosis, median (range) | ||
| Hemoglobin (g/dL) | 13.7 (8.7-17.3) | NA |
| Leukocyte count (× 109/L) | 9.5 (1.3-45.4) | — |
| Platelet count (× 109/L) | 976 (404-2525) | — |
| Palpable spleen | 33 (20.1%) | — |
| Palpable liver | 6 (3.7%) | — |
| Laboratory values/examinations at bone marrow study, median (range) | ||
| Hemoglobin (g/dL) | 13.6 (8.4-17.3) | 10.3 (8.5-13.3) |
| Leukocyte count (× 109/L) | 8.9 (1.3-45.4) | 11 (6.7-24.4) |
| Platelet count (× 109/L) | 930 (144-2525) | 643 (500-1103) |
| Palpable spleen | 34 (20.7%) | 0 |
| Palpable liver | 6 (3.7%) | 0 |
| Vascular event (? yes) | 63 (38.4%) | 0 |
| Number of vascular events | ||
| 0 | 104 (63.4%) | NA |
| 1 | 53 (32.3%) | — |
| 2 | 8 (4.9%) | — |
| 3 | 2 (1.2%) | — |
| Type of vascular event | ||
| Thrombosis | 44 (69.8%) | NA |
| Bleeding | 19 (30.2%) | — |
| Labs at first vascular event, median (range) | ||
| Hemoglobin (g/dL) | 13.6 (9.3-16.4) | NA |
| Leukocyte count (× 109/L) | 11.5 (3.6-45) | — |
| Platelet count (× 109/L) | 920 (151-2620) | — |
| . | Essential thrombocythemia . | Reactive thrombocytosis . |
|---|---|---|
| No. patients | 164 | 19 |
| Cause of thrombocytosis | ET (n = 164) | Inflammatory disorder (n = 8) |
| Lymphoma (n = 5) | ||
| Iron deficiency (n = 2) | ||
| Malignancy (n = 2) | ||
| Liver disease (n = 2) | ||
| Sex | ||
| Male | 63 (38.4%) | 10 (52.6%) |
| Female | 101 (61.6%) | 9 (47.4%) |
| Age at diagnosis, median (y) | 63.2 (range, 12-91.7) | NA |
| Age at bone marrow study, median (y) | 64.9 (range, 18-91.7) | 54.6 (range, 2.5-74.8) |
| Duration of follow-up from diagnosis, median (mo) | 71.6 (range, 0.03-403) | NA |
| Duration of follow-up from bone marrow study, median (mo) | 58.9 (range, 0-148) | 9 (1-98) |
| Interval, diagnosis to bone marrow study, median (mo) | 0 (range, 0-397) | NA |
| < 3 mos from diagnosis | 113 (68.9%) | NA |
| Status at last follow-up | ||
| Alive | 128 (78.0%) | 17 (89.5%) |
| Deceased | 36 (22.0%) | 2 (10.5%) |
| Cause of death | 5 ET related (3.0%) | NA |
| Chemotherapy naive at time of bone marrow study | 127 (77.4%) | NA |
| Laboratory values/examinations at diagnosis, median (range) | ||
| Hemoglobin (g/dL) | 13.7 (8.7-17.3) | NA |
| Leukocyte count (× 109/L) | 9.5 (1.3-45.4) | — |
| Platelet count (× 109/L) | 976 (404-2525) | — |
| Palpable spleen | 33 (20.1%) | — |
| Palpable liver | 6 (3.7%) | — |
| Laboratory values/examinations at bone marrow study, median (range) | ||
| Hemoglobin (g/dL) | 13.6 (8.4-17.3) | 10.3 (8.5-13.3) |
| Leukocyte count (× 109/L) | 8.9 (1.3-45.4) | 11 (6.7-24.4) |
| Platelet count (× 109/L) | 930 (144-2525) | 643 (500-1103) |
| Palpable spleen | 34 (20.7%) | 0 |
| Palpable liver | 6 (3.7%) | 0 |
| Vascular event (? yes) | 63 (38.4%) | 0 |
| Number of vascular events | ||
| 0 | 104 (63.4%) | NA |
| 1 | 53 (32.3%) | — |
| 2 | 8 (4.9%) | — |
| 3 | 2 (1.2%) | — |
| Type of vascular event | ||
| Thrombosis | 44 (69.8%) | NA |
| Bleeding | 19 (30.2%) | — |
| Labs at first vascular event, median (range) | ||
| Hemoglobin (g/dL) | 13.6 (9.3-16.4) | NA |
| Leukocyte count (× 109/L) | 11.5 (3.6-45) | — |
| Platelet count (× 109/L) | 920 (151-2620) | — |
NA indicates not available.
Bone marrow angiogenesis
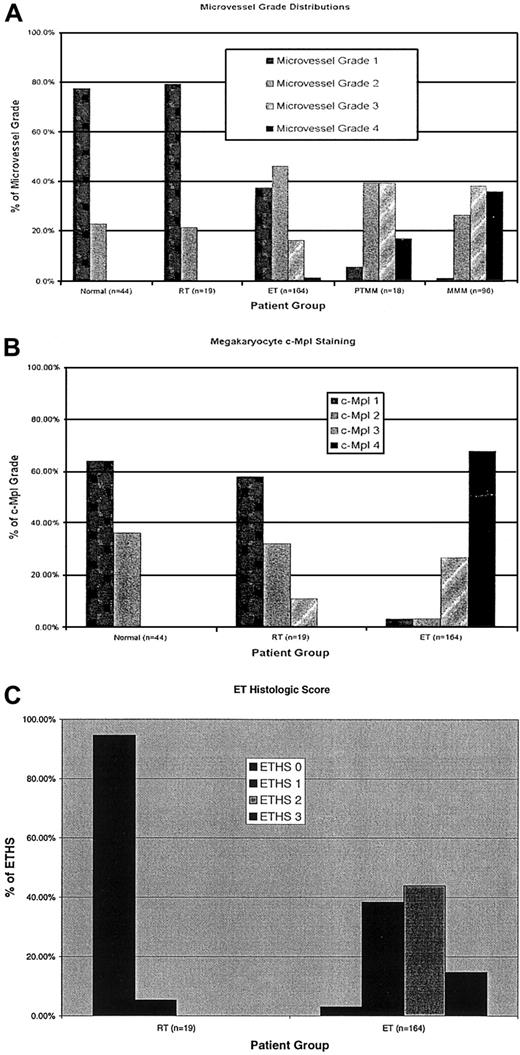
An increase in angiogenesis, as manifested by increased microvessel density, was observed in patients with ET compared with marrows from healthy controls (P < .0001) and patients with RT (P = .006). Although the degree of increase in microvessel density was variable among the ET patients (Table2), no patient with RT had a significant increase in microvessel density. Blinded interobserver variation was minimal, with only 5 of 164 patients (3%) having an initial discrepancy in microvessel density, which was later corrected by open review and consensus between reviewers. We found no correlation between increased microvessel density and clinical presentation, platelet count, or other laboratory studies, vascular events (thrombosis or hemorrhage), or survival (Table 3) in the ET cohort. However, increases in microvessel density did correlate with the presence of palpable splenomegaly (P = .004) and with reticulin fibrosis (P = .005) and osteosclerosis (P = .008) (both in the absence of known transformation to postthrombocythemic myeloid metaplasia) and marked bone marrow megakaryocyte proliferation (P = .002) (Table 3). We then compared the frequency and distribution of increased microvessel density between our ET cohort and patients with either ET-associated myelofibrosis with myeloid metaplasia (postthrombocythemic myeloid metaplasia [PTMM], n = 19) or de novo myelofibrosis with myeloid metaplasia (MMM) (n = 96). Although increased microvessel density was frequently observed in ET, increased angiogenesis was more prevalent in PTMM (P = .0002) and MMM (P < .0001) (Figure2A). Multivariate analysis of microvessel density was significant for progressive disease features, with the presence of reticulin fibrosis, increased megakaryocyte numbers, and palpable splenomegaly remaining significant.
Bone marrow features in 183 patients with thrombocytosis
| Diagnosis . | Essential thrombocythemia (n = 164) no. (%) . | Reactive thrombocytosis (n = 19) no. (%) . |
|---|---|---|
| Bone marrow karyotype | ||
| Available | 151 (92.1) | 14 (73.7) |
| Normal | 125 (82.5) | 14 (100) |
| Abnormal | 26 (17.5) | 0 |
| Megakaryocyte number | ||
| 1 | 29 (17.7) | 10 (52.6) |
| 2 | 68 (41.5) | 9 (47.4) |
| 3 | 46 (28.0) | 0 |
| 4 | 21 (12.8) | 0 |
| Megakaryocyte clustering | ||
| 0 | 16 (9.8) | 18 (94.7) |
| 1 | 60 (36.6) | 1 (5.3) |
| 2 | 88 (53.6) | 0 |
| Reticulin fibrosis | ||
| 0 | 76 (46.3) | 19 (100) |
| 1 | 54 (33.0) | 0 |
| 2 | 24 (14.6) | 0 |
| 3 | 10 (6.1) | 0 |
| 4 | 0 | 0 |
| Osteosclerosis grade | ||
| 0 | 146 (89.1) | 19 (100) |
| 1 | 15 (9.2) | 0 |
| 2 | 3 (1.7) | 0 |
| Microvessel grade | ||
| 1 | 61 (37.2) | 15 (79.0) |
| 2 | 75 (45.8) | 4 (21.0) |
| 3 | 26 (15.8) | 0 |
| 4 | 2 (1.2) | 0 |
| c-Mpl grade | ||
| 1 | 5 (3.0) | 11 (57.9) |
| 2 | 5 (3.0) | 6 (31.6) |
| 3 | 43 (26.2) | 2 (10.5) |
| 4 | 111 (67.8) | 0 |
| Diagnosis . | Essential thrombocythemia (n = 164) no. (%) . | Reactive thrombocytosis (n = 19) no. (%) . |
|---|---|---|
| Bone marrow karyotype | ||
| Available | 151 (92.1) | 14 (73.7) |
| Normal | 125 (82.5) | 14 (100) |
| Abnormal | 26 (17.5) | 0 |
| Megakaryocyte number | ||
| 1 | 29 (17.7) | 10 (52.6) |
| 2 | 68 (41.5) | 9 (47.4) |
| 3 | 46 (28.0) | 0 |
| 4 | 21 (12.8) | 0 |
| Megakaryocyte clustering | ||
| 0 | 16 (9.8) | 18 (94.7) |
| 1 | 60 (36.6) | 1 (5.3) |
| 2 | 88 (53.6) | 0 |
| Reticulin fibrosis | ||
| 0 | 76 (46.3) | 19 (100) |
| 1 | 54 (33.0) | 0 |
| 2 | 24 (14.6) | 0 |
| 3 | 10 (6.1) | 0 |
| 4 | 0 | 0 |
| Osteosclerosis grade | ||
| 0 | 146 (89.1) | 19 (100) |
| 1 | 15 (9.2) | 0 |
| 2 | 3 (1.7) | 0 |
| Microvessel grade | ||
| 1 | 61 (37.2) | 15 (79.0) |
| 2 | 75 (45.8) | 4 (21.0) |
| 3 | 26 (15.8) | 0 |
| 4 | 2 (1.2) | 0 |
| c-Mpl grade | ||
| 1 | 5 (3.0) | 11 (57.9) |
| 2 | 5 (3.0) | 6 (31.6) |
| 3 | 43 (26.2) | 2 (10.5) |
| 4 | 111 (67.8) | 0 |
Results of statistical analysis: P values
| Variable . | Microvessel grade . | c-Mpl grade . | ET histologic score . |
|---|---|---|---|
| Age | .86 | .65 | .79 |
| Sex | .04 | .65 | .32 |
| Counts at diagnosis | |||
| Hemoglobin | .31 | .12 | .98 |
| Leukocyte count | .21 | .39 | .23 |
| Platelet count | .18 | .46 | .38 |
| Palpable spleen | .0013-150,3-151 | .61 | .0013-150,3-151 |
| Counts at bone marrow study | |||
| Hemoglobin | .04 | .12 | .26 |
| Leukocyte count | .56 | .09 | .02 |
| Platelet count | .09 | .47 | .0023-151 |
| Palpable spleen at bone marrow study | .0043-151 | .88 | .0073-151 |
| Vascular event | |||
| Yes/no | .11 | .70 | .30 |
| Number | .28 | .59 | .65 |
| Type (thrombosis vs hemorrhage) | .37 | .37 | .37 |
| Fatal | .74 | .74 | .74 |
| Bone marrow features | |||
| Abnormal karyotype | .72 | .65 | .90 |
| Cellularity | .01 | .44 | .0013-151 |
| Megakaryocyte number | .0023-150,3-151 | .04 | .0013-150,3-151 |
| Megakaryocyte clustering | .0093-151 | .20 | .0013-151 |
| Osteosclerosis | .0083-151 | .48 | .0013-151 |
| Reticulin fibrosis | .0053-150,3-151 | .35 | .03 |
| Variable . | Microvessel grade . | c-Mpl grade . | ET histologic score . |
|---|---|---|---|
| Age | .86 | .65 | .79 |
| Sex | .04 | .65 | .32 |
| Counts at diagnosis | |||
| Hemoglobin | .31 | .12 | .98 |
| Leukocyte count | .21 | .39 | .23 |
| Platelet count | .18 | .46 | .38 |
| Palpable spleen | .0013-150,3-151 | .61 | .0013-150,3-151 |
| Counts at bone marrow study | |||
| Hemoglobin | .04 | .12 | .26 |
| Leukocyte count | .56 | .09 | .02 |
| Platelet count | .09 | .47 | .0023-151 |
| Palpable spleen at bone marrow study | .0043-151 | .88 | .0073-151 |
| Vascular event | |||
| Yes/no | .11 | .70 | .30 |
| Number | .28 | .59 | .65 |
| Type (thrombosis vs hemorrhage) | .37 | .37 | .37 |
| Fatal | .74 | .74 | .74 |
| Bone marrow features | |||
| Abnormal karyotype | .72 | .65 | .90 |
| Cellularity | .01 | .44 | .0013-151 |
| Megakaryocyte number | .0023-150,3-151 | .04 | .0013-150,3-151 |
| Megakaryocyte clustering | .0093-151 | .20 | .0013-151 |
| Osteosclerosis | .0083-151 | .48 | .0013-151 |
| Reticulin fibrosis | .0053-150,3-151 | .35 | .03 |
Significant on multivariate analysis.
Significant on univariate analysis.
Histograms.
Histogram comparisons of patients with ET and RT and of healthy controls with regard to (A) angiogenesis (microvessel grade), (B) megakaryocyte c-Mpl staining, and (C) ET histologic score.
Histograms.
Histogram comparisons of patients with ET and RT and of healthy controls with regard to (A) angiogenesis (microvessel grade), (B) megakaryocyte c-Mpl staining, and (C) ET histologic score.
c-Mpl
Megakaryocyte c-Mpl staining was clearly decreased overall in ET patients than in the healthy comparison group (P < .0001) and in patients with RT (P < .0001) (Figure 2B). However, as we have previously reported in polycythemia vera,20staining intensity was variable among the megakaryocytes of ET patients. When a semiquantitative grading system was used to categorize the c-Mpl staining in the individual patients, neither decreases nor heterogeneity of megakaryocyte staining correlated with clinical presentation, laboratory studies, vascular events, or survival (Table3). Masked interobserver variation was minimal, with only 7 of 164 patients (4.3%) having an initial discrepancy in megakaryocyte c-Mpl grade, which was later corrected by open review and consensus between reviewers.
ET histologic score
The ET histologic score (ETHS), which combined information from microvessel density, megakaryocyte c-Mpl staining, and evidence of megakaryocyte proliferation, was a sensitive and specific means of distinguishing ET from RT (P < .0001) (sensitivity 97%, specificity 95% for ETHS grade 1 or higher; sensitivity 100%, specificity 58% for ETHS grade 2 or higher). ETHS did not directly correlate with vascular events or survival (Table 3). However, an ETHS of grade 2 or higher corresponded with higher platelet count (P = .002) (ETHS grade 1 or lower, median 841 × 109/L [range, 151 × 109/L to 1815 × 109/L]; ETHS grade 2 or higher, median 975 × 109/L [range, 144 × 109/L to 2525 × 109/L]) and palpable splenomegaly (P = 0.007) and with reticulin fibrosis (P = .03) and osteosclerosis (P = .001) (each in the absence of known PTMM) (Table 3). In multivariate analysis, megakaryocyte proliferation (megakaryocyte number) and palpable splenomegaly were significant.
Discussion
In a large retrospective review of 164 patients with confirmed ET, we reviewed the diagnostic and prognostic values of characterizing the degree of bone marrow angiogenesis, megakaryocyte c-Mpl expression, and megakaryocyte proliferation. The observed patterns of angiogenesis and megakaryocyte c-Mpl expression were helpful in distinguishing between patients with ET and those with secondary forms of thrombocytosis. In addition, increases in microvessel density may correlate with the progression of ET to PTMM, the myelofibrotic stage of the disease. However, the investigated histologic features had no prognostic relevance for survival or for vascular events. We fully concede that the follow-up of the study at this juncture may be inadequate to identify the prognostic relevance of the histologic features described herein. The question then arises how the evaluation of histologic features in ET complements what is currently understood concerning the diagnosis and therapy of this disease.
Diagnostic criteria for ET1 23 have 2 main features, the demonstration of sustained myeloproliferation involving at least the megakaryocyte–platelet lineage and the exclusion of secondary causes of thrombocytosis. The lack of an absolute diagnostic marker leads to difficulty in the scientific study of ET and can be a barrier to appropriate management. To aid in discriminating ET from secondary forms of thrombocytosis or from other myeloproliferative disorders, investigators have proposed a variety of methods of diagnostic testing for ET. These can be grouped into serologic, cytogenetic, in vitro culture, and histologic methods.
Direct serologic measurement of acute-phase reactants involved in the inflammatory response—IL-6 and C-reactive protein3—can aid in distinguishing the thrombocytosis associated with inflammatory states from the primary marrow disorder. However, we found that although increased levels of these reactive cytokines strongly suggest RT, normal levels were not specific for ET.
Another proposed diagnostic method is to evaluate patients with thrombocytosis for evidence of clonal versus polyclonal hematopoiesis by using X-chromosome inactivation. A prospective study evaluating patterns of clonality in patients with thrombocytosis found that patients with a clinical diagnosis of ET were heterogeneous in clonality testing.24 There are many technical limitations to an analysis of clonality in ET, including restriction to female patients (X chromosome inactivation patterns), possible existence of clonal and polyclonal hematopoiesis (thereby requiring a sensitive method to detect the clone), and restriction of clonality to a certain lineage (such as megakaryocytes). Because of these limitations, no solid conclusions can be drawn from an analysis of clonality in ET with respect to diagnosis or prognosis.
Spontaneous colony formation of erythroid colonies has been well described in polycythemia vera and MMM25,26 and has led to the evaluation of spontaneous megakaryocyte colony formation in ET.27 The spontaneous growth of megakaryocyte colonies, in the absence of the prerequisite cytokine TPO, has been described as a specific method for distinguishing ET from RT.4 28However, the variability in interpretation of these colony assays, the potential effect of concurrent platelet-reducing therapy on spontaneous growth, and the lack of laboratory infrastructure limit these in vitro methods to centers that specialize in myeloid disorders. Therefore, until we have a reliable and widely applicable molecular marker for ET, we must make maximal use of the information we can obtain from histologic analysis of the bone marrow.
Vascular proliferation in the bone marrow, as manifested by neo-angiogenesis, has been reported in many primary marrow disorders. Included in this growing list are acute myeloid11 and lymphoid leukemia10 and a spectrum of chronic myeloid disorders, including myelodysplasia12 and MMM.13 In this report, we describe angiogenesis as clearly increased in patients with ET compared with controls with healthy marrow and patients with RT; however, the increases were not universal. In addition, as angiogenesis increased, features suggestive of disease progression toward PTMM became appreciable, such as splenomegaly and the presence of reticulin fibrosis and osteosclerosis. However, when compared with our previously published results on patients with PTMM or MMM,13 angiogenesis is still less prominent in ET. Our study was not structured to evaluate increased angiogenesis as a prognostic factor for progression to PTMM; nevertheless, our data suggest that disease progression in ET may in fact correspond to vascular proliferation. Apart from the potential diagnostic value of measuring angiogenesis in ET, the lack of prognostic value, as can be determined with the current duration of follow-up, for vascular events or survival raises the question whether increased angiogenesis is an epiphenomenon or a direct cause of disease progression. Definitive conclusions regarding the prognostic value of increased angiogenesis may require additional follow-up. It is important for us to elucidate the role of angiogenesis in primary bone marrow disorders as we evaluate angiogenesis inhibitors in the therapy of these disorders.
The binding of TPO to its ligand c-Mpl on megakaryocytes is responsible for megakaryocyte growth and development in normal hematopoiesis29; therefore, the TPO–c-Mpl axis has been a subject of scrutiny in thrombocytosis. Various studies have been performed to determine whether serum levels of TPO are increased in ET, with the speculation that such an increase would be the cause of the thrombocytosis. Published reports show that TPO levels in ET may be increased or normal but not decreased.30,31 The occasionally inappropriately elevated levels of TPO in ET led to direct analysis of the TPO gene and gene product for evidence of structural abnormalities or constitutive activation. Only familial ET has been associated with a structural abnormality of the TPO gene,32 and no abnormality of TPO itself has been demonstrated in sporadic ET.33,34 It was logical then to investigate the role of the TPO ligand, c-Mpl, which had previously been found to be decreased in platelets in PV35 and ET.17 We have previously reported that decreased c-Mpl expression can be measured by immunohistochemical methods on bone marrow megakaryocytes across a spectrum of chronic myeloid disorders (PV, ET, agnogenic myeloid metaplasia, and myelodysplasia).19
In this large cohort of patients with thrombocytosis, we found that megakaryocyte c-Mpl expression was markedly diminished in patients with ET compared with healthy controls and patients with RT. In addition, we found heterogeneity of c-Mpl expression in the ET patients, which was consistent with prior reports of interpatient variability in platelet c-Mpl expression in ET17 and megakaryocyte staining in PV.20 This phenomenon may be attributed to variations in staining, to varying degrees of affinity between the antibody used and the c-Mpl receptor, or perhaps to heterogeneity in c-Mpl expression between normal and abnormal megakaryocytes. The mechanism by which c-Mpl is decreased in ET has not been shown.36 In PV, Moliterno and Spivak18 demonstrated a defect in the posttranslational glycosylation of c-Mpl that retards transport from the Golgi apparatus to the cell membrane. One might speculate that a similar mechanism exists in ET, but there are no confirmatory data. The decrease in surface expression of c-Mpl in ET may be responsible for elevated TPO levels through lack of ligand binding; in fact, an inverse correlation between TPO and c-Mpl has been reported in ET.37 However, TPO levels increased in secondary thrombocytosis, perhaps as an acute-phase reactant.38,39We observed normal c-Mpl expression in patients with RT; hence, the increased TPO levels observed in these patients might be the cause of their thrombocytosis. We found no prognostic significance, at least as can be determined by the current duration of follow-up, to the degree of c-Mpl expression in ET, similar to the lack of prognostic value for c-Mpl in PV.20 At this juncture, we believe that the value of megakaryocyte c-Mpl expression is in distinguishing ET from RT but that it will probably not be applicable in distinguishing among the various chronic myeloid disorders.
Megakaryocyte proliferation as manifested by increased absolute numbers of megakaryocytes and their subsequent clumping has been described as a traditional bone marrow feature in ET.40 When we combined all 3 histologic markers investigated in this report (megakaryocyte proliferation, c-Mpl expression, and angiogenesis), we found a highly sensitive and specific diagnostic tool for distinguishing between ET and RT. The advantage of using histologic features to complement the diagnosis of ET is that marrow trephine specimens obtained in any institution may be sent for immunohistochemical analysis to centers experienced in this disorder. When the cumulative histologic information is combined with physical examination findings, clinical course, and laboratory values, the hematologist should be well aided in distinguishing ET from RT. The limitation in using histologic examination as a diagnostic tool in distinguishing ET from other chronic myeloid disorders is that the features found to be present in ET (decreased megakaryocyte c-Mpl expression and increased angiogenesis) have been described in PV20 and may be seen in agnogenic myeloid metaplasia. If additional follow-up demonstrates prognostic relevance of histologic features of c-Mpl and increased angiogenesis in ET patients, these features may influence subsequent management of the disease.
The diagnosis of ET will continue to be uncertain until we find a pathogenetic mechanism. Using available diagnostic information, perhaps we can evaluate homogeneous groups of patients by using modern techniques, such as gene microarray analysis, to begin screening for potential causes of this disorder. The era of targeted pharmacologic therapy will rely on an improved molecular understanding of the stem cell defect that leads to the aberrant cell growth in myeloproliferative disorders.
Supported in part by National Cancer Institute grant CA85818. S.V.R. is a Leukemia and Lymphoma Society Translational Research Awardee.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ruben A. Mesa, Division of Hematology and Internal Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail:mesa.ruben@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal