The t(9;22) chromosomal translocation results in expression of P210BCR-ABL, a fusion protein necessary for the development of chronic myelogenous leukemia (CML). The constitutive activation of the P210BCR-ABL tyrosine kinase results in phosphorylation of multiple signaling pathways leading to the transformed phenotype. Additionally, extracellular interactions between P210BCR-ABL–expressing progenitor cells and bone marrow stroma may provide external signals that facilitate CML development. In contrast to the intracellular signaling pathways involved in CML, little is known about how P210BCR-ABLexpression modifies cell-cell and cell-substratum interactions. To investigate the role of P210BCR-ABL in modulating cellular adhesion, we used a highly sensitive and quantitative cell detachment apparatus that measures the strength of association between a population of cells and an adhesive matrix. Our findings show that P210BCR-ABL expression increased adhesion nearly 2-fold between the myeloblastic cell line, 32D, and fibronectin compared to a control vector. We then investigated whether abnormal adhesion due to P210BCR-ABL expression was caused by its tyrosine kinase activity. A quantitative analysis of cell-fibronectin adhesion found that neither expression of a kinase-inactive P210BCR-ABL mutant in 32D cells or attenuation of kinase activity by STI571 (imatinib mesylate) in 32D cells transduced with wild-type P210BCR-ABL could correct the nearly 2-fold increase in cell-fibronectin adhesion. Similarly, STI571 treatment of Meg-01 cells, a P210BCR-ABL–expressing cell line derived from a patient in blast crisis, failed to inhibit adhesion to fibronectin. Together, our results indicate that changes in adhesion induced by P210BCR-ABL are independent of its tyrosine kinase activity.
Introduction
P210BCR-ABL is a 210-kd nonreceptor tyrosine kinase that is the product of the t(9;22) balanced translocation between the bcr gene on chromosome 22 and theabl gene on chromosome 9.1 The translocation results in the formation of the Philadelphia chromosome (Ph), which is the diagnostic marker for chronic myelogenous leukemia (CML), a disease characterized by abnormal accumulation of hematopoietic cells in the bone marrow, elevated peripheral white blood cell counts, and defective progenitor-stromal adhesion in vitro.2,3 There is intense interest in the observation that P210BCR-ABLexpression in cell lines and bone marrow progenitor cells leads to defective cell binding to fibronectin and stromal cells because abnormal adhesion in transformed cells may contribute to loss of contact inhibition and proliferation in the bone marrow of patients with CML.3-5 However, the mechanism by which P210BCR-ABL alters cellular adhesion is poorly understood.
P210BCR-ABL is primarily found in the cytoplasm and colocalizes with actin through a C-terminal actin-binding domain.6,7 It is unknown if P210BCR-ABLdirectly modifies actin or recruits signaling molecules to the cytoskeleton. However, transformed cells exhibit increased motility and enhanced membrane ruffling and proteins of the focal adhesion complex, such as focal adhesion kinase and paxillin, are phosphorylated in P210BCR-ABL cells.8,9 Together, these observations suggest that P210BCR-ABL induces abnormal cell-substratum binding that is likely involved in the pathogenesis of CML. One possible mechanism is that P210BCR-ABL regulates an inside-out signaling pathway that stimulates integrin-mediated adhesion in a manner similar to growth factors, such as interleukin 3 (IL-3).10 The tyrosine kinase activity is critical for the oncogenic effects of P210BCR-ABL because expression of P210BCR-ABL mutants with either temperature-sensitive tyrosine kinase activity or an inactive tyrosine kinase domain are not oncogenic in vitro or in vivo.11 12Accordingly, the P210BCR-ABL constitutively activated tyrosine kinase is a plausible focal point for inside-out signaling.
Differentiation of the hematopoietic compartment from stem cells through progenitors is a tightly regulated process that relies on specific interactions between stromal and hematopoietic cells for proper cues governing proliferation and differentiation.13,14 In the case of CML, defective progenitor-stromal interactions have been documented using both bone marrow from CML patient donors3,4 and transformed cell lines5,15; yet these studies have yielded equivocal results. It is unclear if these disparate findings are due to methodologic differences or the nature of the cells assayed. Most adhesion studies are limited to panning-style assays that use an uncharacterized and inconsistent washing technique to detach cells. To overcome the inherent variability of panning-style assays to measure adhesion, we adapted a quantitative adhesion assay that relies on fluid flow to remove bound cells using a measurable detachment force.16 This assay provides a quantitative analysis of cell-ligand interactions, and thus provides the capability to measure small changes in adhesion.17 The cell detachment device has previously been used to examine the role of α5β1 integrins in facilitating adhesion between the K562 erythroleukemia cell line and fibronectin to show that antibody-mediated integrin activation stimulates adhesion and increases the force needed to detach adherent cells.18 Radial flow detachment assays using a similar hydrodynamic environment have shown that neutrophils bind more tightly to fibrinogen than to kininogen19 and that osteoblasts adhere to biomaterial implants coated with Arg-Gly-Asp (RGD) sequences.20
To investigate the role of P210BCR-ABL in cellular adhesion, we used the cell detachment device to measure adhesive changes in 32D cells following transduction by P210BCR-ABL–expressing retroviruses. The 32D cells expressing P210BCR-ABL were 1.7 times more adhesive to fibronectin than cells transduced with a control vector. We then used either a tyrosine kinase dead P210BCR-ABL mutant with a Lys1176Arg (K1176R) amino acid substitution12 or wild-type P210BCR-ABL cells treated with the specific P210BCR-ABL tyrosine kinase inhibitor, STI571 (imatinib mesylate, Gleevec),21 to evaluate the role of tyrosine kinase activity in regulating cellular adhesion. The importance of the P210BCR-ABL catalytic activity in contributing to CML is underscored by recent studies indicating that the P210BCR-ABL tyrosine kinase inhibitor STI571 normalizes white blood cell counts in patients with CML.22 We found that neither the tyrosine kinase inactive mutant nor treatment of wild-type P210BCR-ABL with STI571 could normalize the increased adhesion observed on expression of P210BCR-ABL. We also found that localization of P210BCR-ABL to F-actin filaments is independent of tyrosine kinase activity. These findings suggest that both altered adhesion to fibronectin and actin localization are tyrosine kinase-independent activities of P210BCR-ABL. These tyrosine kinase-independent properties of P210BCR-ABL may synergize with its tyrosine kinase-dependent activity to induce the transformed phenotype.
Materials and methods
Plasmid construction and cell culture
Full-length P210BCR-ABL (Pear et al)23and the kinase dead K1176R mutant12 were ligated into pK1, an MSCV-based vector that is identical to MigR1 except that an IRES-puromycin resistance gene has been inserted in place of the IRES-GFP (kind gift from Karen Ehrmann and Stephen Emerson, University of Pennsylvania). High-titer retroviral supernatants were generated.23 BCR-ABL constructs were transduced into 32D cells24 by spinoculation.25 BCR-ABL or control pK1 cells were selected with puromycin (1.5 μg/mL) and maintained in 32D media (RPMI media, 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% glutamate) containing 10% WEHI as a source of IL-3. Meg-01 cells26(CRL-2021, American Type Culture Collection, Rockville, MD), a Ph+ megakaryoblastic cell line, were maintained in Meg-01 media (Dulbecco modified Eagle medium [DMEM] supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% glutamate).
Fifteen to 17 hours prior to assessing adhesion, pK1 and/or BCR-ABL–expressing 32D cells were diluted into 32D medium containing 1% WEHI with or without 10 μM STI571, as indicated. Meg-01 cells were treated with 10 μM STI571 for 3.5 hours or an equal volume of the drug carrier (phosphate-buffered saline [PBS]) for 3.5 hours or 1.5 hours prior to assaying binding. Cells were washed 2 times in PBS and resuspended in adhesion buffer (24 mM Tris, 137 mM NaCl, 1 mM MgCl2, 3 mM KCl, 2 mM glucose) at 1 × 106cells/mL. In some control experiments, Meg-01 cells were treated with 2.125 mM RGD peptide (catalogue no. 03-34-0035, Calbiochem, San Diego, CA) for 10 minutes prior to seeding on fibronectin substrates. Adhesion assays were carried out in the absence of growth factors and were completed within 2 hours of washing. Controls showed that adhesion levels did not change during the period in which multiple samples were assayed (data not shown).
Adhesion assays
Circular glass coverslips (Fisher, Pittsburgh, PA) were adsorbed with 10 μg/mL fibronectin (Becton Dickinson, San Jose, CA) and blocked with 1% bovine serum albumin (BSA). The cell detachment device was operated as described.16 Coverslips were analyzed by recording the adherent fraction of cells at various radial distances from the center. The shear stress, τ, that is imparted on the cells is calculated by the following equation: τ = 0.800 r (ρμω3)1/2where τ is proportional to r, the radial distance; ρ and μ are the fluid density and dynamic viscosity, respectively; and ω is the angular velocity of the spinning disk. The convective fluid flow that is established by a spinning disk has been characterized,27,28 and the fraction of beads or cells under shear flow that remain bound to an adhesive surface is known to follow a sigmoid-shaped profile.17,29,30 Adhesion profiles for this study were prepared16 and were accepted forR2 ≥ 0.80, with the exception of runs using matrices consisting of BSA alone or when Meg-01 cells were treated with the RGD peptide due to the few cells that remain attached in the absence of fibronectin. The critical shear stress, τ50, was measured as the surface shear stress, τ, at an adherent fraction of 0.50. Determination of significance between mean values of critical shear stress, τ50, was determined using a 2-tailed Student t test with significance defined asP ≤ .05. Data are presented as mean ± SD.
Western blots and flow cytometry
Western blots were performed on lysates from 32D cells expressing BCR-ABL or pK1 that were incubated overnight in 32D media with 10% WEHI with or without decreasing concentrations of STI571. Meg-01 cells were treated with 10 μM STI571 or an equal volume of PBS alone for 3 hours. Equal volumes of lysates were assayed on 7% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels to detect P210BCR-ABL, 12.5% SDS-PAGE gels to detect grb2, or 10% SDS-PAGE gels to detect tyrosine phosphorylated proteins. Proteins were transferred from gels to nitrocellulose membranes and were blotted with an anti-abl antibody (24-11, Santa Cruz Biotechnology, Santa Cruz, CA), an antiphosphotyrosine antibody (PY20, Pharmingen, San Diego, CA), or an anti-grb2 antibody (81, Transduction Laboratories, Lexington, KY). Anti-α4 (R-2, Pharmingen) and anti-α5 (5H10-27, Pharmingen) antibodies were used to assess integrin expression by flow cytometry. All samples and controls were run in triplicate. The mean fluorescence index (MFI) was calculated as the difference in geometric mean fluorescence between experimental samples stained with primary and secondary antibodies and negative control samples stained with the secondary antibody alone; this value was normalized to the geometric mean fluorescence of the negative control to give the MFI.
Microscopy
NIH3T3 or 32D cells were transduced with retroviral constructs encoding P210BCR-ABL, K1176R, or the pK1 control plasmid and selected for 4 days with puromycin. NIH3T3 cells were cultured overnight on fibronectin coverslips with or without 10 μM STI571 as indicated. 32D cells were plated onto fibronectin coverslips at a concentration of 1 × 105 cells/mL by following the same procedure outlined above for adhesion assays. Cells were fixed with 2% paraformaldehyde, permeablized with 0.1% Triton-X, and blocked with 1% denatured BSA (NIH3T3 cells) or 5% normal goat serum diluted in 1% denatured BSA (32D cells). Coverslips were stained with 5 μg/mL anti-abl antibody (8E9, Pharmingen). Bound anti-abl antibody was revealed with goat antimouse Texas red antibody (NIH3T3 cells) or goat antimouse Cy3 antibody (32D cells, Jackson ImmunoResearch, West Grove, PA). Actin was stained with 0.1 μg/mL fluorescein isothiocyanate–phalloidin (Sigma, St Louis, MO) and the nucleus was revealed with 4′,6-diamidino-2-phenylindole (DAPI) staining (1:10 000 dilution). Cells were imaged using confocal microscopy at the Bioengineering Confocal and Multiphoton Imaging Facility at the University of Pennsylvania.
Results
The cell detachment device quantitatively measures the strength of binding between cells and fibronectin
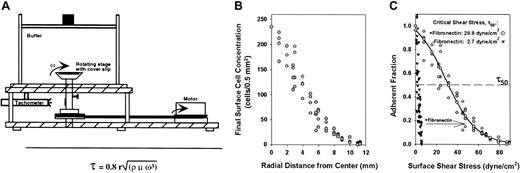
The cell detachment device presents a 2-dimensional matrix of fibronectin and BSA to a monolayer of cells seeded at a constant surface density throughout the coverslip (data not shown). This well-characterized surface is mounted onto a rotating circular stage that creates convective fluid flow within the chamber that dislodges bound cells (Figure 1A). The detachment force is calculated (equation in “Materials and methods”) from the properties of the buffer, the dimensions of the coverslip, and the rotational speed that is measured by a digital tachometer. The detachment force is zero at the center of the matrix and increases linearly to reach a maximum near the circumference, allowing for a range of detachment forces to be tested during a single experiment. Accordingly, few cells remained near the edge due to the higher force at this location than closer to the center (Figure 1B).
Diagram of the cell detachment device and characteristic detachment profile.
(A) Cells are allowed to settle onto a fibronectin-coated matrix atop the rotating stage. Motion of the stage and matrix induces fluid flow that generates a range of detachment forces that dislodges cells. (B) The surface density of pK1 after a 10-minute spin is plotted versus radial position showing that fewer cells remain attached near the circumference where the detachment force, τ, is high compared to the center where the detachment force approaches zero. (C) Fraction of adherent pK1 cells in panel B, normalized to the cell count at the center after a 10-minute spin is plotted versus surface shear stress, τ. Cells were incubated on BSA alone (▾) or in the presence of fibronectin ( ). (–○–) represents a curve fitted to the experimental points. The dashed line represents an adherent fraction of 0.5. The critical shear stress (τ50) is defined as the point on the abscissa (shear stress) that corresponds to an adherent fraction of 0.5 along the fitted curve.
). (–○–) represents a curve fitted to the experimental points. The dashed line represents an adherent fraction of 0.5. The critical shear stress (τ50) is defined as the point on the abscissa (shear stress) that corresponds to an adherent fraction of 0.5 along the fitted curve.
Diagram of the cell detachment device and characteristic detachment profile.
(A) Cells are allowed to settle onto a fibronectin-coated matrix atop the rotating stage. Motion of the stage and matrix induces fluid flow that generates a range of detachment forces that dislodges cells. (B) The surface density of pK1 after a 10-minute spin is plotted versus radial position showing that fewer cells remain attached near the circumference where the detachment force, τ, is high compared to the center where the detachment force approaches zero. (C) Fraction of adherent pK1 cells in panel B, normalized to the cell count at the center after a 10-minute spin is plotted versus surface shear stress, τ. Cells were incubated on BSA alone (▾) or in the presence of fibronectin ( ). (–○–) represents a curve fitted to the experimental points. The dashed line represents an adherent fraction of 0.5. The critical shear stress (τ50) is defined as the point on the abscissa (shear stress) that corresponds to an adherent fraction of 0.5 along the fitted curve.
). (–○–) represents a curve fitted to the experimental points. The dashed line represents an adherent fraction of 0.5. The critical shear stress (τ50) is defined as the point on the abscissa (shear stress) that corresponds to an adherent fraction of 0.5 along the fitted curve.
The fraction of cells that remain on the surface after a spin (normalized to the count at the center where r = τ = 0) is plotted versus the shear stress applied to detach bound cells at each radial position. Each data point indicates the degree of cell-fibronectin binding (Figure 1C). These data trace a sigmoid-shaped profile that allows a curve to be fitted to the observed data.16 Adhesion of cells to BSA-coated coverslips, representing the level of nonspecific binding, is minimal and is just below or at the detection threshold of the device (Figure 1C). Addition of fibronectin to the matrix significantly increases cell adhesion as indicated by the higher fraction of cells remaining attached to the matrix at every given shear stress, effectively shifting the adhesion profile to the right (Figure 1C). Addition of fibronectin-blocking antibodies to the monolayer or treatment of 32D cells with integrin-blocking antibodies or RGD-containing peptides significantly reduces or completely abrogates cell binding (J.A.W., manuscript in preparation). This shows that the interaction between 32D cells and the adhesive matrix is specific for fibronectin-integrin linkages.
P210BCR-ABL expression leads to increased adhesion
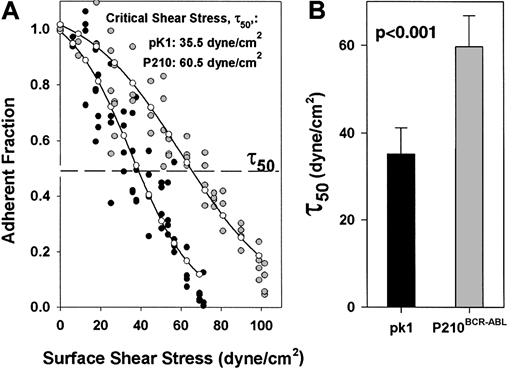
The advantage of the spinning disk adhesion assay is its ability to quantitatively measure the strength by which cells bind fibronectin over a range of defined forces. Accordingly, we asked whether expression of P210BCR-ABL influenced cell adhesion to fibronectin compared to pK1 control cells expressing an empty vector. In the presence of fibronectin, expression of P210BCR-ABLin 32D cells increased the fraction of bound cells at nearly all shear stresses tested compared to pK1 control cells, indicating that expression of P210BCR-ABL led to increased cell adhesion (Figure 2A). The critical shear stress, τ50, (ie, adhesion strength) is the force required to dislodge half of the cells and is a measurement of the strength of association between cells and fibronectin.29 30 The τ50 is averaged for a set of identical experiments and can be compared over different conditions, such as with and without P210BCR-ABL expression (Figure 2B). Expression of P210BCR-ABL led to an increase in the average critical shear stress, τ50, from 35.1 dyne/cm2 to 59.6 dyne/cm2, indicating that adhesion increased approximately 1.7-fold. We also observed nearly a 2-fold increase in adhesion when P210BCR-ABL is expressed from a retroviral construct coexpressing green fluorescent protein (data not shown).
Expression of P210BCR-ABL increases adhesion to fibronectin compared to control pK1 cells.
(A) Fraction of adherent cells after a 10-minute spin is plotted versus surface shear stress, τ, for P210BCR-ABL( ) or pK1 control cells (●). A higher proportion of P210BCR-ABLcells remain attached at nearly every given shear stress compared to pK1 cells suggesting that P210BCR-ABL leads to increased binding to fibronectin. (–○–) represents a curve fitted to the experimental points for P210BCR-ABL or vector control pK1. The dashed line represents an adherent fraction of 0.5. The critical shear stress (τ50) is defined as the point on the abscissa (shear stress) that corresponds to an adherent fraction of 0.5 along the fitted curve. (B) τ50 was determined for spins with cells expressing P210BCR-ABL (n = 5) or pK1 control (n = 12), indicating that an average of 1.7 times as much force is needed to detach 50% of P210BCR-ABL–expressing cells from fibronectin compared to pK1 cells (P < .001). Nonspecific binding between P210BCR-ABL 32D cells to BSA matrices was minimal and similar to pK1 32D control cells (data not shown).
) or pK1 control cells (●). A higher proportion of P210BCR-ABLcells remain attached at nearly every given shear stress compared to pK1 cells suggesting that P210BCR-ABL leads to increased binding to fibronectin. (–○–) represents a curve fitted to the experimental points for P210BCR-ABL or vector control pK1. The dashed line represents an adherent fraction of 0.5. The critical shear stress (τ50) is defined as the point on the abscissa (shear stress) that corresponds to an adherent fraction of 0.5 along the fitted curve. (B) τ50 was determined for spins with cells expressing P210BCR-ABL (n = 5) or pK1 control (n = 12), indicating that an average of 1.7 times as much force is needed to detach 50% of P210BCR-ABL–expressing cells from fibronectin compared to pK1 cells (P < .001). Nonspecific binding between P210BCR-ABL 32D cells to BSA matrices was minimal and similar to pK1 32D control cells (data not shown).
Expression of P210BCR-ABL increases adhesion to fibronectin compared to control pK1 cells.
(A) Fraction of adherent cells after a 10-minute spin is plotted versus surface shear stress, τ, for P210BCR-ABL( ) or pK1 control cells (●). A higher proportion of P210BCR-ABLcells remain attached at nearly every given shear stress compared to pK1 cells suggesting that P210BCR-ABL leads to increased binding to fibronectin. (–○–) represents a curve fitted to the experimental points for P210BCR-ABL or vector control pK1. The dashed line represents an adherent fraction of 0.5. The critical shear stress (τ50) is defined as the point on the abscissa (shear stress) that corresponds to an adherent fraction of 0.5 along the fitted curve. (B) τ50 was determined for spins with cells expressing P210BCR-ABL (n = 5) or pK1 control (n = 12), indicating that an average of 1.7 times as much force is needed to detach 50% of P210BCR-ABL–expressing cells from fibronectin compared to pK1 cells (P < .001). Nonspecific binding between P210BCR-ABL 32D cells to BSA matrices was minimal and similar to pK1 32D control cells (data not shown).
) or pK1 control cells (●). A higher proportion of P210BCR-ABLcells remain attached at nearly every given shear stress compared to pK1 cells suggesting that P210BCR-ABL leads to increased binding to fibronectin. (–○–) represents a curve fitted to the experimental points for P210BCR-ABL or vector control pK1. The dashed line represents an adherent fraction of 0.5. The critical shear stress (τ50) is defined as the point on the abscissa (shear stress) that corresponds to an adherent fraction of 0.5 along the fitted curve. (B) τ50 was determined for spins with cells expressing P210BCR-ABL (n = 5) or pK1 control (n = 12), indicating that an average of 1.7 times as much force is needed to detach 50% of P210BCR-ABL–expressing cells from fibronectin compared to pK1 cells (P < .001). Nonspecific binding between P210BCR-ABL 32D cells to BSA matrices was minimal and similar to pK1 32D control cells (data not shown).
The constitutive tyrosine kinase activity is not required for P210BCR-ABL–mediated adhesion
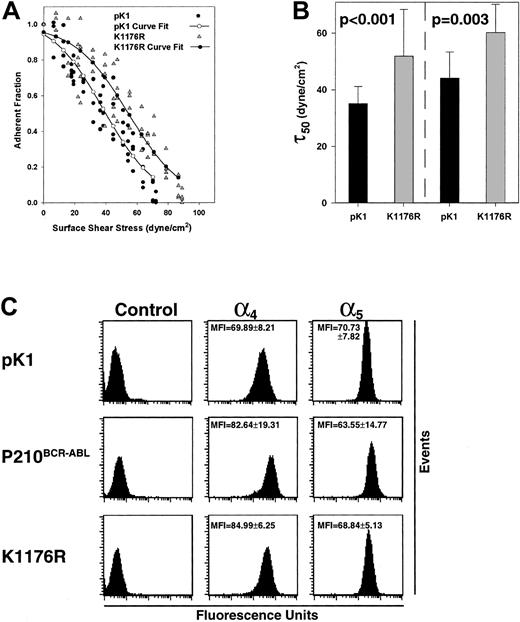
The finding that P210BCR-ABL expression leads to increased cell adhesion in our system may potentially be explained by inside-out signaling initiated by the P210BCR-ABL tyrosine kinase. To assess the role of tyrosine kinase activity on cell adhesion to fibronectin, a P210BCR-ABL mutant lacking tyrosine kinase activity, K1176R, was transduced into 32D cells. Similar to wild-type P210BCR-ABL 32D cells, K1176R 32D cells bound more tightly to fibronectin than control pK1 cells (Figure3A). Moreover, we found that the critical shear stress of a series of spins using 2 separate populations of K1176R-expressing cells were not statistically different from P210BCR-ABL (P = .14, P = .89, not shown), but were significantly elevated compared to the control pK1 cells (P < .001, P = .003, Figure3B).
The kinase dead mutant retains the ability to induce P210BCR-ABL–mediated elevated adhesion.
(A) The profile of a typical spin using K1176R ( ) expressing 32D cells is depicted along with pK1 control cells for comparison (●). (–●–) represents a curve fitted to the experimental points for K1176R while (–○–) represent a curve fitted to the experimental points for pK1 cells. (B) The critical shear stress was calculated for a series of spins using 2 independent populations of cells (left, right) expressing pK1 (n = 12 from Figure 2B, n = 8) or K1176R (n = 18, n = 9) and shows that adhesion is increased in the absence of kinase activity (P < .001,P = .003). Nonspecific binding between K1176R 32D cells to BSA matrices was minimal and similar to pK1 32D control cells (data not shown). (C) Measurement of α4β1- and α5β1-integrin expression in transduced cells 32D. Surface expression of α4- and α5-integrin subunits (corresponding to the α4β1 and α5β1integrins that bind fibronectin) in pK1-, P210BCR-ABL–, or K1176R-transduced cells 32D are displayed. Cells stained with the secondary phycoerythrin-conjugated antibody alone are shown as negative controls. The studies were performed in triplicate, and the MFI and SDs are shown. Representative histograms showing the level of integrin expression are presented.
) expressing 32D cells is depicted along with pK1 control cells for comparison (●). (–●–) represents a curve fitted to the experimental points for K1176R while (–○–) represent a curve fitted to the experimental points for pK1 cells. (B) The critical shear stress was calculated for a series of spins using 2 independent populations of cells (left, right) expressing pK1 (n = 12 from Figure 2B, n = 8) or K1176R (n = 18, n = 9) and shows that adhesion is increased in the absence of kinase activity (P < .001,P = .003). Nonspecific binding between K1176R 32D cells to BSA matrices was minimal and similar to pK1 32D control cells (data not shown). (C) Measurement of α4β1- and α5β1-integrin expression in transduced cells 32D. Surface expression of α4- and α5-integrin subunits (corresponding to the α4β1 and α5β1integrins that bind fibronectin) in pK1-, P210BCR-ABL–, or K1176R-transduced cells 32D are displayed. Cells stained with the secondary phycoerythrin-conjugated antibody alone are shown as negative controls. The studies were performed in triplicate, and the MFI and SDs are shown. Representative histograms showing the level of integrin expression are presented.
The kinase dead mutant retains the ability to induce P210BCR-ABL–mediated elevated adhesion.
(A) The profile of a typical spin using K1176R ( ) expressing 32D cells is depicted along with pK1 control cells for comparison (●). (–●–) represents a curve fitted to the experimental points for K1176R while (–○–) represent a curve fitted to the experimental points for pK1 cells. (B) The critical shear stress was calculated for a series of spins using 2 independent populations of cells (left, right) expressing pK1 (n = 12 from Figure 2B, n = 8) or K1176R (n = 18, n = 9) and shows that adhesion is increased in the absence of kinase activity (P < .001,P = .003). Nonspecific binding between K1176R 32D cells to BSA matrices was minimal and similar to pK1 32D control cells (data not shown). (C) Measurement of α4β1- and α5β1-integrin expression in transduced cells 32D. Surface expression of α4- and α5-integrin subunits (corresponding to the α4β1 and α5β1integrins that bind fibronectin) in pK1-, P210BCR-ABL–, or K1176R-transduced cells 32D are displayed. Cells stained with the secondary phycoerythrin-conjugated antibody alone are shown as negative controls. The studies were performed in triplicate, and the MFI and SDs are shown. Representative histograms showing the level of integrin expression are presented.
) expressing 32D cells is depicted along with pK1 control cells for comparison (●). (–●–) represents a curve fitted to the experimental points for K1176R while (–○–) represent a curve fitted to the experimental points for pK1 cells. (B) The critical shear stress was calculated for a series of spins using 2 independent populations of cells (left, right) expressing pK1 (n = 12 from Figure 2B, n = 8) or K1176R (n = 18, n = 9) and shows that adhesion is increased in the absence of kinase activity (P < .001,P = .003). Nonspecific binding between K1176R 32D cells to BSA matrices was minimal and similar to pK1 32D control cells (data not shown). (C) Measurement of α4β1- and α5β1-integrin expression in transduced cells 32D. Surface expression of α4- and α5-integrin subunits (corresponding to the α4β1 and α5β1integrins that bind fibronectin) in pK1-, P210BCR-ABL–, or K1176R-transduced cells 32D are displayed. Cells stained with the secondary phycoerythrin-conjugated antibody alone are shown as negative controls. The studies were performed in triplicate, and the MFI and SDs are shown. Representative histograms showing the level of integrin expression are presented.
One possible mechanism by which P210BCR-ABL increases adhesion is by influencing integrin expression. The α4β1 and α5β1integrins are the major cell surface receptors for fibronectin and bind to different, conserved sequences on the macromolecule.31 32 Expression of α4β1 and α5β1integrins on P210BCR-ABL, K1176R, and pK1 control cells was measured by flow cytometry. An increase in α4β1 expression was observed; however, there was a simultaneous decrease in α5β1expression (Figure 3C). The changes in expression of α4β1 and α5β1integrins were similar in the presence or absence of the tyrosine kinase activity of P210BCR-ABL (Figure 3C). In parallel studies using α4- and α5-blocking antibodies, we found that anti-α4 had minimal affects on the magnitude of P210BCR-ABL induced adhesion, whereas anti-α5 significantly diminished the P210BCR-ABL–induced increase in adhesion (J.A.W., manuscript in preparation). These results suggest that the changes in α4β1- and α5β1-integrin expression are unlikely to account for the nearly 2-fold increase in fibronectin binding induced by P210BCR-ABL expression.
Treatment of P210BCR-ABL cells with STI571 fails to normalize P210BCR-ABL–mediated adhesion
To confirm our previous finding using the tyrosine kinase–inactive K1176R mutant, we treated P210BCR-ABL–expressing cells with STI571 as an additional method to evaluate the contribution of kinase activity on adhesion. STI571 is a specific inhibitor of the P210BCR-ABL tyrosine kinase by nature of the unique adenosine triphosphate-binding cleft in the catalytic domain.21,33 Although STI571 is effective at inhibiting the kinase of the platelet-derived growth factor receptor and the c-kit receptor,34 we do not expect these receptors to play a role in our assay because their ligands are absent from the adhesion buffer.
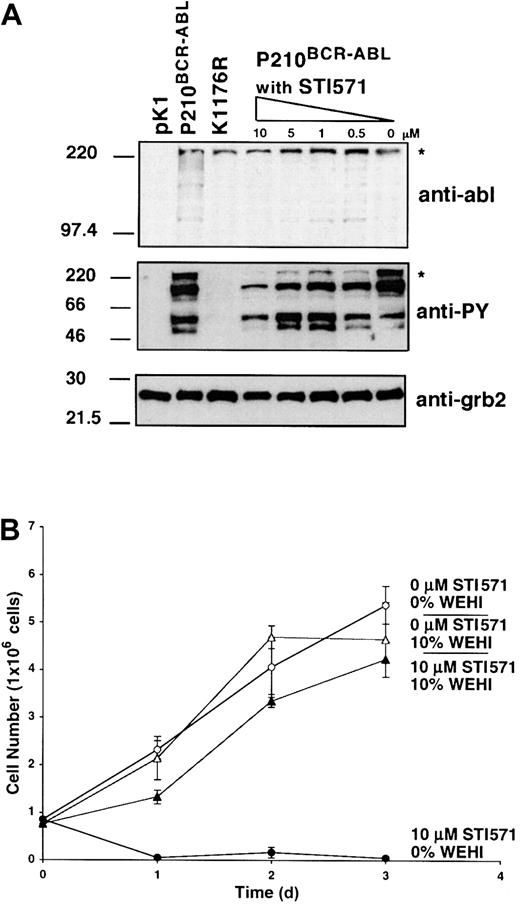
P210BCR-ABL expression dramatically increases phosphotyrosine levels in hematopoietic cells lines leading to cell transformation and IL-3 independence.35 We confirmed that K1176R expression or treatment of P210BCR-ABL cells with STI571 abrogated both the kinase activity and transforming ability of P210BCR-ABL. Specifically, the K1176R mutant or overnight treatment of P210BCR-ABL–expressing cells with 10 μM STI571 or less reduced the total cellular phosphotyrosine level in a dose-dependent manner, even though P210BCR-ABL expression was unchanged (Figure 4A). Furthermore, expression of K1176R was also comparable to untreated or STI571-treated wild-type P210BCR-ABL in our system (Figure 4A). K1176R failed to rescue 32D cells from IL-3 withdrawal (data not shown) and treatment with STI571 reversed the growth factor independence of wild-type P210BCR-ABL–expressing 32D cells leading to apoptosis in the absence of IL-3 (Figure 4B). However, STI571-treated P210BCR-ABL cells survived in 10% WEHI medium containing IL-3, showing that STI571 was not cytotoxic at the concentrations used in this study.
Treatment of wild-type P210BCR-ABL–expressing cells with 10 μM STI571 overnight reverses the IL-3 independence and decreases phosphotyrosine levels but does not affect P210BCR-ABL expression.
(A) Western blots of whole cell lysates showing expression of P210BCR-ABL and phosphotyrosine expression in pK1 control, untreated P210BCR-ABL, K1176R, or P210BCR-ABLcells treated overnight with 10, 5, 1, 0.5, or 0 μM STI571. The BCR-ABL–specific band is indicated by an asterisk. Expression of grb2 was used as a loading control. (B) P210BCR-ABL 32D cells were allowed to proliferate in triplicate in media containing (▴, ▵) or free (●, ○) from WEHI as a source of IL-3 and were treated with (▴, ●) or without (▵, ○) 10 μM STI571, as indicated. Cell viability was determined by trypan blue exclusion. The 32D cells expressing P210BCR-ABL proliferate in the absence of IL-3, but die if treated with STI571. However, cells treated with STI571 remain viable if supplemented with IL-3 showing that STI571 is not toxic at the levels used.
Treatment of wild-type P210BCR-ABL–expressing cells with 10 μM STI571 overnight reverses the IL-3 independence and decreases phosphotyrosine levels but does not affect P210BCR-ABL expression.
(A) Western blots of whole cell lysates showing expression of P210BCR-ABL and phosphotyrosine expression in pK1 control, untreated P210BCR-ABL, K1176R, or P210BCR-ABLcells treated overnight with 10, 5, 1, 0.5, or 0 μM STI571. The BCR-ABL–specific band is indicated by an asterisk. Expression of grb2 was used as a loading control. (B) P210BCR-ABL 32D cells were allowed to proliferate in triplicate in media containing (▴, ▵) or free (●, ○) from WEHI as a source of IL-3 and were treated with (▴, ●) or without (▵, ○) 10 μM STI571, as indicated. Cell viability was determined by trypan blue exclusion. The 32D cells expressing P210BCR-ABL proliferate in the absence of IL-3, but die if treated with STI571. However, cells treated with STI571 remain viable if supplemented with IL-3 showing that STI571 is not toxic at the levels used.
As an independent method to confirm our finding that K1176R exhibits increased adhesion in the absence of tyrosine kinase activity, we treated P210BCR-ABL–expressing 32D cells with STI571. We compared the adhesion of P210BCR-ABL cells that were untreated or treated with STI571 overnight at the same conditions used to evaluate proliferation and tyrosine kinase activity. We found that attenuation of catalytic activity not only failed to decrease cell adhesion compared to untreated cells, but may have slightly increased the ability of these cells to bind fibronectin (Figure5). Taken together, these results suggest that the kinase activity is not a major contributor to the increased cell adhesion to fibronectin brought about by P210BCR-ABLexpression.
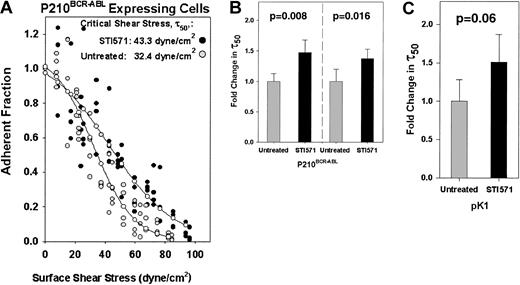
STI571 treatment of P210BCR-ABL 32D cells fails to decrease cell adhesion.
(A) P210BCR-ABL 32D cells were incubated with (●) or without ( ) 10 μM STI571 overnight prior to assaying adhesion. Typical profiles are shown and indicate that adhesion was not reduced, and may have slightly increased, as a result of tyrosine kinase attenuation by STI571. (–○–) represents a curve fitted to the experimental points. The critical shear stress (τ50) is defined as the point on the abscissa (shear stress) that corresponds to an adherent fraction of 0.5 along the fitted curve. (B) The critical shear stress was calculated for a series of spins and is represented as the fold change in τ50 relative to untreated P210BCR-ABLcells. We tested 2 separate populations of P210BCR-ABLcells (left, right) treated with (n = 5, n = 4), or without (n = 3, n = 4) 10 μM STI571 overnight (P = .008,P = .016). (C) pK1 control cells were incubated with (n = 4) or without (n = 10) 10 μM STI571 as in (B) (P = .06) and the data are represented as the fold change in τ50 relative to untreated pK1 cells. Treatment of transduced 32D cells with STI571 did not affect nonspecific binding to BSA matrices (data not shown).
) 10 μM STI571 overnight prior to assaying adhesion. Typical profiles are shown and indicate that adhesion was not reduced, and may have slightly increased, as a result of tyrosine kinase attenuation by STI571. (–○–) represents a curve fitted to the experimental points. The critical shear stress (τ50) is defined as the point on the abscissa (shear stress) that corresponds to an adherent fraction of 0.5 along the fitted curve. (B) The critical shear stress was calculated for a series of spins and is represented as the fold change in τ50 relative to untreated P210BCR-ABLcells. We tested 2 separate populations of P210BCR-ABLcells (left, right) treated with (n = 5, n = 4), or without (n = 3, n = 4) 10 μM STI571 overnight (P = .008,P = .016). (C) pK1 control cells were incubated with (n = 4) or without (n = 10) 10 μM STI571 as in (B) (P = .06) and the data are represented as the fold change in τ50 relative to untreated pK1 cells. Treatment of transduced 32D cells with STI571 did not affect nonspecific binding to BSA matrices (data not shown).
STI571 treatment of P210BCR-ABL 32D cells fails to decrease cell adhesion.
(A) P210BCR-ABL 32D cells were incubated with (●) or without ( ) 10 μM STI571 overnight prior to assaying adhesion. Typical profiles are shown and indicate that adhesion was not reduced, and may have slightly increased, as a result of tyrosine kinase attenuation by STI571. (–○–) represents a curve fitted to the experimental points. The critical shear stress (τ50) is defined as the point on the abscissa (shear stress) that corresponds to an adherent fraction of 0.5 along the fitted curve. (B) The critical shear stress was calculated for a series of spins and is represented as the fold change in τ50 relative to untreated P210BCR-ABLcells. We tested 2 separate populations of P210BCR-ABLcells (left, right) treated with (n = 5, n = 4), or without (n = 3, n = 4) 10 μM STI571 overnight (P = .008,P = .016). (C) pK1 control cells were incubated with (n = 4) or without (n = 10) 10 μM STI571 as in (B) (P = .06) and the data are represented as the fold change in τ50 relative to untreated pK1 cells. Treatment of transduced 32D cells with STI571 did not affect nonspecific binding to BSA matrices (data not shown).
) 10 μM STI571 overnight prior to assaying adhesion. Typical profiles are shown and indicate that adhesion was not reduced, and may have slightly increased, as a result of tyrosine kinase attenuation by STI571. (–○–) represents a curve fitted to the experimental points. The critical shear stress (τ50) is defined as the point on the abscissa (shear stress) that corresponds to an adherent fraction of 0.5 along the fitted curve. (B) The critical shear stress was calculated for a series of spins and is represented as the fold change in τ50 relative to untreated P210BCR-ABLcells. We tested 2 separate populations of P210BCR-ABLcells (left, right) treated with (n = 5, n = 4), or without (n = 3, n = 4) 10 μM STI571 overnight (P = .008,P = .016). (C) pK1 control cells were incubated with (n = 4) or without (n = 10) 10 μM STI571 as in (B) (P = .06) and the data are represented as the fold change in τ50 relative to untreated pK1 cells. Treatment of transduced 32D cells with STI571 did not affect nonspecific binding to BSA matrices (data not shown).
STI571 treatment of a Ph+ human megakaryoblastic cell line, Meg-01, abrogates kinase activity but fails to alter cell adhesion
To determine whether human Ph+ cells also exhibit increased adhesion to fibronectin, we investigated the role of P210BCR-ABL kinase activity in modulating adhesion in Meg-01 cells. The Meg-01 cell line was derived from the bone marrow of a 55-year-old man in the blast crisis phase of CML.26Treatment of these cells with 10 μM STI571 led to a marked reduction in cell viability by 48 hours following treatment initiation (Figure6A). Similar to expression of wild-type P210BCR-ABL in 32D cells, STI571 treatment did not effect expression of P210BCR-ABL but led to a reduction in the whole cell phosphotyrosine levels compared to Meg-01 cells treated with PBS (Figure 6B). Attenuation of tyrosine kinase activity was observed as early as 3 hours after treatment of Meg-01 cells. At this time, binding of kinase attenuated Meg-01 cells to fibronectin was assessed and was found to be unchanged (P = .81) when compared to cells treated with an equal volume of PBS.
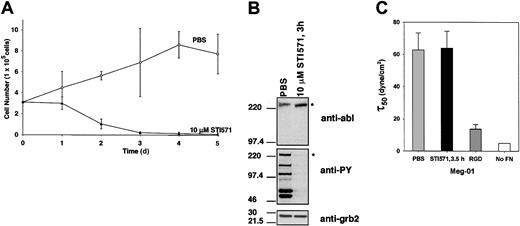
Adhesion of human Meg-01 leukemia cells to fibronectin is unresponsive to tyrosine kinase attenuation by STI571.
(A) Meg-01 cells were treated in triplicate with 10 μM STI571 (▴) or an equal volume of PBS used as the delivery vehicle (○) and were allowed to proliferate in Meg-01 media. Cell viability was determined by trypan blue exclusion. (B) Western blots of whole cell lysates were run to show expression of P210BCR-ABL and tyrosine-phosphorylated proteins in Meg-01 cells treated with 10 μM STI571 or PBS for 3 hours. The P210BCR-ABL–specific band is indicated by an asterisk. Expression of grb2 was used as a loading control. (C) τ50 was determined for spins with Meg-01 cells treated with 10 μM STI571 for 3.5 hours (n = 11) or an equal volume of PBS for 1.5 hours or 3.5 hours (n = 12). No difference in adhesion was observed between 1.5 hours and 3.5 hours for control Meg-01 cells treated with only PBS (data not shown). The magnitude of binding was not significantly affected when the kinase activity was arrested by STI571 (P = .81); however, decreased adhesion could be measured by blocking α5β1integrins with a soluble RGD fragment (n = 4) that prevents binding to fibronectin. Meg-01 cells treated with STI571 or PBS were assayed on BSA only (no FN), which represents nonspecific binding and is very small, at or below the minimum detection threshold of the device (≤ 5 dyne/cm2). At times, profiles could not be determined because of the small number of cells remaining after a spin in the absence of fibronectin.
Adhesion of human Meg-01 leukemia cells to fibronectin is unresponsive to tyrosine kinase attenuation by STI571.
(A) Meg-01 cells were treated in triplicate with 10 μM STI571 (▴) or an equal volume of PBS used as the delivery vehicle (○) and were allowed to proliferate in Meg-01 media. Cell viability was determined by trypan blue exclusion. (B) Western blots of whole cell lysates were run to show expression of P210BCR-ABL and tyrosine-phosphorylated proteins in Meg-01 cells treated with 10 μM STI571 or PBS for 3 hours. The P210BCR-ABL–specific band is indicated by an asterisk. Expression of grb2 was used as a loading control. (C) τ50 was determined for spins with Meg-01 cells treated with 10 μM STI571 for 3.5 hours (n = 11) or an equal volume of PBS for 1.5 hours or 3.5 hours (n = 12). No difference in adhesion was observed between 1.5 hours and 3.5 hours for control Meg-01 cells treated with only PBS (data not shown). The magnitude of binding was not significantly affected when the kinase activity was arrested by STI571 (P = .81); however, decreased adhesion could be measured by blocking α5β1integrins with a soluble RGD fragment (n = 4) that prevents binding to fibronectin. Meg-01 cells treated with STI571 or PBS were assayed on BSA only (no FN), which represents nonspecific binding and is very small, at or below the minimum detection threshold of the device (≤ 5 dyne/cm2). At times, profiles could not be determined because of the small number of cells remaining after a spin in the absence of fibronectin.
Localization of BCR-ABL in the cytoplasm to F-actin is unchanged by K1176R expression or in response to STI571
We sought to determine other characteristics of P210BCR-ABL that may be kinase-independent. Because adhesion to fibronectin did not correlate with kinase activity, we asked if the kinase activity of P210BCR-ABL affects its subcellular localization in NIH3T3 fibroblasts or 32D cells when detected by confocal microscopy. Like wild-type P210BCR-ABL, abrogation of the kinase activity by either the K1176R point mutation or STI571 treatment did not alter the cytoplasmic localization of P210BCR-ABL in either cell line (Figure 7A,B). In NIH3T3 cells, P210BCR-ABL was found along membrane ruffles, actin filaments, and strongly stained close to the cellular membrane in the presence or absence of kinase activity regardless of the method by which it was attenuated (Figure 7A). The 32D cells remained rounded and did not spread on fibronectin during the 15-minute incubation period used in both the immunohistochemistry analysis and the adhesion assays. P210BCR-ABL and actin both displayed a cortical staining pattern along the membrane of 32D cells, and this pattern did not vary in the presence or absence of tyrosine kinase activity (Figure7B).
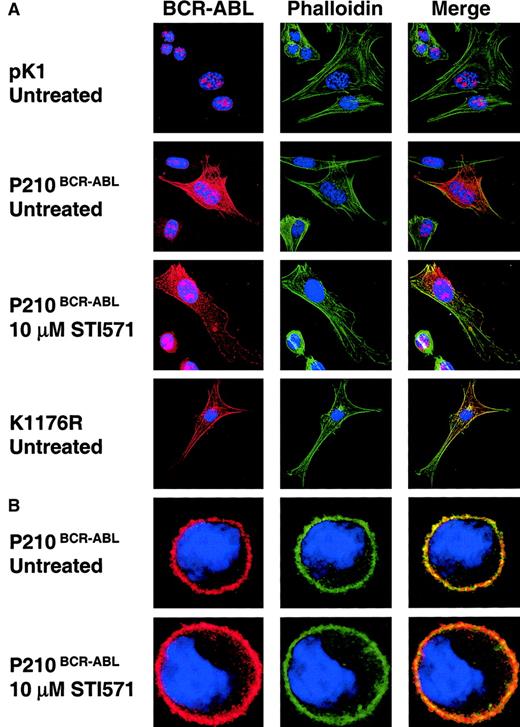
The subcellular localization of P210BCR-ABL is independent of tyrosine kinase activity.
(A) NIH3T3 cells were transduced with pK1, P210BCR-ABL, or K1176R retroviral vectors and selected with puromycin to determine if F-actin colocalization is also independent of tyrosine kinase activity. Cells were attached to fibronectin-coated coverslips overnight in the presence or absence of 10 μM STI571 as indicated. (B) 32D cells expressing P210BCR-ABL or control pK1 (data not shown) were treated with 10 μM STI571 or an equal volume of PBS overnight in the presence of WEHI media containing IL-3. Cells were allowed to bind fibronectin-coated coverslips for 15 minutes prior to fixing and staining cells according to the same protocol used to prepare 32D cells for adhesion assays. 32D and NIH3T3 cells were imaged using confocal microscopy. Colocalization of BCR-ABL with F-actin appears yellow or orange when BCR-ABL (red) and actin (green) are merged. All cells were stained with DAPI to visualize the nucleus (blue). Positive nuclear staining observed in pK1-transduced control NIH3T3 cells is likely due to endogenous c-abl expression. Total magnification, × 120.
The subcellular localization of P210BCR-ABL is independent of tyrosine kinase activity.
(A) NIH3T3 cells were transduced with pK1, P210BCR-ABL, or K1176R retroviral vectors and selected with puromycin to determine if F-actin colocalization is also independent of tyrosine kinase activity. Cells were attached to fibronectin-coated coverslips overnight in the presence or absence of 10 μM STI571 as indicated. (B) 32D cells expressing P210BCR-ABL or control pK1 (data not shown) were treated with 10 μM STI571 or an equal volume of PBS overnight in the presence of WEHI media containing IL-3. Cells were allowed to bind fibronectin-coated coverslips for 15 minutes prior to fixing and staining cells according to the same protocol used to prepare 32D cells for adhesion assays. 32D and NIH3T3 cells were imaged using confocal microscopy. Colocalization of BCR-ABL with F-actin appears yellow or orange when BCR-ABL (red) and actin (green) are merged. All cells were stained with DAPI to visualize the nucleus (blue). Positive nuclear staining observed in pK1-transduced control NIH3T3 cells is likely due to endogenous c-abl expression. Total magnification, × 120.
Discussion
To investigate the role of P210BCR-ABL in modulating cell adhesion to fibronectin, a major component of the bone marrow extracellular matrix,36 we used a highly sensitive and quantitative adhesion assay to measure the hydrodynamic forces used to detach bound cells. This device has advantages over conventional plate and wash assays by directly measuring the strength by which a population of cells is anchored to the extracellular matrix. The standardized detachment forces used in this system eliminate operator bias and variability in washing techniques that may occur in studies using panning-style adhesion assays. In contrast to long-term culture-initiating cell assays,37 or similar adhesion assays that rely on secondary colony formation to enumerate adherent and nonadherent progenitor cells in a heterogeneous population of primary bone marrow,3 our adhesion apparatus directly measures the magnitude of binding between a homogeneous population of cells and fibronectin. Due to the unique geometry of the detachment device, we assay a range of shear stresses during each spin and observe changes in adhesion throughout the entire range of forces that are tested. The cell detachment device generates a range of detachment forces (0-100 dyne/cm2) on a single fibronectin-coated coverslip, allowing our data to be fitted to known models of cell adhesion,16,17,29,30 38 and recapitulates shear stresses commonly found throughout the human body. It should be noted that the precise hydrodynamic environment of the bone marrow is unknown; however, it is likely that some of the forces generated by the detachment device exceed forces found in the bone marrow. Nevertheless, we observe differences in adhesion between P210BCR-ABL–expressing 32D cells and control cells at all shear stresses assayed, suggesting that increased adhesion induced by P210BCR-ABL occurs at physiologic forces.
We expressed P210BCR-ABL in the myeloblastic cell line, 32D, which affords a homogenous population of cells to study changes in adhesion in the predominant cell type affected by CML. Using this model system, we found that P210BCR-ABL expression led to a 1.7-fold increase in adhesion. Consistent with our findings, there have been 2 other reports demonstrating that P210BCR-ABLpromotes increased adhesion to fibronectin.5,15 Our results are also consistent with studies showing that adhesion between P210BCR-ABL–expressing cells and fibronectin promotes cell proliferation, cell cycle progression, and protects cells from DNA damage-induced apoptosis.15 39 In this scenario, increased adhesion may both promote the accumulation of progenitor cells and enhance their survival, 2 features associated with the pathogenesis of CML.
P210BCR-ABL phosphorylates paxillin, focal adhesion kinase, and other members of the focal adhesion complex, suggesting a link between the catalytic activity of P210BCR-ABL and regulation of integrin-mediated adhesion.9 To assess whether the tyrosine kinase activity of P210BCR-ABLinitiates inside-out signaling leading to increased adhesion, we tested the binding of 32D cells transduced with both the K1176R mutant or wild-type P210BCR-ABL cells treated with STI571 at concentrations shown to attenuate tyrosine kinase activity, reverse 32D cell transformation, and kill human leukemia cells over a period of days. Using these 2 independent methods to attenuate kinase activity in 32D cells transduced with P210BCR-ABL, we found that P210BCR-ABL–mediated increased adhesion remains intact in the absence of tyrosine kinase activity. Adhesion to fibronectin was also shown to be independent of tyrosine kinase activity in a second cell line, Meg-01, a Ph+ cell line derived from a patient in the blast crisis phase of CML. Together, these results support our hypothesis that altered adhesion due to P210BCR-ABLexpression is kinase-independent. Consistent with our results describing tyrosine kinase–independent functions of P210BCR-ABL, a kinase inactive mutant of Drosophila abl rescued pupal lethality and defects in eye development of flies lacking abl function.40 Also in support of our findings, P210BCR-ABL has been reported to localize to F-actin in a noncatalytic manner.7,41 Furthermore, expression of a kinase dead c-src mutant restored cell spreading of src−/− fibroblasts on fibronectin.42Together, these findings suggest an important functional role for the tyrosine-kinase independent activities of P210BCR-ABL.
Two previous studies found an inverse relationship between P210BCR-ABL–induced transformation and adhesion. p3T3 fibroblasts, which become anchorage independent on transformation by P210BCR-ABL, return to the adherent layer of cells after treatment with STI571.43 Bhatia and colleagues found that treatment of CML bone marrow with tryphostin AG957, a tyrosine kinase inhibitor, enriched adherent cells with progenitors that populated colony-forming cell assays, suggesting that P210BCR-ABLdecreased adhesion in a kinase-dependent manner.44 In contrast to these assays, our rotating disk system directly measures the magnitude of cell-fibronectin binding, which is independent of the transformation status of the cells and does not require a read-out step that may be sensitive to processes that are known to require tyrosine kinase activity such as proliferation or differentiation.
One significant implication of our results is that altered binding to fibronectin is likely to be a direct consequence of P210BCR-ABL expression and not a side effect of cellular transformation because increased adhesion does not correlate with cell transformation in our system. Two functions of P210BCR-ABL, actin binding and activation of Ras signaling, are potential mechanisms by which P210BCR-ABL may modulate adhesion. P210BCR-ABL is found in the cytoplasm, colocalized with F-actin filaments,6 and cannot be removed from F-actin filaments by treatment with either 10 μM STI571 alone or by complete abrogation of kinase activity by the K1176R mutant. Using confocal microscopy we show that P210BCR-ABL is found along membrane ruffles, actin filaments, and close to the cellular membrane irrespective of its kinase activity or the method by which the tyrosine kinase activity was attenuated. This suggests that F-actin localization is kinase independent, and a recent report has shown that the Abl-related gene-1 (Arg-1) also binds F-actin even after deletion of its kinase domain.45 An intact actin cytoskeleton is required for cell adhesion in our system because treatment with cytochalasin D severely attenuates binding of 32D cells and other cells to fibronectin (data not shown).46 One possibility is that P210BCR-ABL may modify the actin cytoskeleton and influence binding. Alternatively, the role of P210BCR-ABL as a docking protein to recruit downstream molecules to actin, rather than its tyrosine kinase activity, may contribute to defective adhesion. Transgenic mice expressing P190BCR-ABL with a deletion of the C-terminal actin-binding domain survive up to 3 times longer and develop hematologic disease with lower frequency compared to wild-type P190BCR-ABL transgenic mice.47 As shown in our immunohistochemistry studies, the association of P210BCR-ABL with actin is independent of its kinase activity. Thus, cell adhesion and proper subcellular localization of P210BCR-ABL may be prerequisites for P210BCR-ABL tyrosine kinase to initiate CML.
Our results are surprising in light of the wealth of evidence showing that the kinase activity of P210BCR-ABL is critical to disease initiation and in vitro cell transformation. Thus, it is unlikely that elevated adhesion due to P210BCR-ABLexpression alone can initiate the cellular changes that lead to CML. The K1176R mutant retains the ability to modulate adhesion to fibronectin exhibited with wt P210BCR-ABL. However, mice receiving bone marrow transduced with the K1176R mutant fail to develop a CML-like disease under conditions that would normally support disease development with wild-type P210BCR-ABL.12 This raises the question of which functions of P210BCR-ABL are required for CML induction. To this end, murine retroviral transduction models of CML have been particularly insightful. Although tyrosine kinase activity is required, mutants that retain tyrosine kinase activity but lack either the oligomerization domain or grb2-binding site are markedly inhibited in their ability to induce CML in murine models.48-50 This suggests that the activated tyrosine kinase activity of P210BCR-ABL is insufficient for CML induction. Furthermore, we have found that even though the oligomerization domain of P210BCR-ABL is unnecessary for tyrosine kinase activity, it is required to maintain actin colocalization and to obtain wild-type levels of binding to fibronectin (J.A.W., manuscript in preparation). One possibility is that tyrosine kinase activity, signaling through grb2 binding, and tyrosine kinase-independent regulation of oligomerization effecting actin localization and cell adhesion, are all required for efficient CML induction in murine models. Utilization of the cell detachment device on additional P210BCR-ABL mutants and primary CML bone marrow should allow us to investigate this and other possibilities.
We thank Martin Carroll, Alan Gewirtz, Ruibao Ren, and members of the Pear Lab for critical reading of the manuscript and helpful suggestions. We are particularly grateful to Andrés Garcı́a and Laura Lynch for their assistance with the spinning disk system and Ruibao Ren and Karen Ehrmann for providing useful reagents.
Supported by National Institutes of Health grants HL18208 (D.A.H.) and CA77570 and a Scholar Award from the Leukemia and Lymphoma Society (W.S.P.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Warren S. Pear, University of Pennsylvania, 611 BRB II/III, 421 Curie Blvd, Philadelphia, PA 19104-6160; e-mail:wpear@mail.med.upenn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal