Induction of tolerance is likely to be essential for successful xenotransplantation because immune responses across xenogeneic barriers are vigorous. Although mixed hematopoietic chimerism leads to stable donor-specific tolerance in allogeneic and closely related xenogeneic (eg, rat-to-mouse) combinations, the ability of this approach to induce tolerance across a highly disparate xenogeneic barrier has not yet been demonstrated. In this study, we investigated the immune responses of murine T cells that developed in mice with pre-established porcine hematopoietic chimerism. Our results show for the first time that induction of porcine hematopoietic chimerism can eliminate the development of antiporcine donor responses in a highly disparate xenogeneic species. Porcine hematopoietic chimeras showed donor-specific nonresponsiveness in the mixed lymphocyte reaction, lack of antidonor IgG antibody production, and acceptance of donor skin grafts. Thus, mixed chimerism is capable of inducing tolerance in a highly disparate xenogeneic combination and may have clinical potential to prevent xenograft rejection.
Introduction
Xenotransplantation provides a possible solution to the severe shortage of allogeneic organs, a major limiting factor in clinical organ transplantation. In view of the ethical issues and impracticalities associated with the use of nonhuman primates, interest has become focused on nonprimates, in particular the pig, as the most suitable organ donor species for humans. However, organ transplants across discordant species barriers are subject to vigorous immunologic rejection.1-4 Transplantation of organs from pigs results in hyperacute rejection in primate recipients due to the presence of anti–Galα1-3Gal natural antibodies in their sera. These antibodies also mediate a delayed form of humoral rejection, acute vascular rejection, if hyperacute rejection is overcome. The cellular immune response to xenografts is also stronger than that to allografts. Thus, tolerance induction is likely to be essential for successful xenotransplantation.
Induction of mixed hematopoietic chimerism by bone marrow transplantation (BMT) leads to stable donor-specific tolerance in allogeneic and closely related xenogeneic (rat-to-mouse) combinations,5-10 but its ability to induce porcine xenograft tolerance has not been demonstrated in any discordant xenogeneic species because of the difficulty in achieving lasting porcine hematopoietic engraftment. Studies using a pig-primate combination have shown prolonged survival of porcine kidney grafts in cynomolgus monkey recipients preinfused with donor marrow cells. However, long-lasting hematopoietic chimerism was not achieved in any of these animals, and all kidney grafts were eventually rejected.11,12 Because donor hematopoietic stem cells may fail to engraft in discordant xenogeneic recipients, even in recipients in which T and B cells are absent,13,14 the rejection of pig kidney grafts in these studies does not distinguish between the failure of donor hematopoietic cells to engraft and an inability of chimerism to induce tolerance across highly disparate species barriers. It has been shown that the persistence of donor cells is essential for maintaining tolerance in mixed chimeras.15-20
Our previous studies showed that lasting porcine hematopoietic chimerism and stem cell engraftment can be induced in nonobese diabetic/severe combined immunodeficiency transgenic (NOD/SCID-Tg) mice expressing pig cytokine transgenes.21 These transgenic mice also demonstrated spontaneous migration of porcine donor antigen-presenting cells (APCs) to an intact recipient thymus, suggesting the possibility of inducing central tolerance in the discordant xenogeneic setting by mixed chimerism. Here we attempted to determine whether or not hematopoietic chimerism is capable of inducing donor-specific tolerance in this pig-to-mouse combination, by comparing the responses of murine T cells that developed in NOD/SCID-Tg mice with or without pre-established porcine chimerism.
Materials and methods
Animals
The NOD/LtSz-SCID/SCID (NOD/SCID) and B10.D2/o mice were purchased from the Jackson Laboratory (Bar Harbor, ME). NOD/SCID-Tg and B10.D2/o-Tg mice that produce porcine cytokines (interleukin 3 [IL-3], granulocyte-macrophage colony-stimulating factor [GM-CSF], and stem cell factor [SCF]) were developed by crossing the transgenic founders with NOD/SCID and B10.D2/o mice for at least 6 generations, respectively, as previously described.21 All Tg mice used in this study were identified by tail DNA polymerase chain reaction (PCR) using a primer for cytomegalovirus (CMV) promoter in combination with a primer for porcine IL-3, GM-CSF, or SCF as described.21 Mice were housed in a specific pathogen-free microisolator environment and were used at 8 to 13 weeks of age. Partially inbred Massachusetts General Hospital (MGH) miniature swine provided by Dr David H. Sachs22 were used as porcine bone marrow donors. All animals were maintained and procedures were performed in accordance with National Institutes of Health guidelines.
Immune reconstitution
The NOD/SCID-Tg mice were conditioned with 3 Gy whole body irradiation (WBI), and injected with T- and B-cell–depleted B10.D2/o-Tg mouse bone marrow cells (BMCs; 1-1.5 × 106/mouse) at week 5, 7, or 11 after WBI. To prepare T- and B-cell–depleted BMCs, B10.D2/o-Tg mouse BMCs were first incubated with antimouse CD4 monoclonal antibody (mAb) GK 1.5 and anti-CD8 mAb 2.43 for 30 minutes, followed by incubation with low-toxicity rabbit complement for 45 minutes, to deplete CD4 and CD8 T cells.23 CD4- and CD8-depleted BMCs were further depleted of T-cell receptor positive cells (TCR+) and B cells by using the MACS system (Miltenyi Biotec, Bergisch-Gladback, Germany) according to the manufacturer's instructions. Briefly, cells were labeled with biotinylated anti-TCRαβ and anti-CD19 (Pharmingen, San Diego, CA), followed by incubation with streptavidin microbeads (Miltenyi Biotec), and negatively selected on a magnetic separation column. The completeness of depletion was confirmed by fluorescence-activated cell sorter (FACS) analysis.
Induction of porcine hematopoietic chimerism in NOD/SCID-Tg mice
The NOD/SCID-Tg mice were treated with 3 Gy WBI followed within 4 to 8 hours by injection of 1 × 108 porcine BMCs (intravenously) and 5 × 107 porcine peripheral blood mononuclear cells (PBMCs, intraperitoneally) prepared from the same donor as previously described.14 21 Some recipient mice were infused with T- and B-cell–depleted B10.D2/o-Tg mouse BMCs (1.5 × 106; intravenously) at week 7 after porcine BMT to achieve murine lymphohematopoiesis.
Skin grafting
Skin grafting was performed as previously described.24 Split thickness (2.3-mm) porcine skin and full thickness tail skin from fully major histocompatibility complex-mismatched B10.RIII (third-party) mice (Jackson Laboratory), were grafted on the lateral thoracic wall 2 weeks after reconstitution with T- and B-cell–depleted B10.D2/o-Tg mouse BMCs. For tolerance studies, porcine skins were harvested from the BM donors and cryopreserved until use. Skin grafts were evaluated daily from day 7 onward to 4 weeks and then at least one inspection every third day thereafter. Grafts were defined as rejected when less than 10% of the graft remained viable.
FACS analysis
White blood cells (WBCs), spleen, and BMCs were prepared from recipient mice and FACS analysis was performed as described.9,21 The completeness of T- and B-cell depletion and in vivo murine immune reconstitution was determined by staining of cells with antimouse CD4, CD8, TCRαβ, CD19, and IgM (all these mAbs were purchased from Pharmingen). To determine porcine chimerism, cells were stained with phycoerythrin (PE)–conjugated antimouse CD45 mAb (rat IgG2b, Pharmingen) in combination with fluorescein isothiocyanate (FITC)–conjugated antipig pantissue mAb (1030H-1-19),21 and porcine marker-positive cells not stained by antimouse CD45 mAb were considered to be of swine (donor) origin. FITC-conjugated porcine lineage marker-specific mAbs (myeloid, CD2, CD3, CD4, CD8, and CD21) were also used to analyze porcine chimerism as described,21 and the majority of in vivo-repopulating porcine cells were of the myeloid lineage (data not shown). Nonspecific binding of labeled mAbs was blocked with 2.4G2 (rat antimouse FcγR mAb). FITC-conjugated HOPC1 (murine IgG2a mAb) and PE-conjugated rat IgG2a (Pharmingen), both with no known reactivity to mouse or pig cells, served as negative control antibodies. Analysis was performed on a FACScan (Becton Dickinson, Mountain View, CA) and dead cells were excluded by gating out low forward scatter plus high propidium iodide–retaining cells.
Mixed leukocyte reaction assay
Mouse splenocytes were prepared and suspended in RPMI 1640 medium supplemented with 15% (vol/vol) controlled processed serum replacement (CPSR-2; Sigma Chemical, St Louis, MO), 2 mMl-glutamine, 0.1 mM nonessential amino acids (Life Technologies, Grand Island, NY), 1 mM sodium pyruvate, 10 U/mL penicillin, 10 μg/mL streptomycin, 1% Hepes buffer, and 10 μM 2-mercaptoethanol. Triplicate wells containing 4 × 105responders with irradiated (30 Gy) stimulators of swine (1 × 105 PBMCs) or mouse (4 × 105B10.RIII splenocytes) in a total volume of 0.2 mL medium were incubated at 37°C in 5% CO2. Cultures were pulsed with 1 μCi (3.7 × 104 Bq) [3H]-TdR on days 3 and 4, and harvested 18 hours later with a Tomtec (Wallac, Gaithersburg, MD) automated harvester. Samples were assayed in a Pharmacia LKB (Piscataway, NJ) Betaplate counter and data are expressed as stimulation index (cpm of stimulated culture/cpm of unstimulated [media control] culture).
Quantitation of antipig IgG and total IgG
Indirect immunofluorescence staining of donor swine PBMCs was used to detect antiporcine donor antibodies.25 Frozen PBMCs prepared from the porcine BM donors were thawed, washed with FACS buffer, and 105 cells were stained for 30 minutes at 4°C with 2 or 10 μL undiluted mouse serum followed by incubation with FITC-conjugated rat antimouse IgG1, IgG2a, IgG2b, or IgG3 mAb (Pharmingen) for 30 minutes at 4°C. Cells were washed with FACS buffer after each incubation and were analyzed on a FACScan (Becton Dickinson). The serum levels of antipig antibody are presented as median fluorescence intensity (MFI). The serum levels of total murine IgG were measured by enzyme-linked immunosorbent assay (ELISA)10 and calibrated with purified mouse IgG.
Results
Administration of T- and B-cell–depleted immunocompetent mouse BMCs led to development of functional T and B cells in NOD/SCID-Tg mice
Previous studies have shown that injection of immunocompetent murine hematopoietic stem cells gives rise to functional T and B cells in SCID mice.25 26 Because demonstration of porcine chimerism prior to immune reconstitution is essential to distinguish failure of porcine marrow engraftment from a failure of tolerance induction, we attempted to establish murine lymphopoiesis after stable porcine chimerism had first been confirmed. To determine the optimal timing for giving immunocompetent murine stem cells, we followed immune recovery in NOD/SCID-Tg mice reconstituted with T- and B-cell–depleted immunocompetent B10.D2/o-Tg mouse BMCs at various times after 3 Gy WBI. Mice were challenged with skin grafts from allogeneic and xenogeneic donors 2 weeks after B10.D2/o mouse BMC reconstitution. As shown in Figure 1A, both allogeneic and xenogeneic skin grafts were rejected in NOD/SCID-Tg mice that were reconstituted within 5 to 11 weeks after WBI with B10.D2/o-Tg mouse BMCs, whereas skin grafts were permanently accepted by NOD/SCID-Tg mice that did not receive B10.D2/o-Tg mouse BMCs. FACS analyses demonstrated the presence of substantial numbers of mouse T (CD4+, CD8+) and B (surface IgM+) cells in the blood and spleen of B10.D2/o-Tg mouse BMC-reconstituted NOD/SCID-Tg mice. In most mice T and B cells became detectable in the WBCs by 2 to 3 weeks and gradually increased to a stable level within 10 to 15 weeks after injection of B10.D2/o-Tg mouse BMCs (Figure 1B and data not shown). Although “leaky” CD4+ or CD8+ cells (but no surface IgM+ B cells) were detected in some NOD/SCID-Tg mice without B10.D2/o-Tg mouse BMC reconstitution, none of these mice were capable of rejecting skin grafts from allogeneic or xenogeneic donors (Figure 1A). Together, these results demonstrate that injection of T- and B-cell–depleted BMCs from immunocompetent mice into 3 Gy WBI-conditioned NOD/SCID-Tg mice by 11 weeks after WBI can efficiently restore immune reactivity against allogeneic and xenogeneic antigens.
Immune reconstitution in NOD/SCID-Tg mice receiving T- and B-cell–depleted B10.D2/o-Tg mouse BMCs.
NOD/SCID-Tg mice were treated with 3 Gy WBI and some of them received subsequent infusion of 1-1.5 × 106 T- and B- cell–depleted B10.D2/o-Tg mouse BMCs at week 5, 7, or 11 after WBI. (A) Rejection of skin grafts from allogeneic mouse and xenogeneic porcine donors by B10.D2/o-Tg mouse BMC-reconstituted NOD/SCID-Tg mice. B10.RIII mouse and porcine skins were grafted simultaneously in NOD/SCID-Tg mice 2 weeks after administration of T- and B-cell–depleted B10.D2/o-Tg mouse BMCs. Survival is shown for B10.RIII (dashed line) and pig (solid line) skin grafts in irradiated NOD/SCID-Tg mice receiving (▴; n = 8) or not receiving (▪; n = 8) a subsequent infusion of B10.D2/o-Tg mouse BMCs. Both B10.RIII and porcine skin grafts were accepted in NOD/SCID-Tg recipients of WBI only for over 100 days or until death (*), but were rejected by B10.D2/o-Tg mouse BMC-reconstituted NOD/SCID-Tg mice by 42 days. (B). Development of mouse CD4+ cells, CD8+ cells, and surface IgM+ cells in NOD/SCID-Tg mice reconstituted with T- and B-cell–depleted B10.D2/o-Tg mouse BMCs. WBCs and splenocytes were prepared from B10.D2/o-Tg mouse BMC-reconstituted mice (●) and control animals that received WBI only (○) during the period of 10 to 15 weeks after B10.D2/o-Tg mouse BMC infusion, and percentages of mouse CD4+, CD8+ and surface IgM+ cells were measured by 2-color FACS analysis. Each symbol represents an individual animal. Data are pooled from 4 independent experiments.
Immune reconstitution in NOD/SCID-Tg mice receiving T- and B-cell–depleted B10.D2/o-Tg mouse BMCs.
NOD/SCID-Tg mice were treated with 3 Gy WBI and some of them received subsequent infusion of 1-1.5 × 106 T- and B- cell–depleted B10.D2/o-Tg mouse BMCs at week 5, 7, or 11 after WBI. (A) Rejection of skin grafts from allogeneic mouse and xenogeneic porcine donors by B10.D2/o-Tg mouse BMC-reconstituted NOD/SCID-Tg mice. B10.RIII mouse and porcine skins were grafted simultaneously in NOD/SCID-Tg mice 2 weeks after administration of T- and B-cell–depleted B10.D2/o-Tg mouse BMCs. Survival is shown for B10.RIII (dashed line) and pig (solid line) skin grafts in irradiated NOD/SCID-Tg mice receiving (▴; n = 8) or not receiving (▪; n = 8) a subsequent infusion of B10.D2/o-Tg mouse BMCs. Both B10.RIII and porcine skin grafts were accepted in NOD/SCID-Tg recipients of WBI only for over 100 days or until death (*), but were rejected by B10.D2/o-Tg mouse BMC-reconstituted NOD/SCID-Tg mice by 42 days. (B). Development of mouse CD4+ cells, CD8+ cells, and surface IgM+ cells in NOD/SCID-Tg mice reconstituted with T- and B-cell–depleted B10.D2/o-Tg mouse BMCs. WBCs and splenocytes were prepared from B10.D2/o-Tg mouse BMC-reconstituted mice (●) and control animals that received WBI only (○) during the period of 10 to 15 weeks after B10.D2/o-Tg mouse BMC infusion, and percentages of mouse CD4+, CD8+ and surface IgM+ cells were measured by 2-color FACS analysis. Each symbol represents an individual animal. Data are pooled from 4 independent experiments.
Porcine skin graft acceptance in immune-reconstituted NOD/SCID-Tg mice with pre-established porcine hematopoietic chimerism
We next investigated whether or not murine T cells that developed in porcine hematopoietic chimeras could be specifically tolerized to the porcine BM donor (Figure 2). Porcine hematopoietic chimeras were prepared by injection of porcine hematopoietic cells into 3 Gy WBI-conditioned NOD/SCID-Tg mice as previously described.21 These chimeras and NOD/SCID-Tg mice that did not receive porcine cell transplantation were then reconstituted with T- and B-cell–depleted B10.D2/o-Tg mouse BMCs 7 weeks later. Skin grafting was performed 2 weeks after infusion of B10.D2/o-Tg mouse BMCs to assess tolerance. Consistent with the results shown in Figure 1A, both allogeneic third-party and porcine skin grafts were permanently accepted by NOD/SCID-Tg mice that did not receive B10.D2/o-Tg mouse BMCs (Figure 3A), whereas they were both rejected by NOD/SCID-Tg mice that did receive B10.D2/o-Tg mouse BMCs (Figure 3B). However, specific acceptance of porcine donor skin grafts was observed in 5 of 8 B10.D2/o-Tg mouse BMC-reconstituted porcine hematopoietic chimeras (ie, animals in which significant porcine chimerism was detected in the WBCs 1 week prior to murine BMC reconstitution; Figure 3C). Although rejection of porcine donor skin grafts occurred in 3 porcine hematopoietic chimeras, rejection in 2 of these 3 mice was delayed. Porcine donor skin rejection in these porcine hematopoietic chimeras might be due to incomplete tolerance, or skin-specific antigens, or both, because all of these mice showed a specific lack of antiporcine donor responses in mixed lymphocyte reaction (MLR; see below). In contrast, skin grafts from the porcine BM donor were rejected in B10.D2/o-Tg mouse BMC-reconstituted NOD/SCID-Tg mice with poor porcine chimerism (ie, mice with undetectable or < 1.5% WBC chimerism at 1 week prior to murine BMC reconstitution). Both B10.D2/o-Tg mouse BMC-reconstituted porcine hematopoietic chimeras and NOD/SCID-Tg mice with poor porcine chimerism rejected allogeneic third-party skin grafts (Figure 3C), demonstrating immune function in animals that accepted porcine skins.
Donor-specific skin graft prolongation in porcine hematopoietic chimeras reconstituted with T- and B-cell–depleted B10.D2/o-Tg mouse BMCs.
Survival is shown for skin grafts from the porcine BMC donor (solid line) and third-party B10.RIII mice (dashed line) in NOD/SCID-Tg mice receiving transplants with porcine cells only (A; n = 2), B10.D2/o-Tg mouse BMC-reconstituted NOD/SCID-Tg mice without pretransplantation of porcine cells (B; n = 2), and B10.D2/o-Tg mouse BMC-reconstituted NOD/SCID-Tg mice that had received porcine BMCs prior to murine BMC reconstitution (C; n = 11). Based on the level of porcine chimerism in the WBCs at 1 week prior to B10.D2/o-Tg mouse BMC reconstitution (6 weeks after porcine BMT), mice in panel C are divided into 2 groups: porcine hematopoietic chimeras (▪ with ≥ 1.5% [1.5%-33%] WBC chimerism; n = 8) and mice with poor porcine chimerism (■ with undetectable or < 1.5% WBC chimerism; n = 3). Porcine chimerism was determined by FACS analysis using an antipig pan-tissue mAb. Because the rejection of third-party skin grafts was virtually identical between porcine hematopoietic chimeras and mice with poor porcine chimerism, combined third-party rejection results for the 2 groups are shown (C;--▾--). Results from 2 similar experiments are combined.
Donor-specific skin graft prolongation in porcine hematopoietic chimeras reconstituted with T- and B-cell–depleted B10.D2/o-Tg mouse BMCs.
Survival is shown for skin grafts from the porcine BMC donor (solid line) and third-party B10.RIII mice (dashed line) in NOD/SCID-Tg mice receiving transplants with porcine cells only (A; n = 2), B10.D2/o-Tg mouse BMC-reconstituted NOD/SCID-Tg mice without pretransplantation of porcine cells (B; n = 2), and B10.D2/o-Tg mouse BMC-reconstituted NOD/SCID-Tg mice that had received porcine BMCs prior to murine BMC reconstitution (C; n = 11). Based on the level of porcine chimerism in the WBCs at 1 week prior to B10.D2/o-Tg mouse BMC reconstitution (6 weeks after porcine BMT), mice in panel C are divided into 2 groups: porcine hematopoietic chimeras (▪ with ≥ 1.5% [1.5%-33%] WBC chimerism; n = 8) and mice with poor porcine chimerism (■ with undetectable or < 1.5% WBC chimerism; n = 3). Porcine chimerism was determined by FACS analysis using an antipig pan-tissue mAb. Because the rejection of third-party skin grafts was virtually identical between porcine hematopoietic chimeras and mice with poor porcine chimerism, combined third-party rejection results for the 2 groups are shown (C;--▾--). Results from 2 similar experiments are combined.
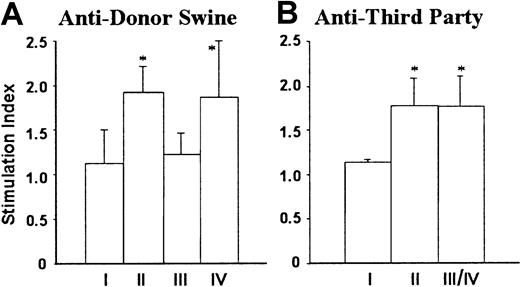
Lack of antiporcine donor MLR responses or antiporcine donor IgG production in B10.D2/o-Tg mouse BMC-reconstituted porcine hematopoietic chimeras
Prolongation of porcine donor skin grafts was accompanied by donor-specific nonresponsiveness in MLRs. MLR assays were performed after skin grafting, because mice do not mount primary antipig MLR responses.27 28 As shown in Figure4, NOD/SCID-Tg mice that did not receive B10.D2/o-Tg mouse BMCs (group I) showed no MLR responses to either porcine donor or third-party donor, whereas responses against both stimulators were measurable in NOD/SCID-Tg mice reconstituted with B10.D2/o-Tg mouse BMCs (group II). However, MLR responses against the porcine donor were undetectable in B10.D2/o-Tg mouse BMC-reconstituted porcine hematopoietic chimeras, including the 3 mice that rejected the porcine donor skin graft (Figure 4A, group III). In contrast, antiporcine donor MLR responses were detected in B10.D2/o-Tg mouse BMC-reconstituted NOD/SCID-Tg mice with poor porcine chimerism (Figure4A, group IV). Anti–third-party MLR responses were detected in both porcine hematopoietic chimeras and NOD/SCID-Tg mice with poor porcine chimerism, and their levels were comparable to those in B10.D2/o-Tg mouse BMC-reconstituted NOD/SCID-Tg mice that did not receive porcine cells (Figure 4B).
Lack of antiporcine donor MLR responses in B10.D2/o-Tg mouse BMC-reconstituted porcine hematopoietic chimeras.
Splenocytes were prepared from NOD/SCID-Tg mice receiving transplants with porcine cells only (I; n = 4), B10.D2/o-Tg mouse BMCs only (II; n = 5), or porcine cells plus B10.D2/o-Tg mouse BMCs (III/IV; n = 11) at week 17 or week 20 after reconstitution with B10.D2/o-Tg mouse BMCs, and antiporcine donor (A) and third-party B10.RIII (B) MLR responses were determined. The recipients of porcine cells plus B10.D2/o-Tg mouse BMCs are divided into porcine hematopoietic chimera (III; n = 8) and poor porcine chimerism (IV; n = 3) groups according to the level of porcine chimerism in the WBCs at 1 week prior to injection of B10.D2/o-Tg mouse BMCs (see the legend to Figure 3). Because the anti–third-party MLR response was virtually identical between porcine hematopoietic chimeras and mice with poor porcine chimerism, combined results for the 2 groups are shown (Figure 4B; III/IV). Data are presented as group means ± SD. *P < .05 for the indicated group compared to group I and to group III in panel A, and to group I in panel B. Results of 2 similar experiments are combined.
Lack of antiporcine donor MLR responses in B10.D2/o-Tg mouse BMC-reconstituted porcine hematopoietic chimeras.
Splenocytes were prepared from NOD/SCID-Tg mice receiving transplants with porcine cells only (I; n = 4), B10.D2/o-Tg mouse BMCs only (II; n = 5), or porcine cells plus B10.D2/o-Tg mouse BMCs (III/IV; n = 11) at week 17 or week 20 after reconstitution with B10.D2/o-Tg mouse BMCs, and antiporcine donor (A) and third-party B10.RIII (B) MLR responses were determined. The recipients of porcine cells plus B10.D2/o-Tg mouse BMCs are divided into porcine hematopoietic chimera (III; n = 8) and poor porcine chimerism (IV; n = 3) groups according to the level of porcine chimerism in the WBCs at 1 week prior to injection of B10.D2/o-Tg mouse BMCs (see the legend to Figure 3). Because the anti–third-party MLR response was virtually identical between porcine hematopoietic chimeras and mice with poor porcine chimerism, combined results for the 2 groups are shown (Figure 4B; III/IV). Data are presented as group means ± SD. *P < .05 for the indicated group compared to group I and to group III in panel A, and to group I in panel B. Results of 2 similar experiments are combined.
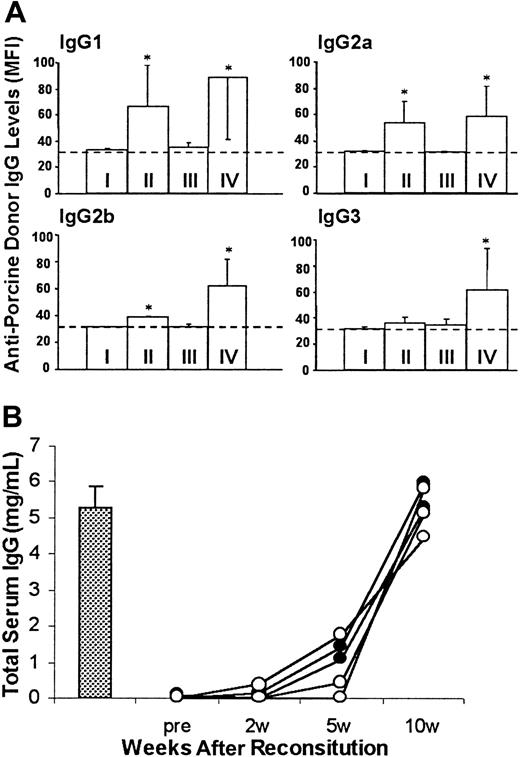
Sera were collected for measuring antiporcine donor IgG antibodies and total murine IgG. Similar to immunodeficient NOD/SCID-Tg mice that did not receive B10.D2/o-Tg mouse BMCs, antidonor swine IgG antibodies were undetectable in B10.D2/o-Tg mouse BMC-reconstituted porcine hematopoietic chimeras that accepted the porcine donor skin graft (Figure 5A). In contrast, the production of all isotypes of antiporcine donor IgG antibodies was stimulated in B10.D2/o-Tg mouse BMC-reconstituted NOD/SCID-Tg mice that rejected porcine donor skin grafts (Figure 5A). Because no difference in serum levels of total murine IgG was detected between mice that accepted or rejected porcine donor skin (Figure 5B), the absence of antiporcine donor IgG production in porcine hematopoietic chimeras reflects tolerance to the porcine hematopoietic donor. Previous studies have shown that antipig IgG antibodies are only produced after exposure to pig antigens, and their production is T cell dependent.25 Thus, these results confirmed tolerance of murine T cells to the porcine BMC donor in B10.D2/o-Tg mouse BMC-reconstituted porcine hematopoietic chimeras.
Lack of antiporcine donor IgG antibodies in sera of B10.D2/o-Tg mouse BMC-reconstituted porcine hematopoietic chimeras that accepted porcine donor skin grafts.
(A) Levels of antiporcine donor IgG antibodies in sera collected at 10 weeks after reconstitution with B10.D2/o-Tg mouse BMCs (8 weeks after skin grafting). Antipig IgGs were measured by staining of porcine donor PBMCs with 2 or 10 μL serum and antibody levels are presented as MFI. The MFI level correlated directly with the volume of antibody-positive serum used for staining in each instance. Data are shown for the MFI levels of porcine PBMCs bound by 10 μL serum (group means ± SD) in NOD/SCID-Tg mice given transplants with porcine cells only (I; n = 4), B10.D2/o-Tg mouse BMCs only (II; n = 5), or with porcine cells plus B10.D2/o-Tg mouse BMCs that accepted (III; n = 5) or rejected (IV, n = 6) the skin graft of the porcine donor. The background level of MFI (ie, MFI of porcine PBMCs stained with FITC-conjugated secondary mAb only) is indicated as a dotted line. *P < .05 for antibody levels in the indicated group compared to the levels of the same IgG isotype in group I and in group III. Results from 2 similar experiments are combined. (B) Serum levels of total murine IgG in representative samples from group III (○; n = 3), group IV (●; n = 2), and normal B10.D2/o-Tg mice (bar, mean ± SD, n = 3) are shown.
Lack of antiporcine donor IgG antibodies in sera of B10.D2/o-Tg mouse BMC-reconstituted porcine hematopoietic chimeras that accepted porcine donor skin grafts.
(A) Levels of antiporcine donor IgG antibodies in sera collected at 10 weeks after reconstitution with B10.D2/o-Tg mouse BMCs (8 weeks after skin grafting). Antipig IgGs were measured by staining of porcine donor PBMCs with 2 or 10 μL serum and antibody levels are presented as MFI. The MFI level correlated directly with the volume of antibody-positive serum used for staining in each instance. Data are shown for the MFI levels of porcine PBMCs bound by 10 μL serum (group means ± SD) in NOD/SCID-Tg mice given transplants with porcine cells only (I; n = 4), B10.D2/o-Tg mouse BMCs only (II; n = 5), or with porcine cells plus B10.D2/o-Tg mouse BMCs that accepted (III; n = 5) or rejected (IV, n = 6) the skin graft of the porcine donor. The background level of MFI (ie, MFI of porcine PBMCs stained with FITC-conjugated secondary mAb only) is indicated as a dotted line. *P < .05 for antibody levels in the indicated group compared to the levels of the same IgG isotype in group I and in group III. Results from 2 similar experiments are combined. (B) Serum levels of total murine IgG in representative samples from group III (○; n = 3), group IV (●; n = 2), and normal B10.D2/o-Tg mice (bar, mean ± SD, n = 3) are shown.
Porcine hematopoietic cells have a competitive disadvantage in murine recipients
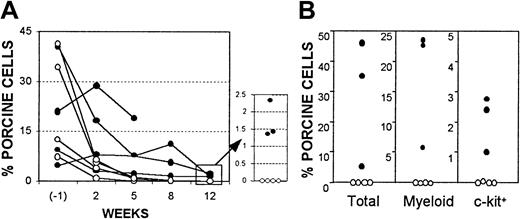
Despite the persistence of donor-specific tolerance in B10.D2/o-Tg mouse BMC-reconstituted porcine hematopoietic chimeras, FACS analysis showed that porcine hematopoietic chimerism in the WBCs and marrow of these mice became undetectable by 17 weeks after injection of B10.D2/o-Tg mouse BMCs (ie, 24 weeks after porcine BMT). Because lasting porcine chimerism was observed in NOD/SCID-Tg mice that received only porcine cells (data not shown),21 these results suggest that infusion of mouse BMCs led to loss of porcine hematopoietic chimerism. Because donor-specific tolerance was maintained after porcine chimerism became undetectable in the recipient WBCs and marrow, the loss of porcine chimerism in these mice was likely mediated by nonimmunologic factors. To determine if infusion of murine BMCs may limit porcine chimerism though nonimmunologic mechanisms, we compared porcine chimerism in NOD/SCID-Tg mouse recipients of porcine BMCs with or without subsequent infusion of immunodeficient mouse BMCs. It has been demonstrated that NOD/SCID mice lack functional T, B, and natural killer cells.29 NOD/SCID-Tg mice were treated with 3 Gy WBI followed within 4 to 8 hours by porcine BMT. Some of these mice were subsequently reconstituted with 5 × 106NOD/SCID-Tg mouse BMCs at week 7 after porcine BMT. Although porcine chimerism in the WBCs declined rapidly at early times in porcine BM recipients, probably due to the death of injected mature porcine cells,21 all NOD/SCID-Tg mice that received only porcine cells (ie, without subsequent murine BMT) maintained long-term (> 19 weeks) chimerism (Figure6). However, injection of NOD/SCID-Tg mouse BMCs at week 7 following porcine BMT completely eliminated pre-established porcine hematopoietic chimerism, despite the inability of these cells to reconstitute lymphopoiesis and to mediate immunologic rejection (Figure 6). Together, these results indicate that the loss of porcine chimerism in B10.D2/o-Tg mouse BMC-reconstituted porcine hematopoietic chimeras, which are tolerant to the porcine donor, was likely due to the competitive effect of infused B10.D2/o-Tg mouse BMCs, but not immunologic rejection.
Competitive advantage for murine hematopoiesis over porcine hematopoiesis in NOD/SCID-Tg mice.
Data are shown for porcine chimerism in porcine BM recipients with (○, n = 4) or without (●, n = 4, 1 mouse died 99 days after porcine BMT) subsequent injection of NOD/SCID-Tg mouse BMCs. (A) Percentages of antipig pan-tissue mAb-positive cells in recipient WBCs at 1 week before (6 weeks after porcine BMT), and weeks 2, 5, 8, and 12 after NOD/SCID-Tg mouse BMT. (B) Levels of porcine chimerism in recipient BM at week 12 after NOD/SCID-Tg mouse BMT. Percentages of antipig pan-tissue mAb+ (total), myeloid/SWC3 (74-22-15+), and c-kit+ (stained by pig SCF) cells are shown. Each symbol represents an individual animal.
Competitive advantage for murine hematopoiesis over porcine hematopoiesis in NOD/SCID-Tg mice.
Data are shown for porcine chimerism in porcine BM recipients with (○, n = 4) or without (●, n = 4, 1 mouse died 99 days after porcine BMT) subsequent injection of NOD/SCID-Tg mouse BMCs. (A) Percentages of antipig pan-tissue mAb-positive cells in recipient WBCs at 1 week before (6 weeks after porcine BMT), and weeks 2, 5, 8, and 12 after NOD/SCID-Tg mouse BMT. (B) Levels of porcine chimerism in recipient BM at week 12 after NOD/SCID-Tg mouse BMT. Percentages of antipig pan-tissue mAb+ (total), myeloid/SWC3 (74-22-15+), and c-kit+ (stained by pig SCF) cells are shown. Each symbol represents an individual animal.
Discussion
In this study we observed that murine T cells developing in porcine hematopoietic chimeras show specific nonresponsiveness to porcine donors, as evidenced by the acceptance of donor skin grafts and lack of antidonor MLR or antidonor IgG production. The capacity to respond to allogeneic antigens indicates that tolerance to the porcine donor has been achieved in these mice. This was further supported by the observation that T cells developing in similarly treated mice without porcine chimerism responded to both porcine donor and allogeneic antigens. It is not clear from the present study whether or not tolerance achieved in this model is swine leukocyte antigen allele specific, because xenoreactivity to a porcine third-party control was not assessed. The capacity of hematopoietic chimerism to induce T-cell tolerance results in large part from the ability of donor cells to induce intrathymic clonal deletion of maturing donor-reactive thymocytes,17,18,30,31 resulting in the generation of a T-cell repertoire that is tolerant of the hematopoietic cell donor. Elimination of antidonor swine responses of murine T cells developing in porcine hematopoietic chimeras suggests that the intrathymic clonal deletion of porcine donor-reactive murine T cells occurs in highly disparate xenogeneic species. Previous studies suggested that the repopulation of host thymus with donor antigen-expressing cells is critical for the deletion of donor-reactive T cells and the maintenance of stable tolerance in mixed chimeras.15-20 Thus, the host thymus-homing capacity of donor cells would be an important determinant for tolerance induction by mixed chimerism across discordant xenogeneic barriers. Although cross-species incompatibilities in adhesion molecules may limit the thymic homing of donor cells in discordant xenogeneic recipients,32-35 such defects have not been detected in the highly disparate pig-to-mouse combination. We have previously shown that long-term (> 20 weeks) repopulation of host thymus with porcine class II+ cells can be achieved in NOD/SCID-Tg mouse recipients of porcine BM (without subsequent mouse BMT).21 In the present study, thymus was prepared at week 24 or week 27 after porcine BMT and analyzed for porcine chimerism by immunohistochemical staining. Despite the fact that transplantation of mouse BMCs into porcine chimeras leads to chronic elimination of porcine chimerism through competition (Figure6), repopulation of host thymus with porcine class II+cells was maintained for over 24 weeks in 1 of 5 B10.D2/o-Tg mouse BMC-reconstituted porcine hematopoietic chimeras that accepted porcine donor skin grafts (data not shown).
To limit the potential for engraftment failure to interfere with the evaluation of the ability of porcine hematopoietic chimerism to induce tolerance in mice, we used a unique model, in which porcine BM recipients maintained immunodeficiency until stable porcine hematopoietic chimerism had been established. Thus, this study assessed only the ability of porcine hematopoietic chimerism to induce tolerance of newly developing, but not mature, murine T cells, and established the principle that such tolerance can be achieved. Studies are in progress to use immunocompetent porcine cytokine transgenic mice to determine what level of immunosuppression is required for induction of porcine hematopoietic chimerism and whether or not mixed chimerism can lead to tolerance in mice with incomplete immunosuppression. Because it is unknown whether it will be clinically feasible and necessary to completely suppress the host cellular and humoral immunity to the level of SCID mice in humans, these studies would be important in determining the applicability of mixed hematopoietic chimerism to clinical xenotransplantation.
Previous studies in a rat-to-mouse BMT model have shown that host hematopoietic stem cells have a competitive advantage that limits xenogeneic donor hematopoietic repopulation.36,37 Here we demonstrate that the competitive advantage for host hematopoiesis is even stronger in pig-to-mouse combination. Injection of immunodeficient NOD/SCID mouse BMCs into porcine hematopoietic chimeras led to complete eradication of porcine hematopoietic chimerism (Figure 6). The disadvantage for porcine hematopoiesis in mice is likely to be the cause for the loss of porcine hematopoietic chimerism in porcine hematopoietic chimeras following injection of B10.D2/o-Tg mouse BMCs, in which donor-specific tolerance was persistent. The maintenance of tolerance in these mice might be due to the persistence of “micro” hematopoietic chimerism. However, it is unclear if such tolerance would be permanently stable. Because active donor hematopoiesis is needed to provide a consistent supply of donor antigens to ensure the ongoing central deletion of donor-reactive T-cell clones,17development of strategies overcoming the nonimmunologic factors that limit donor hematopoiesis would be important for xenograft tolerance induction through hematopoietic chimerism. Incompatibilities between the donor and host in adhesion molecules38-40 and hematopoietic cytokines, which are required for adequate homing, stromal interaction, self-renewal, and differentiation of hematopoietic stem cells, are important nonimmune factors limiting donor hematopoietic engraftment in discordant xenogeneic species. Previous studies in both murine14,21 and primate12,41 42 models have shown that donor cytokines can improve the engraftment of porcine hematopoietic cells across highly disparate xenogeneic barriers. We have recently observed that the use of mAbs that specifically block the adhesion of host but not xenogeneic hematopoietic stem cells to BM stroma could overcome the competitive advantage of host hematopoiesis and thereby facilitate the induction of porcine hematopoietic chimerism in xenogeneic recipients (our unpublished data; April 2001).
In this report, we demonstrate that mixed chimerism induces donor-specific tolerance in a highly disparate xenogeneic combination, pig-to-mouse. We have recently observed that implantation of fetal human thymus and liver fragments into NOD/SCID-Tg mice with pre-established porcine chimerism led to the development of human T cells in these mice. Interestingly, human T cells generated in these chimeras did not reject porcine cells and stable triple hematopoietic chimerism (human/swine/mouse) was maintained until killed at week 20 after transplantation (our unpublished data, October 2000). Additional studies are clearly necessary, but these results suggest that induction of mixed chimerism may also overcome the rejection of porcine xenografts by human T cells.
The authors thank Drs Christene A. Huang and Ping Lan for helpful review of the manuscript, Dr David H. Sachs for his advice and helpful discussion, and Sharon Titus for her expert secretarial assistance.
Supported by Juvenile Diabetes Foundation International grant 1-1999-573.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yong-Guang Yang, Transplantation Biology Research Center, Massachusetts General Hospital, MGH East, Bldg 149-5202, 13th St, Boston, MA 02129; e-mail: yongguang.yang@tbrc.mgh.harvard.edu



![Fig. 3. Donor-specific skin graft prolongation in porcine hematopoietic chimeras reconstituted with T- and B-cell–depleted B10.D2/o-Tg mouse BMCs. / Survival is shown for skin grafts from the porcine BMC donor (solid line) and third-party B10.RIII mice (dashed line) in NOD/SCID-Tg mice receiving transplants with porcine cells only (A; n = 2), B10.D2/o-Tg mouse BMC-reconstituted NOD/SCID-Tg mice without pretransplantation of porcine cells (B; n = 2), and B10.D2/o-Tg mouse BMC-reconstituted NOD/SCID-Tg mice that had received porcine BMCs prior to murine BMC reconstitution (C; n = 11). Based on the level of porcine chimerism in the WBCs at 1 week prior to B10.D2/o-Tg mouse BMC reconstitution (6 weeks after porcine BMT), mice in panel C are divided into 2 groups: porcine hematopoietic chimeras (▪ with ≥ 1.5% [1.5%-33%] WBC chimerism; n = 8) and mice with poor porcine chimerism (■ with undetectable or < 1.5% WBC chimerism; n = 3). Porcine chimerism was determined by FACS analysis using an antipig pan-tissue mAb. Because the rejection of third-party skin grafts was virtually identical between porcine hematopoietic chimeras and mice with poor porcine chimerism, combined third-party rejection results for the 2 groups are shown (C;--▾--). Results from 2 similar experiments are combined.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/10/10.1182_blood.v99.10.3823/6/m_h81022575003.jpeg?Expires=1767729339&Signature=oWrcETbSuUvz6ArK2B~jw9q2dBOmssLhu~u21~unY4M88MVTkggHP7cw1sRZ~jxGoB0F03G8N-s8dJJBc3xRWUqlZT2z0HA-H-90kyd4FO-UE2sGs6cjkaQAURcG0wEY8691GU011HFndFe4NicpxTNWYm0x4xQ~cGDcn8-SRBrXwczbCaTo-8zSAzBSuh7C-BZpfn~serfNvn~2tzylmuSu-VloeytcPlYRwPimM3XAxI66qiiX7N8J3IYIxDX07nPJ4z8PZLoFFNy05DU4SWdBxJgHeK9HZE9E~ZjmQO-60gf91ORlSD1OGVreFcxjx2IIF3KvMlmTg9vJJNuHUA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal