Previously it was shown that β2-integrins are necessary for slow leukocyte rolling in inflamed venules. In this study, mice that are deficient for either one of the β2-integrins, αLβ2 (LFA-1) or αMβ2 (Mac-1), were used to determine which of the β2-integrins are responsible for slowing rolling leukocytes. The cremaster muscles of these mice were treated with tumor necrosis factor-α and prepared for intravital microscopy. The average rolling velocities in venules were elevated in LFA-1−/−mice (11.0 ± 0.7 μm/s) and Mac-1−/− mice (10.1 ± 1.1 μm/s) compared to wild-type mice (4.8 ± 0.3 μm/s;P < .05), but were lower than in CD18−/−mice (28.5 ± 2.1 μm/s). When both LFA-1 and Mac-1 were absent or blocked, rolling velocity became dependent on shear rate and approached that of CD18−/− mice. In addition, leukocyte adhesion efficiency was decreased in LFA-1−/− mice to near CD18−/− levels, but decreased only slightly in Mac-1−/− mice. Thus, both LFA-1 and Mac-1 contribute to slowing down rolling leukocytes, although LFA-1 is more important than Mac-1 in efficiently inducing firm adhesion.
Introduction
During an inflammatory response, neutrophils roll along the wall of inflamed venules before they come to a stop, adhere, and transmigrate. The original paradigm of leukocyte adhesion held that rolling and adhesion were separate, sequential steps.1However, recent work has shown that the β2-integrins and E-selectin cooperate in the conversion from rolling to firm adhesion.2-6 Efficient conversion from rolling to firm adhesion is dependent on the time a leukocyte spends in close contact with the endothelium.2 In mice lacking β2-integrins, the mean rolling velocity of leukocytes in tumor necrosis factor-α (TNF-α)–treated cremaster venules is elevated 3-fold over wild-type mice.2 Neutrophils in β2-integrin–deficient mice fail to slow down and arrest in response to TNF-α.5 As a leukocyte rolls, the interaction between selectins and their ligands7 and between chemokines presented on the endothelium8 and G-protein–coupled receptors on the leukocyte surface are thought to activate β2-integrins, enabling them to interact with their ligands.9,10 Ligation of G-protein–coupled receptors by chemokines or other chemoattractants triggers intracellular signaling events that result in integrin activation9-12 and may trigger a systematic slowing down of rolling leukocytes.5 13
Although it is known that the β2-integrins are necessary for slow rolling2,6 and aid in the leukocyte deceleration process,5 it is unknown which of the 4 β2-integrins is responsible. LFA-1 (CD11a/CD18) is the predominant β2-integrin on lymphocytes and neutrophils and is also expressed on other leukocytes. Mac-1 (CD11b/CD18) is exclusive to granulocytes and monocytes. Some p150,95 (CD11c/CD18) is also expressed on granulocytes and monocytes. CD11d/CD18, expressed predominantly on myelomonocytic cells,14 is probably not involved in neutrophil trafficking. Therefore, LFA-1 and Mac-1 are the most likely candidates that may be responsible for the slowing down of leukocytes on inflamed endothelia.
Both LFA-1 and Mac-1 are known to bind to a number of ligands. One LFA-1 ligand on endothelial cells is intracellular adhesion molecule-1 (ICAM-1).15 In addition, LFA-1 also binds to ICAM-2 on endothelial cells and platelets and ICAM-3 on lymphocytes.16 Mac-1 binds to ICAM-1, ICAM-2, and a number of other ligands, including iC3b, factor X, fibrinogen, and many denatured proteins.17,18 Although ICAM-1 is necessary for neutrophil adhesion to unstimulated endothelium, it is probably not significantly involved in either slow rolling3,19 or chemoattractant-induced firm adhesion of leukocytes in inflamed venules.20
Materials and methods
Mice
All mice appeared healthy in vivarium conditions. Experiments were conducted under a protocol approved by the University of Virginia institutional animal care and use committee.
Gene-targeted mice lacking CD1823 were obtained from Dr Arthur L. Beaudet (Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, TX). Gene-targeted mice lacking LFA-121 or Mac-122 were obtained from Dr Christie M. Ballantyne (Department of Medicine, Baylor College of Medicine, Houston, TX). All mice were back-crossed into the C57BL/6 strain for at least 7 generations. Wild-type C57BL/6 mice were from Hilltop Labs (Scottsdale, PA) or Jackson Laboratories (C57BL/6J) (Bar Harbor, ME). We used male mice with a mean age of 17 weeks and a mean weight of 27 g. Six groups of mice were studied: wild-type, CD18−/−, Mac-1−/− , LFA-1−/−, Mac-1−/− + LFA-1 monoclonal antibody (mAb), and LFA-1−/− + Mac-1 mAb (Table1).
Experimental groups and hemodynamic parameters
| Mouse type . | Number of mice . | Number of venules . | Number of cells . | ||
|---|---|---|---|---|---|
| 200-500 s−1 . | 500-1000 s−1 . | 200-500 s−1 . | 500-1000 s−1 . | ||
| Wild-type | 5 | 11 | 11 | 110 | 110 |
| CD18 −/− | 2 | 5 | 5 | 50 | 50 |
| Mac-1 −/− | 9 | 16 | 7 | 159 | 70 |
| Mac-1 −/− + TIB217 | 6 | 9 | 9 | 90 | 90 |
| LFA-1 −/− | 8 | 16 | 24 | 160 | 239 |
| LFA-1 −/− + M1/70 | 8 | 17 | 16 | 164 | 154 |
| Mouse type . | Number of mice . | Number of venules . | Number of cells . | ||
|---|---|---|---|---|---|
| 200-500 s−1 . | 500-1000 s−1 . | 200-500 s−1 . | 500-1000 s−1 . | ||
| Wild-type | 5 | 11 | 11 | 110 | 110 |
| CD18 −/− | 2 | 5 | 5 | 50 | 50 |
| Mac-1 −/− | 9 | 16 | 7 | 159 | 70 |
| Mac-1 −/− + TIB217 | 6 | 9 | 9 | 90 | 90 |
| LFA-1 −/− | 8 | 16 | 24 | 160 | 239 |
| LFA-1 −/− + M1/70 | 8 | 17 | 16 | 164 | 154 |
| Mouse type . | Average diameter (μm) . | Centerline blood flow velocity (mm/s) . | Wall shear rate (s−1) . | |||
|---|---|---|---|---|---|---|
| 200-500 s−1 . | 500-1000 s−1 . | 200-500 s−1 . | 500-1000 s−1 . | 200-500 s−1 . | 500-1000 s−1 . | |
| Wild-type | 59 ± 7 | 41 ± 5 | 2.1 ± 0.1 | 2.8 ± 0.4 | 408 ± 20 | 692 ± 30 |
| CD18 −/− | 47 ± 4 | 41 ± 6 | 1.5 ± 0.2 | 2.8 ± 0.4 | 330 ± 40 | 730 ± 30 |
| Mac-1 −/− | 38 ± 2 | 42 ± 4 | 1.4 ± 0.1 | 2.7 ± 0.3 | 390 ± 20 | 690 ± 50 |
| Mac-1 −/− + TIB217 | 52 ± 5 | 39 ± 3 | 1.9 ± 0.2 | 2.7 ± 0.2 | 390 ± 20 | 740 ± 40 |
| LFA-1 −/− | 44 ± 3 | 41 ± 3 | 1.4 ± 0.1 | 2.6 ± 0.2 | 340 ± 20 | 680 ± 30 |
| LFA-1 −/− + M1/70 | 41 ± 3 | 44 ± 2 | 1.3 ± 0.1 | 3.0 ± 0.2 | 350 ± 20 | 700 ± 30 |
| Mouse type . | Average diameter (μm) . | Centerline blood flow velocity (mm/s) . | Wall shear rate (s−1) . | |||
|---|---|---|---|---|---|---|
| 200-500 s−1 . | 500-1000 s−1 . | 200-500 s−1 . | 500-1000 s−1 . | 200-500 s−1 . | 500-1000 s−1 . | |
| Wild-type | 59 ± 7 | 41 ± 5 | 2.1 ± 0.1 | 2.8 ± 0.4 | 408 ± 20 | 692 ± 30 |
| CD18 −/− | 47 ± 4 | 41 ± 6 | 1.5 ± 0.2 | 2.8 ± 0.4 | 330 ± 40 | 730 ± 30 |
| Mac-1 −/− | 38 ± 2 | 42 ± 4 | 1.4 ± 0.1 | 2.7 ± 0.3 | 390 ± 20 | 690 ± 50 |
| Mac-1 −/− + TIB217 | 52 ± 5 | 39 ± 3 | 1.9 ± 0.2 | 2.7 ± 0.2 | 390 ± 20 | 740 ± 40 |
| LFA-1 −/− | 44 ± 3 | 41 ± 3 | 1.4 ± 0.1 | 2.6 ± 0.2 | 340 ± 20 | 680 ± 30 |
| LFA-1 −/− + M1/70 | 41 ± 3 | 44 ± 2 | 1.3 ± 0.1 | 3.0 ± 0.2 | 350 ± 20 | 700 ± 30 |
Data are presented as mean ± SEM.
Reagents
Murine recombinant TNF-α (0.5 μg/mouse) was obtained from R & D Systems (Minneapolis, MN). The blocking mAb M1/70, specific for the αM subunit of Mac-1 (rat IgG2b, 30 μg/mouse) was obtained from Pharmingen (San Diego, CA).24 The LFA-1 mAb TIB-217 (rat IgG2aκ, 30 μg/mouse) was purified at the Lymphocyte Culture Center at the University of Virginia from hybridoma supernatant (American Type Culture Collection, Rockville, MD).25
Intravital microscopy
Two hours before exteriorization of the cremaster muscle, all mice were injected intrascrotally with 0.5 μg TNF-α in 0.30 mL isotonic saline. Mice were anesthetized with ketamine hydrochloride (125 mg/kg; Sanofi Winthrop Pharmaceuticals, New York, NY), xylazine (12.5 mg/kg TranquiVed; Phoenix Scientific, St Joseph, MO), and atropine sulfate (0.025 mg/kg; Fujisawa USA, Deerfield, IL) intraperitoneally. Mice were kept at 37°C; trachea, jugular vein (for additional anesthetic), and one carotid artery (for blood sampling and mAb injections) were cannulated with polyethylene tubing (Becton Dickinson, Sparks, MD). After surgery, the cremaster muscle was prepared for intravital microscopy, the epididymis and testis were pinned to the side, and the cremaster was superfused with a thermocontrolled (37°C) bicarbonate-buffered saline (131.9 mM NaCl, 18 mM NaHCO3, 4.7 mM KCl, 2.0 mM CaCl2 · 2H2O, and 1.2 mM MgCl2) equilibrated with 5% CO2 in N2. All microscopic observations were made on a Zeiss intravital microscope (Axioskop, Carl Zeiss, Thornwood, NY), with a saline immersion objective (SW 40/0.75 numerical aperture). Venules between 20 and 90 μm were videotaped through a CCD camera system (model VE-1000CD, Dage-MTI, Michigan City, IN) for approximately 90 s/venule on a VHS recorder (Panasonic AG-W1) for off-line analysis of leukocyte rolling velocity and adhesion data. The vessel centerline blood velocity was measured using a dual photodiode and a digital on-line cross correlation program as previously described.26 Mean blood flow velocity, Vb, was approximated by multiplying the centerline blood velocity by a factor of 0.625.17 Wall shear rate, γw, was estimated asγw = 2.12 × 8 × [Vb/d], where d is the diameter of the vessel and 2.12 is a median empirical correction factor obtained from velocity profiles measured in microvessels in vivo.28 Systemic leukocyte counts were taken from 10 μL carotid blood samples throughout the experiment and stained in 90 mL Kimura stain (11 mL toludine blue, 0.8 mL 0.03% light green SF yellowish [Sigma, St Louis, MO], 0.5 mL saturated saponin [Sigma] in 50% ethanol, and 5 mL 1/15 M phosphate buffer, pH 6.4) in a hemocytometer to obtain absolute numbers of leukocytes per microliter and a 2-part differential count (Table2).
Total leukocyte counts after TNF-α and differential analysis
| Genotype . | Blood leukocyte concentration (cells/μL) . | % PMNs . | PMNs (fold over wild-type) . |
|---|---|---|---|
| Wild-type | 2 300 ± 600 | 45 | 1 |
| CD18−/− | 18 900 ± 1 200 | 70 | 13 |
| LFA-1−/− | 8 800 ± 1 100 | 61 | 5 |
| Mac-1−/− | 7 400 ± 1 500 | 62 | 4 |
| Genotype . | Blood leukocyte concentration (cells/μL) . | % PMNs . | PMNs (fold over wild-type) . |
|---|---|---|---|
| Wild-type | 2 300 ± 600 | 45 | 1 |
| CD18−/− | 18 900 ± 1 200 | 70 | 13 |
| LFA-1−/− | 8 800 ± 1 100 | 61 | 5 |
| Mac-1−/− | 7 400 ± 1 500 | 62 | 4 |
Data are presented as mean ± SEM.
Flow cytometry
Heparinized whole mouse blood was incubated with either LFA-1 mAb (M1/70) or Mac-1 mAb (TIB217) followed by a fluorescein isothiocyanate (FITC)–labeled secondary antibody (polyclonal antirat Ig, Pharmingen). Appropriate FITC-labeled isotype controls were used for each mAb (R35-95 for M1/70 and G15-337 for TIB217, both from Pharmingen).
Data analysis
Vessel diameter and length were measured using electronic calipers. Adherent leukocytes were defined being stationary for more than 30 seconds. Adhesion numbers are expressed per unit surface area of the vessel, assuming cylindrical geometry. In vessels larger than 40 μm, only one half of the vessel is in sharp focus, and the sampled surface area was approximated as a half-cylinder. Rolling velocities were measured for 10 leukocytes per vessel picked at random by viewing the translation during 2 seconds.
For each of the groups studied, mean leukocyte rolling velocity and the distribution of rolling velocities were determined. Quartile averages were calculated and compared. To study the effects of shear rate on rolling velocity and adhesion, individual venules for each group were stratified into 2 shear rate ranges, low (200-500 s−1) and high (500-1000 s−1). Peripheral leukocyte counts were determined and differentiated into polymorphonuclear cells (PMNs) and mononuclear cells. The mean was calculated for each group. Overall adhesion efficiency was determined by dividing the number of adherent leukocytes/mm2 by the number of peripheral leukocytes/μL as described.2 Statistical significance between groups was determined by a one-way ANOVA test followed by a t test at a significance level of P less than .05.
Results
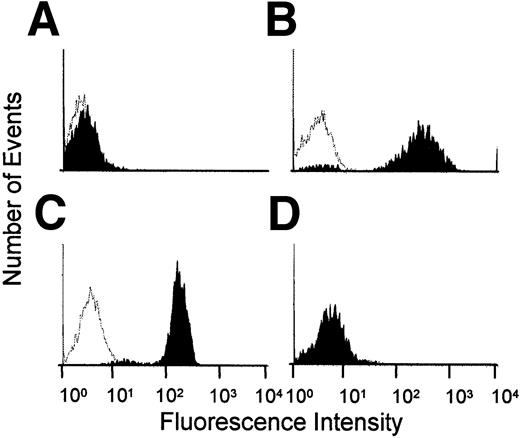
To confirm that LFA-1 and Mac-1 proteins were absent in LFA-1−/− and Mac-1−/− mice, respectively, we used flow cytometry. In Mac-1−/− mice, anti-LFA-1 mAb, TIB217, but not anti-Mac-1 mAb, M1/70 showed binding at the same level as in wild-type mice. Conversely, in LFA-1−/− mice, M1/70 showed binding at the same level as in wild-type, but TIB217 did not exhibit binding above isotype control (Figure1). Flow cytometry also showed that injecting TIB217 and M1/70 at 30 μg/mouse was sufficient to stain essentially all neutrophils.
Expression of Mac-1 and LFA-1 on neutrophils.
(A) LFA-1−/− mouse stained with LFA-1 mAb, TIB217. (B) LFA-1−/− mouse stained with Mac-1 mAb, M1/70. (C) Mac-1−/− mouse stained with TIB217. (D) Mac-1−/− mouse stained with M1/70. Clear areas represent background (isotype control).
Expression of Mac-1 and LFA-1 on neutrophils.
(A) LFA-1−/− mouse stained with LFA-1 mAb, TIB217. (B) LFA-1−/− mouse stained with Mac-1 mAb, M1/70. (C) Mac-1−/− mouse stained with TIB217. (D) Mac-1−/− mouse stained with M1/70. Clear areas represent background (isotype control).
Rolling velocity distribution
To determine whether LFA-1 and/or Mac-1 modulated leukocyte rolling velocity, rolling velocity was measured in each of the experimental groups after 2 hours of stimulation with TNF-α (Figure2). Leukocytes in wild-type mouse venules treated with TNF-α rolled at an average velocity of 4.8 ± 0.3 μm/s. Leukocytes in CD18−/− venules rolled significantly faster than wild-type, with an average rolling velocity of 28.5 ± 2.1 μm/s, confirming a key role for β2-integrins in slow rolling.2 5
Rolling velocity histograms.
(A) Wild-type, n = 220; (B) Mac-1−/−, n = 229; (C) LFA-1−/−, n = 399; and (D) CD18−/−, n = 100 mice. Not stratified by wall shear rate. Arrows indicate mean value.
Rolling velocity histograms.
(A) Wild-type, n = 220; (B) Mac-1−/−, n = 229; (C) LFA-1−/−, n = 399; and (D) CD18−/−, n = 100 mice. Not stratified by wall shear rate. Arrows indicate mean value.
To discern which of the β2-integrins was responsible for slowing down rolling leukocytes, the rolling velocities of mice lacking either Mac-1 or LFA-1 were investigated. The average rolling velocity in cremaster venules of Mac-1−/− mice was 10.1 ± 1.1 μm/s, while it was 11.0 ± 0.7 μm/s in LFA-1−/−mice. Both of these values are significantly higher than those in wild-type mice and significantly lower than those in CD18−/− mice. Based on the partial effect of either integrin on rolling velocity, we hypothesized that Mac-1 and LFA-1 may act cooperatively to modulate leukocyte rolling in inflammation. When Mac-1−/− mice were treated with a mAb to LFA-1 (TIB217), average rolling velocity increased to 20.7 ± 1.5 μm/s (significantly higher than untreated Mac-1−/− mice,P < .05). Similarly, when LFA-1−/− mice were treated with a mAb to Mac-1 (M1/70), rolling velocity increased to 15.4 ± 0.7 μm/s (significantly higher than untreated LFA-1−/− mice, P < .05). These data suggest that both LFA-1 and Mac-1 contribute to slow rolling in an additive fashion.
Influence of wall shear rate
To determine if shear rate had an effect on rolling velocity, rolling velocity data were stratified by shear rate into 2 groups, low shear rate (200-500 s−1) and high shear rate (500-1000 s−1) (Figure 3). Cumulative frequency histograms for all experimental groups were created to observe the dependence of rolling velocity on wall shear rate. In wild-type mice, the median rolling velocity did not differ from the low shear rate range (3.9 μm/s) to the high shear rate range (3.5 μm/s), suggesting that there is little dependence of rolling velocity on shear rate in these mice. In CD18−/− mice, rolling velocity increased significantly (P < .05) from the low shear rate range to the high shear rate range, almost doubling the median rolling velocity from 17.3 μm/s to 33.4 μm/s. The median rolling velocity in Mac-1−/− mice increased from 4.7 μm/s in the low shear rate range to 8.5 μm/s in the high shear rate range. In LFA-1−/− mice, rolling velocities increased from 5.7 μm/s in the low shear rate range to 6.5 μm/s in the high shear rate range. At low shear rates (200-500 s−1), rolling velocities in LFA-1−/− mice averaged 9.3 ± 0.9 μm/s (Figure 3E) and were significantly higher than the rolling velocity in Mac-1−/− mice (6.8 ± 0.8 μm/s, Figure 3C).
Cumulative frequency rolling velocity histograms.
(A) Wild-type, (B) CD18−/−, (C) Mac-1−/− , (D) Mac-1−/− + mAb to LFA-1, (E) LFA-1−/−, and (F) LFA-1−/− + mAb to Mac-1. Open circles represent leukocytes in the low shear rate range (200-500 s−1), and closed triangles represent leukocytes in the high shear rate range (500-1000 s−1). Table 1 lists numbers of mice, venules, and cells.
Cumulative frequency rolling velocity histograms.
(A) Wild-type, (B) CD18−/−, (C) Mac-1−/− , (D) Mac-1−/− + mAb to LFA-1, (E) LFA-1−/−, and (F) LFA-1−/− + mAb to Mac-1. Open circles represent leukocytes in the low shear rate range (200-500 s−1), and closed triangles represent leukocytes in the high shear rate range (500-1000 s−1). Table 1 lists numbers of mice, venules, and cells.
To investigate the cooperative effect of Mac-1 and LFA-1 on shear rate-dependent rolling velocity, the rolling velocity was measured in venules of Mac-1−/− mice treated with mAb TIB217 and of LFA-1−/− mice treated with mAb M1/70. In Mac-1−/− + TIB217 mice, rolling velocity significantly increased from 7.5 μm/s in the low shear rate range to 21.8 μm/s in the high shear rate range (P < .05). Similarly, in LFA-1−/− treated with mAb M1/70 mice, rolling velocity more than doubled from 7.8 μm/s in the low shear rate range to 19.7 μm/s in the high shear rate range. These data show that rolling velocity is largely independent of wall shear rate in wild-type mice and only slightly shear rate dependent in LFA-1−/− mice and Mac-1−/− mice, but becomes shear rate dependent in CD18−/− mice or in mice lacking both LFA-1 and Mac-1 function.
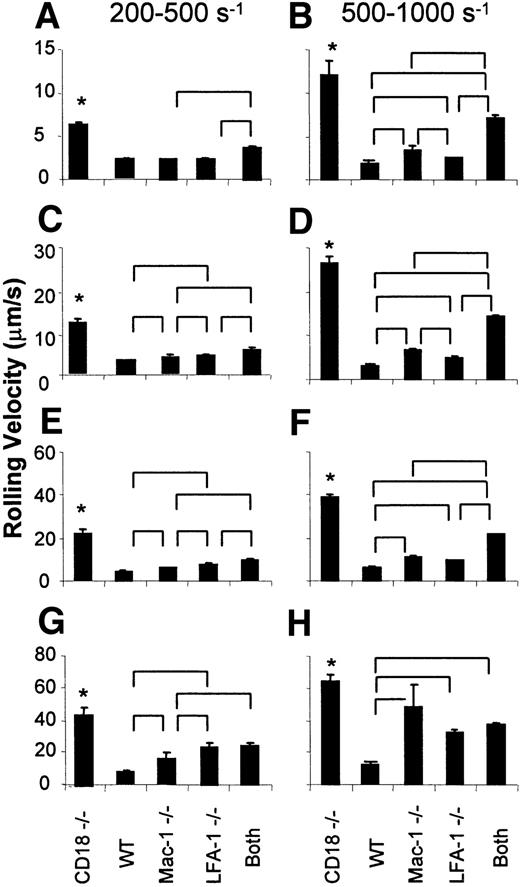
To statistically analyze rolling velocities in more detail, averages were calculated for the lowest, second, third, and highest quartiles for both the low and higher shear rate venules (Figure4). In all quartiles and both shear rate ranges, the rolling velocity was highest in CD18−/− mice. At low shear rate, the lowest quartile of rolling cells in LFA-1−/− and in Mac-1−/− mice were not faster than in wild-type mice. However, at low shear rates, the highest 3 quartiles of leukocytes in LFA-1−/− mice rolled significantly faster than in Mac-1−/− mice. In the third quartile, the means ± SEM were 6.1 ± 0.2 μm/s and 7.4 ± 0.3 μm/s for Mac-1−/− and LFA-1−/−, respectively. The difference was even larger in the fourth quartile, with 15.1 ± 3.1 and 23.4 ± 2.4 μm/s, respectively. Therefore, the fastest-rolling leukocytes in low shear venules rolled significantly faster in LFA-1−/− than Mac-1−/− mice. In the venules with higher shear rate (500-1000 s−1), leukocytes in all quartiles rolled significantly faster in LFA-1−/− and Mac-1−/− mice than in wild-type mice. In the lower 3 quartiles, blocking LFA-1 in Mac-1−/− mice or Mac-1 in LFA-1−/− mice had an additional effect beyond the absence of each single adhesion molecule, but in the fastest-rolling cells (fourth quartile), it was sufficient to remove either LFA-1 or Mac-1 to produce rolling at a velocity similar to that seen with additional antibody blockade. In the lower 2 quartiles, leukocyte rolling was significantly faster in Mac-1−/− than LFA-1−/− mice, suggesting that Mac-1 is necessary to stabilize the velocity of slow-rolling leukocytes in venules with high shear rate. Taken together, these data suggest that LFA-1 appears to be more important than Mac-1 in stabilizing the velocities of fast-rolling leukocytes in low shear venules, and Mac-1 appears to dominate in stabilizing the velocity of slow-rolling leukocytes in high shear venules.
Quartile averages.
Panels A and B represent the first quartile for the low shear rate range (200-500 s−1) and high shear rate range (500-1000 s−1), respectively. Similarly, panels C and D represent the second quartile, E and F represent the third quartile, and G and H represent the fourth quartile for the low and high shear rate ranges, respectively. “Both” indicates Mac-1−/−mice + LFA-1 mAb or LFA-1−/− mice + Mac-1 mAb (pooled). Data presented as mean ± SEM. In both shear rate groups, rolling velocity in CD18−/− mice was significantly higher than in all other groups (*). Other significant differences among groups are indicated by brackets above bars. WT indicates wild-type mice.
Quartile averages.
Panels A and B represent the first quartile for the low shear rate range (200-500 s−1) and high shear rate range (500-1000 s−1), respectively. Similarly, panels C and D represent the second quartile, E and F represent the third quartile, and G and H represent the fourth quartile for the low and high shear rate ranges, respectively. “Both” indicates Mac-1−/−mice + LFA-1 mAb or LFA-1−/− mice + Mac-1 mAb (pooled). Data presented as mean ± SEM. In both shear rate groups, rolling velocity in CD18−/− mice was significantly higher than in all other groups (*). Other significant differences among groups are indicated by brackets above bars. WT indicates wild-type mice.
Role of LFA-1 and Mac-1 in leukocyte adhesion
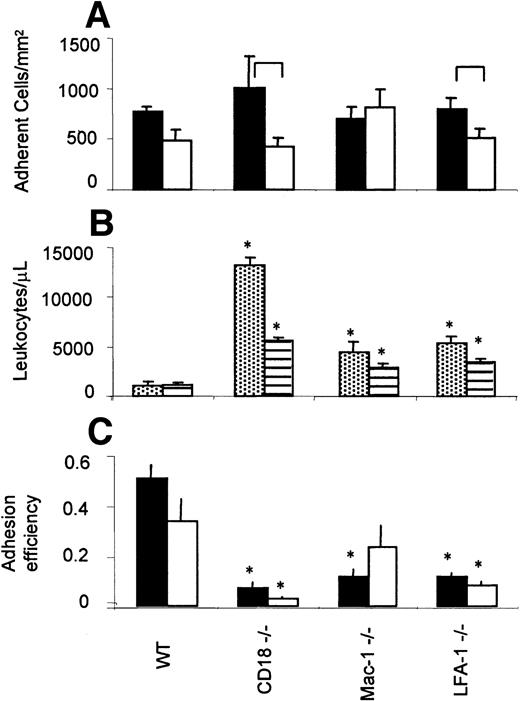
To assess the role of the β2-integrins on adhesion, the number of adherent cells (stationary > 30 seconds) was determined in all venules, stratified by shear rate into low (200-500 s−1) and high (500-1000 s−1) groups. In CD18−/− mice, adhesion was influenced by shear rate, decreasing from 1009 ± 311 adherent leukocytes/mm2 in the low shear group to 422 ± 86 adherent leukocytes/mm2in the high shear group. In wild-type mice, adhesion tended to decrease from 770 ± 45 adherent leukocytes/mm2 in the low shear group to 486 ± 104 adherent leukocytes/mm2 in the high shear group, but the difference was not statistically significant. Interestingly, the absolute numbers of adherent leukocytes were not different among the groups tested, including CD18−/−mice. This is consistent with previous findings in that mouse2 and suggests that mice lacking β2-integrins compensate for the severe adhesion defect by increased systemic leukocyte counts.
Leukocyte adhesion efficiency
The efficiency of neutrophil adhesion can be expressed as a ratio of the number of leukocytes available to adhere and those that do adhere.2 Consistent with previous reports,21-23 CD18−/− mice showed a nearly 13-fold increase in circulating neutrophils compared to wild-type, LFA-1−/− mice showed almost a 5-fold increase, and Mac-1−/− showed a 4-fold increase (Figure5B). Overall adhesion efficiency was calculated by dividing the number of adherent by the number of circulating neutrophils.2 We found that adhesion efficiency was markedly and significantly decreased in CD18−/− mice as compared to wild-type mice. A similar significant decrease is seen in the LFA-1−/− mice. In Mac-1−/− mice, adhesion efficiency was significantly decreased in venules with low wall shear rate only (Figure 5C). Taken together, these data show that the adhesion impairment is more severe in LFA-1−/− than in Mac-1−/− mice, suggesting that LFA-1 is more important for leukocyte arrest than Mac-1.
Adhesion efficiency.
(A) Number of adherent (> 30 seconds) leukocytes/mm2. Closed bars indicate low shear venules (200-500 s−1); open bars, high shear venules (500-1000 s−1). (B) Systemic leukocyte counts measured in TNF-α–treated mice. Dotted, PMN, striped, mononuclear cells. (C) Overall adhesion efficiency. The number of adhered leukocytes/mm2 was normalized by the systemic leukocyte counts to determine overall adhesion efficiency. Closed bars represent low shear rate range (200-500 s−1); open bars represent high shear rate range (500-1000 s−1). Data presented as mean ± SEM. Significant differences between shear rate groups indicated by brackets in panel A and differences from wild-type mice indicated by asterisk in panels B and C (P < .05). Table 1 lists numbers of mice and venules.
Adhesion efficiency.
(A) Number of adherent (> 30 seconds) leukocytes/mm2. Closed bars indicate low shear venules (200-500 s−1); open bars, high shear venules (500-1000 s−1). (B) Systemic leukocyte counts measured in TNF-α–treated mice. Dotted, PMN, striped, mononuclear cells. (C) Overall adhesion efficiency. The number of adhered leukocytes/mm2 was normalized by the systemic leukocyte counts to determine overall adhesion efficiency. Closed bars represent low shear rate range (200-500 s−1); open bars represent high shear rate range (500-1000 s−1). Data presented as mean ± SEM. Significant differences between shear rate groups indicated by brackets in panel A and differences from wild-type mice indicated by asterisk in panels B and C (P < .05). Table 1 lists numbers of mice and venules.
Discussion
This study shows that removing either Mac-1 or LFA-1 from mice leads to significantly increased average leukocyte rolling velocities in inflamed venules, but these velocities were still significantly lower than in CD18−/− mice. The rolling velocity increase was due mostly to elevated rolling velocity of the fastest-rolling leukocytes (upper quartile). When LFA-1 was blocked in Mac-1−/− mice or when Mac-1 was blocked in LFA-1−/− mice, leukocyte rolling velocities increased further, but did not quite reach the velocity seen in CD18−/− mice. Leukocyte rolling velocity increased with increasing wall shear rate in CD18−/− mice, but remained unchanged in wild-type mice. Similarly, wall shear rate had little effect on leukocyte rolling velocity in Mac-1−/− mice or LFA-1−/− mice alone. However, when LFA-1 was blocked in Mac-1−/− mice or when Mac-1 was blocked in LFA-1−/− mice, leukocyte rolling velocity became dependent on wall shear rate, approaching the dependence seen in CD18−/− mice.
Obviously, both Mac-1 and LFA-1 are important to slow down rolling leukocytes. However, in the low shear rate range, LFA-1 is more important to slow down the fastest rolling leukocytes, as additionally blocking Mac-1 in LFA-1−/− mice has no effect on rolling velocity for the fastest rolling leukocytes. In the high shear rate range, both Mac-1 and LFA-1 have an equally important role in stabilizing the fastest rolling cells as shown by a similar increase of rolling velocity.
These results show that Mac-1 and LFA-1 work cooperatively to slow down rolling leukocytes. Although many studies have examined the relative roles of these molecules in the later stages of inflammation, namely, firm adhesion and transmigration, this is the first study to elucidate the roles of Mac-1 and LFA-1 in leukocyte rolling. In previous work from our laboratory, we have shown that CD18 integrins are important for slow rolling2 and the conversion of leukocytes from rolling to adhesion.5 To address this conversion, we determined the impact of the absence of LFA-1 or Mac-1 on firm leukocyte adhesion. These data suggest that LFA-1 is more important than Mac-1 for inducing firm adhesion, although both LFA-1 and Mac-1 are involved in slow rolling. This is consistent with a recent study that determined the relative roles of Mac-1 and LFA-1 in adhesion to ICAM-1. Neutrophils were preincubated with saturating concentrations of anti–LFA-1 or anti–Mac-1 mAbs or both and then allowed to interact with ICAM-1–expressing cells in a cone-plate viscometer. LFA-1 accounted for the majority of cell adhesion capture under shear conditions, whereas Mac-1 supported stable adhesion over several minutes of chemotactic stimulation, suggesting that LFA-1 and Mac-1 may serve sequential rather than parallel functions.29
During an inflammatory response, mAbs to LFA-1 effectively inhibit adhesion of PMNs to endothelial cells in culture that express ligands for LFA-1.30 In an in vitro static adhesion study with neutrophils from Mac-1−/− mice, LFA-1−/− mice, and CD18−/− mice, stimulated neutrophils from these mice were allowed to adhere to ICAM-1 and to endothelial cells. On both substrates, LFA-1−/−neutrophils showed decreased adhesion as compared to wild-type neutrophils, similar to CD18−/− neutrophils. Mac-1−/− neutrophils exhibited slightly increased adhesion as compared to LFA-1−/− neutrophils and CD18−/− neutrophils, but this adhesion did not reach wild-type levels. In addition, LFA-1−/− neutrophils adhering to purified ICAM-1 showed decreased adhesion as compared to LFA-1−/− neutrophils adhering to endothelial cells, suggesting that there is a ligand other than ICAM-1 for LFA-1 on endothelial cells.21 This is also suggested by a recent in vivo study where blocking ICAM-1 had no effect on chemoattractant-induced leukocyte adhesion to inflamed venules.20
Previous studies have suggested that adhesion via LFA-1 and Mac-1 proceed through independent pathways. Chimeras with extracellular and transmembrane regions of the α chain of Mac-1 linked to the cytoplasmic domain of the α chain of LFA-1 or vice versa, showed that the cytoplasmic domains were responsible for different modes of activation. The cytoplasmic domain of Mac-1 was found to induce a sustained adhesion whereas LFA-1 triggered a rapid but transient activation.11 In another study, Neelamegham and coworkers used a cone-plate viscometer to show a more rapid time course for adhesion mediated by LFA-1 than Mac-1.31 Our data suggest that rapid activation of LFA-1 may support more efficient adhesion in inflamed venules in vivo. In interpreting these data, it is important to recognize that secondary changes occur in adhesion molecule-deficient mice that lead to increased leukocyte numbers and possibly other changes.
In conclusion, either Mac-1 alone or LFA-1 alone is sufficient to slow down rolling leukocytes in TNF-α–treated cremaster venules. Rolling velocity increases and becomes shear rate-dependent when both molecules are absent or blocked. The striking defect in adherent leukocytes in LFA-1−/− mice but not Mac-1−/− mice at higher shear rates suggests that firm adhesion in TNF-α–treated venules requires LFA-1, although the slowing down of rolling leukocytes can be achieved by either Mac-1 or LFA-1.
We thank Nick Douris, Jennifer Bryant, and Michele Kirkpatrick for animal husbandry. We would also like to thank Jim White for genotyping.
Supported by National Institutes of Health grants HL-54136 (K.L.), HL-42550 and HL-62243 (C.M.B.), and AI-32177 (A.L.B.). J.L.D. is supported by National Institutes of Health Training Grant T32 HL-07284 to B. R. Duling.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Klaus Ley, Department of Biomedical Engineering, University of Virginia, PO Box 800759, Charlottesville, VA 22908; e-mail: klausley@virginia.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal