Abstract
B-cell chronic lymphocytic leukemia (B-CLL) follows heterogeneous clinical courses, and several biological parameters need to be added to the current clinical staging systems to predict which patients will experience an indolent or an aggressive outcome. This study analyzed CD38 expression by flow cytometry and soluble APO1/Fas (sAPO1/Fas), Bcl-2 (sBcl-2), and CD23 (sCD23) proteins by immunoenzymatic methods to evaluate their effect on the clinical course of 168 unselected B-CLL patients. Intermediate/high risk modified Rai stages were characterized by a higher CD38+ B-cell number (P = .0002) and higher sCD23 levels (P < .0001). Moreover, CD38+ B-cell percentages were significantly and directly associated both with β2-microglobulin and sCD23 concentrations (P < .0001 and P = .002, respectively). Both a higher tumor burden (lymphadenopathy/splenomegaly) and a lymphocyte doubling time less than 12 months were significantly associated with higher CD38+ percentages (P < .0001 and P = .0001, respectively). With regard to clinical outcome, progression-free survival was significantly longer (75% versus 37% at 5 years; P = .00006) in patients with lower CD38+ B-cell percentages. Furthermore, the risk of partial or no response to fludarabine increased with increasing CD38 expression (P = .003), and a shorter overall survival (50% versus 92% at 8 years; P < .00001) characterized patients with more than 30% CD38+ B-cell number. The predictive value of CD38 expression was maintained among the patients within the Rai intermediate risk group and was confirmed in multivariate analysis. Thus, the percentage of CD38+ B cells appears to be an accurate predictor of clinical outcome and therefore could be used to indicate when more novel chemotherapeutic approaches are needed.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is the most common leukemia in the Western world,1 characterized by the relentless accumulation of monoclonal B cells typically positive for CD5, CD23, and CD19 and negative for surface CD22 and FMC7.2 These cells overexpress the Bcl-2 gene product and are resistant to apoptosis; however, examining the relative levels of this anti-apoptotic protein has not been particularly helpful in predicting clinical outcome.3,4 The clinical course of patients with B-CLL can be quite variable, with many patients surviving for prolonged periods without any therapy, whereas others succumb rapidly despite aggressive treatment.5 Although the 2 major staging systems have provided valuable information in addressing this clinical heterogeneity,6,7 they have been unable to predict an indolent or aggressive course within the intermediate risk category. For this reason, several parameters such as lymphocyte doubling time (LDT),8 serum levels of β2-microglobulin,9 soluble CD23 (sCD23),10 serum thymidine kinase levels,11bone marrow histology,12 and cytogenetic abnormalities13 have been added to the current staging systems to differentiate prognostic subsets.
Despite having several characteristics of naive B cells, such as sequences of VH genes in germline configuration (unmutated), B-CLL cells have been shown to have somatically mutated immunoglobulin variable region genes in at least half of the cases, indicating that the cell of origin has passed through the germinal center (GC). Recent data on VH and VLsequencing suggest that approximately 60% of B-CLL cases can be considered post-GC memory B cells; thus B-CLL patients can be divided into 2 subgroups according to the mutational status of the immunoglobulin V gene.14 Along this line, Damle et al15 demonstrated that the percentages of CD38+ B-CLL cells in the unmutated and mutated groups were significantly different (means, 63.9% versus 7.3%, respectively;P = .00001). The positive predictive value of more than 30% CD38+ B cells, indicative of the unmutated genotype, was between 95% and 100%; conversely, the predictive value of less than 30% CD38+ B cells, indicating the mutated genotype, was 82%. Importantly, both the unmutated and the more than 30% CD38+ groups were characterized by a poor response to chemotherapy and a shorter survival.15 16
In this study we investigated whether a multiparametric determination of CD38 antigen by flow cytometry in a large cohort of unselected B-CLL patients has a predictive value in clinical course and outcomes. If so, then analysis of CD38 antigen might be added to current staging systems because of its easy determination in clinical laboratories and its strong value as a prognostic determinant of poor response to conventional treatment.
Patients, materials, and methods
Patients
One hundred sixty-eight consecutive and unselected B-CLL patients were enrolled in this study, from 1989 to 1999, all fulfilling the recommended diagnostic criteria with dim surface immunoglobulins and CD5+/CD23+ immunologic pattern. There were 94 men and 74 women with a median age of 64.2 years (range, 37 to 84). The median age of the more than 30% CD38+ (median, 63; range, 37-81) and less than 30% CD38+ (median, 64.5; range, 43-84) patients was similar. Fresh or cryopreserved B-CLL cells were available for CD38 marker analysis in 168 patients. Cell lysates were used for soluble Bcl-2 (sBcl-2) in 151 cases. Serum samples were obtained for sCD23 and soluble APO1/Fas (sAPO1/Fas) from 167 and 161 patients, respectively. All the samples were collected on a single day for each patient and were evaluated at initial diagnosis before any chemotherapeutic approach. We tried to set empirically various cut-off points for each biological variable (sCD23, sBcl-2, sAPO1/Fas, and β2-microglobulin), and the selected thresholds were sufficient to severely affect progression and survival, identifying accurately patients at poor prognosis. Moreover, we applied a discriminant function analysis based on the squared Mahalanobis distances of each case from its group centroids (β2-microglobulin, sBcl-2, CD38, sCD23, and sAPO1/Fas) to verify the selected cut points. The percentage correct (observed classifications versus predicted classifications) was more than 90% for all variables. Forty-five patients had low-modified Rai stage, 116 had intermediate stage, and 7 had high stage. Because of the very low number of patients belonging to the high-modified Rai stage, we pooled intermediate and high-modified Rai stages for statistical evaluations. Ninety-six of 168 patients received chemotherapy for their disease. Of the 96 treated patients, 48 were intermittently treated with a combination of chlorambucil at conventional doses and prednisone. The remaining 48 patients received and completed 6 courses of fludarabine monophosphate (Fludara; Schering AG, Berlin, Germany) at 25 mg/m2/d for 5 days.
Cellular immunophenotypic analysis
The following antibody conjugates were used: anti-CD23–phycoerythrin (PE), anti-CD5–fluorescein isothiocyanate (FITC), anti-CD38–PE, anti-CD19–allophycocyanin (APC), anti-CD45–FITC, and anti-CD14–PE, anti-CD95–PE, anti-CD10–FITC (all from Becton Dickinson Immunocytometry Systems, San Jose, CA). Peripheral blood mononuclear cells, separated from heparinized venous blood by density gradient centrifugation (Histopaque-1077; Sigma Diagnostics, St Louis, MO), were analyzed for surface expression of CD19/CD5/CD38 and CD19/CD5/CD23 by triple-color immunofluorescence. Isotype-matched negative controls were used in all the assays to determine positive from negative results. Flow cytometric analyses were performed on a FACS Calibur flow cytometer (Becton Dickinson Immunocytometry Systems) equipped with argon and red diode lasers. The CellQuest software system (Becton Dickinson Immunocytometry Systems) was used to acquire and to analyze data.
Bcl-2 enzyme-linked immunosorbent assay
Peripheral blood mononuclear cells were separated by density gradient centrifugation, and CD19+ cells were highly purified by positive selection, using both CD19 Microbeads (Miltenyi Biotec, Bergish Gladbach, Germany) and the Variomacs magnetic cell separator, according to the manufacturer's instructions. This system achieved enriched CD19+ populations (92% ± 5%), as demonstrated by restaining CD19+ cells with anti-CD19–PE monoclonal antibody (Becton Dickinson Immunocytometry Systems). Cell suspension densities were adjusted to 2.5 × 106 ± 0.5 × 106 cells/mL, as recommended by the manufacturer (Endogen Human Bcl-2 ELISA; Endogen, Woburn, MA). Therefore, 250 μL of each sample was combined with 50 μL Lysis Reagent (Endogen, Woburn, MA) and then frozen at −70°C. Before running the assay, lysate samples were brought gently to room temperature and diluted 1:10 by using the sample diluent provided. The Bcl-2 enzyme-linked immunosorbent assay (ELISA) procedure was performed according to the manufacturer's instructions, and the results were given as units per milliliter, taking into account that sBcl-2 levels in whole blood lysates collected from 20 apparently healthy individuals were all under 50 U/mL. The threshold of positivity was set at the sBcl-2 median value of more than 240 U/mL.
Cytoscreen human APO-1/Fas (CD95) ELISA immunoassay
This solid-phase sandwich ELISA was performed on serum samples frozen at −70°C and then brought to room temperature, according to the manufacturer's instructions (Biosource International, Camarillo, CA). The normal human APO-1/Fas values ranged from 3.3 to 7 ng/mL. Values higher than 7 ng/mL were considered positive.
Soluble CD23 immunoenzymometric assay
The procedure was performed according to the manufacturer's instructions (Biosource Europe, Nivelles, Belgium) for the quantitative determination of heterogeneous bioactive fragments (sCD23) derived from the cleavage of the CD23 membrane molecule. As reference interval, the mean of 118 normal plasma samples was 1.3 U/mL (SD = 0.6), ranging between 0.15 U/mL and 3.3 U/mL. The threshold of positivity was set at the sCD23 value of more than 60 U/mL.
Statistical methods
Study statistical power.
Because we observed 4 deaths in the group with low CD38 and 14 deaths in the group with high CD38, for an α level (2-sided) = 0.05, our study identified the following mortality hazard ratios for the overall survival at 5 years: 3 with a power 1-β = 0.49, 4 with a power 1-β = 0.69, 5 with a power 1-β = 0.81, 6 with a power 1-β = 0.88, and 7 with a power 1-β = 0.92.17
Data analysis.
All statistical analyses were performed at the end of data collection. Associations between the CD38 percentages and some clinical variables (modified Rai stages and response to fludarabine) were assessed by the Mann-Whitney test and the proportional odds ordered logistic regression model for continuous and categorical variables, respectively. To quantify the degree of association between CD38 percentages and other biological variables, such as sCD23, sBcl-2, sAPO1/Fas, and β2-microglobulin, the Spearman coefficient was calculated. Correlations between lymphadenopathy/splenomegaly or LDT and the CD38 percentages or the sAPO1/Fas levels were based on the two-tailed Fisher exact test. The assessment of response was based on the National Cancer Institute Working Group criteria.18Progression-free survival and overall survival, measured from the diagnosis, were estimated by the Kaplan-Meier method and compared by the log-rank test. Logistic regression model and Cox proportional hazards regression model were used to assess the independent effect of covariables, treated as dichotomous, on the complete remission (CR) and on the overall survival.
Results
Characteristics of CD38+ B cells in B-CLL
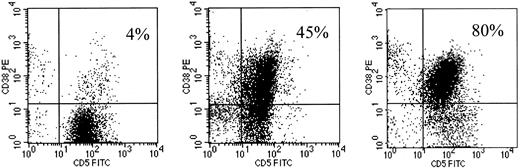
CD38 antigen showed variable patterns of fluorescence intensity and percentages on CD19+CD5+ B cells (Figure1). When the percentages of CD38+ B-CLL cells and the modified Rai stages were represented as a biparametric dot-plot (Figure2), 2 distinct sets could be separated, empirically based on the observed distributions, 1 with more than 30% CD38+ cells (CD38+) and the other with less than 30% CD38+ cells (CD38−). Interestingly, more than 30% CD95 and CD10 expression was observed in 5 of 50 (10%) and in 1 of 50 (2%) CD38+ patients, respectively, indicating that these molecules usually found on GC B cells are consistently low in the CD38+ B-CLL subset. Immunoglobulin M (IgM) mean fluorescence intensity expression detected on the CD19+ B cells was significantly and directly correlated with the CD38 percentages (Spearman test; r = 0.20;P = .025). The Spearman correlation between the percentages of CD38+ cells and the serum levels of β2-microglobulin or sCD23 was r = 0.49 (P < .0001) and r = 0.24 (P = .002), indicating a moderate and a low direct relationship, respectively. On the contrary, no significant correlation was found between the CD38+ percentages and the sAPO1/Fas or sBcl-2 levels (r = 0.13; P = .089 andr = −0.019; P = .81, respectively).
Flow cytometric profiles of CD38 expression on 3 CD5+CD19+ B-CLL cases.
B-CLL cases were analyzed by flow cytometry after exposure to anti-CD19–APC, anti-CD5–FITC, and anti-CD38–PE. The events represented were obtained by gating on cells expressing CD19. The variable percentages of CD5+CD38+ cells are reported on the upper-right quadrant region of each dot-plot.
Flow cytometric profiles of CD38 expression on 3 CD5+CD19+ B-CLL cases.
B-CLL cases were analyzed by flow cytometry after exposure to anti-CD19–APC, anti-CD5–FITC, and anti-CD38–PE. The events represented were obtained by gating on cells expressing CD19. The variable percentages of CD5+CD38+ cells are reported on the upper-right quadrant region of each dot-plot.
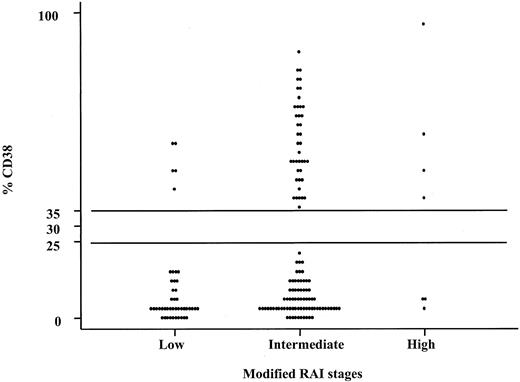
Percentages of CD38+CD19+ cells among the modified Rai stages.
Note that there was a clear distinction between the cases that had more than 30% CD38+ B-CLL cells and those expressing low numbers (< 30%) of CD38+ B-CLL cells within each modified Rai stage (low, intermediate, and high). These comparisons were statistically significant (P = .0002; Mann-Whitney test).
Percentages of CD38+CD19+ cells among the modified Rai stages.
Note that there was a clear distinction between the cases that had more than 30% CD38+ B-CLL cells and those expressing low numbers (< 30%) of CD38+ B-CLL cells within each modified Rai stage (low, intermediate, and high). These comparisons were statistically significant (P = .0002; Mann-Whitney test).
Clinical course and outcome of B-CLL cases
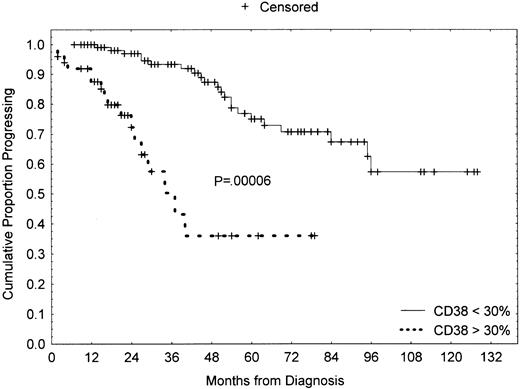
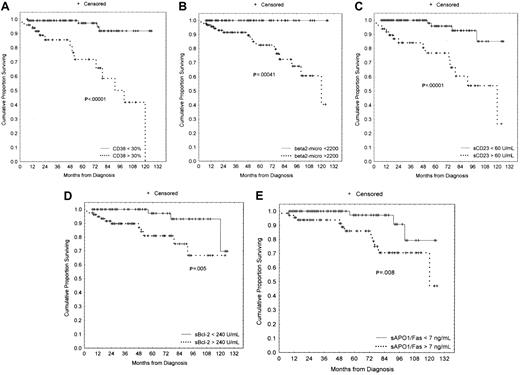
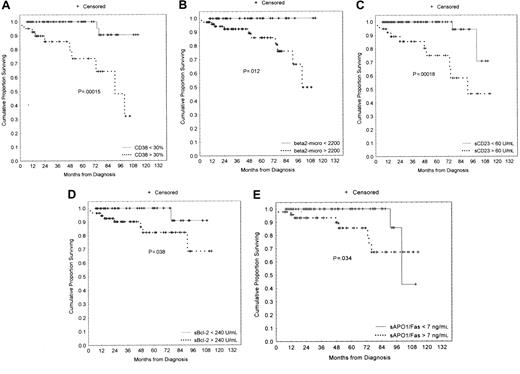
We compared the clinical stage at the time of diagnosis by using the modified Rai system as a function of the percentages of CD38+ cells. Both higher CD38 and sCD23 levels were closely associated with the intermediate/high-modified Rai stages (P = .0002 and P < .0001, respectively; Table 1). Moreover, the presence of multiple (3 or more) intrathoracic and/or abdominal lymphadenopathies (> 3 cm in diameter) and/or splenomegaly were strongly correlated both with more than 30% CD38+ B cells and more than 7 ng/mL sAPO1/Fas levels (P < .0001 andP = .0022, respectively; Table2). LDT less than 12 months was observed in 14 patients: 10 of 14 showed more than 30% CD38+ B cells (P = .0001; Table 2). Furthermore, CD38 was investigated as a predictor of clinical outcome. To this purpose, we used as an end point disease progression, defined as the appearance of an intermediate/high stage from a low Rai stage. The progression-free survival was 37% (confidence interval [CI], 18-65) in the CD38+ and 75% (CI, 59-84) in the CD38− cases at 5 years from the diagnosis (P = .00006; Figure3). Forty-eight patients started and completed as first-line approach 6 courses of fludarabine monophosphate at 25 mg/m2/d for 5 days, achieving a global CR rate of 35% (17 of 48). There was a significant correlation between higher CD38 percentages and the lack of a complete response to chemotherapy (P = .003; Table 3). Similarly, a significantly lower CR rate was observed in patients with higher β2-microglobulin (P = .018; Table 3), higher sCD23 (P = .001; Table 3), and higher sBcl-2 (P = .012; Table 3). A significantly shorter survival was found in CD38+ patients as compared with CD38−patients [50% (CI, 25-71) versus 92% (CI, 78-97) at 8 years;P < .00001; Figure 4A]. Likewise a shorter survival was observed in patients with β2-microglobulin more than 2200 μg/mL (P = .00041, Figure 4B), sCD23 more than 60 U/mL (P = .00001, Figure 4C), sBcl-2 more than 240 U/mL (P = .005, Figure 4D), and sAPO1/Fas more than 7 ng/mL (P = .008, Figure 4E). These significant differences in survival were maintained when the analysis was restricted to only the Rai intermediate risk group with regard to CD38 (P = .00015; Figure 5A), β2-microglobulin (P = .012; Figure 5B), sCD23 (P = .00018; Figure 5C), sBcl-2 (P = .038; Figure 5D), and sAPO1/Fas (P = .034; Figure 5E). In a logistic multivariate regression analysis of CR after fludarabine treatment, only CD38 more than 30% (odds ratio, 7.7; CI, 1.1-56.4; P = .036) was a significant independent prognostic factor. Finally, using a multivariate Cox regression analysis of overall survival, we found the mortality hazard ratios were 6.86 for CD38 more than 30% (P = .001) and 5.39 for sCD23 more than 60 U/mL (P = .004), respectively (Table4). Other variables, such as modified Rai stages, β2-microglobulin, sBcl-2, and sAPO1/Fas, were not of prognostic significance showing colinearity with CD38 and sCD23.
Comparisons of CD38 percentages and sCD23 levels between samples from patients with low and intermediate/high-modified Rai stage at diagnosis
| Variables . | Low Rai stage . | Intermediate/high Rai stage . | P . |
|---|---|---|---|
| CD38+(%) | |||
| Median | 3 | 10 | .0002* |
| P25-P75 | 2-11 | 3-50 | |
| No. of samples | 45 | 123 | |
| sCD23 (U/mL) | |||
| Median | 10.9 | 51.75 | < .0001* |
| P25-P75 | 4.9-28.7 | 22-120 | |
| No. of samples | 45 | 122 |
| Variables . | Low Rai stage . | Intermediate/high Rai stage . | P . |
|---|---|---|---|
| CD38+(%) | |||
| Median | 3 | 10 | .0002* |
| P25-P75 | 2-11 | 3-50 | |
| No. of samples | 45 | 123 | |
| sCD23 (U/mL) | |||
| Median | 10.9 | 51.75 | < .0001* |
| P25-P75 | 4.9-28.7 | 22-120 | |
| No. of samples | 45 | 122 |
P25 indicates the 25th percentile; P75, the 75th percentile.
As determined by the Mann-Whitney test.
Lymphadenopathy/splenomegaly or lymphocyte doubling time and CD38 percentages or soluble APO1/Fas levels
| . | Lymphadenopathy/splenomegaly . | LDT* . | ||
|---|---|---|---|---|
| Present (n = 65) . | Absent (n = 103) . | Less than 12 mo (n = 14) . | More than 12 mo (n = 154) . | |
| CD38† | ||||
| More than 30% (n = 50) | 86.0% (73.3-94.2)2-153 | 14.0% (5.8-26.7) | 71.4% (41.9-91.6) | 28.6% (8.4-58.1) |
| Less than 30% (n = 118) | 18.6% (12.1-26.9) | 81.3% (73.1-87.9) | 26% (19.2-33.6) | 74% (66.3-80.7) |
| sAPO1/Fas‡ | ||||
| More than 7 ng/mL (n = 68) | 52.9% (40.4-65.1) | 47.1% (34.8-59.5) | 77.8% (39.9-97.2) | 22.2% (2.8-60) |
| Less than 7 ng/mL (n = 93) | 25.8% (17.3-35.9) | 74.2% (64.1-82.7) | 40.1% (32.3-48.4) | 59.8% (51.6-67.7) |
| . | Lymphadenopathy/splenomegaly . | LDT* . | ||
|---|---|---|---|---|
| Present (n = 65) . | Absent (n = 103) . | Less than 12 mo (n = 14) . | More than 12 mo (n = 154) . | |
| CD38† | ||||
| More than 30% (n = 50) | 86.0% (73.3-94.2)2-153 | 14.0% (5.8-26.7) | 71.4% (41.9-91.6) | 28.6% (8.4-58.1) |
| Less than 30% (n = 118) | 18.6% (12.1-26.9) | 81.3% (73.1-87.9) | 26% (19.2-33.6) | 74% (66.3-80.7) |
| sAPO1/Fas‡ | ||||
| More than 7 ng/mL (n = 68) | 52.9% (40.4-65.1) | 47.1% (34.8-59.5) | 77.8% (39.9-97.2) | 22.2% (2.8-60) |
| Less than 7 ng/mL (n = 93) | 25.8% (17.3-35.9) | 74.2% (64.1-82.7) | 40.1% (32.3-48.4) | 59.8% (51.6-67.7) |
LDT indicates lymphocyte doubling time; n, the number of samples.
As determined by the two-tailed Fisher exact test.
For lymphadenopathy/splenomegaly, P < .0001; for LDT, P = .0001.
For lymphadenopathy/splenomegaly, P = .0022; for LDT, P = .036.
CI 95% throughout.
Progression-free survival curves based on CD38 expression.
Kaplan-Meier plot comparing progression-free survival (from low to intermediate/high-modified Rai stage) based on the detection of more than 30% or less than 30% CD38+ B-CLL cells (> 30%, 50 cases; < 30%, 118 cases). As shown, CD38− patients experienced a highly significant longer progression-free survival (P = .00006).
Progression-free survival curves based on CD38 expression.
Kaplan-Meier plot comparing progression-free survival (from low to intermediate/high-modified Rai stage) based on the detection of more than 30% or less than 30% CD38+ B-CLL cells (> 30%, 50 cases; < 30%, 118 cases). As shown, CD38− patients experienced a highly significant longer progression-free survival (P = .00006).
Complete, partial, or no response to fludarabine and CD38, soluble CD23, β2-microglobulin, soluble APO1/Fas, and soluble Bcl-2 levels
| Variable . | CR (n = 17) . | PR (n = 19) . | NR (n = 12) . |
|---|---|---|---|
| CD38+ (%)3-150 | |||
| More than 30 (n = 28) | 18% (6-37)3-154 | 46% (27-66) | 36% (19-56) |
| Less than 30 (n = 20) | 60% (36-81) | 30% (12-54) | 10% (1.2-32) |
| sCD23 (U/mL)3-151 | |||
| More than 60 (n = 20) | 15% (3-38) | 35% (15-59) | 50% (27-73) |
| Less than 60 (n = 28) | 50% (31-69) | 43% (24-63) | 7% (0.8-23) |
| β2-micro (μg/mL)3-152 | |||
| More than 2.2 (n = 36) | 25% (12-42) | 47% (30-64) | 28% (14-45) |
| Less than 2.2 (n = 10) | 70% (35-93) | 20% (2.5-56) | 10% (0.2-44) |
| sAPO1/Fas (ng/mL)3-153 | |||
| More than 7 (n = 20) | 35% (15-59) | 30% (12-54) | 35% (15-59) |
| Less than 7 (n = 24) | 38% (19-59) | 54% (33-74) | 8% (1-27) |
| sBcl-2 (U/mL)3-155 | |||
| More than 240 (n = 17) | 23.5% (7-50) | 23.5% (7-50) | 53% (28-77) |
| Less than 240 (n = 25) | 48% (28-69) | 40% (21-61) | 12% (2.5-31) |
| Variable . | CR (n = 17) . | PR (n = 19) . | NR (n = 12) . |
|---|---|---|---|
| CD38+ (%)3-150 | |||
| More than 30 (n = 28) | 18% (6-37)3-154 | 46% (27-66) | 36% (19-56) |
| Less than 30 (n = 20) | 60% (36-81) | 30% (12-54) | 10% (1.2-32) |
| sCD23 (U/mL)3-151 | |||
| More than 60 (n = 20) | 15% (3-38) | 35% (15-59) | 50% (27-73) |
| Less than 60 (n = 28) | 50% (31-69) | 43% (24-63) | 7% (0.8-23) |
| β2-micro (μg/mL)3-152 | |||
| More than 2.2 (n = 36) | 25% (12-42) | 47% (30-64) | 28% (14-45) |
| Less than 2.2 (n = 10) | 70% (35-93) | 20% (2.5-56) | 10% (0.2-44) |
| sAPO1/Fas (ng/mL)3-153 | |||
| More than 7 (n = 20) | 35% (15-59) | 30% (12-54) | 35% (15-59) |
| Less than 7 (n = 24) | 38% (19-59) | 54% (33-74) | 8% (1-27) |
| sBcl-2 (U/mL)3-155 | |||
| More than 240 (n = 17) | 23.5% (7-50) | 23.5% (7-50) | 53% (28-77) |
| Less than 240 (n = 25) | 48% (28-69) | 40% (21-61) | 12% (2.5-31) |
CR indicates complete remission; PR, partial remission; NR, no response or progression; n, number of observations.
P = .003. P values are determined by the proportional odds ordered logistic regression univariate model.
P = .001.
P = .018.
P = .238.
P = .012.
CI 95% throughout.
Survival curves based on CD38 expression, β2-microglobulin, soluble CD23 (sCD23), soluble Bcl-2 (sBcl-2), and soluble APO1/Fas (sAPO1/Fas) levels.
(A) Kaplan-Meier plot comparing survival based on the detection of more than 30% (CD38+) or less than 30% CD38+(CD38−) B-CLL cells (> 30%, 50 cases; < 30%, 118 cases). Less than 30% CD38+ patients experienced a significantly longer survival (P < .00001). (B) B-CLL patients with β2-microglobulin less than 2200 μg/mL (71 cases) showed a significant longer survival as compared with patients with β2-microglobulin more than 2200 μg/mL (94 cases;P = .00041). None of the patients in the group with β2-microglobulin less than 2200 μg/mL died during the follow-up period. (C) A very significant longer survival (P = .00001) was found in 83 patients with less than 60 sCD23 U/mL as compared with 84 cases with more than 60 U/mL. (D) Seventy-nine patients with more than 240 U/mL sBcl-2 showed a worse outcome (P = .005) in comparison with 72 cases with less than 240 U/mL sBcl-2. (E) Lack of sAPO1/Fas expression identified 93 patients with a longer survival (P = .008) in comparison with 68 cases with sAPO1/Fas+ expression.
Survival curves based on CD38 expression, β2-microglobulin, soluble CD23 (sCD23), soluble Bcl-2 (sBcl-2), and soluble APO1/Fas (sAPO1/Fas) levels.
(A) Kaplan-Meier plot comparing survival based on the detection of more than 30% (CD38+) or less than 30% CD38+(CD38−) B-CLL cells (> 30%, 50 cases; < 30%, 118 cases). Less than 30% CD38+ patients experienced a significantly longer survival (P < .00001). (B) B-CLL patients with β2-microglobulin less than 2200 μg/mL (71 cases) showed a significant longer survival as compared with patients with β2-microglobulin more than 2200 μg/mL (94 cases;P = .00041). None of the patients in the group with β2-microglobulin less than 2200 μg/mL died during the follow-up period. (C) A very significant longer survival (P = .00001) was found in 83 patients with less than 60 sCD23 U/mL as compared with 84 cases with more than 60 U/mL. (D) Seventy-nine patients with more than 240 U/mL sBcl-2 showed a worse outcome (P = .005) in comparison with 72 cases with less than 240 U/mL sBcl-2. (E) Lack of sAPO1/Fas expression identified 93 patients with a longer survival (P = .008) in comparison with 68 cases with sAPO1/Fas+ expression.
Survival curves based on CD38 expression, β2-microglobulin, sCD23, sBcl-2, and sAPO1/Fas levels within the intermediate Rai stage.
(A) Kaplan-Meier plot comparing numbers of CD38+ B-CLL cells (> 30%, 41 cases; < 30%, 75 cases) with overall survival. The difference between these 2 groups remained highly significant (P = .00015). (B) The significant difference between less than 2200 μg/mL (38 cases) and more than 2200 μg/mL (75 cases) β2-microglobulin was maintained within the Rai intermediate risk category (P = .012). (C) The difference between less than 60 U/mL (39 cases) and more than 60 U/mL sCD23 (76 cases) was also confirmed within the intermediate Rai stage (P = .00018). (D) Within the Rai intermediate risk group, patients with higher sBcl-2 levels (57 patients) had a shorter survival (P = .038) than those with lower sBcl-2 levels (44 patients). (E) Within the intermediate Rai risk group, lower sAPO1/Fas expression (63 patients) was characterized with a better outcome (P = .034) than higher sAPO1/Fas expression (48 patients).
Survival curves based on CD38 expression, β2-microglobulin, sCD23, sBcl-2, and sAPO1/Fas levels within the intermediate Rai stage.
(A) Kaplan-Meier plot comparing numbers of CD38+ B-CLL cells (> 30%, 41 cases; < 30%, 75 cases) with overall survival. The difference between these 2 groups remained highly significant (P = .00015). (B) The significant difference between less than 2200 μg/mL (38 cases) and more than 2200 μg/mL (75 cases) β2-microglobulin was maintained within the Rai intermediate risk category (P = .012). (C) The difference between less than 60 U/mL (39 cases) and more than 60 U/mL sCD23 (76 cases) was also confirmed within the intermediate Rai stage (P = .00018). (D) Within the Rai intermediate risk group, patients with higher sBcl-2 levels (57 patients) had a shorter survival (P = .038) than those with lower sBcl-2 levels (44 patients). (E) Within the intermediate Rai risk group, lower sAPO1/Fas expression (63 patients) was characterized with a better outcome (P = .034) than higher sAPO1/Fas expression (48 patients).
Discussion
CD38+ B-CLLs are tumors of naive B cells
Our present study identifies a distinct subset of B-CLL, expressing higher CD38 percentages together with higher β2-microglobulin and sCD23 levels. This close relationship between CD38 expression and β2-microglobulin or sCD23 concentrations emphasizes that our CD38+ cases are authentic B-CLL showing a greater disease activity.19Damle et al15 investigated the expression of CD38 in relation to the mutational status of the immunoglobulin variable region genes in B-CLL. They found, looking at more than 30% CD38+expression in B-CLL, that these cases contained unmutated V genes, whereas samples expressing less than 30% CD38+ contained all the mutated cases and only 3 unmutated cases. This finding argues that most of our cases are tumors of memory B cells and not of naive B cells14 because 2 of 3 of our samples express lower than 30% CD38. In addition, it has been demonstrated that B-CLLs having mutated IgVH genes show generally a relative high frequency of BCL-6 mutations (9 of 30; 30%), further evidence supporting their origin from GC-related B cells.20 However, Hamblin et al21 found no significant association between CD38 expression and mutational status. In any case, both markers (CD38 and somatic mutations) probably reflect a common feature, such as maturation stage or signaling dependence (eg, T dependent versus T independent) of the leukemic B cells.16 Theoretically, a less differentiated CD38+ lymphocyte precursor should give rise to a subtype of CLL that has a greater malignant potential and better probability of being resistant to chemotherapeutic-induced apoptosis and, thus in general, a poorer prognosis.22Conversely, Zupo et al23 have reported that CD38+ B-CLL cells can be easily induced to undergo apoptosis in vitro after exposure to anti-immunoglobulin antibodies, whereas CD38− B-CLL cells are resistant to these effects. The explanation for this apparent disagreement could be that the quality of the antigen receptor stimulus may lead to diverse endpoints (survival versus apoptosis). Previous studies suggest that CD38 expression identifies those B-CLL clones that are capable of transducing signals through their antigen receptors that may increase or decrease their chance for survival.24 It might be the case that mature B cells can be rescued from apoptosis by CD38 triggering,25 whereas anti-CD38–mediated signaling results in the death of immature B cells.26 In fact, Yamashita et al27 reported that a monoclonal antibody against a murine CD38 homologue, such as CS/2, could deliver a signal to B cells for prolongation of survival and protection against apoptosis in vitro. In their study, stimulation of spleen cells with CS/2 induced a blastoid morphology in B cells and a significant uptake of [3H] thymidine.
Apoptotic molecules in B-CLL
From the literature, it is well known that soluble Fas molecules are able to block Fas/Fas ligand interaction and thus prevent apoptosis.28,29 Moreover, in vitro, it has been demonstrated that soluble forms of Fas (sFas) can be detected in human serum and are able to inhibit apoptosis induced by either an agonistic antibody or, more importantly, by the natural Fas ligand in Fas+ cell lines.30,31 Interestingly, higher sAPO1/Fas levels were correlated with lymphadenopathy/splenomegaly (Table 2) and a shorter survival (Figures 4E and 5E) in our B-CLL patients. In these cases, a more crucial block in apoptosis could explain both the advanced disease and the poor prognosis.28 Moreover, the higher levels of sBcl-2 found in our B-CLL patients with a less favorable prognosis reinforces this hypothesis (Figures 4D and 5D). Besides, several studies show the expression of multiple apoptosis-regulating proteins in B-CLLs and suggest that the relative levels of some of these, such as Mcl-1, may provide information about in vivo responses to chemotherapy.32 Furthermore, given that many B-CLLs have been reported to be resistant to apoptosis induced by a variety of stimuli, including anti-Fas antibodies and transforming growth factor β,33 it could be of interest to explore whether cross-resistance to several apoptotic stimuli can be used to segregate B-CLLs into apoptosis-sensitive and -resistant subgroups and further to attempt correlating this information with clinical outcome. In any case, the biological and clinical interrelations between these apoptotic proteins (sAPO1/Fas and sBcl-2) and CD38 expression should be clarified by further in vitro and in vivo studies.
Clinical course of CD38+ B-CLLs
From a clinical point of view, higher CD38 percentages and higher sCD23 levels were significantly correlated with more advanced Rai stages (Table 1). Moreover, our CD38+ patients most often presented with large intrathoracic/abdominal lymphadenopathies, splenomegaly (P < .0001), and a LDT less than 12 months (P = .0001), showing an active and aggressive disease. Complete response to fludarabine as initial therapy34 was significantly correlated with CD38 percentages (P = .003) and with sCD23 (P = .001) and sBcl-2 (P = .012) levels, demonstrating that these biological factors might be used to predict the chemosensitivity of B-CLL patients. In any case, only CD38 more than 30% resulted in being an independent prognostic factor with regard to CR achievement in multivariate analysis (P = .036). Moreover, CD38+ patients progressed more rapidly to advanced Rai stages (P = .00006) and showed a very significant shorter survival (P < .00001). Accordingly, both Damle et al15 and Hamblin et al21 demonstrated that the CD38+ patients responded poorly to continuous multiregimen chemotherapy and had a significantly poorer prognosis. Besides, in a series of 131 patients with B-CLL, the same investigators demonstrated that the B-CLL cases with unmutated VH genes had a distinctly more malignant disease and a much shorter survival than those with somatic mutations.15 35 Finally we performed these same biological determinations (CD38, β2-microglobulin, sCD23, sBcl-2, and sAPO1/Fas) among those patients who presented initially in the Rai intermediate stage. It is often difficult for clinicians to choose which patients in this group have to be treated because they can show either an indolent course or a rapid downhill outcome despite aggressive treatment. In univariate analysis, CD38 positivity as well as the other biological variables was able to segregate those Rai intermediate risk patients who experienced a shorter survival. The prognostic effect of CD38 expression on clinical outcome of B-CLL patients was also corroborated by the results of the multivariate analysis. Therefore, our study proposes CD38 expression as a novel and significant prognostic indicator that delineates similar overlapping groups of B-CLL patients. In addition, this parameter can be determined easily and rapidly by flow cytometry in most hematologic laboratories, and, consequently, it may be a very useful addition to the current staging systems. Actually, this simple test may enable physicians to accurately predict a favorable or an unfavorable clinical course. Last, our experience suggests that CD38+ B-CLL patients are more likely than CD38− patients to have a progressive and an unfavorable disease, and, therefore, they should be considered as candidates for experimental and more tailored therapeutic approaches.
We thank Dr Andrea Stoler for revising the style and the presentation of this paper and the members of our Department of Hematology clinical staff for their support to our B-CLL clinical research program.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Giovanni Del Poeta, Cattedra e Divisione di Ematologia, Università “Tor Vergata,” Via Fiume Giallo, 430 MA, 00144 Roma, Italy; e-mail: g.delpoeta@tin.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal