Abstract
Few reports are available on the treatment of patients with Waldenström macroglobulinemia (WM) and primary or secondary resistance to alkylating-agent–based regimens. From December 1993 through December 1997, 92 patients with WM resistant to first-line therapy (42) or with first relapse (50) after alkylating-agent therapy were randomly assigned to receive fludarabine (25 mg/m2 of body-surface area on days 1-5) or cyclophosphamide, doxorubicin (Adriamycin), and prednisone (CAP; 750 mg/m2cyclophosphamide and 25 mg/m2 doxorubicin on day 1 and 40 mg/m2 prednisone on days 1-5). The first end point evaluated was the response rate after 6 treatment courses. Forty-five patients received CAP and 45 received fludarabine. Two patients died before the first course of chemotherapy. No statistical differences were observed between the 2 treatment arms with respect to hematologic toxicity or infections. Mucositis and alopecia occurred significantly more often in patients treated with CAP. Partial responses were obtained in 14 patients (30%) treated with fludarabine and 5 patients (11%) treated with CAP (P = .019). Responses were more durable in patients treated with fludarabine (19 months versus 3 months), and the event-free survival rate was significantly higher in this group (P < .01). Forty-four patients died, 22 in the fludarabine group and 22 in the CAP group. There was no statistical difference in the median overall survival time in the 2 study arms. Fludarabine was thus more active than CAP in salvage therapy of WM and should be tested as first-line therapy in a randomized comparison with alkylating agents.
Introduction
Waldenström macroglobulinemia (WM) results from malignant proliferation of well-differentiated plasmacytic lymphocytes that produce monoclonal IgM.1 According to the revised European-American lymphoma classification of lymphoid neoplasms, WM accounts for most diagnoses of lymphoplasmacytoid lymphoma/immunocytoma.2 WM is characterized by a highly variable clinical picture, with complications being determined by the physicochemical properties and antibody activity of the monoclonal IgM.3,4 Chemotherapy with alkylating agents alone or combined with corticosteroids has been the standard primary treatment for patients with symptomatic macroglobulinemia. The agent most commonly employed is oral chlorambucil, which is used in both daily low-dose schedules and intermittent high-dose schedules.3,5,6 Others7 8 have used combinations of alkylating agents given with or without a vinca alkaloid or nitrosurea, but there is no evidence that such combinations are more beneficial.
Preliminary studies suggested that purine analogs such as fludarabine and 2-chlorodeoxyadenosine (2-CdA) are effective in most patients with WM. Approximately 50% of previously untreated patients have a response to conventional therapies, whereas more than 75% of patients may respond to nucleoside analogs.9-15 The few available reports on treatment of patients with primary or secondary resistance to first-line therapies showed a benefit with interferon,16,17 doxorubicin,18 and nucleoside analogs. Kantarjian et al,14 Dimopoulos et al,19 and Zinzani et al20 reported responses to fludarabine salvage therapy in about one third of patients with resistance to previously administered treatments, and these results were confirmed by other studies. We previously reported a series of 71 patients with advanced and resistant WM in whom fludarabine yielded a 30% response rate and a median overall survival time of 23 months; unfortunately, toxicity was severe in these heavily pretreated patients.21 Here, we describe a randomized, multicenter study comparing the efficacy of fludarabine with that of the combination of cyclophosphamide, doxorubicin (Adriamycin), and prednisone (CAP) in 92 WM patients with first relapse or primary refractory disease.
Patients and methods
Patients and treatment
From December 1993 through December 1997, 92 patients who fulfilled the following inclusion criteria were enrolled in this randomized study in 37 centers: men or women aged 18 to 75 years with WM indicated by infiltration with well-differentiated lymphocytes and atypical or lymphocytic plasma cells in biopsy samples of involved organs, a monoclonal IgM component of at least 5 g/L on electrophoresis study, and primary resistance or first relapse despite administration of treatment regimens based on alkylating agents. Patients were treated according to a randomized phase II protocol approved by an institutional review board and a local ethics committee. All eligible patients provided written consent to their participation in the study. Clinical and biologic characteristics of the patients are summarized in Table 1. All patients had received alkylating-agent monotherapy (chlorambucil, cyclophosphamide, or melphalan) with or without a corticosteroid. None of the patients had received purine analogs or doxorubicin. Fifty patients had first relapse (8 were receiving therapy when relapse occurred) and 42 had primary resistant disease.
Patients' characteristics at enrollment (n = 92)
| Characteristic . | Value . |
|---|---|
| Median age (range), y | 64.3 (34.6-75) |
| No. (%) of patients with hepatomegaly | 10 (11) |
| No. (%) of patients with splenomegaly | 16 (17) |
| No. (%) of patients with ≥1 lymph node involved | 33 (36) |
| Mean (range) no. of mo between diagnosis and enrollment | 47 (2-224) |
| Mean (range) no. of mo between end of first-line treatment and enrollment | 3 (0-158) |
| No. of patients with first relapse | 50 |
| No. of patients with resistant disease | 42 |
| No. (%) of patients with hemoglobin level < 100 g/L | 47 (51) |
| No. (%) of patients with platelet count < 100 × 109/L | 20 (22) |
| No. (%) patients with β2 microglobulin level > 2.5 mg/L | 48 (52) |
| Median (range) IgM (g/L) | 30.5 (8.4-101) |
| No. of patients with lactate dehydrogenase level > normal | 11 |
| Characteristic . | Value . |
|---|---|
| Median age (range), y | 64.3 (34.6-75) |
| No. (%) of patients with hepatomegaly | 10 (11) |
| No. (%) of patients with splenomegaly | 16 (17) |
| No. (%) of patients with ≥1 lymph node involved | 33 (36) |
| Mean (range) no. of mo between diagnosis and enrollment | 47 (2-224) |
| Mean (range) no. of mo between end of first-line treatment and enrollment | 3 (0-158) |
| No. of patients with first relapse | 50 |
| No. of patients with resistant disease | 42 |
| No. (%) of patients with hemoglobin level < 100 g/L | 47 (51) |
| No. (%) of patients with platelet count < 100 × 109/L | 20 (22) |
| No. (%) patients with β2 microglobulin level > 2.5 mg/L | 48 (52) |
| Median (range) IgM (g/L) | 30.5 (8.4-101) |
| No. of patients with lactate dehydrogenase level > normal | 11 |
Patients were randomly assigned to receive fludarabine (25 mg/m2 of body-surface area given intravenously during 30 minutes daily for 5 consecutive days) or CAP (750 mg/m2cyclophosphamide and 25 mg/m2 doxorubicin given intravenously on day 1, and 40 mg/m2 prednisone given orally on days 1-5). Treatment courses were repeated every 4 weeks, with a target of 6 courses. Oral trimethoprim-sulfamethoxazole (200 mg/day and 40 mg/day) or pentamidine aerosols (300 mg once a month) and oral acyclovir (800 mg/day) were given to patients treated with fludarabine to prevent Pneumocystis carinii and herpesvirus infection, respectively, during all courses of chemotherapy.
Evaluation of responses
Tumor burden was assessed by means of computed tomographic assessment of the chest, abdomen, and pelvis, with measurement of all involved lymph nodes, the liver, and the spleen. The pretreatment assessment included complete blood cell counts, kidney and liver function tests, serum and urine protein electrophoresis and immunoelectrophoresis, and assays of serum immunoglobulin, lactate dehydrogenase, β2 microglobulin, and C-reactive protein. Bone marrow aspirates or bone marrow trephine biopsy samples were obtained to determine the percentage of lymphocytes, lymphocytoid plasma cells, and plasma cells. The response criteria were adapted from those used for multiple myeloma and from previous studies of WM. A partial response was defined as a sustained decrease of more than 50% in the IgM production rate (on densitometry tracing of the serum electrophoretic pattern) on at least 2 occasions 2 months apart and a more than 50% reduction in the size of all involved organs. A complete response was defined as disappearance of the monoclonal IgM (on immunofixation study), resolution of lymphadenopathy and splenomegaly, and a finding of less than 20% lymphocytes in the bone marrow. Patients with a response were subsequently monitored without treatment until disease progression, defined as a more than 25% increase in tumor mass relative to the lowest level achieved. Treatment was considered to have failed in patients with a mixed response, no response, or withdrawal from the trial for any reason.
Assessment of adverse effects
All patients were included in the evaluation of adverse effects, and toxicity was analyzed according to World Health Organization criteria. Hematologic values and other clinical and laboratory indicators of adverse effects were recorded at least monthly by the data-management office and were reported at each round of treatment.
Statistical analysis
The cut-off date for this analysis was April 15, 2000. Results were assessed on an intent-to-treat basis. The primary end point was the response after 6 treatment courses. The sample size was computed in 88 patients per group on the basis of an expected intergroup difference in response rate of 25% (25% with CAP and 50% with fludarabine), a type 1 error of 5%, a power of 90%, and an interim analysis after enrollment of half of the 196 patients. The trial was stopped after the interim analysis. Other end points were the response after 3 courses, event-free survival (EFS) rate, duration of response, and overall survival (OS) rate. EFS was measured from enrollment to disease progression, death from any cause, or the date of the last follow-up visit. Duration of response was measured from the date of response to disease progression or death, only in responding patients. OS was measured from enrollment to death or the last follow-up visit. Survival curves for EFS and OS in the 2 treatment groups were plotted by using the Kaplan-Meier method and compared by using the log rank test.22 All analyses were done with SAS (SAS Institute, Cary, NC) and Splus software (Mathsoft, Seattle, WA).
Results
Responses and survival
Forty-six patients were randomly assigned to each arm, and 45 patients in each arm received the first round of chemotherapy. There were 50 patients with first relapse and 42 with primary resistant disease. Clinical and biologic characteristics of the patients in the 2 treatment groups are shown in Table 2. There were no significant differences between the groups in clinical disease manifestations, duration of previous treatment, or disease status at enrollment. The only significant biologic differences between the 2 groups were in their hemoglobin and C-reactive protein levels. One patient in each arm died before the first round of chemotherapy.
Clinical and biologic characteristics in the 2 study groups at enrollment
| Characteristic . | Study group . | |
|---|---|---|
| FAMP (n = 46) . | CAP (n = 46) . | |
| Median age (range), y | 64 (34.6-74) | 64.7 (39.5-75) |
| Sex (M/F) | 34/12 | 28/18 |
| No. of patients with tumors | 27 | 31 |
| Mean (range) no. of mo between diagnosis and enrollment | 44.6 (5.3-207.9) | 58.9 (3.15-220) |
| Mean (range) no. of mo between end of first-line treatment and enrollment | 2.7 (1.4-13.7) | 3 (0.6-57.2) |
| No. of patients with first relapse | 26 | 24 |
| No. of patients with resistant disease | 20 | 22 |
| Mean (range) IgM, g/L | 36.3 (8.4-46) | 28.9 (10-73.7) |
| Mean (range) hemoglobin level, g/L | 107 (50-149) | 94 (63-144)* |
| Mean (range) platelet count, × 109/L | 188 (9-429) | 155 (5-460) |
| Mean (range) lactate dehydrogenase level, U/L | 272 (79-1950) | 242 (95-1725) |
| Mean (range) C-reactive protein level, mg/L | 19.2 (2-122) | 18.5 (2-108)* |
| Mean (range) β2 microglobulin level, mg/L | 2.58 (1.6-13) | 3.1 (1.4-9.3) |
| Characteristic . | Study group . | |
|---|---|---|
| FAMP (n = 46) . | CAP (n = 46) . | |
| Median age (range), y | 64 (34.6-74) | 64.7 (39.5-75) |
| Sex (M/F) | 34/12 | 28/18 |
| No. of patients with tumors | 27 | 31 |
| Mean (range) no. of mo between diagnosis and enrollment | 44.6 (5.3-207.9) | 58.9 (3.15-220) |
| Mean (range) no. of mo between end of first-line treatment and enrollment | 2.7 (1.4-13.7) | 3 (0.6-57.2) |
| No. of patients with first relapse | 26 | 24 |
| No. of patients with resistant disease | 20 | 22 |
| Mean (range) IgM, g/L | 36.3 (8.4-46) | 28.9 (10-73.7) |
| Mean (range) hemoglobin level, g/L | 107 (50-149) | 94 (63-144)* |
| Mean (range) platelet count, × 109/L | 188 (9-429) | 155 (5-460) |
| Mean (range) lactate dehydrogenase level, U/L | 272 (79-1950) | 242 (95-1725) |
| Mean (range) C-reactive protein level, mg/L | 19.2 (2-122) | 18.5 (2-108)* |
| Mean (range) β2 microglobulin level, mg/L | 2.58 (1.6-13) | 3.1 (1.4-9.3) |
FAMP indicates fludarabine; CAP, cyclophosphamide, doxorubicin, and prednisone.
Significant difference between the 2 groups.
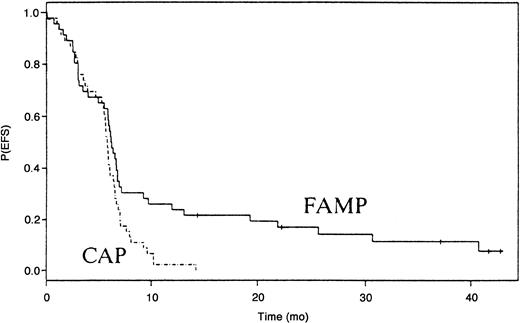
Treatment response could be assessed in 90 patients. A partial response was observed after 3 courses of treatment in 3 of 45 patients treated with fludarabine and 2 of 45 patients treated with CAP. After 6 courses, 14 patients (30%) treated with fludarabine and 5 patients (11%) patients treated with CAP had a partial response (P=.02). No complete remissions were observed in either group. The median duration of response was 19 months after fludarabine therapy and 3 months after CAP therapy (P < .01). EFS was significantly higher in patients treated with fludarabine (P < .01; Figure 1). The median follow-up time among survivors was 52 months (range, 29 to 86 months).
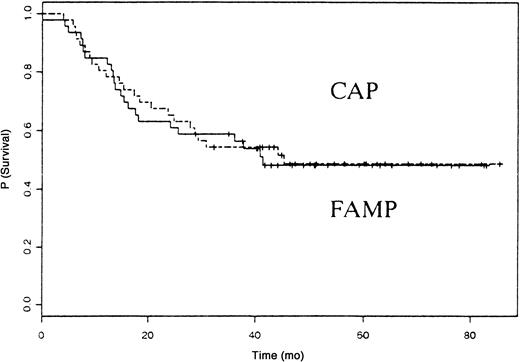
Forty-four patients died, 22 in the fludarabine group and 22 in the CAP group. The median survival time was 41 months in patients treated with fludarabine and 45 months in those treated with CAP (P not significant). The OS rate was similar in the 2 groups (Figure2). The causes of death are listed in Table 3. Eight patients in the fludarabine group and 14 in the CAP group died from disease progression. Four cases of secondary acute myeloid leukemia/myelodysplastic syndrome (AML/MDS) and 3 cases of disease transformation into diffuse large-cell lymphoma (Richter syndrome) occurred in the fludarabine group, and 2 cases of secondary AML/MDS and 2 cases of disease transformation into diffuse large-cell lymphoma occurred in the CAP group.
Deaths in the 2 study groups
| Cause of death . | Study group . | |
|---|---|---|
| FAMP (total deaths = 22) . | CAP (total deaths = 22) . | |
| Toxicity during treatment | 1 | 1 |
| High-grade lymphoma | 3 | 2 |
| Secondary AML/MDS | 4 | 2 |
| Infection | 2 | 0 |
| Disease progression | 8 | 14 |
| Other | 4 | 1 |
| Unknown | 0 | 2 |
| Cause of death . | Study group . | |
|---|---|---|
| FAMP (total deaths = 22) . | CAP (total deaths = 22) . | |
| Toxicity during treatment | 1 | 1 |
| High-grade lymphoma | 3 | 2 |
| Secondary AML/MDS | 4 | 2 |
| Infection | 2 | 0 |
| Disease progression | 8 | 14 |
| Other | 4 | 1 |
| Unknown | 0 | 2 |
Values are number of deaths.
AML/MDS indicates acute myeloid leukemia/myelodysplastic syndrome.
Adverse effects
There were no significant differences between the 2 treatment groups in hematologic toxicity. Mucositis and alopecia occurred significantly more often in patients treated with 25 mg/m2doxorubicin in the CAP regimen, with 10 episodes of grade 1 or higher mucositis. We did not observe any instance of grade 1 mucositis or higher in patients treated with fludarabine. Eleven patients treated with CAP and 3 treated with fludarabine had grade 1 or higher alopecia. The frequency and severity of infections were identical in the 2 groups (Table 4). A quality-of-life study based on quality-adjusted time without symptoms or toxicity was reported previously.23
Toxicity in the 2 study groups
| Area of abnormality/ grade of toxicity4-150 . | FAMP (total courses = 216) . | CAP (total courses = 227) . | P (χ2 or Fisher exact test) . |
|---|---|---|---|
| Hemoglobin > 2 | 32 | 49 | .065 |
| Leukocytes > 2 | 69 | 54 | .059 |
| PMNC > 2 | 63 | 63 | .72 |
| Platelets > 2 | 11 | 15 | .51 |
| Infection > 2 | 3 | 5 | .52 |
| Mucositis ≥ 1 | 0 | 10 | < .014-151 |
| Alopecia ≥ 1 | 3 | 11 | < .014-151 |
| Area of abnormality/ grade of toxicity4-150 . | FAMP (total courses = 216) . | CAP (total courses = 227) . | P (χ2 or Fisher exact test) . |
|---|---|---|---|
| Hemoglobin > 2 | 32 | 49 | .065 |
| Leukocytes > 2 | 69 | 54 | .059 |
| PMNC > 2 | 63 | 63 | .72 |
| Platelets > 2 | 11 | 15 | .51 |
| Infection > 2 | 3 | 5 | .52 |
| Mucositis ≥ 1 | 0 | 10 | < .014-151 |
| Alopecia ≥ 1 | 3 | 11 | < .014-151 |
Values are number of cases unless otherwise indicated.
PMNC indicates peripheral blood mononuclear cells.
According to World Health Organization criteria.
Significant difference in toxicity.
Discussion
Salvage treatment of patients with WM and primary or secondary resistance to first-line therapy has not previously been studied in a randomized trial. During the past decade, the newer nucleoside analogs (2-CdA and fludarabine) have shown activity in this setting, even in patients with resistant disease. Studies found that 2-CdA yielded a high response rate (75%) in untreated patients with WM, although few complete remissions were observed.14,24,25 Fludarabine has also been studied as front-line treatment of WM: a European multicenter phase II study of 20 previously untreated patients with WM showed objective responses in 79%, with a median time to disease progression of 40 months.15 However, the response rate was lower in a preliminary study by Dhodapkar et al,26 with only 33% of responses occurring in previously untreated patients.
Approximately one third of previously treated patients are expected to have an objective response to purine nucleoside analogs.10,12,13,25 We recently reported a retrospective study of 71 patients with advanced disease treated with fludarabine.21 As in previous studies, 30% of patients had a response, and the median survival time among such patients was 30 months. Unfortunately, toxicity was severe, with 5 deaths due to infection after only 1 or 2 courses of treatment and 2 deaths from infection in patients with a response. The median survival time in the entire group was 23 months.
We thus conducted a randomized study to test the efficacy of fludarabine in patients with primary resistance or first relapse after alkylating-agent–based regimens. The upper age limit of 75 years was chosen because of the high toxicity rate associated with fludarabine in heavily pretreated patients in our previous study.21 As in the largest reported series, a monoclonal IgM component of at least 5 g/L on electrophoresis study was chosen as the cut-off value for inclusion in the study.27-29 Previous trials of second-line treatments involved doxorubicin, high-dose corticosteroids, γ interferon, and splenectomy, but these studies were limited and the results not particularly noteworthy.16-18,30,31 We chose CAP as the comparative regimen in this study because of its efficacy in low-grade lymphoid malignant diseases such as chronic lymphocytic leukemia and low-grade lymphoma.32,33 Furthermore, Dimopoulos and Alexanian3 observed similar response rates in 20 patients who received first-line treatment with cyclophosphamide, hydroxydaunomycin, vincristine, and prednisone and in a historical series of patients treated with a combination of chlorambucil and prednisone, but there was significant difference in median survival rates. Claman et al18 reported successful doxorubicin treatment of 2 patients with alkylating-agent–resistant WM. Vincristine was not used to avoid worsening the peripheral neuropathy often observed in WM.
Our prospective study found that fludarabine was significantly more effective than CAP, on the basis of the number and duration of responses and the EFS rate. However, the median survival time was similar in the 2 treatment groups. This not the first trial to show no difference in survival rates among patients with chronic lymphoproliferative disorders, even with a large number of patients. Rai et al34 reported a randomized trial in patients with chronic lymphocytic leukemia in whom the response rate and median duration of remission were better in the group given fludarabine but the survival rate was not, despite enrollment of 178 patients in the fludarabine group. Similar results were observed in a trial that tested the efficacy of 2-CdA and prednisone compared with chlorambucil.35
The lack of difference in OS rate in our study might be explained by efficient salvage treatment after relapse. It was not due to treatment switches after failure of the regimen to which patients had been randomly assigned: only 10 patients in the CAP group received fludarabine and only 1 had a response; likewise, 5 patients with no response to fludarabine received CAP, and only 1 had a response. Thus, the lack of any difference in OS rate between the 2 groups seems rather to have been due to the higher rate of deaths unrelated to disease progression in the fludarabine group, in which 4 patients died from myocardial infarction, 1 from myocardial infarction, 1 from lung cancer, 1 from a trauma-related pulmonary complication, and 1 from acute graft-versus-host disease after allogeneic bone marrow transplantation. Tolerability was good, but mucositis and alopecia occurred more frequently with the 25 mg/m2 dose of doxorubicin in the CAP regimen. Few patients had severe infections during treatment, and there was no significant difference between the 2 treatment groups in progression to higher-grade lymphomas such as large-cell immunoblastic lymphoma, a finding that confirmed, in this randomized study, the data of Cheson et al,36 who found no significantly increased risk of secondary malignant disease in patients treated with nucleoside analogs.
Dimopoulos et al19 24 found that disease status at the outset of salvage treatment with a nucleoside analog was a predictor of the likelihood and durability of responses. Although we administered salvage treatment early in the disease course, we did not obtain a higher response rate than in our previous study, which included 63 patients with refractory relapse and 8 patients with primary refractory disease. However, the median OS time in the current trial (45 months) was longer than that in the previous study (23 months).
These results suggest that treatment with nucleoside analogs should be started without delay in patients with macroglobulinemia and first relapse or primary resistance to alkylating agents. The efficacy of fludarabine used as first-line therapy is controversial.15,26 These results have prompted establishment of a European multicenter trial comparing the efficacy of fludarabine with that of chlorambucil in untreated patients. Patients with resistance to nucleoside analogs need new therapeutic strategies, such as anti-CD20 monoclonal antibodies, which may be active in about 30% of previously treated patients.37 38
Factors indicating a poor prognosis at diagnosis of WM were described by Facon et al27 and Morel et al,28 who defined subgroups of WM patients with significantly different survival rates on the basis of age, albumin level, and severity of cytopenia. High-risk patients had a 5-year survival rate of 27%. Furthermore, resistance to first-line therapy with alkylating-agent–based regimens was shown to have an adverse effect on survival.3,5,27Thus, young patients with indicators of a poor prognosis at diagnosis or who have resistance to first-line therapy (including some with resistance to nucleoside analogs) might benefit from high-dose therapy with autologous stem-cell rescue used early during the disease course.39-41
The following departments of hematology of the French Cooperative Group on Chronic Lymphocytic Leukemia and Macroglobulinemia participated actively in this study. The number of patients enrolled is indicated in parentheses: Centre Hospitalier Universitaire, Strasbourg: F. Maloisel (9); Centre Hospitalier Universitaire, Lille: B. Cazin (5); Hôpital Saint-Louis, Paris: J. P. Fermand (5); Centre Hospitalier Universitaire-Hotel Dieu, Nantes: J. L. Harousseau (5); Hôpital Pitié-Salpétrière, Paris: V. Leblond (5); Centre Hospitalier Universitaire, Limoges: L. Remeneiras (5); Centre Hospitalier Universitaire, Angers: M. Gardembas (4); Centre Hospitalier Universitaire, Besançon: E. Deconinck (4); Centre Hospitalier Universitaire, Pessac: G. Marit (4); Centre Hospitalier, Amiens: B. Desablens (3); Centre Hospitalier Universitaire, Poitiers: F. Guilhot (3); Centre Hospitalier, Pontoise: G. Philippe (3); Centre anti-cancéreux Becquerel, Rouen: A. Stamatoullas (3); Centre Hospitalier, Suresnes: A. Baumelou (2); Hôpital Necker, Paris: C. Bélanger (2); Hôpital Saint-Louis, Paris: J. P. Clauvel (2); Hôpital Henri Mondor, Créteil: M. Divine (2); Centre Hospitalier, Nancy: N. Feugier (2); Centre Hospitalier, Martigues: M. Nezri (2); Hôpital Kremlin-Bicêtre, Kremlin: G. Tertian (2); Centre anti-cancéreux Lacassagne, Nice: A. Thyss (2); Centre Hospitalier, Avignon: A. M. Touchais (2); Hôpital Saint-Louis, Paris: P. Brice (1); Centre Hospitalier, Briis sous Forge: N. Cheron (1); Hôpital Hotel-Dieu, Paris: A. Delmer (1); Centre Hospitalier, Troyes: G. Dine (1); Institut Bergonie-CRLCC, Bordeaux: H. Eghbali (1); Centre Hospitalier Universitaire, Lyon: D. Fière (1); Centre Hospitalier Dr Schaffner, Lens: P. Morel (1); Hôpital Percy, Clamart: G. Nedelec (1); Centre Hospitalier, Valenciennes: J. P. Pollet (1); Hôpital Charles-Foix, Ivry: O. Saint-Jean (1); Clinique Victor Hugo, Le Mans: P. Solal-Celigny (1); Centre Hospitalier, Clermont-Ferrand: P. Travade (1); Centre Hospitalier, Caen: X. Troussard (1); Hôpital Beaujon, Clichy: P. Turlure (1); and Centre Hospitalier, Compiègne: D. Zylberait (1).
Also participating was Laboratoire Schering, Lys Lez Lannoy: O. Guibon. The coordinating center was Hôpital Pitié-Salpétrière, Paris: V. Leblond. The statistical center was Département de biostatistique et informatique médicale, Hôpital Saint-Louis, Paris: V. Lévy and R. Porcher.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Véronique Leblond, Département d'hématologie, Hôpital Pitié-Salpêtrière, 47 boulevard de l'Hôpital, 75013 Paris, France; e-mail: veronique.leblond@psl.ap-hop-paris.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal