Abstract
Anticancer treatment using cytotoxic drugs is considered to mediate cell death by activating key elements of the apoptosis program and the cellular stress response. While proteolytic enzymes (caspases) serve as main effectors of apoptosis, the mechanisms involved in activation of the caspase system are less clear. Two distinct pathways upstream of the caspase cascade have been identified. Death receptors, eg, CD95 (APO-1/Fas), trigger caspase-8, and mitochondria release apoptogenic factors (cytochrome c, Apaf-1, AIF), leading to the activation of caspase-9. The stressed endoplasmic reticulum (ER) contributes to apoptosis by the unfolded protein response pathway, which induces ER chaperones, and by the ER overload response pathway, which produces cytokines via nuclear factor-κB. Multiple other stress-inducible molecules, such as p53, JNK, AP-1, NF-κB, PKC/MAPK/ERK, and members of the sphingomyelin pathway have a profound influence on apoptosis. Understanding the complex interaction between different cellular programs provides insights into sensitivity or resistance of tumor cells and identifies molecular targets for rational therapeutic intervention strategies.
Introduction
In an overall scenario, the development of malignant tumors results from deregulated proliferation or an inability of cells to undergo apoptotic cell death.1,2Anticancer drugs inhibit proliferation and induce apoptosis in sensitive tumor cells.3 4 The cellular targets for different cytotoxic agents are diverse. Thus, anticancer drugs are classified as DNA-damaging agents (cyclophosphamide, cisplatin, doxorubicin), antimetabolites (methotrexate, 5-fluorouracil), mitotic inhibitors (vincristine), nucleotide analogs (6-mercaptopurine), or inhibitors of topoisomerases (etoposide). The common underlying mechanism for chemotherapy-induced apoptosis might be damage to DNA, lipid components of cell membranes, and cellular proteins causing an imbalance of the cellular homeostasis commonly designated as cellular stress. This in turn initiates a complex cascade of stress-inducible signaling molecules in an attempt to return the cell to its previous equilibrium. As for the response to DNA damage, this may include cell-cycle regulation and repair mechanisms. The type and dose of stress within the cellular context appears to dictate the outcome of the cellular response, which is intimately converted to complex pathways mediating cell-cycle control or cell death. Apoptosis seems to be induced if damage exceeds the capacity of repair mechanisms. Here, we review mechanisms of cellular stress signaling with respect to their integration into apoptosis pathways.
The cell death machinery
Caspases as death effectors
Apoptosis signaling induced by anticancer drugs converges in the activation of intracellular caspases and their modification of protein substrates within the nucleus and cytoplasm (Figure1). Currently more than 14 caspases have been cloned and partially characterized in mammals, some of which are not involved in apoptosis but rather mediate cytokine processing. Caspases are cysteine proteases produced as inactive zymogens that cleave their substrates at aspartic acid residues contained within a tetrapeptide recognition motif. Activation of initiator caspases (procaspase-8, -9, -10) leads to the proteolytic activation of downstream effector caspases (caspase-3, -6, -7) that cleave specific substrates. For example, cleavage of the nuclear lamin is required for nuclear shrinking and budding. Loss of overall cell shape is probably caused by the cleavage of cytoskeletal proteins, such as fodrin, gelsolin, plectin, actin, and cytokeratin. DNA fragmentation is due to cleavage and inactivation of ICAD, the initiator of CAD (caspase-activated DNase). In addition, the activation of several kinases by caspase cleavage, including PAK2—a member of the p21-activated kinase family—and the Ste20-related kinases MST1 and SLK, contributes to the membrane remodeling and active blebbing observed in apoptotic cells.5-7 Two independent initiator pathways lie immediately upstream of these effector events: cross-linking of death receptors by their ligands and the release of apoptogenic factors from mitochondria.5,8 9
The cell death machinery.
The death receptor pathway (left) is triggered by members of the death receptor superfamily such as CD95. Binding of CD95-L to its receptor induces trimerization of CD95 and formation of a death-inducing complex. This complex recruits, via the adaptor molecule FADD, multiple procaspase-8 molecules, resulting in caspase-8 activation. Caspase-8 activation can be blocked by recruitment of c-FLIP. The mitochondrial death pathway (right) is controlled by members of the Bcl-2 family, including the proapoptotic Bax and Bid proteins and the antiapoptotic Bcl-2 and Bcl-XL proteins. Death stimuli induce the release of cytochrome c, AIF, Apaf-1, Smac/DIABLO, and possibly other factors from mitochondria. Cytochrome c associates with Apaf-1 and caspase-9 to form the apoptosome. The death receptor and mitochondrial pathways converge at the level of caspase-3 activation. Caspase-3 activation and activity is antagonized by the IAP proteins, which themselves are antagonized by the Smac/DIABLO protein released from mitochondria. Active caspase-3 activates downstream caspases, which results in cleavage of cellular substrates and apoptosis. Crosstalk between the death receptor and mitochondrial pathways is provided by Bid, a proapoptotic Bcl-2 family member. Caspase-8–mediated cleavage of Bid greatly increases its prodeath activity and results in its translocation to mitochondria, where it promotes cytochrome c exit.
The cell death machinery.
The death receptor pathway (left) is triggered by members of the death receptor superfamily such as CD95. Binding of CD95-L to its receptor induces trimerization of CD95 and formation of a death-inducing complex. This complex recruits, via the adaptor molecule FADD, multiple procaspase-8 molecules, resulting in caspase-8 activation. Caspase-8 activation can be blocked by recruitment of c-FLIP. The mitochondrial death pathway (right) is controlled by members of the Bcl-2 family, including the proapoptotic Bax and Bid proteins and the antiapoptotic Bcl-2 and Bcl-XL proteins. Death stimuli induce the release of cytochrome c, AIF, Apaf-1, Smac/DIABLO, and possibly other factors from mitochondria. Cytochrome c associates with Apaf-1 and caspase-9 to form the apoptosome. The death receptor and mitochondrial pathways converge at the level of caspase-3 activation. Caspase-3 activation and activity is antagonized by the IAP proteins, which themselves are antagonized by the Smac/DIABLO protein released from mitochondria. Active caspase-3 activates downstream caspases, which results in cleavage of cellular substrates and apoptosis. Crosstalk between the death receptor and mitochondrial pathways is provided by Bid, a proapoptotic Bcl-2 family member. Caspase-8–mediated cleavage of Bid greatly increases its prodeath activity and results in its translocation to mitochondria, where it promotes cytochrome c exit.
Mitochondria and activation of caspases
Mitochondria are organelles with 2 well-defined compartments: the matrix, surrounded by the inner membrane, and the intermembrane space, surrounded by the outer membrane. Mitochondria are induced to release cytochrome c in response to most anticancer drugs and other cellular stresses, either by opening of channels in the outer membrane or because of the organellar swelling and rupture that occurs following permeability transition pore opening.10,11 Although release of cytochrome c through the outer membrane is mostly associated with a permanent loss of the mitochondrial membrane potential, this may be transient due to resealing of the inner membrane.10Release of cytochrome c into the cytosol results in activation of the caspase adaptor Apaf-1 and procaspase-9, which form a holoenzyme complex termed “apoptosome.” Caspase-9 in context with this holoenzyme activates downstream caspases—most importantly caspase-3, but also caspase-8—which results in DNA fragmentation and apoptosis. Cytochrome c exit is but one of a host of mitochondrial prodeath compounds. Also present in mitochondria and released upon induction of apoptosis is Smac/DIABLO (second mitochondria-derived activator of caspases/direct IAP-binding protein with a low isoelectric point), a molecule of the inhibitors of apoptosis (IAP) family (see below) and apoptosis-inducing factor (AIF), which exhibits a potent but apparently caspase-independent apoptotic activity.5 9
Bcl-2 family proteins play a central role in controlling the mitochondrial pathway. In humans, more than 20 members of this family have been identified to date, including proteins that suppress (Bcl-2, Bcl-XL, Mcl-1, Bfl-1/A1, Bcl-W, Bcl-G) and proteins that promote (Bax, Bak, Bok, Bad, Bid, Bik, Bim, Bcl-Xs, Krk, Mtd, Nip3, Nix, Noxa, Bcl-B)12-15 apoptosis. Bcl-2 proteins localize or translocate to the mitochondrial membrane and modulate apoptosis by permeabilization of the inner and/or outer membrane, leading to release of cytochrome c or by stabilizing barrier function. Most Bcl-2 family proteins are capable of physically interacting, forming homodimers or heterodimers, and functioning as agonists or antagonists of each other.12 Additionally, Bcl-XL binds and inactivates Apaf-1, whereas proapoptotic members can displace Bcl-XL from Apaf-1, allowing Apaf-1 to activate caspase-9. Thus, Bcl-2 family members can directly influence the response to cellular stress.8,11 Control of chemotherapy-induced apoptosis by Bcl-2 or Bcl-XL has been suggested by a number of experimental and clinical studies. Increased Bax levels in several tumor cells have been associated with favorable responses to chemotherapy in vivo. Human colorectal cancer cells lacking functional Bax genes were found to be partially resistant to the apoptotic effects of chemotherapeutic agents. Vice versa, resistance to chemotherapy was found to be related to increased levels of expression of Bcl-2 and Bcl-XL16,17 in some studies.18
Death receptors and activation of caspases
The second pathway that leads to direct caspase activation originates from death receptor signaling, eg, through CD95 (APO-1/Fas) and its ligand CD95-L (APO-1–L/Fas-L). CD95 and other death receptors contain an intracellular death domain. CD95-L is a type II membrane protein that can be proteolytically shed into the intercellular space as a soluble form that is less potent in inducing apoptosis than the membrane-bound form.19 Binding of CD95-L and similar tumor necrosis factor (TNF)–family ligands (eg, TNF-α or TNF-related apoptosis-inducing ligand [TRAIL]) to their respective death-inducing receptors (CD95/APO-1/FAS, TNF-R1, DR4 [TRAIL-R1], and DR5 [TRAIL-R2], respectively) leads to receptor trimerization and recruitment of adaptor proteins to the cytoplasmic death domain. This death-inducing signaling complex (DISC) forms within seconds of receptor engagement. First, specific adaptor proteins (FADD [Fas-associated death domain] for CD95 and DR4/5; TRADD [TNF-R–associated death domain] for TNF-R1) bind via their own death domain to the death domain of the respective receptor. FADD carries a death effector domain (DED) and recruits the DED-containing procaspase-8 (FLICE) into the DISC. Next, procaspase-8 is activated proteolytically and cleaves various proteins, including procaspase-3, which results in its activation and the completion of the cell death program.9
The mitochondrial and caspase apoptotic pathways are intimately connected. For example, caspase-8 cleaves the cytosolic proapoptotic protein Bid. Bid is a member of the BH3 domain–only subgroup of Bcl-2 family members. This set of proapoptotic proteins shares its only sequence homology within the BH3 amphipathic α-helical domain that is essential for killing activity and heterodimerization with other Bcl-2 family members. Upon cleavage, Bid translocates to mitochondrial membranes and binds to Bad, which is another Bcl-2 family protein, and induces release of cytochrome c from mitochondria. Cytochrome c in turn causes Apaf-1 to activate caspase-9. Under most conditions, this crosstalk is minimal, and the 2 pathways operate largely independently of each other.5
Inhibitors of caspase-action: IAP proteins
A family of endogenous direct inhibitors of caspases (IAPs) are conserved throughout animal evolution with homologies in viruses, yeast, flies (Drosophila), worms (C elegans),mice, and humans.25-28 All IAPs contain 1 to 3 baculovirus IAP repeat (BIR) domains, which may be involved in the inhibition of caspase activity. In addition, most also possess a carboxy-terminal RING finger motif. The mammalian IAPs, X-IAP, cIAP-1 (MHIB), cIAP-2 (MIHC), ML-IAP, and livin, inhibit active caspase-3 and -7 and the activation of caspase-9 mediated by Apaf-1–cytochrome c, while survivin has been implicated in regulation of cell cycle and mitosis. Because survivin, ML-IAP, and livin are overexpressed in many cancers but not in normal adult tissues, these molecules represent possible targets for the development of drugs that selectively eliminate cancer cells.29-31
A mammalian IAP inhibitor known as Smac or DIABLO binds to IAP family members and neutralizes their antiapoptotic activity. This regulatory effect seems to be part of a mitochondrial positive feedback loop because Smac/DIABLO is a mitochondrial protein that is released together with cytochrome c into the cytosol in apoptotic cells.5
Ligation of death receptors and cellular stress–induced apoptosis
Recent data from our own laboratory and others suggested that anticancer drug–induced cell death may involve the CD95 system. CD95 and CD95-L are constitutively expressed in many tissues and further induced by the appropriate stimuli. Mutations or failure to up-regulate CD95 and CD95-L may result in apoptosis defects of tumor cells.32 Mutation of CD95 in humans or lpr mice results in a lymphoproliferative syndrome caused by the inability to delete long-term activated T cells.33-37 Splenocytes from lpr mice have been found to exhibit decreased sensitivity toward γ-irradiation– or heat shock–induced apoptosis38corresponding to a suggested function of the CD95 system in cellular stress–induced apoptosis. Several investigations have shown that cell lines derived from leukemia, hepatoma, neuroblastoma, colon, breast cancer, brain tumors, and small lung cell carcinoma increase expression of CD95-L upon treatment with chemotherapeutic drugs or radiation.39-52 Drugs that have been observed to enhance CD95-L messenger RNA levels include doxorubicin, etoposide, teniposide, methotrexate, cytarabine, cisplatin, bleomycin, and 5-fluorouracil. Elevated levels of CD95-L protein have also been detected after many of these treatments, although the increase sometimes appears smaller in magnitude than the increase in messenger RNA.53Furthermore, expression of the CD95 receptor increased after drug treatment, especially in cells bearing wild-type p53.44-46,50,54,55 A direct correlation was observed between CD95 receptor density and drug sensitivity, and mutant cell lines resistant to agonistic antibodies to CD95 were also resistant to anticancer drugs.43,56-58 The most significant result from these studies was that drug-induced apoptosis was prevented in some instances by soluble blocking CD95 receptors, neutralizing CD95-L antibodies, or dominant negative FADD, which prevents signaling of death receptors.39-43,45-48,50-52 Thus, cellular stress may involve interaction of CD95 with its ligand and may lower the threshold for the induction of apoptotic signals. Although CD95/CD95-L interaction may regulate certain types of stress-induced apoptosis, CD95-L–independent oligomerization of the CD95 receptor by cytotoxic drugs and UV irradiation can be sufficient to activate caspase-8 in a FADD-dependent manner.59-61 Other death systems, such as the TNF or TRAIL system, may be involved in stress-induced apoptosis, thereby contributing to the redundancy of the apoptosis network under conditions in which one system is blocked.47
However, other results are incompatible with the view that death receptor signaling is essential for drug-induced apoptosis. Comparison of CD95-sensitive and CD95-resistant Jurkat T-lymphoma cells revealed no difference in their sensitivity to a broad range of chemotherapeutic drugs. Blockade of CD95 signaling by antibodies that neutralize either the receptor or CD95-L did not protect lymphoma cells against drug-induced cell death.62,63 Also, CD95-deficient thymocytes from lpr mice or cells that express FLIP or a dominant-negative construct of FADD did not exhibit increased proliferation to γ-irradiation and chemotherapeutic drugs compared with control cells.22,64,65 Furthermore, overexpression of the serpin crmA, which inhibits caspase-8, had no effect on drug-induced apoptosis.63,66 Finally, some groups failed to detect increased levels of CD95-L protein in drug-treated tumor cells,54,67 and there have been problems with the specificity of certain commercially available antibodies to CD95-L.68-71 Other studies in mice containing targeted gene disruptions are consistent with the conclusion that drug-induced apoptosis, at least in nontransformed cell types, is independent of the CD95 system. In particular, FADD−/− and caspase-8−/− fibroblasts are resistant to death mediated by death receptor triggering but not to drug and cellular stress–induced apoptosis.72,73 In contrast, caspase-9−/− embryonic stem cells are sensitive to death receptor–mediated apoptosis but exhibit marked decreases in drug-induced apoptosis.74,75 Likewise, Apaf-1−/− thymocytes are sensitive to CD95 but resistant to cellular stress–induced apoptosis.76,77 Collectively, these observations suggest that most anticancer drugs can trigger apoptosis in the absence of a functional CD95/CD95-L pathway, although the CD95/CD95-L pathway might contribute under certain circumstances to apoptosis in response to drug treatment. In this context, Jiang et al78 recently contributed a surprising finding to the ongoing discussion. HepG2 cells, which do or do not constitutively express CD95, were used. Agonistic CD95 antibody induced apoptosis only in the CD95-expressing cell line. However, apoptosis could be induced by cytotoxic drugs in both CD95+ and CD95−cell lines. A blocking anti-CD95 antibody inhibited drug-induced apoptosis in CD95+ but not in CD95− cells. Thus, drug-induced apoptosis may be induced via both CD95-dependent and CD95-independent pathways, eg, mitochondrial death signaling.
The relative contribution of death receptor versus mitochondrial pathways in stress-induced apoptosis may vary depending on the dose and kinetics but may also reflect the existence of 2 different cell types with respect to CD95 signaling: Type I cells undergo CD95-mediated apoptosis without the involvement of mitochondria, whereas type II cells require the release of cytochrome c from mitochondria in order for CD95 to exert its apoptotic effect. At the molecular level, these 2 cell types differ principally in the amount of caspase-8 recruited to CD95 via the adapter molecule FADD to form the DISC. Whereas type I cells contain large amounts of DISC in response to anti-CD95 antibodies, type II cells do not and thus are dependent on stimulation of the intrinsic apoptotic pathway to undergo cell death. Mitochondria are activated in both type I and type II cells but are dispensable for the death of type I cells.5,9,79 With respect to drug-induced apoptosis, a type I response (depending on cross-linked CD95 receptors) has been found in some cell types.80
Caspase-independent apoptosis
Cell death is generally classified into 2 large categories: apoptosis, representing “active” programmed cell death, and necrosis, representing “passive” cell death without (known) underlying regulatory mechanisms. However, there are forms of cell death that cannot be readily classified as apoptosis or necrosis—for example, when cells die by cytoplasmic and membrane changes seen in apoptosis but do not exhibit DNA and/or nuclear fragmentation.81-83 Also, z-VAD-fmk could not rescue cells from apoptosis induced by the overexpression of Bax, although caspase-3 activation and nuclear fragmentation were clearly blocked.84,85 In addition, a mixture of apoptotic and necrotic morphology has been found in some cells, eg, in TNF- and CD95-induced cytotoxicity.86,87 This necroticlike cell death depends on an intact downstream intracellular signaling pathway most likely involving generation of reactive oxygen species (ROS),88 whereas activation of caspases seems to be dispensable.87
The cellular components of caspase-independent apoptosis are not identified so far. In most apoptotic systems z-VAD-fmk does not block mitochondrial changes, such as loss of the membrane potential, production of ROS, or the release of apoptogenic factors such as cytochrome c and AIF.8 Thus, despite caspase inhibition, apoptotic morphology may still be induced by 2 factors: AIF and Bax or Bax-like proteins. How AIF and other released proteins trigger apoptosis in the absence of caspases is unknown. These molecules may activate proteases such as the calcium-dependent calpain proteinases or the lysosomal cathepsins, which can partially substitute caspases but in a less efficient way.81 Cathepsins released from lysosomes cleave Bid, which activates the mitochondrial apoptosis pathway,89 whereas calpains cleave Bax, which promotes cytochrome c release.90-92 Furthermore, calpains are known to cleave and thereby inactivate the cytoprotective endoplasmic reticulum (ER) chaperone glycoprotein GRP94, which contributes to apoptosis.93
Cellular stress and apoptosis
JNK signaling and cellular stress–induced apoptosis
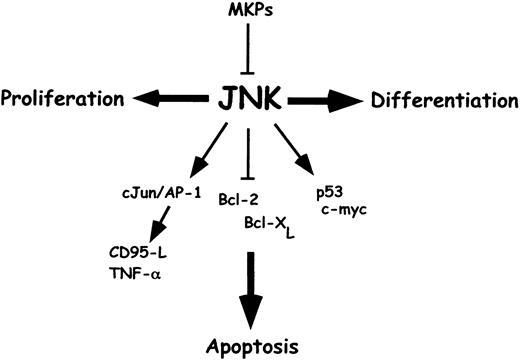
Jun N-terminal kinase (JNK) signaling and c-Jun/AP-1 have been implicated in various, often opposing cellular responses, including proliferation, differentiation, and cellular stress–induced apoptosis (Figure 2). The AP-1 family of transcription factors consists of members of the Jun, Fos, and ATF-2 subfamilies.94 Mammalian Jun proteins include c-Jun, Jun B, and Jun D. Fos proteins include c-Fos, FosB, Fra-1, and Fra-2. ATF proteins that participate in forming AP-1 dimers are ATF-2 and ATF-a. Depending on the stimulus and cellular context, the composition of the dimeric AP-1 complex varies between different members of the Jun, Fos, and ATF family. The activity of individual AP-1 subunits can be regulated either by transcription increasing the intracellular concentration of the proteins or by posttranslational modifications and interaction with other proteins such as members of the mitogen-activated protein kinase (MAPK) signaling pathways.
JNK and cellular stress–induced apoptosis.
JNK signaling has been implicated in proliferation, differentiation, and cellular stress–induced apoptosis. The effects of JNK on cellular apoptosis depend strongly on the cell type and the context of other regulatory influences. JNK signaling can be turned off by dual-specificity MAPK phosphatases. JNK activation results in phosphorylation of AP-1 transcription factor family members such as c-Jun and ATF-2, which then bind to AP-1 binding sites in the promoters of multiple target genes. JNK may contribute to death receptor transcription-dependent apoptotic signaling via c-Jun/AP-1 (leading to promoter induction of CD95-L, TNF-α, and p53) to transcription-independent apoptotic signaling by phosphorylation-dependent posttranslational proapoptotic processes (leading to cytochrome c release, stabilization of p53 protein, inactivation of Bcl-2, Bcl-XL, and activation of c-myc). These mechanisms may function separately or cooperate in induction of apoptosis. JNK signaling in combination with other factors, eg, the suppression of proliferation pathways, may mediate cellular stress–induced apoptosis.
JNK and cellular stress–induced apoptosis.
JNK signaling has been implicated in proliferation, differentiation, and cellular stress–induced apoptosis. The effects of JNK on cellular apoptosis depend strongly on the cell type and the context of other regulatory influences. JNK signaling can be turned off by dual-specificity MAPK phosphatases. JNK activation results in phosphorylation of AP-1 transcription factor family members such as c-Jun and ATF-2, which then bind to AP-1 binding sites in the promoters of multiple target genes. JNK may contribute to death receptor transcription-dependent apoptotic signaling via c-Jun/AP-1 (leading to promoter induction of CD95-L, TNF-α, and p53) to transcription-independent apoptotic signaling by phosphorylation-dependent posttranslational proapoptotic processes (leading to cytochrome c release, stabilization of p53 protein, inactivation of Bcl-2, Bcl-XL, and activation of c-myc). These mechanisms may function separately or cooperate in induction of apoptosis. JNK signaling in combination with other factors, eg, the suppression of proliferation pathways, may mediate cellular stress–induced apoptosis.
The MAPK pathway includes the subfamilies extracellular signal–regulated kinase (ERK), JNK, and p38. These different MAPKs are members of separate modules and are regulated by distinct extracellular stimuli. For example, ERKs are activated by receptor tyrosine kinases and provide proliferation or differentiation signals. JNK and p38-type MAPKs are activated predominantly by stress stimuli and pathogenic insults but in some cell types also by mitogens. All 3 classes of MAPKs are involved in the regulation of distinct AP-1 components. c-Jun is regulated by JNK phosphorylation and in some cell types also by ERK-mediated mechanisms. c-Fos is a substrate for regulatory phosphorylations by ERK, and ATF-2 is regulated by JNK and p38 kinases.95 Due to this complex regulation of AP-1 factors, the range of biological responses is broad. In the following, we focus on how activation of JNK and c-Jun/AP-1 contributes to cellular stress–induced apoptosis.
JNK protein kinases are encoded by 3 genes. While Jnk1 and Jnk2 genes are ubiquitously expressed, expression of the Jnk3 gene is restricted to the brain, heart, and testis. Alternative splicing generates at least 10 different JNK isoforms, which might differ in their substrate specificity. JNK signaling can be turned off by dual-specificity MAPK phosphatases, which often function in a negative feedback loop.96-99
JNK signaling may contribute to apoptosis100-112 or may be dispensable for apoptosis113 and even inhibit apoptosis to promote proliferation and differentiation.114,115 Thus, the effects of JNK on cellular responses appear to depend on the cell type and the context of other signals received by the cell. A clear proapoptotic role of JNK has been demonstrated in studies of mice with targeted disruption of the neuronal gene Jnk3.110JNK3−/− mice are developmentally normal but are defective in the apoptotic response to excitotoxins. Disruption of the ubiquitously expressed Jnk1 or Jnk2 genes in mice causes no obvious phenotype or apoptosis defect. In contrast, compound mutation of Jnk1 plus Jnk2 leads to early embryonic death associated with defects in neuronal apoptosis and exencephaly.116,117 An apoptosis defect was also observed in fibroblasts derived from mice with a mutation in the c-Jun gene.118 119
Among the proapoptotic targets of c-Jun are the promoters of CD95-L and TNF-α, which both contain essential binding sites for AP-1. Expression of these death-inducing ligands is activated by the sequential signaling of JNK and c-Jun/AP-1 following cellular stress and is involved in the induction of cellular stress–induced apoptosis.102 119-128
Substrates for JNK activity also include p53. Dependent on the cellular context, JNK either destabilizes p53 by binding, promoting ubiquitin-mediated degradation, or stabilizes p53 by phosphorylation, whereby inhibiting ubiquitin-mediated degradation.129,130Furthermore, JNK may be involved in regulating transcription of the p53 gene because c-Jun can repress the p53 promoter.131 These data suggest that JNK may be important for controlling the level of p53 expression by regulating the half-life of p53, although these data are discussed with controversy.
An additional potential target of proapoptotic signaling by JNK is the transcription factor c-Myc. Recent studies indicate that c-Myc interacts with JNK and is phosphorylated at Ser62 and Thr71.132 Apoptosis induced by ectopic c-Myc expression in serum-starved cells is associated with increased JNK activity, as concluded from dominant-negative experiments leading to inhibition of JNK signaling and c-Myc–stimulated apoptosis. However, because JNK-induced apoptosis does not require either ectopic c-Myc expression or serum starvation, the role of c-Myc phosphorylation by JNK is unclear.
Despite a function of c-Jun in the regulation of CD95-L, TNF-α, p53, and c-Myc, JNK might enable apoptosis by interfering with mitochondria, resulting in the release of cytochrome c.133 Potential targets of JNK that may regulate cytochrome c release include members of the Bcl-2 group of apoptotic regulatory proteins. Exposure of cells to cellular stress resulted in translocation of JNK to mitochondria.134 In vitro, JNK phosphorylates Bcl-2 and Bcl-XL and may thereby inactivate the death protective function,135 causing cytochrome c release following cellular stress.134 However, Bcl-2 and Bcl-XLmay not be physiologic substrates of JNK because this kinase did not phosphorylate Bcl-2 in vivo.96 Primary fibroblasts prepared from Jnk1−/− Jnk2−/− mouse embryos lack expression of both JNK protein and JNK activity107 and represent a powerful model for the analysis of JNK-induced apoptosis. These JNK null cells exhibit profound defects in cellular stress–induced apoptosis (UV irradiation, anisomycin, MMS [methyl methane-sulfonate]). The defect in apoptosis was caused by defective activation of effector caspases, including caspase-3.107 However, CD95-induced apoptosis was intact, indicating that JNK is not essential for signaling downstream of death receptors and caspase-8. In contrast, JNK null fibroblasts exhibited impaired mitochondrial depolarization and release of cytochrome c,107 suggesting that apoptotic JNK signaling is mediated via mitochondria. However, because JNK activates death ligands (which bind to death receptors to induce apoptosis), JNK may be dispensable but contributes to death receptor–mediated apoptosis.
In conclusion, JNK may induce apoptosis by transcription-dependent signaling (leading to secretion of death ligands), by transcription-independent signaling (leading to cytochrome c release from mitochondria), or by phosphorylation-dependent posttranslational proapoptotic signaling yet to be identified. It is possible that these mechanisms may function separately, but these mechanisms may also cooperate to induce death. Taken together, JNK signaling in combination with other factors, such as the suppression of proliferation pathways, may induce apoptosis following cellular stress.
Endoplasmic reticulum and cellular stress–induced apoptosis
As a protein-folding compartment, the ER is exquisitely sensitive to alterations in homeostasis, for example, induced by cellular stress. Different stimuli signal through several protein kinases to up-regulate the protein-folding capacity of the ER by activation of 2 signaling pathways: the unfolded protein response pathway, leading to the induction of ER chaperones such as grp78/Bip via the C/EBP homologous transcription factor CHOP/GADD153,136 and the ER overload response pathway, leading to the production of cytokines via nuclear factor κB (NF-κB) (Figure 3). Both pathways help the cell to cope with incorrectly folded or accumulated proteins in the ER but may also contribute to its elimination when abnormalities become too extensive.137Consistent with this idea, both CHOP/GADD153 138 and NF-κB have been implicated in apoptosis regulation.139Another mediator of death signaling may be caspase-12, which is localized to the ER and is proteolytically activated by ER stress. Mice that are deficient in caspase-12 are resistant to ER stress-induced apoptosis, but their cells undergo apoptosis in response to other death stimuli.140
Endoplasmic reticulum and cellular stress–induced apoptosis.
The endoplasmic reticulum regulates protein synthesis, N-linked glycosylation, trafficking, and intracellular Ca++ levels. Alterations in homeostasis such as induced by cellular stress induce the unfolded protein response and the ER overload response pathways, which may cope with incorrectly folded proteins in the ER but may also contribute to its elimination when abnormalities become too intensive. The unfolded protein response pathway leads to induction of chaperones such as grp78/Bip via the transcription factor CHOP/GADD153. The ER overload response pathway leads to production of cytokines via NF-κB. Several ER membrane proteins interact with Bcl-2 family members, such as the antiapoptotic Bax inhibitor I and Bap31 and the proapoptoticS pombe calnexin chaperone homolog Cnx1, the reticulon proteins (RTN) NSP-C/RTN1-C, and RTN-XS, or the calcium pump SERCA. Calreticulin, an ER luminal protein, promotes the release of cytochrome c from mitochondria, caspase-3 activity, and DNA fragmentation. Stress in the ER also activates JNKs in several cell types. Thus, the ER, via specific components of its luminal environment, may play an important role in the modulation of cell sensitivity to apoptosis.
Endoplasmic reticulum and cellular stress–induced apoptosis.
The endoplasmic reticulum regulates protein synthesis, N-linked glycosylation, trafficking, and intracellular Ca++ levels. Alterations in homeostasis such as induced by cellular stress induce the unfolded protein response and the ER overload response pathways, which may cope with incorrectly folded proteins in the ER but may also contribute to its elimination when abnormalities become too intensive. The unfolded protein response pathway leads to induction of chaperones such as grp78/Bip via the transcription factor CHOP/GADD153. The ER overload response pathway leads to production of cytokines via NF-κB. Several ER membrane proteins interact with Bcl-2 family members, such as the antiapoptotic Bax inhibitor I and Bap31 and the proapoptoticS pombe calnexin chaperone homolog Cnx1, the reticulon proteins (RTN) NSP-C/RTN1-C, and RTN-XS, or the calcium pump SERCA. Calreticulin, an ER luminal protein, promotes the release of cytochrome c from mitochondria, caspase-3 activity, and DNA fragmentation. Stress in the ER also activates JNKs in several cell types. Thus, the ER, via specific components of its luminal environment, may play an important role in the modulation of cell sensitivity to apoptosis.
Although the effects of Bcl-2 on the mitochondria have been studied intensively, little is known about the effects of Bcl-2 on the ER, where antiapoptotic Bcl-2 family proteins are also localized. Several ER membrane proteins have been reported to interact with Bcl-2 family members to enhance their antiapoptotic effect. Among them is Bax inhibitor I141 and the Bcl-2/Bcl-XL–associated Bap31.21,142,143 Similarly, but in a proapoptotic manner, the Schizosaccharomyces pombe calnexin chaperone homolog Cnx1 interacts with Bak,144 whereas the calcium pump SERCA (sarcoplasmic/endoplasmic reticulum calcium-ATPase) interacts with Bcl-2,145 and members of the ER-anchored reticulon family such as NSP-C and RTN-XS bind to Bcl-XL and Bcl-2,146 thereby contributing to apoptosis.
Apoptotic agents perturbing ER functions such as brefeldin A induce the release of cytochrome c from mitochondria that is blocked by Bcl-2 derived from either mitochondria or ER. Brefeldin A–induced cytochrome c release occurred in a caspase-8– and Bid-independent manner and was followed by caspase-3 activation and DNA/nuclear fragmentation.147 Overexpression of calreticulin, an ER luminal protein, sensitized cells to apoptosis induced by thapsigargin (an agent that promotes ER stress by depletion of luminal calcium stores) and staurosporine (a potent inhibitor of phospholipid/calcium-dependent protein kinase). This correlated with an increased release of cytochrome c from mitochondria. Calreticulin-deficient cells were significantly resistant to apoptosis, correlating to a decreased release of cytochrome c from mitochondria and low levels of caspase-3 activity.148
ER stress may also activate JNKs.149 Lysates from ER-stressed rat pancreatic cells treated with thapsigargin, tunicamycin (which block protein glycoslyation), or dithiothreitol (which interferes with disulfide bond formation) all exhibited increased JNK activity.150 Activation of JNKs by ER stress, although always present, varies in magnitude depending on cell type and is particularly pronounced in cells with a well-developed ER. Coupling of ER stress to JNK activation may be mediated by a mammalian homolog of yeast IRE1, which activates chaperone genes. Overexpression of IRE1 or its mammalian homolog leads to JNK activation, and IRE1α−/− fibroblasts were impaired in JNK activation by ER stress. The cytoplasmic part of IRE1 binds TNF receptor–associated factor-2 (TRAF2), an adaptor protein that couples plasma membrane receptors such as TNF to JNK activation.150 Another hierarchical model for activation of JNKs by ER stress suggests induction of the JNK pathway in Jurkat cells downstream of cytochrome c release and caspase-3.151
Together, these findings implicate that the ER, via specific components of its luminal environment or by interaction among ER, mitochondria, and JNK, may play an important role in the modulation of cell sensitivity toward apoptosis.
p53 and cellular stress–induced apoptosis
Various stress stimuli such as cytotoxic drugs, γ-irradiation, heat shock, hypoxia, osmotic shock, and DNA-damaging agents stabilize the tumor suppressor protein p53, which promotes cell-cycle arrest to enable DNA repair or apoptosis to eliminate defective cells152 (Figure 4). However, it is still largely unknown how p53 selects the pathways of G1 arrest or apoptosis. In this context, the proline-rich domain (residues 64-92)153,154 and a recently identified transcriptional activation domain (residues 43-63)155 have been suggested to be necessary for mediation of apoptosis because deletion of either of these 2 domains abolishes this activity. On the other hand, it has been shown that phosphorylation and acetylation play important roles for regulating biological activities of p53.156,157Although the roles of these modifications are not fully characterized, they are likely to play roles in regulating the binding of p53 with its negative regulator, Mdm2. Other negative regulatory mechanisms involve binding of JNK to p53, which mediates ubiquitination and proteolytic removal of p53,129 and the retinoblastoma gene product (Rb), which prevents the apoptotic function of p53.158,159Both p53 inhibitors, Rb and Mdm2, are cleaved by caspases during apoptosis,160-162 suggesting a positive self-regulation of programmed cell death and close connection to key cell-cycle regulators.
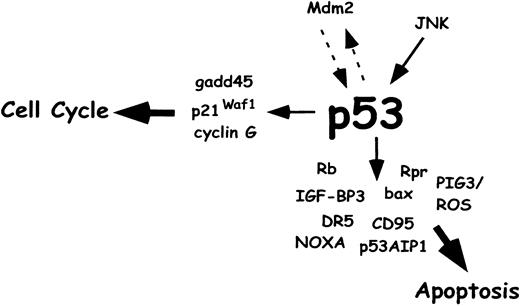
p53 and cellular stress–induced apoptosis.
Various stress stimuli activate the p53 protein, which promotes cell-cycle arrest to enable DNA repair or apoptosis to eliminate defective cells. A key player in the regulation of p53 is Mdm2, which binds to p53 and inhibits the DNA binding activity as well as the transcription rate of the p53 gene. In a negative feedback loop, p53 binds to the mdm2 gene and stimulates its transcription. Another inhibitor is the Rb protein, which prevents the apoptotic function of p53. p53 induces various target genes, such as the cell-cycle regulators p21Waf1, GADD45, and cyclin G. Proapoptotic p53 target proteins include Bax, CD95, DR5, IGF-BP3, NOXA, p53AIP1, and (in Drosophila) Rpr. ROS production may be mediated by the p53-inducible gene PIG3 and may contribute to cytochrome c release from mitochondria. Taken together, the apoptotic target genes of p53 may need to act in concert by activating parallel apoptotic pathways to mount a full apoptotic response.
p53 and cellular stress–induced apoptosis.
Various stress stimuli activate the p53 protein, which promotes cell-cycle arrest to enable DNA repair or apoptosis to eliminate defective cells. A key player in the regulation of p53 is Mdm2, which binds to p53 and inhibits the DNA binding activity as well as the transcription rate of the p53 gene. In a negative feedback loop, p53 binds to the mdm2 gene and stimulates its transcription. Another inhibitor is the Rb protein, which prevents the apoptotic function of p53. p53 induces various target genes, such as the cell-cycle regulators p21Waf1, GADD45, and cyclin G. Proapoptotic p53 target proteins include Bax, CD95, DR5, IGF-BP3, NOXA, p53AIP1, and (in Drosophila) Rpr. ROS production may be mediated by the p53-inducible gene PIG3 and may contribute to cytochrome c release from mitochondria. Taken together, the apoptotic target genes of p53 may need to act in concert by activating parallel apoptotic pathways to mount a full apoptotic response.
Whereas we do not entirely understand how p53 exerts its effects on cells, it is clear that the transcriptional activating function of p53 is a major component of its biological effects. Many p53 target genes have been identified, and those functions have been characterized. Cell-cycle arrest that is dependent on p53 requires transactivation of p21Waf1, GADD45, and cyclin G. Proapoptotic p53 target proteins include Bax, PIG genes, CD95, DR5 (a receptor for the death ligand TRAIL), IGF-BP3, Rpr (in Drosophila), Cdc42 (a Ras-like GTPase), Noxa (a Bcl-2 family protein), and p53AIP1.15,152 163-169
The mechanism of p53-induced apoptosis has been extensively studied and involves activation of the mitochondrial Apaf-1/caspase-9 pathway,170 death receptor signaling,50,171,172 and cleavage of downstream caspases.173 For example, cells expressing mutant p53 fail to induce CD95 and are less sensitive to drug-induced apoptosis.50,174 Independently of transcription, p53 may facilitate the transport of CD95 from Golgi stores to the membrane, leading to death receptor aggregation.171 However, in some cases CD95 is not essential for p53-mediated apoptosis, and p53-dependent up-regulation of CD95 does not induce apoptosis per se.175 An additional route by which p53 may signal apoptosis is through the production of ROS, which influence the mitochondrial membrane potential without involving cytochrome c release.173,176 In particular, the p53-inducible gene PIG3 shares homology with an NADPH-quinone oxidoreductase, which generates ROS. When overexpressed alone, PIG3 failed to initiate apoptosis, implying that other signals must be activated in parallel.177 Recently, p53 itself was shown to cause caspase activation in cell-free extracts from E1A/ras-transformed, but not normal, fibroblasts by a mechanism independent of transcription or presence of Bax or cytochrome c.178 Oncogene-dependent activation of caspases by p53 was also mediated by the c-Myc oncogene, a finding consistent with the requirement of caspase-9 and Apaf-1 in p53-dependent Myc-induced apoptosis.179 Thus, p53 can transduce apoptotic signals through protein-protein interactions, thereby modulating p53-dependent caspase activation. Another mechanism by which p53 promotes apoptosis is through activation of the Ras-like GTPase Cdc42, which activates the JNK1-induced phosphorylation of Bcl-2.168
Taken together, apoptosis mediated by or involving p53 consists of parallel or sequential activation of a set of different molecules and pathways that may need to act in concert to activate a full death response.
NF-κB and cellular stress–induced apoptosis
NF-κB activity is required for the induction of more than 150 genes involved in cell growth, differentiation, development, apoptosis, and adaptive responses to changes in cellular redox balance (Figure5). A wide variety of external stimuli including cytokines, pathogens, stress, and chemotherapeutic agents can lead to the activation of NF-κB.180 These stimuli induce phosphorylation and subsequent degradation of IκB inhibitory proteins, thereby releasing NF-κB proteins for translocation to the nucleus to function as transcription factors.181Phosphorylation of IκB is mediated by a protein complex containing 2 kinases, IκB kinase α and β (IKK-1 and IKK-2), and a noncatalytic regulatory subunit called IKKγ.182 NF-κB transcription factors are heterodimer and homodimer complexes of related proteins that contain a Rel homology domain involved in specific DNA binding, protein dimerization, and nuclear import.180 The Rel proteins predominantly found in mammalian cells consist of 2 transcriptionally inactive forms, NF-κB1 (p50) and NF-κB2 (p52), and 3 transcriptionally active subunits known as RelA (p65), c-Rel, and RelB.180
NF-κB and cellular stress–induced apoptosis.
NF-κB activity is required for the induction of more than 150 genes involved in cell growth, differentiation, development, apoptosis, and adaptive responses to changes in cellular redox balance. NF-κB is bound by IκB, which prevents NF-κB activity. NF-κB target genes with antiapoptotic function include the IAP family, TRAF1 and TRAF2, thought to suppress caspase-8 activation, the prosurvival Bcl-2 homologs Bfl1/A1 and Bcl-XL, and nitrous oxide synthase–inducible genes. The apoptotic signaling of NF-κB may be due to the promoter activation of death receptors and ligands such as CD95, CD95-L, TNF-α, and the TRAIL receptors DR4 and DR5.
NF-κB and cellular stress–induced apoptosis.
NF-κB activity is required for the induction of more than 150 genes involved in cell growth, differentiation, development, apoptosis, and adaptive responses to changes in cellular redox balance. NF-κB is bound by IκB, which prevents NF-κB activity. NF-κB target genes with antiapoptotic function include the IAP family, TRAF1 and TRAF2, thought to suppress caspase-8 activation, the prosurvival Bcl-2 homologs Bfl1/A1 and Bcl-XL, and nitrous oxide synthase–inducible genes. The apoptotic signaling of NF-κB may be due to the promoter activation of death receptors and ligands such as CD95, CD95-L, TNF-α, and the TRAIL receptors DR4 and DR5.
Different NF-κB transcription factors may play diverse and even opposing roles in modulating cell death by apoptosis. In certain settings, c-Rel has been associated with promoting apoptosis. Increased expression of c-Rel protein and its accumulation in the nucleus correlate with induction of apoptosis in various tissues.128,183-185 In contrast, a variety of studies in knockout mice have demonstrated the importance of RelA and c-Rel in prevention of apoptosis because mice lacking NF-κB activity die during embryogenesis.186,187 Similarly, overexpression of RelA/NF-κB protects cells from TNF-α or chemotherapy-mediated apoptosis,114,186,188-191 whereas inhibition of NF-κB restored apoptosis sensitivity of drug-resistant primary leukemic cells and leukemic cell lines.189 Thus, activation of NF-κB transcription factors in different settings can control apoptosis in quite opposite manners.
NF-κB target genes that may provide antiapoptotic function include the IAP family of caspase inhibitory proteins, TRAF1 and TRAF2, thought to suppress caspase-8 activation, the prosurvival Bcl-2 homolog proteins Bfl1/A1 and Bcl-XL,192,193 and inducible nitrous oxide synthase genes194 whose metabolites have been linked to inhibiton of apoptosis.195 The apoptotic signaling of NF-κB may be due to the promoter activation of death receptors and death ligands such as CD95, CD95-L,120and the TRAIL receptors DR4 and DR5.179 However, although diverse studies describe the requirement of NF-κB for induction of CD95-L,120,196-198 the NF-κB signaling pathway is not required for CD95-L induction in other circumstances.199 Also, crosstalk between NF-κB and the caspase pathway has been found. For instance, RIP, the adaptor for induction of NF-κB by TNF-R1, can be cleaved by caspase-8 and this cleavage may play a role in regulating the balance between life and death in response to TNF.200
NF-κB is also able to function in concert with other transcription factors, such as AP-1, whose transcriptional activation involves phosphorylation of JNK. Therefore, the signal transduction cascade following cellular stress results in the activation of parallel kinase cascades regulating AP-1 and NF-κB.201 This dual pathway enhances production of proapoptotic and antiapoptotic proteins dependent on the cellular context.
Ceramide and cellular stress–induced apoptosis
Ceramide, a sphingolipid-derived second messenger molecule, has been described as an important bioeffector molecule involved in cellular stress responses implicated in apoptosis, growth inhibition, and cellular differentiation (Figure 6). Stress stimuli such as TNF, CD95-L, oxidative stress, growth factor withdrawal, chemotherapeutic agents, ionizing or UV radiation, and heat shock induce an elevation in the endogenous levels of ceramide, and exogenous ceramide analogs mimic these biological responses in specific cell types.202-207
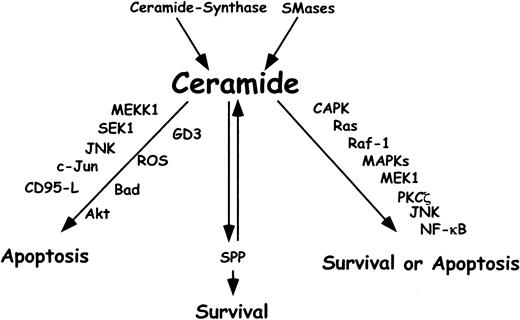
Ceramide and cellular stress–induced apoptosis.
Ceramide is involved in cellular stress responses implicated in apoptosis, growth inhibition, and differentiation. The major source of ceramide is hydrolysis of sphingomyelin by SMases. De novo synthesis via ceramide synthase may also lead to the generation of ceramide. Ceramide acts as a catalyst for apoptosis through the consecutive activation of MEKK1, SEK1, JNK, c-Jun, and death-inducing ligands such as CD95-L. BAD, a proapoptotic Bcl-2 family member, is induced by a ceramide-mediated pathway involving CAPK, Ras, c-Raf-1, and MEK1. Other mediators of ceramide-induced apoptosis are ROS and the ganglioside GD3, which both affect mitochondria. Ceramide acts also upstream of the antiapoptotic kinase Akt, leading to a decrease in its activity. Activation of the stress response by ceramide leads to either survival or apoptosis. Members of this signaling cascade are CAPK, Ras, c-Raf-1, MEK1, PKC-ζ, JNK, and NF-κB. SPP results from the catabolic pathway for ceramide and acts as a second messenger in cellular proliferation and survival.
Ceramide and cellular stress–induced apoptosis.
Ceramide is involved in cellular stress responses implicated in apoptosis, growth inhibition, and differentiation. The major source of ceramide is hydrolysis of sphingomyelin by SMases. De novo synthesis via ceramide synthase may also lead to the generation of ceramide. Ceramide acts as a catalyst for apoptosis through the consecutive activation of MEKK1, SEK1, JNK, c-Jun, and death-inducing ligands such as CD95-L. BAD, a proapoptotic Bcl-2 family member, is induced by a ceramide-mediated pathway involving CAPK, Ras, c-Raf-1, and MEK1. Other mediators of ceramide-induced apoptosis are ROS and the ganglioside GD3, which both affect mitochondria. Ceramide acts also upstream of the antiapoptotic kinase Akt, leading to a decrease in its activity. Activation of the stress response by ceramide leads to either survival or apoptosis. Members of this signaling cascade are CAPK, Ras, c-Raf-1, MEK1, PKC-ζ, JNK, and NF-κB. SPP results from the catabolic pathway for ceramide and acts as a second messenger in cellular proliferation and survival.
Hydrolysis of sphingomyelin, a main lipid in plasma membranes of mammalian cells, is the major source of ceramide. Sphingomyelin hydrolysis may occur via the action of sphingomyelin-specific forms of phospholipase C, termed sphingomyelinases (SMases), which are defined by their pH optima as neutral (nSMase) or acid (aSMase). These enzymes are activated in response to TNF and other cytokines. De novo synthesis via ceramide synthase may also lead to the generation of ceramide.203 205
The catabolic pathway for ceramide involves deacetylation by ceramidases to generate sphingosine, which is phosphorylated by sphingosine kinase to form sphingosine-1-phosphate (SPP). SPP in turn acts as a second messenger in cellular proliferation and survival induced by platelet-derived growth factor or serum. Previously, a model has been proposed in which the dynamic balance between the intracellular levels of ceramide and SPP is an important factor that determines whether a cell survives or dies. According to this model, stress stimuli such as TNF activate SMases, leading to increased intracellular ceramide levels and thus to increased cell death, whereas platelet-derived growth factor and other growth factors stimulate ceramidase and sphingosine kinase and elevate SPP levels, resulting in cellular survival and proliferation.208 209
Mechanisms by which ceramide induces multiple signaling pathways involve the sequential activation of different kinases such as ceramide-activated protein kinase (CAPK), phosphorylation of Raf-1, and the MAPK cascade. Protein kinase C-ζ (PKC-ζ) has been identified as another CAPK that is a critical element in ceramide-induced JNK activation and NF-κB translocation.206,210 Thus, ceramide may act as a catalyst for the stress response kinase cascade through the consecutive involvement of MEKK1, SEK1, JNK, and c-Jun.206 Concomitant activation NF-κB may contribute to enhanced expression of proapoptotic TNF superfamily members such as CD95-L, TRAIL, and TNF-α.211,212 Ceramide also influences mitochondrial apoptosis signaling by the proapoptotic Bcl-2 family member BAD through a pathway involving CAPK, Ras, c-Raf-1, and MEK1.213 ROS generated at the ubiquinone site of the mitochondrial respiratory chain seem also to be necessary for ceramide-induced apoptosis and transcription factor activation.214 215
Another metabolizing pathway for ceramide was proposed by Testi and coworkers.216 Ceramide can be shuttled to the Golgi complex, where it is converted to gangliosides. It was found that CD95 ligation or treatment with ceramide resulted in the accumulation of the ganglioside GD3, an event that was prevented by caspase inhibitors. Antisense oligonucleotides toward GD3 synthetase, which is localized in the Golgi complex, attenuated apoptosis, whereas overexpression of the wild-type enzyme was associated with massive cell death. Thus, GD3 ganglioside may be targeted to mitochondria, where it alters mitochondrial function and causes cell death during CD95-mediated apoptosis.
While the role of ceramide for apoptosis induction, eg, through death receptors, is highly controversial, there is a substantial evidence for a role of ceramide in the initiation of the apoptosis response by cytotoxic drugs and γ-irradiation. Cell lines resistant to γ-irradiation– or doxorubicin-induced apoptosis fail to generate ceramide following these treatments.47,217,218 The phenotype of acid SMase knockout mice resembles the type A form of human Niemann-Pick disease. Lung endothelial cells from knockout mice, as well as lymphoblasts and fibroblasts from Niemann-Pick patients, display defective ceramide generation and are resistant to stress-induced apoptosis although thymocytes are still susceptible.204,212 219
Despite these findings, much confusion remains about the role of endogenous ceramide in apoptosis. Whereas some publications place ceramide upstream of caspases,108,212,220-222 others suggest that it acts downstream of caspases, because it can be blocked by caspase inhibitors.223-226 A possible reason for the discrepancy on the role of ceramides may lie in methodologic problems. Ceramide production is mostly determined in assays using diacylglycerol kinase. In a recent investigation, no ceramide production in response to CD95 ligation could be detected using mass spectroscopy, whereas an apparent increase of ceramide was measured by the classical diacylglycerol kinase assay.227 It was suggested that lysates from apoptotic cells may stimulate diacylglycerol kinase activity directly, which then increases ceramide production.
Thus, depending on the cell line used and the experimental setup, the effects of ceramide generation ranged from induction of apoptosis and cell-cycle arrest to proliferation and terminal differentiation.
Conclusion
Cytotoxic drugs have been developed for the treatment of leukemia and malignant tumors based on their capacity to inhibit cellular proliferation.228 While an overwhelming amount of data indicate that cytotoxic drugs induce and activate molecules of the apoptosis and cellular stress response pathway, a number of key questions are still open and unresolved. For example, the view that apoptosis represents the main mechanism by which tumor cells are killed by cancer therapy may not be universally true.229,230Problems in identifying apoptosis signaling as a key event may be related to the assay used to detect drug-induced cell death. For example, cells that have received sufficient DNA damage to be unable to proliferate may die because of mitotic disaster, which is not detected in apoptosis assays but only in clonogenicity assays. Key elements of the cell death pathway are closely linked to other complex signaling systems such as the DNA damage response and cell-cycle control, which complicates the identification of individual compounds in the clinical setting.231,232 Most importantly, while in vitro assays have convincingly demonstrated that deregulated expression of apoptosis-mediating molecules may confer drug resistance,17 53 results of clinical studies in patients are less clear. Mutations of caspases are rarely found in tumors, although these molecules are the crucial effectors of the apoptosis response and would be ideal targets for mutations providing a survival advantage for tumor cells.
Taken together, research on regulation of apoptosis, growth arrest, and DNA repair initiated by the cellular stress response has provided a detailed insight into fundamental cellular mechanisms with widespread clinical implications. Given the importance of cell death, including apoptosis as one possible outcome of the stress response in cells treated by cytotoxic drugs, further studies are required to identify the role of individual regulators of stress signaling and cell death for sensitivity to anticancer therapy. These will include analysis of gene expression profiles, novel proteomic approaches, as well as functional in vitro and in vivo studies in malignant cells from patients undergoing cancer therapy.
We apologize to those whose work was not cited or discussed because of space limitations. We thank R. Zwacka for critical reading and V. Krok-Szwed for assistance in preparing the manuscript.
Supported by the Deutsche Forschungsgemeinschaft, Deutsche Krebshilfe, EU grant, Deutsche Leukämieforschungshilfe, and Sander Stiftung.
@ 2001 by The American Society of Hematologycharge
References
Author notes
Klaus-Michael Debatin, Universitäts Kinderklinik, Prittwitzstr 43, D-89075 Ulm, Germany; e-mail:klaus-michael.debatin@medizin.uni-ulm.de.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal