Abstract
It is here reported that mesenchymal stem cells known to give rise to limb-bud mesoderm can, at the single-cell level, also differentiate into cells of visceral mesoderm and can be expanded extensively by means of clinically applicable methods. These cells were named mesodermal progenitor cells (MPCs). MPCs were selected by depleting bone marrow mononuclear cells from more than 30 healthy human donors of CD45+/glycophorin-A (GlyA)+ cells. Cells were cultured on fibronectin with epidermal growth factor and platelet-derived growth factor BB and 2% or less fetal calf serum. It was found that 1/5 × 103CD45−GlyA− cells, or 1/106 bone marrow mononuclear cells, gave rise to clusters of small adherent cells. Cell-doubling time was 48 to 72 hours, and cells have been expanded in culture for more than 60 cell doublings. MPCs are CD34−, CD44low, CD45−, CD117 (cKit)−, class I–HLA−, and HLA-DR−. MPCs differentiated into cells of limb-bud mesoderm (osteoblasts, chondrocytes, adipocytes, stroma cells, and skeletal myoblasts) as well as visceral mesoderm (endothelial cells). Retroviral marking was used to definitively prove that single MPCs can differentiate into cells of limb bud and visceral mesoderm. Thus, MPCs that proliferate without obvious senescence under clinically applicable conditions and differentiate at the single-cell level not only into mesenchymal cells but also cells of visceral mesoderm may be an ideal source of stem cells for treatment of genetic or degenerative disorders affecting cells of mesodermal origin.
Introduction
Embryonic stem (ES) cells are pluripotent cells derived from blastocysts that can be propagated indefinitely undifferentiated in vitro, can differentiate to all cell lineages in vivo, and can be induced to differentiate to most cell types in vitro.1-4 Although ES cells have been isolated from humans,2,3 their use in research as well as therapeutics is encumbered by ethical considerations.5 The ability to purify, culture, and manipulate multipotent stem cells from nonembryonic origin would provide investigators with an invaluable cell source to study cell and organ development. In addition, such cells could serve to develop replacement tissues for congenital or degenerative disorders. Stem cells have been identified in most organ tissues, including hematopoietic,6 neural,7gastrointestinal,8 epidermal,9hepatic,10 and mesenchymal stem cells (MSCs).11-14
MSCs were first identified by Fridenshtein,11 who demonstrated that when bone marrow (BM) is plated in fetal calf serum (FCS)–containing medium, colonies of adherent fibroblastlike cells develop that differentiate into bone and adipocytes. Since then, several investigators have shown that these cells can also differentiate into chondrocytes, adipocytes, and, at least in rodents, skeletal myocytes.13-15 MSCs can be purified on the basis of their ability to adhere to plastic or with monoclonal antibodies (SH2 and SH4, or Stro1).13 14 Here, we describe for the first time the isolation and ex vivo expansion of cells from postnatal BM that can differentiate at the single-cell level not only into MSCs, but also into cells of visceral mesodermal origin, such as endothelium, which we termed mesodermal progenitor cells (MPCs).
Materials and methods
Bone marrow
BM was obtained from 30 healthy donors (ages 2 to 50 years) following informed consent according to guidelines from the University of Minnesota Committee on the Use of Human Subjects in Research. BM mononuclear cells (BMMNCs), obtained by Ficoll-Paque density gradient centrifugation (Sigma Chemical, St Louis, MO), were depleted of CD45+ and glycophorin-A–positive (GlyA+) cells by means of micromagnetic beads (Miltenyi Biotec, Sunnyvale, CA). The eluted cells were 99.5% CD45p−GlyA−.
Cytokines
Epidermal growth factor (EGF) was from Sigma and platelet-derived growth factor BB (PDGF-BB), insulinlike growth factor (IGF), transforming growth factor β (TGF-β1), basic fibroblast growth factor (bFGF), acidic FGF (aFGF), bone morphogenetic protein 4 (BMP-4), interleukin-1α (IL-1α), IL-3, and IL-6 were from R&D Systems (Minneapolis, MN). Fetal liver tyrosine kinase 3 (Flt3) and granulocyte-macrophage colony-stimulating factor (GM-CSF) were from Immunex (Seattle, WA), and stem cell factor (SCF), granulocyte CSF (G-CSF), erythropoietin, and thrombopoietin were from Amgen (Thousand Oaks, CA). Vascular endothelial growth factor B (VEGF-B) was a gift from Dr S. Ramakrishnan, University of Minnesota.
Antibodies
Antibodies against fast-twitch skeletal myosin, sarcomeric actin, β-actin, fibroblast surface antigen (1B10), and control mouse immunoglobulin G (IgG) were from Sigma. Antibodies against Tie, Tek, Flt1, fms-related tyrosine kinase gene 1 (Flk1), type II collagen, type I collagen, peroxisome proliferator activated receptor (PPAR)–γ, von Willebrand factor (vWF), MYO-D, and myogenin were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against CD13, CD31, CD34, CD36, CD38, CD44, CD49b, CD49e, CD50, CDw90, CD117, HLA-DR, β2-microglobulin, and class I HLA were from Becton Dickinson (Mountain View, CA). Antibodies against osteonectin (LF-7), osteopontin (BON-1), and bone sialoprotein (LF-100) were a gift from Dr L. Fisher, National Institutes of Health (Bethesda, MD). H1P12 was a gift from Dr R. Hebbel, University of Minnesota. Secondary goat anti–mouse or goat anti–rabbit antibodies were from Dako (Carpinteria, CA).
Cell lines
AFT024, a fetal liver myofibroblast feeder, kindly provided by Dr I. Lemishka, Princeton University, was maintained and subcultured as described.16 The HT85 human osteosarcoma cell line was obtained from ATCC (Manassas, VA). The cell line was maintained in high-glucose Dulbecco modified essential medium (DMEM; Gibco BRL, Grand Island, NY) with 10% FCS (Hyclone Laboratories, Logan, UT) until use.
MPC and differentiation culture conditions
MPC cultures.
We plated 5 to 10 × 103CD45−GlyA− cells in 1 mL expansion medium supplemented with 10 ng/mL EGF and 10 ng/mL PDGF-BB in wells of 96-well plates coated with 10 ng/mL fibronectin (FN), laminin, or type IV collagen (Sigma). Expansion medium consisted of 60% low-glucose DMEM (Gibco BRL), 40% MCDB-201 (Sigma), 1 × insulin transferrin selenium, 1 × linoleic acid bovine serum albumin (BSA), 10−9 M dexamethasone (Sigma), and 10−4 M ascorbic acid 2-phosphate (Sigma), 100 U penicillin, and 1000 U streptomycin (Gibco). In some conditions, we added 2% FCS, 10% FCS, 10 ng/mL IGF-1, 10 ng/mL aFGF, 10 ng/mL bFGF, or 10 ng/mL BMP-4. Once adherent cells were more than 50% confluent, they were detached with 0.25% trypsin-EDTA (Sigma) and replated at a 1:4 dilution under the same culture conditions.
Limiting dilutions.
To assess MPC frequency, we plated CD45−GlyA−cells at 5 × 104/mL (n = 2 to 4 replicates), 1 × 104/mL (n = 3 to 5 replicates), 5 × 103/mL (n = 5 to 10 replicates), 1 × 103/mL (n = more than 10 replicates), and 0.5 × 103/mL in FN-coated wells of 96-well plates. We determined the frequency of MPC precursors in CD45−GlyA− BMMNCs by means of Poisson statistics.17
Differentiation cultures.
To induce differentiation, we used MPC expansion medium without serum, EGF, and PDGF-BB unless otherwise indicated, supplemented with factors as indicated.
Western blotting
Protein lysates, obtained from MPCs or differentiated progeny as previously described,18 were separated on precast 7.5% to 10% polyacrylamide gel electrophoresis gels (Bio-Rad, Hercules, CA). After blocking, blots were incubated with rabbit polyclonal antibodies against Tek, type II collagen, β-actin at 1:200, goat polyclonal antibodies against PPAR-γ, vWF at 1:100, and mouse monoclonal antibodies against fast-twitch skeletal myosin at 1:2000, washed and incubated with a goat antimouse or antirabbit horseradish peroxidase–conjugated antibody (1:10 000 dilution) (E. I. du Pont de Nemours, Boston, MA). Bands were visualized by means of an electrogenerated chemiluminescence (ECL) detection system.
Immunohistochemistry and immunofluorescence
Immunohistochemistry.
Proteins were visualized by means of the labeled streptavidin biotin (LSAB+) immunoperoxidase system (Dako). Cells were fixed with 4% paraformaldehyde at 20°C, and endogenous peroxidase activity was quenched with 3% peroxide for 5 minutes. Slides were incubated sequentially for 30 minutes each with rabbit polyclonal antibodies against LF-7, BON-1, and LF-100 (1:400); rabbit polyclonal antibodies against type I and II collagen (1:50); and rabbit polyclonal antibodies against fast-twitch myosin (1:50), followed by biotin-labeled anti–rabbit or anti–goat IgG antibody and streptavidin and then for 10 minutes with 3-amino-9-ethylcarbazole.
For Von Kossa staining, cells were fixed with methanol at −20°C for 2 minutes, treated with 2% silver nitrate (Sigma) in a clear glass coplin jar placed directly in front of a 60-W lamp for 1 hour. Slides were rinsed in dH2O, fixed with 2.5% sodium thiosulphate (Sigma) for 5 minutes, and washed in dH2O. Cells were counterstained with 1% neutral red (Sigma) for 1 minute and rinsed in tap water.
For toluidine blue staining, cells were fixed with methanol at −20°C for 2 minutes and stained in 1% toluidine blue (Sigma) in 50% isopropanol at 37°C for 30 minutes. Slides were dehydrated in 100% isopropanol and mounted.
For oil-red staining, cells were fixed with methanol at −20°C for 2 minutes and rinsed in 50% alcohol. Slides were stained in oil-red-O (Sigma) for 10 minutes and rinsed in 50% alcohol. After rinsing in dH2O, slides were counterstained with Mayer hematoxylin (Sigma) for 1 minute.
For alkaline phosphatase staining, cells were fixed with methanol at −20°C for 2 minutes and washed in 100 mM Tris-HCl, pH 9.5, 100 mM NaCl, and 10 mM MgCl2 buffer (all from Sigma) for 10 minutes. Slides were then stained with fast 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium alkaline phosphatase substrate (Sigma) for 5 to 10 minutes and rinsed in dH2O.
Immunofluorescence.
For staining of cytoskeletal proteins, cells were fixed with methanol at −20°C for 2 minutes and permeabilized with 0.1% Triton X-100 for 10 minutes. For other intracellular molecules, cells were fixed with 4% paraformaldehyde at 20°C for 10 minutes and permeabilized with 0.1% triton X-100 for 10 minutes. For cell surface receptors, cells were fixed with 4% paraformaldehyde at 20°C for 10 minutes. Blocking and diluent solution consisted of phosphate-buffered saline (PBS), 1% BSA, and 1% serum (Sigma) from species similar to the species in which the primary antibody was raised. Slides were blocked for 30 minutes, incubated sequentially for 30 minutes each with antibodies against CD34 (1:50), CD36 (1:50), vWF (1:1:50), or fast-twitch myosin (1:50) and fluorescein- or phycoerythrin-coupled anti–mouse or anti–rabbit IgG antibody. Between each step, slides were washed with PBS plus 0.3% BSA.
Fluorescence-activated cell sorting.
For fluorescence-activated cell sorting (FACS), cells were detached and stained sequentially with primary antibodies and immunofluorescent secondary antibodies and fixed with 2% paraformaldehyde until analysis with a FACSCalibur (Becton Dickinson). For detection of intracellular proteins, cells were permeabilized with methanol/PBS for 2 minutes at −20°C.
Hematopoietic assays
MPCs were plated at 20 000/cm2, treated with 10 U IL-1 for 24 hours in expansion medium, and maintained afterward in Iscoves modified Dulbecco medium (IMDM) (Gibco BRL) plus 12.5% FCS plus 12.5% horse serum and 10−7 M hydrocortisone. MPC-derived stroma and confluent AFT024 cells were irradiated at 2 Gy as described.16 Cord blood CD34+ cells, purified as previously described,19 were plated in contact with irradiated MPC-derived stroma or AFT024 feeders in IMDM plus 12.5% FCS plus 12.5% horse serum (Hyclone) and 10−7 M hydrocortisone (Sigma) for 2 weeks. After 2 weeks, cells were harvested and plated in clonogenic methylcellulose medium, and colony-forming cells (CFCs) were enumerated.16
Karyotyping
Telomere length measurements
Telomere length was measured by means of the Telomere Length Assay Kit from Pharmingen (Franklin Lakes, NJ) according to the manufacturer's recommendations.
Transduction
Retroviral supernatant was produced by incubating a recombinant variant of Moloney murine leukemia virus (MFG) and enhanced green fluorescent protein (eGFP) containing PG13 cells (provided by Dr G. Wagemaker, University of Rotterdam, The Netherlands)21with MPC expansion medium for 48 hours, filtered and frozen at −80°C. MPCs were incubated with retroviral supernatants and 8 μg/mL protamine (Sigma) for 6 hours. This was repeated 24 hours later. Transduction efficiency was analyzed by FACS.
Southern blot analysis for retroviral insert
DNA from 0.5 to 5 × 106 MPCs or endothelial- or myoblast-differentiated progeny was prepared from cells by standard methods and digested overnight with NcoI (a single cutter in eGFP) or EcoRI (a single cutter between eGFP and the 3′ long terminal repeat). Fragments were separated by gel electrophoresis, blotted to nylon, and probed with biotin-labeled eGFP–complementary DNA (cDNA) or MFG–internal ribosome entry site (IRES)–eGFP-cDNA. Hybridization of DNA digested withEcoRI with the eGFP-cDNA probe should yield a single band, whereas hybridization of DNA digested with NcoI with the MFG-IRES-eGFP-cDNA probe should yield 2 bands per retroviral insert. Detection was by ECL.
Results and discussion
Culture of undifferentiated MPCs
Our initial hypothesis was that mesodermal progenitors are present in marrow but differ from hematopoietic progenitors. We therefore selected cells that do not express hematopoietic markers (CD45 and GlyA). Following 2 depletion steps using Macs immunomagnetic beads (Miltenyi Biotec), mononuclear cells were greater than 99.5% CD45−GlyA−. CD45−GlyA− cells constitute 0.1% to 0.5% of BMMNCs. CD45−GlyA− cells were plated in wells coated with 10 μg/mL FN with expansion medium. After 7 to 21 days, small clusters of adherent cells developed (Figure1A). Limiting dilution assays (n = 5) showed that the frequency of cells giving rise to these adherent clusters is 0.02% to 0.08% of CD45−GlyA−cells. CD45+ or GlyA+ BMMNCs did not give rise to adherent cells.
Morphology of MPCs.
CD45−GlyA− cells were plated on FN in expansion medium with 2% FCS, 10 ng/mL EGF, and 10 ng/mL PDGF-BB and passaged for 15 cell doublings (A) and 53 cell doublings (B) to maintain a cell density between 2 and 8 × 103/cm2. Alternatively, MPCs were maintained for 15 cell doublings in MPC medium containing 10% FCS (C) or at a density greater than 104/cm2 (D). MPCs cultured at low density and with 2% or less FCS are small (10 to 15 μm diameter) and have scant cytoplasm with few vacuoles or granules. In contrast, MPCs cultured either with 10% FCS or at higher density are significantly larger (greater than 20 μm diameter) and often contain vacuoles or granules. Shown are phase-contrast pictures at 80 × magnification. A representative example of more than 5 experiments is shown.
Morphology of MPCs.
CD45−GlyA− cells were plated on FN in expansion medium with 2% FCS, 10 ng/mL EGF, and 10 ng/mL PDGF-BB and passaged for 15 cell doublings (A) and 53 cell doublings (B) to maintain a cell density between 2 and 8 × 103/cm2. Alternatively, MPCs were maintained for 15 cell doublings in MPC medium containing 10% FCS (C) or at a density greater than 104/cm2 (D). MPCs cultured at low density and with 2% or less FCS are small (10 to 15 μm diameter) and have scant cytoplasm with few vacuoles or granules. In contrast, MPCs cultured either with 10% FCS or at higher density are significantly larger (greater than 20 μm diameter) and often contain vacuoles or granules. Shown are phase-contrast pictures at 80 × magnification. A representative example of more than 5 experiments is shown.
When clusters appeared (about 103 cells), cells were replated every 3 to 5 days at a 1:4 dilution under the same culture conditions. Cell densities were maintained between 2 and 8 × 103/cm2. Cell-doubling time was 48 to 60 hours for the initial 20 to 25 cell doublings and increased to 72 hours after 25 to 30 cell doublings. Immunophenotypic analysis by FACS of cells obtained after 10 to 15 cell doublings (n = more than 15) showed that cells did not express CD10, CD31, CD34, CD36, CD38, CD50, CD62E, CD106, CD117, H1P12, fibroblast surface antigen (1B10), HLA-DR, class I HLA (Figure 2A), CD45, Tie, or Tek (not shown). Cells expressed low levels of β2-microglobulin, CD44, CDw90, KDR, and Flt1 and high levels of CD13 and CD49b. MPC morphology (Figure 1B) and phenotype (Figure 2B) remained unchanged for more than 40 cell doublings (n = 10). We have established cultures with MPCs capable of proliferating beyond 30 cell doublings and differentiating to all mesodermal cell types (see below) from greater than 85% of donors, ages 2 to 50 years, and MPCs have been expanded for more than 50 cell doublings in at least 10 donors.
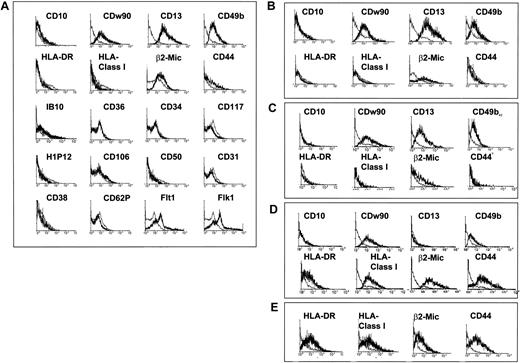
Phenotype of MPCs.
CD45−GlyA− cells were plated on FN in expansion medium with 10 ng/mL EGF and 10 ng/mL PDGF-BB, and passaged for 15 to 40 cell doublings. Cells were harvested and labeled with antibodies against CD10, CD13, CD31, CD34, CD36, CD38, CD44, CD49b, CD50, CD62P, CDw90, CD106, CD117, H1P12, IB10, KDR, Flt1, HLA-DR, class I HLA, and β2-microglobulin or control IgGs, as indicated and analyzed by FACS. Plots show isotype control IgG-staining profile (thin line) versus specific antibody staining profile (thick line). A representative example of more than 5 experiments is shown. (A) Cells were cultured with 2% FCS at a cell density between 2 and 8 × 103/cm2 for 15 cell doublings. (B) cells were cultured with 2% FCS at a cell density between 2 and 8 × 103/cm2 for 40 cell doublings. (C) Cells were cultured without FCS but with 10 ng/mL IGF-1 at a cell density between 2 and 8 × 103/cm2 for 15 cell doublings. (D) Cells were plated with 10% FCS, at a cell density between 2 and 8 × 103/cm2 for 15 cell doublings. (E) Cells were plated with 2% FCS at a cell density between greater than 104/cm2 for 15 cell doublings.
Phenotype of MPCs.
CD45−GlyA− cells were plated on FN in expansion medium with 10 ng/mL EGF and 10 ng/mL PDGF-BB, and passaged for 15 to 40 cell doublings. Cells were harvested and labeled with antibodies against CD10, CD13, CD31, CD34, CD36, CD38, CD44, CD49b, CD50, CD62P, CDw90, CD106, CD117, H1P12, IB10, KDR, Flt1, HLA-DR, class I HLA, and β2-microglobulin or control IgGs, as indicated and analyzed by FACS. Plots show isotype control IgG-staining profile (thin line) versus specific antibody staining profile (thick line). A representative example of more than 5 experiments is shown. (A) Cells were cultured with 2% FCS at a cell density between 2 and 8 × 103/cm2 for 15 cell doublings. (B) cells were cultured with 2% FCS at a cell density between 2 and 8 × 103/cm2 for 40 cell doublings. (C) Cells were cultured without FCS but with 10 ng/mL IGF-1 at a cell density between 2 and 8 × 103/cm2 for 15 cell doublings. (D) Cells were plated with 10% FCS, at a cell density between 2 and 8 × 103/cm2 for 15 cell doublings. (E) Cells were plated with 2% FCS at a cell density between greater than 104/cm2 for 15 cell doublings.
When MPCs were cultured in serum-free medium supplemented with 10 ng/mL IGF-1, cell doubling was slower (longer than 60 hours), but more than 50 cell doublings could be obtained. As was seen for cells cultured with 2% FCS, MPCs in serum-free cultures with IGF-1 were small (not shown), did not express HLA-DR and class I HLA, expressed low levels of CD44 (Figure 2C), and differentiated into all mesodermal phenotypes (see below). Thus, in contrast to the phenotype reported for MSCs,12-14 MPCs cultured at low density and with 2% or lower FCS are class I–HLA− and HLA-DR− and express only low levels of CD44.
Average telomere length of MPCs from 5 donors (ages 2 to 50 years) cultured for 35 cell doublings on FN with 2% FCS, 10 ng/mL EGF, and 10 ng/mL PDGF at densities between 2 and 8 × 103/cm2 was between 11 and 15 kilobases (kb) (Figure 3A). In 3 of the 5 donors from whom blood mononuclear cells were available, this was approximately 3 kb longer than the telomere length in blood lymphocytes (not shown). Telomere length of cells from one donor evaluated after 15, 35, and 45 cell doublings remained unchanged. Cytogenetic analysis of MPCs recovered after 40 cell doublings (n = 2) showed a normal karyotype (Figure4B). Although this suggests that MPCs may have unlimited cell expansion potential, more extensive culture to beyond 100 cell doublings will be required to fully prove that MPCs are not subject to the Hayflick phenomenon.22 Studies are ongoing to determine telomerase activity in these cells.
Characteristics of culture-expanded MPCs.
Culture-expanded MPCs have long telomeres and are cytogenetically stable. (A) CD45−GlyA− cells from 4 donors ages 2 to 50 were plated on FN in expansion medium with 2% FCS, 10 ng/mL EGF, and 10 ng/mL PDGF-BB, and passaged for 35 cell doublings. Cells were harvested and telomere lengths evaluated. Lanes 1 = 22 years; lane 2 = 35 years; lane 3 = 50 years; lane 4 = 2 years; lane 5 = 293 cells; lane 6 = HL60 cells. ATL indicates average telomere length. (B) CD45−GlyA− cells were plated on FN in expansion medium with 2% FCS, 10 ng/mL EGF, and 10 ng/mL PDGF-BB and passaged for 40 cell doublings. Cells were harvested 24 hours after replating and were cytogenetically analyzed. A representative example of 4 experiments is shown.
Characteristics of culture-expanded MPCs.
Culture-expanded MPCs have long telomeres and are cytogenetically stable. (A) CD45−GlyA− cells from 4 donors ages 2 to 50 were plated on FN in expansion medium with 2% FCS, 10 ng/mL EGF, and 10 ng/mL PDGF-BB, and passaged for 35 cell doublings. Cells were harvested and telomere lengths evaluated. Lanes 1 = 22 years; lane 2 = 35 years; lane 3 = 50 years; lane 4 = 2 years; lane 5 = 293 cells; lane 6 = HL60 cells. ATL indicates average telomere length. (B) CD45−GlyA− cells were plated on FN in expansion medium with 2% FCS, 10 ng/mL EGF, and 10 ng/mL PDGF-BB and passaged for 40 cell doublings. Cells were harvested 24 hours after replating and were cytogenetically analyzed. A representative example of 4 experiments is shown.
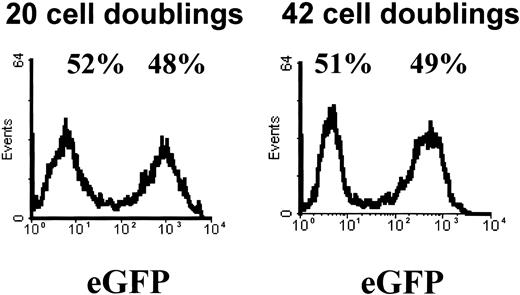
Retroviral transduction of MPCs.
MPCs were subcultured at a 1:4 dilution and then subjected to 2 sequential transductions 24 hours apart with a gibbon ape leukemia virus (GALV)–pseudotyped MFG-eGFP vector. Transduction efficiency was examined by FACS analysis for eGFP. Shown is the result of a representative example of more than 10 experiments on day 3 after transduction and again after 22 additional population doublings.
Retroviral transduction of MPCs.
MPCs were subcultured at a 1:4 dilution and then subjected to 2 sequential transductions 24 hours apart with a gibbon ape leukemia virus (GALV)–pseudotyped MFG-eGFP vector. Transduction efficiency was examined by FACS analysis for eGFP. Shown is the result of a representative example of more than 10 experiments on day 3 after transduction and again after 22 additional population doublings.
When plated on type IV collagen or laminin instead of FN, cells expressed CD44 and class I HLA (not shown), similar to the phenotype described for MSCs, and proliferated poorly beyond 30 cell doublings. This suggests that signaling through the α4β1 or α5β1 integrin is required for maintaining the more primitive MPC phenotype. When EGF or PDGF was omitted, cells did not proliferate and died; increased concentrations of EGF or PDGF allowed initial growth of MPCs but caused loss of proliferation beyond 30 cell doublings (not shown). Addition of higher concentrations of dexamethasone also caused loss of proliferation beyond 30 cell doublings. This might be due to initial commitment to the osteoblast and adipocyte lineage, as concentrations of dexamethasone in the 10−6 to 10−7 M range are commonly used to induce these mesenchymal lineages. Addition of other cytokines, such as bFGF, BMP-4, or aFGF, also induced high-level CD44 expression and loss of proliferation potential beyond 30 cell doublings (not shown).
Culture with greater than 2% FCS (Figures 1C, 2D) or culture at high density (greater than 8 × 103/cm2) (Figures1D, 2E) yielded cells that were significantly larger and more vacuolated, and caused acquisition of high levels of CD44, HLA-DR, and class I HLA and decreased levels of CD13 and CD49b. This was associated with loss of proliferation potential beyond 30 cell doublings and loss of differentiation potential (see below).
High-efficiency retroviral transduction of MPCs
MPCs were subcultured at a 1:4 dilution and then subjected to 2 sequential transductions 24 hours apart with a GALV-pseudotyped MFG-eGFP vector. Transduction efficiency ranged between 30% and 70% (Figure 4). GFP expression remained relatively unchanged for at least 20 population doublings, as is shown in Figure 4. In addition, the percentage of eGFP+ cells was unchanged following cryopreservation (not shown) or following differentiation (Figure 12).
MPCs cultured at low density and with 2% FCS differentiate to mesenchymal cell types and skeletal myoblasts
Differentiation to tissue-specific cells required addition of induction agents or cytokines. This differs from what has been seen for ES cells that differentiate spontaneously when cells are cultured as embryoid bodies (EBs). The cytokines/inducers were chosen on the basis of what has been described for differentiation of MSCs to the osteoblast, cartilage, and adipocyte lineage13,23 and for differentiation of EB to the skeletal myoblast15 lineage and endothelium.24 Differentiation to all mesodermal lineages required that cells be replated at high confluency (greater than 2 × 104/cm2). Whether this causes exit from cell cycle, required for differentiation, or whether cell-cell contact itself or factors secreted following cell-cell contact are needed for differentiation is not known.
Osteoblasts
MPCs, replated at 2 × 104/cm2, were cultured in DMEM with 10 mM β-glycerophosphate, 10−7 M dexamethasone, and 0.2 mM ascorbic acid,13 23 with media changes every 3 to 4 days. After 14 days, more than 80% of MPCs acquired markers of osteoblasts as shown by FACS for osteopontin, bone sialoprotein, and osteonectin (Figure5A). We could not detect cells expressing skeletal muscle markers (fast-twitch myosin) or endothelial markers (vWF) (FACS analysis; data not shown). These results were confirmed by immunohistochemistry (Figure 5B) and Western blot (Figure 5C). We also used Von Kossa staining to demonstrate the presence of calcium phosphate after culture conditions were changed and showed that differentiation to osteoblasts is associated with increased alkaline phosphatase staining (Figure 5D).
Osteoblast differentiation from MPCs.
MPCs were cultured at 2 × 104/cm2 on FN in DMEM without serum, EGF, or PDGF-BB, but with β-glycerophosphate, 10−7 M dexamethasone, and 10 mM ascorbic acid. Differentiation to osteoblasts was shown by presence after 14 days of osteopontin, bone sialoprotein, and osteonectin by FACS (A; specific antibody, thick line; control IgG, thin line). This was confirmed by immunohistochemistry (B; enlargement = 10 ×; arrows indicate nuclei) and Western blot (C; clones nos. 1, 2, and 3; see also Figure 12; β-actin serves as loading control) on day 14. Positive control on Western blot (C+): HT85 cells were induced to differentiate to osteoblasts. Presence of CaPo4 crystals was shown by Von Kossa staining, and presence of osteoblast differentiation was further confirmed by increased alkaline phosphatase staining (D; enlargement = 10 ×). A representative example of more than 5 experiments is shown.
Osteoblast differentiation from MPCs.
MPCs were cultured at 2 × 104/cm2 on FN in DMEM without serum, EGF, or PDGF-BB, but with β-glycerophosphate, 10−7 M dexamethasone, and 10 mM ascorbic acid. Differentiation to osteoblasts was shown by presence after 14 days of osteopontin, bone sialoprotein, and osteonectin by FACS (A; specific antibody, thick line; control IgG, thin line). This was confirmed by immunohistochemistry (B; enlargement = 10 ×; arrows indicate nuclei) and Western blot (C; clones nos. 1, 2, and 3; see also Figure 12; β-actin serves as loading control) on day 14. Positive control on Western blot (C+): HT85 cells were induced to differentiate to osteoblasts. Presence of CaPo4 crystals was shown by Von Kossa staining, and presence of osteoblast differentiation was further confirmed by increased alkaline phosphatase staining (D; enlargement = 10 ×). A representative example of more than 5 experiments is shown.
Chondroblasts
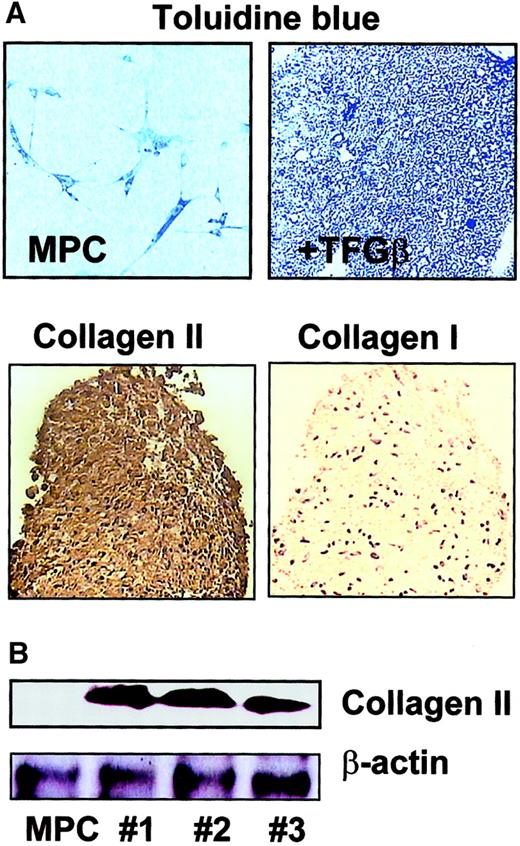
To induce chondroblast differentiation, MPCs were trypsinized, and 1 × 106 MPCs were cultured in 1 mL serum-free expansion medium without EGF and PDGF-BB, but with 100 ng/mL TGF-β1, in the tip of a 15-mL conical tube to allow aggregation of the cells in micromass suspension culture.13,23 Medium was exchanged every 3 to 4 days. Development of chondroblasts was followed by staining the micromasses with toluidine blue, which stains the proteoglycan extracellular matrix,13,14 and with antibodies against type II and type I collagen.25 We found that small aggregates of cartilage formed in the bottom of the tubes that stained positive with toluidine blue from day 14 on (Figure6A). Expression of type II collagen was detected from day 5 on, and by day 21, type II collagen but not type I collagen staining could be detected throughout the micromass (Figure6A). Results were confirmed by Western blot analysis (Figure6B).
Chondroblast differentiation from MPCs.
MPCs were trypsinized and cultured in expansion medium without serum, EGF, or PDGF-BB, but with 100 ng/mL TGF-β1 in micromass culture. For micromass cultures, 1 × 106 MPCs were diluted in 1 mL culture medium and added to a 15-mL conical tube, where close cell-cell interaction was induced. Differentiation to chondroblasts was shown by toluidine blue staining and positive staining for type II collagen but not type I collagen (A; enlargement = 10 ×). Presence of type II collagen was confirmed by Western blot on day 14 (B; clones nos. 1, 2, and 3; see also Figure 12; β-actin serves as loading control). A representative example of more than 5 experiments is shown.
Chondroblast differentiation from MPCs.
MPCs were trypsinized and cultured in expansion medium without serum, EGF, or PDGF-BB, but with 100 ng/mL TGF-β1 in micromass culture. For micromass cultures, 1 × 106 MPCs were diluted in 1 mL culture medium and added to a 15-mL conical tube, where close cell-cell interaction was induced. Differentiation to chondroblasts was shown by toluidine blue staining and positive staining for type II collagen but not type I collagen (A; enlargement = 10 ×). Presence of type II collagen was confirmed by Western blot on day 14 (B; clones nos. 1, 2, and 3; see also Figure 12; β-actin serves as loading control). A representative example of more than 5 experiments is shown.
Adipocytes
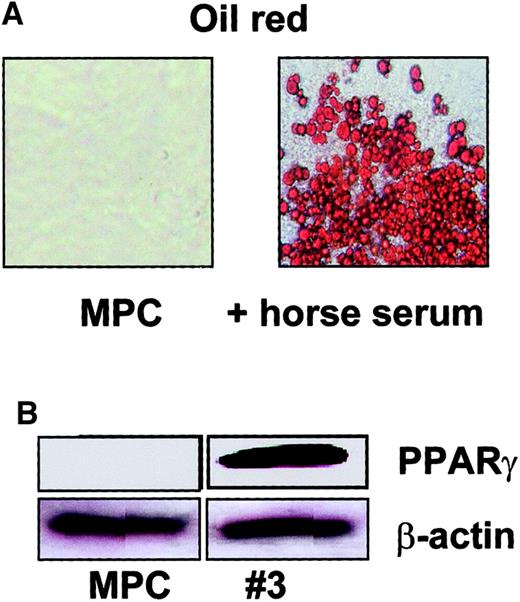
To induce adipocyte differentiation, MPCs were cultured at 2 × 104/cm2 with 10% horse serum or 100 ng/mL insulin (not shown). Cultures were maintained for 14 days with medium exchanges every 3 to 4 days. After 14 days, more than 80% of cells differentiated into lipid-laden cells that stained with oil-red (Figure 7A). Using Western blot, we also showed that cells expressed PPAR-γ (Figure 7B).13Although such markers are also expressed in hepatic epithelium, the differentiated cells did not have epithelial morphology and did not stain for other hepatocyte-specific markers, such as albumin.
Adipocyte differentiation from MPCs.
MPCs were cultured at 2 × 104/cm2 in expansion medium without FCS, EGF, and PDGF-BB, but with 10% horse serum. Adipocyte differentiation was shown by oil-red staining (A; enlargement = 20 ×) and presence of PPAR-γ (B, clone no. 3; see also Figure 12; β-actin serves as loading control) on day 14. A representative example of more than 5 experiments is shown.
Adipocyte differentiation from MPCs.
MPCs were cultured at 2 × 104/cm2 in expansion medium without FCS, EGF, and PDGF-BB, but with 10% horse serum. Adipocyte differentiation was shown by oil-red staining (A; enlargement = 20 ×) and presence of PPAR-γ (B, clone no. 3; see also Figure 12; β-actin serves as loading control) on day 14. A representative example of more than 5 experiments is shown.
Hematopoietic supportive stroma
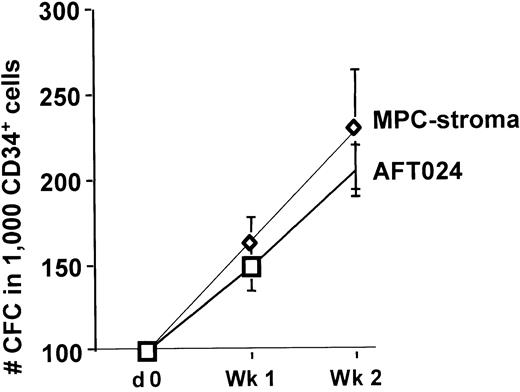
We induced “stromal” differentiation by incubating MPCs subcultured at 2 × 104/cm2 with 10 U IL-1α in expansion medium for 24 hours, after which they were switched to IMDM without EGF and PDGF-BB, but with 12.5% FCS, 12% horse serum, and 10−7 M hydrocortisone.26 On day 7, 50% of the medium was replaced with fresh medium. To demonstrate that these cells support hematopoiesis, feeders were irradiated at 2 Gy, and allogeneic cord blood CD34+ cells were plated in contact with the feeder in IMDM with FCS, horse serum, and hydrocortisone. After 2 weeks, progeny was replated in methylcellulose assay to enumerate CFCs. A 3- to 5-fold expansion of CFCs was seen, which was similar to cultures of CD34+ cells in contact with the murine fetal liver feeder, AFT02416 (Figure8). We did not test whether treatment of MPCs with IL-1α is required to elucidate the hematopoietic supportive ability of MPCs. It is also not yet known if MPCs, such as, for example, the AFT024 feeder, will support repopulating stem cells.19 27
MPC support of human hematopoiesis in vitro.
MPCs plated at 2 × 104/cm2 were treated with 10 U/mL IL-1β for 24 hours and maintained with 12.5% FCS and 12.5% horse serum. Following irradiation at 2 Gy, cord blood CD34+ cells were plated in contact with the MPCs. The number of CFCs present in fresh CD34+ cells or in progeny from CD34+ cells cultured on MPCs was enumerated by methylcellulose assay (n = 3). As control, cells were plated on irradiated confluent AFT024 feeders.
MPC support of human hematopoiesis in vitro.
MPCs plated at 2 × 104/cm2 were treated with 10 U/mL IL-1β for 24 hours and maintained with 12.5% FCS and 12.5% horse serum. Following irradiation at 2 Gy, cord blood CD34+ cells were plated in contact with the MPCs. The number of CFCs present in fresh CD34+ cells or in progeny from CD34+ cells cultured on MPCs was enumerated by methylcellulose assay (n = 3). As control, cells were plated on irradiated confluent AFT024 feeders.
Skeletal myoblasts
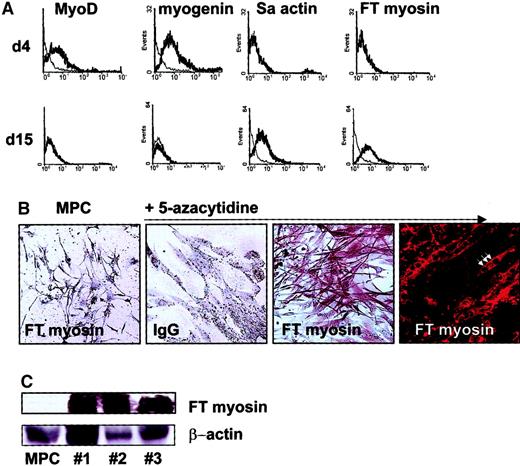
Caplan showed that rat MSCs can be induced to differentiate into skeletal myoblastlike cells.15 We therefore tested whether human MPCs can differentiate into cells of the myoblast lineage. MPCs were plated at 2 × 104/cm2 in expansion medium containing 2% FCS, 10 ng/mL EGF, and 10 ng/mL PDGF-BB as well as 3 μM 5-azacytidine15 for 24 hours. Afterward, cells were maintained in expansion medium with 2% FCS, EGF, and PDGF-BB, but without 5-azacytidine, and medium was exchanged every 3 to 4 days. We evaluated the cultures by FACS (Figure9A), immunohistochemistry (Figure 9B), and Western blot (Figure 9C) to detect transcription factors and skeletal proteins found during muscle development.28-30 At 4 days after induction, we detected the MYO-D and myogenin transcription factors. After 15 days, we no longer detected MYO-D or myogenin, but could detect fast-twitch myosin and sarcomeric actin. When cultures were evaluated by immunohistochemistry and FACS, we found that greater than 80% of cells expressed skeletal muscle–specific transcription factors and mature muscle proteins (Figure 9A). Cells treated with 5-azacytidine did not express osteoblast-specific markers (bone sialoprotein) or endothelial markers (vWF) (FACS analysis; not shown). When cells treated with 5-azacytidine and maintained in EGF- and PDGF-BB–containing medium for 14 days were subjected to culture conditions used for osteoblast differentiation (DMEM and β-glycerophosphate, see above), cells died and no osteoblast differentiation was seen, demonstrating that the majority of cells had lost their multilineage potential.
Myoblast differentiation from MPCs.
MPCs plated at 2 × 104/cm2 were treated with 3 μM 5-azacytidine in MPC expansion medium with 10 ng/mL EGF and 10 ng/mL PDGF-BB for 24 hours and maintained in the same expansion medium with 2% FCS, 10 ng/mL EGF, and 10 ng/mL PDGF-BB, but without 5-azacytidine for up to 15 days. After 5 and 14 days, cultures were analyzed by FACS for MYO-D, myogenin, fast-twitch myosin, and sarcomeric actin (A; specific antibody, thick line; control IgG, thin line) and on day 15 by immunohistochemistry and immunofluorescence for fast-twitch myosin (B, MPCs stained with anti–fast-twitch myosin antibody; 5-azacytidine–induced cells stained with control IgG, with anti–fast-twitch myosin antibody; arrow indicates multinucleated cell; 10 × and 40 × enlargement). Presence of fast-twitch myosin was confirmed by Western blot (C, clones nos. 1, 2, and 3; see also Figure12; β-actin serves as loading control). A representative example of more than 5 experiments is shown.
Myoblast differentiation from MPCs.
MPCs plated at 2 × 104/cm2 were treated with 3 μM 5-azacytidine in MPC expansion medium with 10 ng/mL EGF and 10 ng/mL PDGF-BB for 24 hours and maintained in the same expansion medium with 2% FCS, 10 ng/mL EGF, and 10 ng/mL PDGF-BB, but without 5-azacytidine for up to 15 days. After 5 and 14 days, cultures were analyzed by FACS for MYO-D, myogenin, fast-twitch myosin, and sarcomeric actin (A; specific antibody, thick line; control IgG, thin line) and on day 15 by immunohistochemistry and immunofluorescence for fast-twitch myosin (B, MPCs stained with anti–fast-twitch myosin antibody; 5-azacytidine–induced cells stained with control IgG, with anti–fast-twitch myosin antibody; arrow indicates multinucleated cell; 10 × and 40 × enlargement). Presence of fast-twitch myosin was confirmed by Western blot (C, clones nos. 1, 2, and 3; see also Figure12; β-actin serves as loading control). A representative example of more than 5 experiments is shown.
Thus, we demonstrate that MPCs cultured under the conditions described here, like MSC, can differentiate into osteoblastlike, chondroblastlike, adipocytelike and stromalike cells as well as skeletal muscle–like cells.
MPCs cultured under low-density conditions and with 2% FCS differentiate to visceral mesoderm–derived endothelial cells
Until now, there has been no evidence that MSCs cultured under conditions initially developed by Pittenger et al14differentiate into cells with endothelial characteristics. Because undifferentiated MPCs express low levels of the VEGF receptors KDR and FLt1 (Figure 10), we tested whether they could be induced to differentiate into endothelial cells. We replated human MPCs at 2 × 104/cm2 in serum-free expansion medium without EGF and PDGF, but with 10 ng/mL VEGF-B. We evaluated progeny for markers of endothelial differentiation. Undifferentiated MPCs do not express CD31, CD34, CD36, CD62E, CD62P, H1P12, Tie, Tek, and vWF, but express low levels of VEGF-R1, Flt1, VEGF-R2, and KDR (Figure 10). Following treatment with VEGF for 14 days, greater than 80% of cells expressed CD34, vWF, CD36, Tek (Figure 10A), CD62E, CD62P, H1P12, and Tie (not shown) and expressed much higher levels of the VEGF receptors KDR (Figure 10A) and Flt1 (not shown). Results were again confirmed by immunofluorescence analysis (Figure 10B) and Western blot (Figure 10C). Whether similar results can be obtained when other VEGF isoforms are used was not evaluated. Endothelial cells differentiated from MPCs could be maintained in culture for at least 2 months when subcultured every 3 to 5 days in the presence of 10% FCS and VEGF.
Endothelial differentiation from MPCs.
MPCs plated at 2 × 104/cm2 were cultured in MPC expansion medium without FCS, EGF, or PDGF, but with 20 ng/mL VEGF-B for 14 days. Differentiation to endothelial cells was evaluated by FACS for KDR, vWF, CD34, CD36, and Tek (A; specific antibody, thick line; control IgG, thin line) and immunofluorescence microscopy for CD34, CD36, and vWF (B). Shown are VEGF-induced cells stained with specific antibodies (upper row) and control IgG (middle row) as well as undifferentiated MPCs stained with specific antibodies (bottom row) (enlargement = 40 ×). Results were confirmed by Western blot for Tek and vWF (C; clones nos. 1, 2, and 3; see also Figure 12; β-actin serves as loading control). A representative example of more than 10 experiments is shown.
Endothelial differentiation from MPCs.
MPCs plated at 2 × 104/cm2 were cultured in MPC expansion medium without FCS, EGF, or PDGF, but with 20 ng/mL VEGF-B for 14 days. Differentiation to endothelial cells was evaluated by FACS for KDR, vWF, CD34, CD36, and Tek (A; specific antibody, thick line; control IgG, thin line) and immunofluorescence microscopy for CD34, CD36, and vWF (B). Shown are VEGF-induced cells stained with specific antibodies (upper row) and control IgG (middle row) as well as undifferentiated MPCs stained with specific antibodies (bottom row) (enlargement = 40 ×). Results were confirmed by Western blot for Tek and vWF (C; clones nos. 1, 2, and 3; see also Figure 12; β-actin serves as loading control). A representative example of more than 10 experiments is shown.
No hematopoietic differentiation from MPCs
Choi et al24 have shown that Flk1-positive cells present in murine EBs can, depending on the culture conditions, be induced to differentiate into CD34+ endothelial cells as well CD34+ hematopoietic cells. Because we had shown that KDRdim MPCs could be induced to differentiate to endothelial cells, we also tested whether KDRdim MPCs could be induced to differentiate into hematopoietic cells. The eGFP-transduced MPCs were cocultured in contact with the AFT024 feeder, or in supernatants from this feeder with different combinations of cytokines, including 10 to 20 ng/mL VEGF, bFGF, BMP-4, Flt3-L, SCF, IL-3, IL-6, G-CSF, GM-CSF, thrombopoietin, and erythropoietin, for 2 to 6 weeks and were analyzed by FACS for expression of CD45 and/or CD34. Although eGFP+ CD34+ cells could be detected, they did not costain with CD45, indicating their endothelial nature. No CD45+ cells have been detected under any of the culture conditions tested. Therefore, we have been unable to induce MPCs to differentiate to CD34+/CD45+ hematopoietic cells despite culture of MPCs on the AFT024 feeder, which is derived from murine fetal liver and is known to support murine hematopoietic stem cells (HSCs) as well as human marrow–, peripheral blood–, and cord blood–derived primitive progenitors.19,27 31 Studies are ongoing to test additional feeders and cytokine combinations for their ability to induce hematopoietic differentiation from MPCs.
Differentiation from MPCs cultured with 10% FCS or without FCS
In contrast to differentiation to the mesenchymal cell types osteoblasts, chondroblasts, adipocytes, and stromal cells, differentiation to skeletal muscle and endothelium required that the initiating cell population be CD44− and class I–HLA−. Thus, when MPC cultures were maintained with greater than 2% FCS (Figure 11) or at high density (greater than 104/cm2) (not shown), no skeletal muscle or endothelial differentiation was seen. Like MPCs cultured with 2% FCS and EGF plus PDGF-BB, MPCs that had been cultured in serum-free MPC medium supplemented with EGF and PDGF-BB but also with IGF could be induced to differentiate to osteoblasts, chondroblasts, adipocytes, and stromal cells, as well as to skeletal myoblasts and endothelium (Figure 11).
Differentiation of MPCs from cultures supplemented with 10% FCS or without FCS.
CD45−GlyA− cells were expanded on FN in expansion medium with EGF and PDGF-BB and with 2% FCS, with 10% FCS, or with no FCS but with 10 ng/mL IGF for 25 cell doublings. Cells were replated at MPCs plated at 2 × 104/cm2 under osteoblast, skeletal muscle, and endothelial conditions for 14 days. Cells were analyzed by FACS for expression of bone sialoprotein, fast-twitch myosin, and vWF (thick line) compared with IgG control phenotype (thin line). Differentiation to the osteoblast but not myoblast or endothelial lineage was observed for cells cultured with 10% FCS. Differentiation to osteoblasts, skeletal myoblasts, and endothelium is seen for cells cultured without FCS but with IGF. A representative example of 3 experiments is shown.
Differentiation of MPCs from cultures supplemented with 10% FCS or without FCS.
CD45−GlyA− cells were expanded on FN in expansion medium with EGF and PDGF-BB and with 2% FCS, with 10% FCS, or with no FCS but with 10 ng/mL IGF for 25 cell doublings. Cells were replated at MPCs plated at 2 × 104/cm2 under osteoblast, skeletal muscle, and endothelial conditions for 14 days. Cells were analyzed by FACS for expression of bone sialoprotein, fast-twitch myosin, and vWF (thick line) compared with IgG control phenotype (thin line). Differentiation to the osteoblast but not myoblast or endothelial lineage was observed for cells cultured with 10% FCS. Differentiation to osteoblasts, skeletal myoblasts, and endothelium is seen for cells cultured without FCS but with IGF. A representative example of 3 experiments is shown.
Single-cell origin of limb-bud and visceral mesoderm progeny
To prove single-cell derivation of the differentiated cell types, we initially plated more than 2000 cells recovered from established cultures as single cells in FN-coated 96-well plates in MPC expansion medium. In no well did we detect cell growth. However, when cells were deposited at 10 cells per well, progeny of 0.2% of wells could be expanded to more than 107 cells. Thus, MPCs themselves or accessory cells may secrete growth factors, cytokines, or extracellular matrix components required for their growth. This is currently under evaluation.
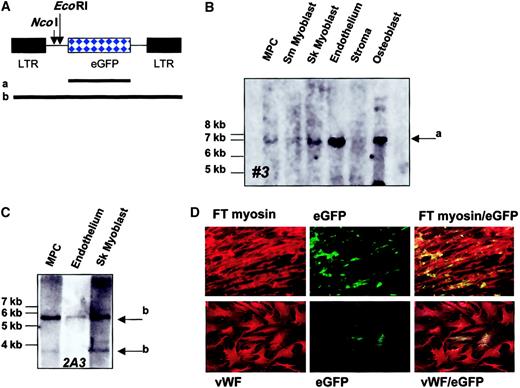
An alternative method for proving single-cell origin of differentiated progeny is retroviral marking.32 33 In the first experiment, MPCs obtained after 20 cell doublings were transduced with an MFG-eGFP retrovirus, and eGFP+ cells were selected by FACS. Cells were deposited as 10 cells per well and expanded in culture until greater than 2 × 107 cells were obtained. Then, 5 × 106 undifferentiated MPCs were collected for Southern blot analysis, and further groups of MPCs, consisting of 5 × 106 cells for each group, were induced to differentiate to osteoblasts, adipocytes, stroma cells, skeletal myoblasts, and endothelium. After 14 days under differentiation conditions, cells were harvested and used for Southern blot (0.5 to 2 × 106 cells) and Western blot (0.5 to 2 × 106 cells) analysis. We examined 3 independent populations. Results for Western blot studies are shown for clones nos. 1, 2, and 3 in Figures 6, 7, 9, and 11. Results from the Southern blot for clone no. 3 is shown in Figure12A. A single retroviral insert was seen in MPCs, skeletal myoblasts, endothelium, and osteoblasts. No insert could be detected in stroma, possibly owing to the fact that DNA from only 0.5 × 106 cells was loaded.
Single-cell origin of muscle and endothelial differentiated progeny.
MPCs obtained after 20 cell doublings were transduced with MFG-eGFP. (A) MFG-eGFP retroviral vector. Shown are EcoRI andNcoI digestion sites. Also shown are probes that span the eGFP gene only (a) or the complete retroviral vector (b). (B) FACS-selected eGFP+ MPCs were cultured at 10 cells per well until approximately 4 × 107 cells was obtained. Then, 5 × 106 MPCs were frozen, and groups of 5 × 106 MPCs each were induced to differentiate to osteoblasts, chondrocytes, adipocytes, stroma, skeletal muscle, and endothelium. Western blot analysis for the different lineages is shown for 3 individual clones in Figures 6, 7, 9, 10, and 11. DNA was extracted from 5 × 106 undifferentiated MPCs and 0.5 to 2 × 106 cells from osteoblast, chondrocyte, adipocyte, stroma, skeletal muscle, and endothelium differentiation cultures. Following digestion overnight with EcoRI, fragments were separated by gel electrophoresis, blotted to (+) nylon, and probed with a biotin-labeled eGFP-cDNA probe (a). Detection was by ECL. A single retroviral insert was detected in clone no. 3 (arrow), which was also present in cells recovered from osteoblast, chondrocyte, adipocyte, skeletal muscle, and endothelium differentiation cultures (representative of 3 clones). (C-D) Cultures were established in 96-well plates with 95 untransduced MPCs and 5 MFG-eGFP–transduced MPCs. Cultures were expanded to 2 × 107 cells; 5 × 106 MPCs were frozen; and groups of 5 × 106 MPCs were each induced to differentiate to skeletal muscle and endothelium. DNA was extracted from 5 × 106 undifferentiated MPCs and from 0.5 and 1 × 106 cells from myoblast and endothelium differentiation cultures. Following digestion overnight withNcoI, fragments were separated by gel electrophoresis, blotted to (+) nylon, and probed with a biotin-labeled MFG-eGFP-cDNA probe (b). Detection was by ECL. A single retroviral insert was detected (clone 2A3; 2 arrows indicate both retroviral fragments), which was also present in cells recovered from the myoblast and endothelium differentiation cultures (C). A fraction of the cells were fixed and stained with antibodies against fast-twitch myosin and vWF and examined by confocal microscopy for costaining of these markers with eGFP (D). A representative example of 2 clones is shown.
Single-cell origin of muscle and endothelial differentiated progeny.
MPCs obtained after 20 cell doublings were transduced with MFG-eGFP. (A) MFG-eGFP retroviral vector. Shown are EcoRI andNcoI digestion sites. Also shown are probes that span the eGFP gene only (a) or the complete retroviral vector (b). (B) FACS-selected eGFP+ MPCs were cultured at 10 cells per well until approximately 4 × 107 cells was obtained. Then, 5 × 106 MPCs were frozen, and groups of 5 × 106 MPCs each were induced to differentiate to osteoblasts, chondrocytes, adipocytes, stroma, skeletal muscle, and endothelium. Western blot analysis for the different lineages is shown for 3 individual clones in Figures 6, 7, 9, 10, and 11. DNA was extracted from 5 × 106 undifferentiated MPCs and 0.5 to 2 × 106 cells from osteoblast, chondrocyte, adipocyte, stroma, skeletal muscle, and endothelium differentiation cultures. Following digestion overnight with EcoRI, fragments were separated by gel electrophoresis, blotted to (+) nylon, and probed with a biotin-labeled eGFP-cDNA probe (a). Detection was by ECL. A single retroviral insert was detected in clone no. 3 (arrow), which was also present in cells recovered from osteoblast, chondrocyte, adipocyte, skeletal muscle, and endothelium differentiation cultures (representative of 3 clones). (C-D) Cultures were established in 96-well plates with 95 untransduced MPCs and 5 MFG-eGFP–transduced MPCs. Cultures were expanded to 2 × 107 cells; 5 × 106 MPCs were frozen; and groups of 5 × 106 MPCs were each induced to differentiate to skeletal muscle and endothelium. DNA was extracted from 5 × 106 undifferentiated MPCs and from 0.5 and 1 × 106 cells from myoblast and endothelium differentiation cultures. Following digestion overnight withNcoI, fragments were separated by gel electrophoresis, blotted to (+) nylon, and probed with a biotin-labeled MFG-eGFP-cDNA probe (b). Detection was by ECL. A single retroviral insert was detected (clone 2A3; 2 arrows indicate both retroviral fragments), which was also present in cells recovered from the myoblast and endothelium differentiation cultures (C). A fraction of the cells were fixed and stained with antibodies against fast-twitch myosin and vWF and examined by confocal microscopy for costaining of these markers with eGFP (D). A representative example of 2 clones is shown.
In the second experiment, a fraction of MPCs obtained after 20 cell doublings was transduced and eGFP+ MPCs were diluted in nontransduced MPCs from the same donor to obtain a final concentration of 5%. These mixtures were plated at 100 cells per well, and culture was expanded until greater than 2 × 107 cells were obtained. Then, 5 × 106 MPCs were collected for Southern blot analysis, and groups of 5 × 106 MPCs were each induced to differentiate to skeletal myoblasts and endothelium. After 14 days under differentiation conditions, cells were harvested and used for Southern blot analysis (0.5 to 2 × 106 cells). Cells were also fixed and subjected to immunofluorescence evaluation for fast-twitch myosin and eGFP (skeletal muscle cultures) or vWF and eGFP (endothelial cultures). We found a single retroviral insertion site in MPCs and skeletal and endothelial differentiated progeny (clone 2A3, Figure 12C). Immunofluorescence evaluation showed that 8% of cells in clone 2A3 that were induced to differentiate with 5-azacytidine stained positive for eGFP and fast-twitch myosin, and 7% of cells that were induced to differentiate to endothelium costained for eGFP and vWF (Figure 12D). These studies therefore definitively prove for the first time that mesodermal progenitors exist in human marrow and that these can differentiate at the single-cell level in cells of limb-bud and visceral mesodermal lineage.
Conclusion
We describe here for the first time the isolation and ex vivo culture of mesodermal progenitor cells that can be expanded beyond 50 cell doublings and can, at the single-cell level, generate both limb-bud and visceral mesodermal cell types. MPCs are CD44−, class I HLA−, and β2-microglobulin−, characteristics of classical mesenchymal stem cells.14 MPCs are also CD34−, CD45−, and CD117− and therefore differ from primitive hematopoietic progenitors, which have recently also been associated with “stem cell plasticity.”34-36 However, as has been suggested in these reports,34-36 we also have preliminary evidence that MPCs may be capable of differentiating into nonmesodermal cell types, such as neuroectoderm, and this at the single-cell level (manuscript in preparation).
In contrast to HSCs, we show that MPCs can be expanded ex vivo for more than 50 population doublings without obvious signs of differentiation or senescence and can be readily transduced with retroviral vectors. Provided that MPCs engraft long term, which is currently being tested, they may be a much better source of cells than HSCs for gene therapy of congenital disorders characterized by enzyme deficiencies, such as Gaucher disease,37mucopolisaccharidosis,38 leukodystrophies,39and others. Like MPCs, ES cells can differentiate into all mesodermal cell types and may have an even greater proliferation potential. However, MPCs but not ES cells can be derived from BM from most healthy donors irrespective of age, providing for an autologous source of stem cells. Thus, because MPCs can be selected and expanded under conditions that should be readily adaptable to production by clinical good manufacturing process and are easily transduced with retroviral vectors, they may be an ideal source of cells for therapy of degenerative or traumatic disorders of mesodermal cells or for therapy of single gene disorders.
Supported by National Institutes of Health grants PO1-CA-65493-1 (C.M.V.) and 1F31AI/GM10291 (M.R.); Children's Cancer Research Fund; and the University of Minnesota Bone Marrow Transplant Research Fund; C.M.V. is a Scholar of the Leukemia Society of America.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Catherine M. Verfaillie, Professor of Medicine, University of Minnesota, MMC 716, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: verfa001@umn.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal