Abstract
Recombinant factor VIII and factor IX concentrates, human-plasma–derived albumin, and samples from previously untreated patients with hemophilia were examined for the presence of TT virus (TTV) by using polymerase chain reaction testing. Blood samples from the patients were obtained prospectively before and every 3 to 6 months after therapy was begun. TTV was detected in 23.5% of the recombinant-product lots and 55.5% of the albumin lots tested. Only first-generation factor VIII recombinant concentrates stabilized with human albumin were positive for TTV, whereas all second-generation (human protein–free) concentrates were negative for the virus. In 59% of patients treated with either first- or second-generation recombinant factor concentrates, TTV infection developed at some point after the initial infusion. Infection with TTV in these patients before and after treatment did not appear to be clinically important. Thus, first-generation recombinant factor VIII concentrates may contain TTV and the source of the viral contamination may be human albumin.
Introduction
TT virus (TTV) is a small, naked, single-stranded DNA virus of the Circoviridae family with a circular genome. It was first detected in the serum of patients with posttransfusion hepatitis of unknown cause.1 It is not known whether TTV is pathogenic.2-4 TTV may be transmitted parenterally through transfusion of blood products,5-7 by the fecal-oral route, and by pregnant women to fetuses.8,9 TTV may also be disseminated by aerosols, since the virus is present in saliva.8,10 A high rate of TTV infection was found in patients given multiple transfusions11 and patients with hemophilia.5,12-14 TTV sequences are frequently detected in clotting factor concentrates prepared from large plasma pools.5 15
First-generation recombinant human factor VIII concentrates stabilized with human-plasma–derived albumin (HPDA) before lyophilization (Recombinate, Baxter, Glendale, CA; and Kogenate, Bayer, Leverkusen, Germany) have been licensed for more than 10 years. They became widely used by patients with hemophilia because of their perceived safety with respect to viral infection. However, parvovirus B19 DNA was detected by polymerase chain reaction (PCR) assay in a few lots of Kogenate,16 and seroconversion occurred in a few patients with hemophilia who were treated with this product.17 The source of the B19 virus contamination in recombinant products was thought to be the HPDA used to stabilize the recombinant protein before lyophilization. Second-generation recombinant factor VIII and factor IX concentrates that do not contain albumin (ReFacto and Benefix, Genetics Institute, Cambridge, MA) were recently licensed for the treatment of hemophilia. A prospective study of the viral safety of these concentrates in accordance with International Society on Thrombosis and Haemostasis guidelines has not yet been conducted. To assess the safety of recombinant concentrates regarding TTV transmission, we tested several such products for TTV DNA by using PCR assays. We also studied the incidence of TTV infection in 24 previously untreated patients before and after treatment with recombinant clotting factor concentrates.
Study design
Recombinant clotting factor concentrates and human albumin
Thirteen lots of Recombinate, 10 lots of Kogenate, 7 lots of ReFacto, and 4 lots of Benefix were studied, as were 9 lots of 20% solution of human albumin designed for clinical use. The lyophilized products were reconstituted with the distilled water provided with the infusion kit for each product, stocked in aliquots of 0.5 mL each, and stored at −20°C until the PCR assay.
Patients
Twenty-four previously untreated patients (22 with hemophilia A and 2 with hemophilia B) given infusions of recombinant factors exclusively were enrolled in this study. Fourteen received first-generation recombinant products and 10 received second-generation, human albumin–free products. Their average age at first treatment was 18 months (range, 0.1-51 months). The first infusion dose was at least 25 U/kg of body weight, and the median amount of concentrate administered to each patient during the study was 60 000 U/year. The dose range was very wide (3500-478 000 U) because some patients were treated on demand and others were treated according to prophylactic or immunotolerance protocols. Blood samples for clinical laboratory evaluations (complete blood count and levels of aspartate aminotransferase, alanine aminotransferase, γ-glutamyltransferase [GGT], and bilirubin) and for the TTV PCR assays were collected before and after the first infusion and at intervals ranging from 3 to 6 months. The total follow-up time was at least 3 years.
PCR for detection of TTV DNA
Before amplification, the samples (recombinant products, HPDA, and serum) were subjected to a procedure for DNA extraction (Roche, Basel, Switzerland) to eliminate PCR inhibitors. Three different nested PCRs (untranslated region [UTR] (A) PCR, UTR (B) PCR, and N22-PCR), which amplify distinct viral DNA regions, were used to detect TTV DNA. The first and second reactions amplify 2 untranslated, more conserved, regions, whereas the third target sequence is in the N22 region,18 is more variable, and is in the coding region of the TTV genome. The UTR (A) PCR was done with NG133 and NG147 as outer primers and NG134 and NG132 as inner primers. For the UTR (B) PCR, the outer primers were NG148 and NG135 and the inner primers were NG149 and NG136; and for the N22 PCR, the outer primers were NG059 and NG063 and the inner primers were NG061 and NG063. The PCR conditions were described previously.18 Samples positive on the UTR (A) PCR assay only were considered positive after repeated control assessments.
Results and discussion
TTV DNA was present in 8 of the 34 recombinant-product lots (Table1). Specifically, TTV DNA was found in 35% of recombinant-product lots to which albumin had been added as a stabilizer before lyophilization (Recombinate and Kogenate). The albumin-free products (Benefix and ReFacto) were negative for TTV DNA. Thus, it appeared that albumin was the source of the TTV contamination. Five of the 9 lots of albumin tested (55.5%) were positive for TTV DNA.
Detection of TTV DNA in recombinant factor VIII and factor IX products
| Product . | Manufacturer . | No. of lots tested . | No. (%) of lots positive for TTV DNA . |
|---|---|---|---|
| Recombinate | Baxter | 13 | 3 (23) |
| Kogenate | Bayer | 10 | 5 (50) |
| Refacto | Genetics Institute | 7 | 0 (0) |
| Benefix | Genetics Institute | 4 | 0 (0) |
| Product . | Manufacturer . | No. of lots tested . | No. (%) of lots positive for TTV DNA . |
|---|---|---|---|
| Recombinate | Baxter | 13 | 3 (23) |
| Kogenate | Bayer | 10 | 5 (50) |
| Refacto | Genetics Institute | 7 | 0 (0) |
| Benefix | Genetics Institute | 4 | 0 (0) |
Of the 8 TTV-positive recombinant products, 2 were positive on the UTR (A) PCR assay only, 5 on both the UTR (A) and UTR (B) PCR assay, and 1 on all 3 PCR tests. Four lots of HPDA were positive on both the UTR (A) and UTR (B) PCR assay, and 1 was positive on the UTR (A) PCR assay only. This result only apparently contradicts findings by Pisani et al,15 who did not detect TTV DNA sequences in HPDA by using primers for the N22 region. The low percentage of positive results on the N22 PCR assay was likely due to the high variability of the viral genome in this region and not to a different sensitivity of the 3 PCR reactions, in agreement with previously reported data.18
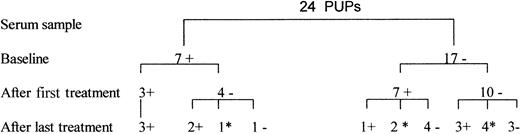
The study of previously untreated patients (Figure1) showed that baseline samples from 7 of the 24 patients (29%) were positive for TTV DNA, probably because of early spread of TTV infection through fecal-oral or maternal-fetal transmission. TTV DNA was detected in 7 of 17 patients negative for TTV (41%) in the first serum sample obtained 3 to 6 months after the first infusion of a recombinant product. Serum samples from 11 of 17 TTV-negative patients obtained after subsequent infusions were available for analysis. In 3 patients with hemophilia who were negative for TTV after the first treatment, TTV viremia developed after subsequent infusions. Overall, serum samples showing TTV viremia were obtained after the first or one of the subsequent infusions in 10 patients (59%)—6 patients treated with first-generation recombinant products and 4 patients treated with second-generation products. At the end of the follow-up period, only 6 patients were positive for TTV DNA; the other 4 had clearance of the virus. Of the 7 patients who were positive for TTV DNA at baseline, 5 were positive at the end of the follow-up period and 2 were negative. TTV viremia persisted intermittently or continually for 1 to 3 years in 7 patients.
Detection by PCR of TTV DNA in serum from 24 previously untreated patients (PUPs) with hemophilia.
The baseline samples were obtained just before the first infusion with recombinant concentrates. Serum samples after the first and the last treatments were obtained 3 to 6 months after the corresponding infusion. A plus sign indicates positive results on PCR assay; a negative sign, negative results; and an asterisk, sample not available.
Detection by PCR of TTV DNA in serum from 24 previously untreated patients (PUPs) with hemophilia.
The baseline samples were obtained just before the first infusion with recombinant concentrates. Serum samples after the first and the last treatments were obtained 3 to 6 months after the corresponding infusion. A plus sign indicates positive results on PCR assay; a negative sign, negative results; and an asterisk, sample not available.
For several reasons, we could not demonstrate that TTV was transmitted by the recombinant products. Moreover, the serum samples showing TTV viremia were obtained from patients after treatment with either first-generation albumin-containing products or second-generation, albumin-free recombinant products. All patients had normal results on clinical chemistry tests at enrollment, and no patient had alterations in levels of transaminases, GGT, or bilirubin. No clinical signs of acute illness were observed during the follow-up period.
TTV may have little or no pathogenicity. However, detection of TTV DNA in recombinant products containing HPDA as a stabilizer, regardless of its clinical relevance, indicates a need for additional procedures to ensure product safety. Furthermore, viral surveillance in noninfected patients with hemophilia must be conducted regardless of whether they are being treated with recombinant clotting factor concentrates, since assuming that these products are completely free of virus may be misleading.
We thank Nadia Ewing, MD, Division of Pediatrics, City of Hope National Medical Center, Duarte, CA, for critically reviewing the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alberta Azzi, Dipartimento di SanitàPubblica (Sezione di Microbiologia e Virologia), Viale Morgagni 48, 50134 Florence, Italy; e-mail: azzi@dsp.igiene.unifi.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal