Abstract
In developing T helper 1 (Th1) and Th2 cells the acquisition of effector function is intimately connected with the acquisition of new migratory capacities, as exemplified by differential expression of chemokine receptors. This study investigates the molecular mechanisms responsible for Th2-restricted expression of the CC-chemokine receptor 3 (CCR3). The minimal promoter in T cells was identified in the −149 base pair (bp) upstream sequence that contains a positive regulatory element. A strong negative element was also localized in the flanking intronic sequence. The study further investigates the role of chromatin remodeling in the regulation of this Th2-specific gene. Drugs that affect the chromatin structure facilitate CCR3 expression in T cells. Furthermore, in differentiating Th2 cells, selected regions are associated with acetylated-H3 histones and become more accessible to DNase I. These results suggest that in Th2 cells both cytokine production and migratory capacity are regulated through a similar mechanism involving chromatin remodeling.
Introduction
Activation of naive T cells results in their clonal expansion and in the generation of polarized effector T cells.1 The T helper 1 (Th1)/Th2 phenotype is not restricted to the cytokine production pattern but involves the acquisition of a different capacity to migrate to inflamed tissues.2 This pattern is exemplified by the differential expression of chemokine receptors.3,4 Although some receptors are expressed by both subsets, others are either specific for Th1, like CXCR3 and CC-chemokine receptor 5 (CCR5), or for Th2, like CCR3 and CCR4. CCR3, originally described in eosinophils and basophils, is selectively expressed by Th2 cells5,6 and binds inflammatory chemokines. CCR3+ T cells (mainly Th2) readily migrate in response to eotaxin.6,7 It may coordinate recruitment of Th2, basophils, and eosinophils in the allergic inflammation.8 In Th2 lines and clones the expression of CCR3 is heterogeneous and correlates with the capacity to produce interleukin 4 (IL-4), which is also a property of a minority of the cells.5 In this study, we asked which structural events are implicated in the regulation of CCR3 expression in T cells, and we investigated the existence of Th2-specific modifications of the chromatin structure along the upstream genomic region of CCR3.
Study design
Cells
Human polarized T-cell lines and clones were maintained and restimulated as described.3 Cell lines were maintained in RPMI 10% fetal calf serum.
Promoter mapping experiments
Gene fragments of varying lengths were cloned in the promoterless pGL3 Basic vector (Promega, Madison, WI) upstream of the Firefly luciferase gene. Cell lines were transiently transfected with reporter constructs by electroporation. Transfection efficiency was controlled with the pGL3 Control and normalized by cotransfecting the Renilla luciferase vector, pRL-TK. Luciferase activities were determined after 48 hours by using the Dual Luciferase Assay System (Promega).
RNA preparation and polymerase chain reaction
Total RNA was prepared, reverse-transcribed, and amplified with the following primers sets: 5′-TTCTTGTGCTTATCCGGGCAAGAAC and 5′-GACGAGGAAGAGCAGGTCCGAAATG (human CCR3); 5′-ACACTGTGCCCATCTACGAGGGG and 5′-ATGATGGAGTTGAAGGTAGTTTCGTGGAT (β-actin). Products were transferred for Southern blot analysis by using a CCR3 5′ complementary DNA probe.
Chromatin immunoprecipitation
Chromatin preparation and immunoprecipitation were performed by using acetyl-histone H3 Immunoprecipitation (ChIP) Assay kit (Upstate Biotechnologies, Lake Placid, NY) as described.9Polymerase chain reaction (PCR) was performed on 1/100 (input and unbound) or 1/50 (bound) by using the following primers: 5′-GCAGCACTAATTGCAAGGATTTCTCAGGTG and 5′-CCTGCTAATTTAGTGAAGTCCTTGATATGTC for the promoter region analysis (−239/+13); 5′-CTGGAAGCAATTTGACAAAATGCATCAAG and 5′-CACACGGACGCGCATGAAAGATAGTACAC for the flanking intronic region (+9284/+9263); 5′-CAGCTAGTAATCAAGGACTTGGCTGCTGAC and 5′-GCTCAGTTTGAGGGTAGTG for the downstream intronic region (+20 540/+20 729). One fifth of the products was separated and transferred for hybridizations with specific probes.
DNase I hypersensitivity
Nuclei aliquots were digested by increasing concentrations (0-10 units) of DNase I (Roche Molecular Biochemicals, Rotkruez, Switzerland) for 2 minutes. Purified DNA was digested with 25 U HindIII (Roche), separated, and transferred for hybridizations.
Results and discussion
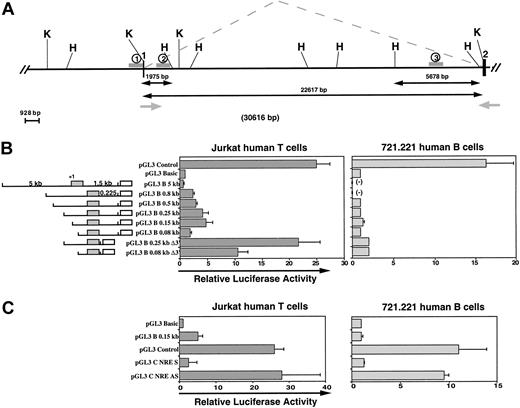
To identify a T-cell promoter activity in the region located immediately upstream of the first noncoding exon of human CCR3, we constructed a series of chimeric reporter genes and tested the ability of these 5′ deletion mutants to drive the expression of theLuciferase gene in Jurkat cells, a human T-cell line, and in 721.221 cells, a human B-cell line. The relative luciferase activity increases in Jurkat cells on 5′ truncation of the 5-kb upstream region (Figure 1B). No CCR3 promoter activity was detected in 721.221 cells. The maximal activity was obtained in Jurkat cells with the −149/+237 fragment (∼4-fold) and decreased with the −78/+237 fragment, suggesting the existence of a T-cell positive regulatory sequence located in the −149/−78 region.
Characterization of the functional promoter for CCR3 in human T cells.
(A) Map of the upstream region of the human CCR3 gene. Black boxes and angle dashed lines represent exons and splicing patterns, respectively. Exon 1 contains the main 5′ UTR part and exon 2 contains the ATG coding region. Grey arrows represent primers subsequently used for PCR in Figure 2A. H and K, respectively, indicate the position ofHindIII and KpnI sites. The 1, 2, and 3 located with gray lines indicate the regions analyzed by ChIP and DNaseI HS experiments. (B) Identification of the T-cell minimal promoter region for CCR3. Construct maps and data for relative luciferase expression in Jurkat human T cells (filled bars) and 721.221 human B cells (open bars). PCR fragments of variable sizes of the CCR3 5′-flanking region were generated and cloned in the Firefly luciferase pGL3 Basic vector. Exon 1 (gray box) and Luciferase (open box) are represented on the maps. Plasmids were transiently transfected by electroporation with a control plasmid pRL-TK (Renillaluciferase) into Jurkat and 721.221 cells. Relative luciferase activities of the cell lysates are representative of at least 5 independent experiments performed with different clones for each construct. Errors bars indicate SDs. (-) indicates not tested. (C) Identification of an NRE located in the flanking intronic region. An intronic PCR fragment (+134 to +237) was cloned either in sense (pGL3 C NRE S) or antisense orientation (pGL3 C NRE AS) into theFirefly luciferase vector pGL3 control containing the SV40 promoter and enhancer. Relative luciferase activities were determined as described in (B).
Characterization of the functional promoter for CCR3 in human T cells.
(A) Map of the upstream region of the human CCR3 gene. Black boxes and angle dashed lines represent exons and splicing patterns, respectively. Exon 1 contains the main 5′ UTR part and exon 2 contains the ATG coding region. Grey arrows represent primers subsequently used for PCR in Figure 2A. H and K, respectively, indicate the position ofHindIII and KpnI sites. The 1, 2, and 3 located with gray lines indicate the regions analyzed by ChIP and DNaseI HS experiments. (B) Identification of the T-cell minimal promoter region for CCR3. Construct maps and data for relative luciferase expression in Jurkat human T cells (filled bars) and 721.221 human B cells (open bars). PCR fragments of variable sizes of the CCR3 5′-flanking region were generated and cloned in the Firefly luciferase pGL3 Basic vector. Exon 1 (gray box) and Luciferase (open box) are represented on the maps. Plasmids were transiently transfected by electroporation with a control plasmid pRL-TK (Renillaluciferase) into Jurkat and 721.221 cells. Relative luciferase activities of the cell lysates are representative of at least 5 independent experiments performed with different clones for each construct. Errors bars indicate SDs. (-) indicates not tested. (C) Identification of an NRE located in the flanking intronic region. An intronic PCR fragment (+134 to +237) was cloned either in sense (pGL3 C NRE S) or antisense orientation (pGL3 C NRE AS) into theFirefly luciferase vector pGL3 control containing the SV40 promoter and enhancer. Relative luciferase activities were determined as described in (B).
This minimal promoter activity could be greatly enhanced by deletion of a short element in the flanking intronic sequence. The activity was again higher in Jurkat cells transfected with the construct containing the positive regulatory element (∼16-fold) compared with the activity obtained with the −78-bp fragment (∼9-fold). These fragments were not able to drive the reporter gene in B cells. This negative regulatory element (NRE) was further analyzed in a heterologous system. When the +134/+237 flanking intronic region was inserted downstream of the SV40 promoter, a complete inhibition was observed in both T and B cells (Figure 1C). In contrast, if the sequence was inserted in the reverse orientation, no negative effect was detected. This NRE acts as a strong negative modulator in different cell types but only in a correct orientation. Cases of NRE in the flanking intronic region have already been described, especially for CCR5.10 However, inspection of the corresponding nucleotide sequence did not reveal binding sites for known candidate repressors, suggesting that this sequence may down-regulate the transcription through an alternate mechanism like a repressive secondary structure.
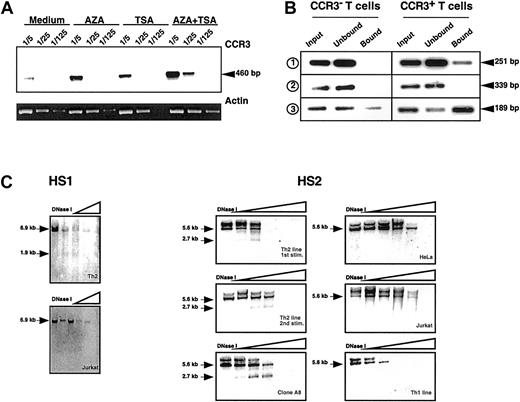
Promoters and enhancers are not the sole elements controlling gene expression, and chromatin remodeling may also participate in the regulation of several eukaryotic tissue-specific genes.11-13 To look for independent evidence for a role of chromatin structure in Th2-specific expression of CCR3, we used 5-aza-2′deoxycytidine (AZA) that promotes demethylation of the CpG sites in replicating DNA14 and Trichostatin A (TSA) that inhibits histone deacetylases, thus leading to a less-compact chromatin structure.15 When developing Th2 cells were restimulated in the presence of AZA and TSA, enhanced transcription of CCR3 was observed, the 2 drugs being at least additive (Figure2A).
Increased accessibility of the CCR3 gene in Th2 cells.
(A) AZA and TSA induce CCR3 expression in activated T cells. Purified naive CD4+ T cells were first stimulated with α-CD3 + α-CD28 in the presence of IL-2 and IL-4. After 10 days, the cells were restimulated under the same conditions in the presence of either 5 μM AZA or 16.5 nM TSA or both drugs (AZA + TSA). After 3 days the cells were washed and expanded for 5 days with IL-2. Semiquantitative PCR experiments were performed with cDNA dilutions (1/5, 1/25, and 1/125). The result of CCR3 amplifications was visualized by Southern blot. β-actin amplification was analyzed by ethidium bromide staining of the agarose gel. The increased receptor expression was also detected by cell surface staining (not shown). (B) Two regions of the human CCR3 gene are associated with acetylated H3 histones in Th2 cells. Primers used are described in “Study design.” The first set (1) covers 251 bp of the promoter region from −239 to +13, the second set (2) covers 339 bp of the upstream intronic region from +9284 to +9623, and the third set (3) covers 189 bp of the downstream intronic region from +20 540 to +20 729. Soluble chromatin was immunoprecipitated with an anti–acetyl H3 antibody from CCR3+ and CCR3− T cells. DNA was purified from the different fractions (input, unbound, bound), and equal amounts were amplified by PCR under stringent and nonsaturating conditions (25 cycles). PCR products were visualized by Southern blot by using specific probes generated by PCR. Similar results were obtained in 3 independent experiments. (C) Increased accessibility to DNaseI of CCR3 gene in Th2 cells. Nuclei were purified from 1 to 2.107 cells and digested with increasing concentrations of DNaseI. Genomic DNA was purified, and each fraction was digested by HindIII. The results were visualized by Southern blot. Left panel: DNAseI HS analysis of the CCR3 promoter region in a Th2 line and Jurkat cells. Membranes were hybridized using a 3′ probe (2) of 339 bp (from +9284 to +9623). The 6.9-kbHindIII fragment analyzed and the 1.9-kb HS site are indicated with arrows. Right panel: DNAseI HS analysis of the 3′ intronic region of CCR3. Nuclei were purified from CCR3+(left) and CCR3− (right) cells, and membranes were hybridized using a probe (3) of 796 bp (from +20 540 to +21 336). The 5.678-kb HindIII fragment analyzed and the 2.7-kb HS site are indicated.
Increased accessibility of the CCR3 gene in Th2 cells.
(A) AZA and TSA induce CCR3 expression in activated T cells. Purified naive CD4+ T cells were first stimulated with α-CD3 + α-CD28 in the presence of IL-2 and IL-4. After 10 days, the cells were restimulated under the same conditions in the presence of either 5 μM AZA or 16.5 nM TSA or both drugs (AZA + TSA). After 3 days the cells were washed and expanded for 5 days with IL-2. Semiquantitative PCR experiments were performed with cDNA dilutions (1/5, 1/25, and 1/125). The result of CCR3 amplifications was visualized by Southern blot. β-actin amplification was analyzed by ethidium bromide staining of the agarose gel. The increased receptor expression was also detected by cell surface staining (not shown). (B) Two regions of the human CCR3 gene are associated with acetylated H3 histones in Th2 cells. Primers used are described in “Study design.” The first set (1) covers 251 bp of the promoter region from −239 to +13, the second set (2) covers 339 bp of the upstream intronic region from +9284 to +9623, and the third set (3) covers 189 bp of the downstream intronic region from +20 540 to +20 729. Soluble chromatin was immunoprecipitated with an anti–acetyl H3 antibody from CCR3+ and CCR3− T cells. DNA was purified from the different fractions (input, unbound, bound), and equal amounts were amplified by PCR under stringent and nonsaturating conditions (25 cycles). PCR products were visualized by Southern blot by using specific probes generated by PCR. Similar results were obtained in 3 independent experiments. (C) Increased accessibility to DNaseI of CCR3 gene in Th2 cells. Nuclei were purified from 1 to 2.107 cells and digested with increasing concentrations of DNaseI. Genomic DNA was purified, and each fraction was digested by HindIII. The results were visualized by Southern blot. Left panel: DNAseI HS analysis of the CCR3 promoter region in a Th2 line and Jurkat cells. Membranes were hybridized using a 3′ probe (2) of 339 bp (from +9284 to +9623). The 6.9-kbHindIII fragment analyzed and the 1.9-kb HS site are indicated with arrows. Right panel: DNAseI HS analysis of the 3′ intronic region of CCR3. Nuclei were purified from CCR3+(left) and CCR3− (right) cells, and membranes were hybridized using a probe (3) of 796 bp (from +20 540 to +21 336). The 5.678-kb HindIII fragment analyzed and the 2.7-kb HS site are indicated.
To investigate for a preferential association of genomic regions of the CCR3 gene with acetylated histones, ChIP assays were performed by using an antibody to acetylated-H3 histones. Two DNA regions of the 3 analyzed, respectively located in the promoter and in the downstream intronic regions, are preferentially associated with acetylated-H3 histones in CCR3+ T cells (Figure 2B). This finding suggests that specific chromatin remodeling events in this upstream region of the CCR3 gene do not occur by a global spread of histone acetylation but rather involve targeted acetylation of regions potentially implicated in the transcriptional regulation.
To provide further evidence for an association of specific chromatin remodeling and transcriptional regulation of CCR3, we looked for local distortions in the chromatin structure of the CCR3 gene in developing Th2 cells by performing DNaseI hypersensitivity (HS) experiments. Two regions, HS1 in the close promoter region and HS2 in the downstream intronic part, were specifically detected in CCR3+ Th2 cell lines and clones (Figure 2C). HS2 region, potentially able to bind GATA factors, becomes readily accessible in Th2 cells, after the first round of polarization when CCR3 transcription is barely detectable. These changes of the chromatin organization in the CCR3 region are strictly correlated to the expression of the receptor because HS regions could not be detected in CCR3− cells like Th1, HeLa, and Jurkat cell lines. HS regions are very often associated to regulatory sequences like promoters and enhancers.16 Recent studies suggested that major alterations of the chromatin are implicated in the specific expression of the IL-4 gene in Th2 cells.17 The CCR3 locus could be regulated in a “2-step fashion” in which an initial stimulus may promote a specific opening of the locus, and the second step would provide the necessary transcription factors.12
The regulation of CCR3 expression in Th2 cells seems to be played at different complementary levels. The positive and negative regulatory elements of the promoter, as well as specific, close, and distal chromatin alterations along the genomic region, are implicated in the control of the chemotactic capacity of Th2 cells.
We thank M. Dessing and T. Hayden for help in cell sorting, F. Sallusto and M. Cella for providing cell lines, and H. J. Fehling for providing plasmids. We thank C. Hernandez-Munain and J. F. McBlane for critical comments.
Supported by the Helmut Horten Foundation (A.L.). The Basel Institute for Immunology was founded and is supported by Hoffmann-La Roche, Basel, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Emmanuel Scotet, INSERM U 463, Institut de Biologie, 9 quai Moncousu, 44093 Nantes Cedex, France; e-mail:emmanuel.scotet@nantes.inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal