Abstract
Human herpesvirus 7 (HHV-7) is endemic in the adult human population. Although HHV-7 preferentially infects activated CD4+ T lymphocytes, the consequence of T-cell infection for viral pathogenesis and immunity are still largely unknown. HHV-7 infection induces apoptosis mostly in uninfected bystander cells but not in productively infected CD4+ T cells. To dissect the underlying molecular events, the role of death-inducing ligands belonging to the tumor necrosis factor (TNF) cytokine superfamily was investigated. HHV-7 selectively up-regulated the expression of TNF-related apoptosis-inducing ligand (TRAIL), but not that of CD95 ligand or TNF-α in lymphoblastoid (SupT1) or primary activated CD4+ T cells. Moreover, in a cell-to-cell-contact assay, HHV-7–infected CD4+ T lymphocytes were cytotoxic for bystander uninfected CD4+ T cells through the TRAIL pathway. By contrast, HHV-7 infection caused a marked decrease of surface TRAIL-R1, but not of TRAIL-R2, CD95, TNF-R1, or TNF-R2. Of note, the down-regulation of TRAIL-R1 selectively occurred in cells coexpressing HHV-7 antigens that became resistant to TRAIL-mediated cytotoxicity. These findings suggest that the TRAIL-mediated induction of T-cell death may represent an important immune evasion mechanism of HHV-7, helping the virus to persist in the host organism throughout its lifetime.
Introduction
Human herpesvirus 7 (HHV-7) is a CD4+ T lymphotropic herpesvirus isolated for the first time in 1990; it belongs to the Betaherpesvirinae subfamily.1 The overall structure of the HHV-7 genome is identical to that of HHV-6, with a long, unique component of approximately 133-kb pairs flanked by a single direct repeat (DR) unit at each end.2-4 HHV-7 is a prevalent virus toward which most (more than 90%) of the population is seropositive by adulthood.5,6 Although the portal of entry of HHV-7 into the human host, the sites of primary infection, and the sites of latency have yet to be fully elucidated, it has been demonstrated that the human salivary system is a source for persistent production of infectious HHV-7.7 Moreover, it has been previously shown that the CD4 antigen, expressed at high levels on the surfaces of a subset of mature T cells, is the high-affinity receptor for HHV-7.8 9
Primary HHV-7 infection has been associated with rash, exanthema subitum, chronic fatigue syndrome, chronic Epstein-Barr–like syndrome, liver dysfunction, and central nervous system manifestations.10-12 More important, because HHV-7 is widespread in the adult population,5,6 it represents a potential opportunistic agent in immunocompromised hosts such as patients undergoing bone marrow or organ transplantation.13-18 In this respect, HHV-7 DNA has been found in up to 50% of bone marrow samples obtained from healthy donors,14 and we have recently shown that it can infect CD34+ hematopoietic progenitors.19 Moreover, it has been demonstrated that reactivation of HHV-7 occurs after renal,16,17 liver,18 and bone marrow15,20 transplantation, and it has been shown that HHV-7 seroconversion represents a risk factor for cytomegalovirus disease in transplant recipients independently of donor-recipient cytomegalovirus serostatus.17,18 At the cellular level, it is conceivable that once reactivated, HHV-7 worsens the state of immunodeficiency because of its selective tropism for CD4+T lymphocytes,8,9,13 whose infection results in cytotoxicity 8,21-23 and immunomodulatory activities.9 24-26
Tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL/Apo2 ligand) is a recently described death-inducing ligand (DIL) belonging to the TNF superfamily that shows structural and functional similarities with CD95 (Apo1/Fas) ligand (L), including the use of Fas-associated death domain as an adaptor molecule.27,28Like other members of the TNF family, TRAIL is a type 2 membrane protein with an intracellular amino-terminal portion, an internal trans-membrane domain, and a carboxyl terminus external to the cell. In addition, a soluble form of TRAIL has been described,29 as also previously shown for TNF-α and CD95L.30 At variance with CD95L, with its long intracellular region of 81 amino acids, TRAIL has a short intracellular tail of 17 amino acids and appears regulated at the cell surfaces of different cell types by proteolysis.29 TRAIL binds with similar affinity to at least 5 receptors: TRAIL-R1/DR4, TRAIL-R2/DR5/TRICK2/killer, TRAIL-R3/TRID/DcR1/LIT, TRAIL-R4/TRUNDD/DcR2, and osteoprotegerin.31,32 TRAIL-R1 and TRAIL-R2 contain a death domain homologous to that in CD95 and TNF-RI. Oligomerization of the death domain in TRAIL-R1 and TRAIL-R2 recruits caspase 8 through the Fas-associated death domain and activates the subsequent cascade of caspase proteases resulting in apoptosis.33,34 On the other hand, TRAIL-R3 (DcR1) and TRAIL-R4 (DcR2) are homologous to TRAIL-R1 and TRAIL-R2 in their cysteine-rich extracellular domains, but they lack an intracellular death domain and apoptosis-inducing capability.31
The aim of this study was to investigate the functional expression of DIL and DIL receptors and, in particular, of TRAIL and its receptors during HHV-7 infection. For this purpose, we have used, as targets for HHV-7 infection, the SupT1 lymphoblastoid CD4+ T-cell line and primary CD4+ T lymphocytes, purified from the peripheral blood of healthy subjects.
Materials and methods
Cells
The CD4+ human T-cell line SupT1 (AIDS Research and Reference Reagent Program) was routinely cultured in RPMI 1640 (Gibco BRL, Gaithersburg, MD) containing 10% fetal calf serum (Gibco BRL). Primary enriched CD4+ and CD8+ T cells were derived from the peripheral blood of 14 healthy blood donors by negative immunomagnetic selection, as previously described.8,21-23 Primary cells were cultured in RPMI 1640 containing 10% fetal calf serum and were activated with 5 μg/mL purified phytohemagglutinin (Sigma Chemical, St Louis, MO) plus 20 U/mL human recombinant interleukin-2 (rIL-2) (Genzyme, Boston, MA). After 3 days of culture, cells were washed twice and seeded again in complete medium plus 5 U/mL human rIL-2 alone, which was re-added every 4 days, as previously described.8 21-23
Viral infections
The HHV-7 isolate and the preparation procedures of viral stocks have been previously described.21,22 Briefly, HHV-7 infections of both SupT1 and preactivated CD4+ T cells were carried out by incubation with 0.45- μm–filtered infectious supernatants obtained from HHV-7–infected SupT1 cells. Supernatants from uninfected SupT1 cells were used for mock infections. The occurrence of a productive HHV-7 infection was monitored by scoring the appearance of balloonlike cells at light microscopy and by indirect immunofluorescence staining on acetone-fixed cells by using a specific HHV-7 monoclonal antibody (5E1 mAb, generously provided by Prof Campadelli-Fiume, University of Bologna, Italy),35 as previously described.21
Western blot analysis
For the analysis of TRAIL protein expression, Western blot analysis was performed on approximately 5 to 10 × 106cells/experimental point. Cells were harvested in lysis buffer containing 1% Triton X-100, sonicated, and processed for Western blot. Protein determination was performed by Bradford assay (Bio-Rad, Richmond, CA). Equal amounts of proteins for each sample were migrated in 12% polyacrylamide gel electrophoresis and blotted onto nitrocellulose filters. Blotted filters were blocked for 60 minutes in a 3% suspension of dried skim milk in phosphate-buffered saline (PBS) and were incubated overnight at 4°C with a 1:200 dilution of anti-TRAIL mAb (clone B35-1; Pharmingen, San Diego, CA). Filters were washed and further incubated for 1 hour at room temperature with a 1:1000 dilution of peroxidase-conjugated anti–mouse immunoglobulin G (Sigma). Specific reactions were revealed with the enhanced chemiluminescence Western blotting detection reagent (Amersham, Arlington Heights, IL). Densitometric analysis of immunoreactive bands was performed with an imaging densitometer (model GS 670; Bio-Rad Italia, Milan, Italy), using the Molecular Analyst software. Results were reported as arbitrary units and as percentage expression in HHV-7–infected samples compared with uninfected ones.
Flow cytometric analysis
Aliquots of 1 × 106 cells/experimental point were subjected to single- or multiple-label staining to examine the presence of surface antigens, as described previously.9 Surface TRAIL expression was analyzed by indirect staining using 1 μg RIK-2 anti-TRAIL mA (a generous gift from Dr Hideo Yagita, Juntendo University School of Medicine, Tokyo, Japan), followed by phycoerythrin (PE)–labeled goat anti–mouse IgG (Sigma). Expression of TRAIL-receptor (R)1, TRAIL-R2, TRAIL-R3, and TRAIL-R4 was analyzed by indirect staining using goat anti–human TRAIL-R1, TRAIL-R2, TRAIL-R3, and TRAIL-R4 antibodies (all from R&D System, Oxon, United Kingdom) followed by PE-conjugated rabbit anti–goat IgG (Sigma). Aspecific fluorescence was assessed by using either normal mouse IgG (for TRAIL) or normal goat IgG (for TRAIL receptors) followed by a second layer as above. CD4 expression was analyzed by using the Cy5-PE–conjugated mAb (Becton Dickinson, San Jose, CA). Analysis was performed by using a FACScan flow cytometer (Becton Dickinson) and the Lysis II software (Becton Dickinson). Data collected from 10 000 cells are reported as either percentage positive cells or mean fluorescence intensity (MFI) values.
JAM test
The JAM assay36 was performed to measure the degree of cell death induced by HHV-7. For this purpose, target cells were labeled with [3H] thymidine (TdR). When cell death occurred in the labeled cell population, DNA fragments were washed through glass fiber filters during cell harvesting. In contrast, DNA from surviving target cells remained intact and was captured by the filters. Percentage cell death was calculated by comparing the amount of [3H]TdR bound to filters in the presence and absence of the apoptosis-inducing events. Briefly, proliferating uninfected SupT1 or primary CD4+ or CD8+ T cells (target) were pulsed overnight with .37 MBq/mL (10 μCi/mL) [3H]TdR (New England Nuclear, Boston, MA), washed with complete medium 3 times, and plated into wells that contained graded numbers of HHV-7–infected (effector) cells or equivalent numbers of uninfected cells as control. After incubation at 37°C, cells were harvested onto glass fiber filters by using vacuum aspiration, and radioactivity was counted. All measurements given represent the mean of 6 wells. When required, blocking antibodies were added to HHV-7–infected effector cells 1 hour before incubation with uninfected (target) cells. Anti-TRAIL neutralizing polyclonal Ab (R&D Systems) was used at 1 μg/mL; anti–TNF-α neutralizing polyclonal Ab (R&D Systems) was used at 1 μg/mL; anti-CD95 Fab′ mAb (kindly provided by Dr Peter Krammer, University of Heidelberg, Germany), which specifically blocks the ability of CD95L to interact with CD95, was used at 0.1 μg/mL. In preliminary titration experiments, the neutralizing activity of 0.01 to 10 μg/mL anti–TNF-α and anti-CD95 Fab′ mAb was tested, and we have used those concentrations of antibodies, which were able to completely inhibit the cell death induced by 10 ng recombinant TNF-α and of anti-CD95 IgM agonistic antibody, respectively. Results are expressed as percentage DNA fragmentation or DNA loss.
Recombinant TRAIL
Both rHis6-tagged TRAIL and rHis6-tag control peptides were produced in bacteria and were purified by affinity chromatography on Ni2+ affinity resin, as previously described.37 In preliminary dose- and time-course experiments performed in SupT1 cells, TRAIL-induced apoptosis was complete by 20 hours and reached a plateau at the concentration of 1 μg/mL. In contrast, equimolar concentrations of rHis6-tag control peptide did not show any significant toxicity. Therefore, a 20-hour incubation period and a concentration of 1 μg/mL TRAIL were chosen for experiments in uninfected and HHV-7–infected SupT1 cells.
Evaluation of apoptosis
The presence of apoptosis was examined by flow cytometry after propidium iodide (PI) staining and by fluorescence microscopy after 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) staining. For flow cytometry studies, cells were harvested by centrifugation at 200g for 10 minutes at 4°C, fixed with cold 70% ethanol for at least 1 hour at 4°C, and treated as previously described.21,38 Samples were then pelleted, treated with 0.5 μg RNAse (type I-A; Sigma), and resuspended in PBS containing 50 μg/mL PI. Analysis was performed by FACScan with the FL2 detector in logarithmic mode using Lysis II software (Becton Dickinson). Data were expressed as percentage of apoptotic versus nonapoptotic cells belonging to all cell cycle phases. It should be emphasized that the percentage of apoptosis was calculated using very strict criteria, which have been previously described,38 to score only apoptotic cells and excluding all cell debris.
For DAPI staining of nuclei, cells were washed with PBS, fixed in paraformaldehyde 4% for 10 minutes, permeabilized in Triton X100 0.1% for 10 minutes, washed again with PBS, and incubated with 500 ng/mL DAPI (Sigma) in PBS for 15 minutes at 37°C in a dark, humidified chamber. After several washes in PBS, the coverslips were mounted on PBS-glycerin, and the intercalation of DAPI was visualized by means of an Axiophot Zeiss fluorescence microscope (Carl Zeiss, Thornwood, NY).
Statistics
Statistical analysis was performed using the 2-tailed Student t test.
Results
HHV-7 infection up-regulates TRAIL protein expression
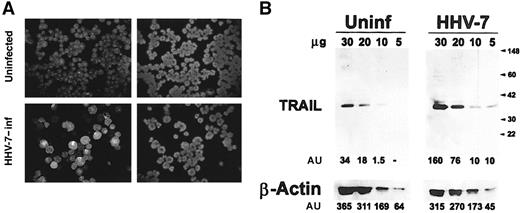
CD4+ T lymphoblastoid SupT1 cells, which show a high susceptibility to HHV-7 infection,8 21-23 were either mock-treated or infected with HHV-7 at a multiplicity of infection of 0.1. The peak of infection was usually observed at day 8 after infection, when most cells were infected with HHV-7, as evaluated by the progressive increase of balloonlike polyploid cells and by specific indirect immunofluorescence for HHV-7 antigens (52.6% ± 9% positive cells; mean ± SD of 5 separate experiments) (Figure 1A). Semiquantitative evaluation of total TRAIL protein expression was performed by Western blot analysis of the protein cell lysates after blotting with a specific anti-TRAIL mAb, and with an anti–β-actin mAb as a control for protein loading (Figure 1B). A 33- to 35-kd protein corresponding to the full-length monomeric TRAIL was already expressed in uninfected SupT1 cells and was up-regulated after HHV-7 infection (Figure 1B). The amount of TRAIL protein, as evaluated by densitometric analysis of the bands, was significantly (P < .01) increased in HHV-7–infected versus uninfected cultures (percentage increase was 390% ± 45% of 5 separate experiments).
Expression of TRAIL in uninfected and HHV-7–infected SupT1 cells, evaluated by immunoblotting analysis of cell lysates.
(A) Virus growth was detected by indirect immunofluorescence using the HHV-7–specific 5E mAb. Representative photographs taken 8 days after infection are shown. (left panels) HHV-7–specific immunofluorescence. (right panels) DAPI staining of the nuclei of the same field. Original magnification is × 250. (B) Equivalent amounts of protein lysates obtained from HHV-7–infected (inf) and uninfected (uninf) SupT1 cells were analyzed by Western blot with anti-TRAIL mAb. Equal loading of protein in each lane was confirmed by staining with the antibody to β-actin. Molecular size markers are indicated on the right in kilodaltons. Relative intensities of the bands were densitometrically quantified and expressed in arbitrary units (AU). Results are representative of 5 independent experiments performed.
Expression of TRAIL in uninfected and HHV-7–infected SupT1 cells, evaluated by immunoblotting analysis of cell lysates.
(A) Virus growth was detected by indirect immunofluorescence using the HHV-7–specific 5E mAb. Representative photographs taken 8 days after infection are shown. (left panels) HHV-7–specific immunofluorescence. (right panels) DAPI staining of the nuclei of the same field. Original magnification is × 250. (B) Equivalent amounts of protein lysates obtained from HHV-7–infected (inf) and uninfected (uninf) SupT1 cells were analyzed by Western blot with anti-TRAIL mAb. Equal loading of protein in each lane was confirmed by staining with the antibody to β-actin. Molecular size markers are indicated on the right in kilodaltons. Relative intensities of the bands were densitometrically quantified and expressed in arbitrary units (AU). Results are representative of 5 independent experiments performed.
Because TRAIL is a type 2 membrane protein,27 the surface expression of TRAIL was next investigated by flow cytometry. SupT1 cells were either mock-treated or were infected with HHV-7 and cultured for 8 days, when the cells were labeled with an anti-TRAIL mAb. Although surface TRAIL was not detectable in SupT1 control cells, a dim but clearly detectable expression of surface TRAIL was observed in HHV-7–infected SupT1 cells (Figure 2A). These findings are consistent with the data of other authors, who demonstrated that TRAIL protein is expressed as a constitutive intra-cytoplasmic protein in lymphoid CD4+ T cells.39 40 Although small, the difference in TRAIL surface expression between HHV-7–infected (MFI, 7.8 ± 1.6) and uninfected (MFI, 5 ± 1.3, corresponding to the background level) cells was reproducible in various experiments (mean ± SD of 5 separate experiments; P < .05). Moreover, the up-regulation of surface TRAIL mediated by HHV-7 was specific; the surface expression of other DIL, such as CD95L and TNF-α, was undetectable in uninfected and HHV-7–infected SupT1 cultures (Figure2B).
Surface expression of DIL analyzed in uninfected and HHV-7–infected SupT1 cells by immunofluorescence staining revealed by flow cytometry.
Representative analysis of surface TRAIL (A), CD95L, and TNF-α (B) performed 8 days after infection is shown. Control (unshadowed) histograms represent the background fluorescence obtained from the staining of the same cultures with an isotype-matched control mAb. On the left side of panel A and in all of panel B, the unshadowed histograms are not visible because they are completely overlapped by the shadowed histograms. In panel A, a shift of the shadowed histogram along the x-axis illustrates the increased level of TRAIL expression in HHV-7–infected cultures. Data shown are from a single experiment representative of 5 independent experiments with similar results.
Surface expression of DIL analyzed in uninfected and HHV-7–infected SupT1 cells by immunofluorescence staining revealed by flow cytometry.
Representative analysis of surface TRAIL (A), CD95L, and TNF-α (B) performed 8 days after infection is shown. Control (unshadowed) histograms represent the background fluorescence obtained from the staining of the same cultures with an isotype-matched control mAb. On the left side of panel A and in all of panel B, the unshadowed histograms are not visible because they are completely overlapped by the shadowed histograms. In panel A, a shift of the shadowed histogram along the x-axis illustrates the increased level of TRAIL expression in HHV-7–infected cultures. Data shown are from a single experiment representative of 5 independent experiments with similar results.
TRAIL is a mediator of HHV-7–induced cytotoxicity
To evaluate whether the up-regulation of TRAIL by HHV-7 was functional, a JAM cytotoxicity assay was performed.36[3H]TdR-labeled SupT1 (target) cells were incubated with increasing amounts of HHV-7–infected or mock-infected cells (effector cells) for 16 hours. HHV-7–infected cells induced marked and specific target cell lysis, as determined by calculating DNA loss,36 that was dose dependent (Figure3A). On the other hand, mock-infected cells and the supernatant of the HHV-7–infected cultures did not induce any significant target cell lysis (Figure 3A). This first group of experiments indicated that HHV-7–induced cell death in SupT1 cells required cell-to-cell contact. Then, to determine whether HHV-7–induced cell death was mediated by TRAIL, uninfected labeled SupT1 cells were incubated with HHV-7–infected cells (target-effector ratio, 1:4) for 16 hours in the absence or presence of anti-TRAIL neutralizing polyclonal Ab. The presence of anti-TRAIL Ab significantly (P < .01) inhibited cell lysis induced by HHV-7–infected SupT1 cells (Figure3B). Conversely, both anti-CD95 Fab′ and anti-TNF-α neutralizing antibodies had modest effects on the cytotoxic activity induced by contact with HHV-7–infected cultures (Figure 3B).
Role of TRAIL in the cytotoxic activity of HHV-7–infected cells.
SupT1 cells were either mock-treated (control) or HHV-7–infected (effector cells) and, at 8 days after infection, cultured with [3H] TdR-labeled SupT1 (target) cells at the indicated ratios. (A) After 16 hours, cells were harvested and [3H] TdR was counted. The x-axis gives the degree of fragmentation of labeled target cell DNA (expressed as percentage DNA loss) in response to the cytotoxic attack, represented either by HHV-7–infected cells or by the supernatant (Supern) of the HHV-7–infected cultures. (B) SupT1 cells were either mock-treated (control) or HHV-7 infected and, at 8 days after infection, cultured with [3H] TdR-labeled SupT1 cells at the E/T ratio of 4:1 either in the absence (nil) or in the presence of neutralizing anti-TRAIL (1 μg/mL), anti–TNF-α (1 μg/mL), or anti-CD95 Fab′ (0.1 μ/mL) antibodies. Data are expressed as mean ± SD of 6 separate experiments performed in duplicate.
Role of TRAIL in the cytotoxic activity of HHV-7–infected cells.
SupT1 cells were either mock-treated (control) or HHV-7–infected (effector cells) and, at 8 days after infection, cultured with [3H] TdR-labeled SupT1 (target) cells at the indicated ratios. (A) After 16 hours, cells were harvested and [3H] TdR was counted. The x-axis gives the degree of fragmentation of labeled target cell DNA (expressed as percentage DNA loss) in response to the cytotoxic attack, represented either by HHV-7–infected cells or by the supernatant (Supern) of the HHV-7–infected cultures. (B) SupT1 cells were either mock-treated (control) or HHV-7 infected and, at 8 days after infection, cultured with [3H] TdR-labeled SupT1 cells at the E/T ratio of 4:1 either in the absence (nil) or in the presence of neutralizing anti-TRAIL (1 μg/mL), anti–TNF-α (1 μg/mL), or anti-CD95 Fab′ (0.1 μ/mL) antibodies. Data are expressed as mean ± SD of 6 separate experiments performed in duplicate.
Parallel experiments were carried out using primary CD4+ T lymphocytes, preactivated with phytohemagglutinin plus IL-2 for 3 days, and then were infected with HHV-7 and cultured in the presence of 5 U/mL IL-2 for an additional 13 days (peak of infection). As expected on the basis of previous studies,8 21-23 the kinetics of infection in CD4+ T cells was slower with respect to that observed in SupT1 lymphoblastoid T cells. However, at day 13 after infection, several cells were infected by HHV-7 (34% ± 11%; mean ± SD of 5 separate experiments), as evaluated by indirect immunofluorescence analysis of HHV-7 antigens (Figure4A). The amount of TRAIL was significantly (P < .01) higher in HHV-7–infected CD4+ T lymphocytes than in uninfected control cells (Figure4B), starting from day 10 after infection (256% ± 55% of increase at day 13 after infection; mean ± SD of 5 separate experiments). Remarkably, when using primary cells, HHV-7–infected cultures induced specific target cell lysis after incubation with labeled uninfected primary CD4+ T cells that was significantly (P < .01) and selectively inhibited by the presence of anti-TRAIL neutralizing Ab (Figure 4C). In some experiments, primary HHV-7–infected CD4+ T cells were cocultured with labeled uninfected CD8+ T cells purified from the same donor. HHV-7–infected CD4+ T cells efficiently killed uninfected CD8+ T cells, but apparently in a DIL-independent manner, as indicated by the failure of anti-TRAIL, anti-CD95 Fab′, and anti–TNF-α neutralizing antibodies to block the killing of CD8+ T cells (Figure 4D). Together, these results demonstrate that HHV-7 infection induces the expression of functional TRAIL on the surfaces of human CD4+ T cells. By using both SupT1 and primary CD4+ T cells as targets, we could also demonstrate that HHV-7–induced cytotoxic activity of lymphoid CD4+ T cells is mediated by TRAIL.
Expression of TRAIL in uninfected and HHV-7–infected activated primary CD4+ T cells.
(A) Virus growth was detected by indirect immunofluorescence assay with the HHV-7–specific 5E mAbs. Representative photographs taken 13 days after infection are shown. (left) HHV-7–specific immunofluorescence. (right) DAPI staining of the nuclei of the same field. Original magnification is × 250. (B) Equivalent amounts of protein lysates obtained from HHV-7–infected and uninfected CD4+ T cells were analyzed by Western blot with an anti-TRAIL mAb. Equal loading of protein in each lane was confirmed by staining with the antibody to β-actin. Molecular size markers are indicated on the right in kilodaltons. Relative intensities of the bands were densitometrically quantified and expressed in arbitrary units. (C, D) CD4+ T cells were either mock-treated (control) or HHV-7 infected and, at 10 to 12 days after infection, cultured with [3H]TdR-labeled CD4+ (C) or CD8+ (D) T cells at the E/T ratio of 4:1, either in the absence (nil) or the presence of neutralizing anti-TRAIL (1 μg/mL), anti–TNF-α (1 μg/mL), or anti-CD95 Fab′ (0.1 μg/mL) Ab. These results are representative of 5 independent experiments performed.
Expression of TRAIL in uninfected and HHV-7–infected activated primary CD4+ T cells.
(A) Virus growth was detected by indirect immunofluorescence assay with the HHV-7–specific 5E mAbs. Representative photographs taken 13 days after infection are shown. (left) HHV-7–specific immunofluorescence. (right) DAPI staining of the nuclei of the same field. Original magnification is × 250. (B) Equivalent amounts of protein lysates obtained from HHV-7–infected and uninfected CD4+ T cells were analyzed by Western blot with an anti-TRAIL mAb. Equal loading of protein in each lane was confirmed by staining with the antibody to β-actin. Molecular size markers are indicated on the right in kilodaltons. Relative intensities of the bands were densitometrically quantified and expressed in arbitrary units. (C, D) CD4+ T cells were either mock-treated (control) or HHV-7 infected and, at 10 to 12 days after infection, cultured with [3H]TdR-labeled CD4+ (C) or CD8+ (D) T cells at the E/T ratio of 4:1, either in the absence (nil) or the presence of neutralizing anti-TRAIL (1 μg/mL), anti–TNF-α (1 μg/mL), or anti-CD95 Fab′ (0.1 μg/mL) Ab. These results are representative of 5 independent experiments performed.
HHV-7 infection selectively down-modulates TRAIL-R1 expression
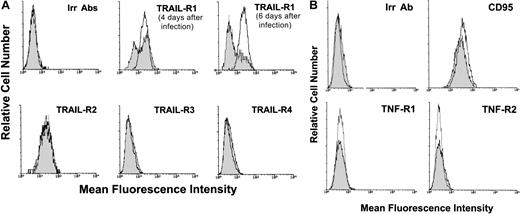
The surface expression of TRAIL receptors was next evaluated at various time points after infection by flow cytometry (Figure5A). Uninfected SupT1 cells showed high levels of surface expression of both TRAIL-R1 and TRAIL-R2, whereas TRAIL-R3 and TRAIL-R4 were not detectable. In HHV-7–infected SupT1 cultures, the expression of surface TRAIL-R1, but not TRAIL-R2, showed a progressive and significant (P < .01) decrease in comparison to uninfected controls (Figure 5A). On the other hand, TRAIL-R3 and TRAIL-R4 surface antigens were unaffected by HHV-7 infection (Figure 5A). The specificity of HHV-7–mediated TRAIL-R1 down-regulation was further confirmed by the analysis of other receptors of the TNF receptor superfamily (CD95, TNF-R1, and TNF-R2), whose expression was unchanged on HHV-7 infection (Figure 5B).
Surface expression of DIL receptors analyzed in uninfected and HHV-7–infected SupT1 cells by immunofluorescence staining revealed by flow cytometry.
Representative analyses of surface TRAIL receptors (A) and of CD95, TNF-R1, and TNF-R2 (B) performed at 8 days after infection are shown. Uninfected cultures, unshadowed histograms. HHV-7–infected cultures, shadowed histograms. In panel A, the shift of the shadowed histograms along the x-axis illustrates the progressive decrease in the number of TRAIL-R1–expressing cells in HHV-7–infected cultures. In panels A and B, the negative control is represented by uninfected (unshadowed histograms) and HHV-7–infected (shadowed histograms) cultures stained with an irrelevant (Irr) isotype control Ab (Irr Ab). Data shown are from a single experiment representative of 6 independent experiments with similar results.
Surface expression of DIL receptors analyzed in uninfected and HHV-7–infected SupT1 cells by immunofluorescence staining revealed by flow cytometry.
Representative analyses of surface TRAIL receptors (A) and of CD95, TNF-R1, and TNF-R2 (B) performed at 8 days after infection are shown. Uninfected cultures, unshadowed histograms. HHV-7–infected cultures, shadowed histograms. In panel A, the shift of the shadowed histograms along the x-axis illustrates the progressive decrease in the number of TRAIL-R1–expressing cells in HHV-7–infected cultures. In panels A and B, the negative control is represented by uninfected (unshadowed histograms) and HHV-7–infected (shadowed histograms) cultures stained with an irrelevant (Irr) isotype control Ab (Irr Ab). Data shown are from a single experiment representative of 6 independent experiments with similar results.
In consideration of the promiscuity of infected and uninfected cells in HHV-7–infected cultures, we next sought to elucidate whether TRAIL-R1 down-regulation occurred in infected or uninfected cells. In this respect, we have previously shown that HHV-7 infection of CD4+ T lymphocytes induces a progressive down-regulation of surface CD4, which represents the high-affinity receptor for HHV-7, and that the expression of HHV-7 antigens correlates with the loss of surface CD4 receptor.9 Therefore, double staining was next performed with anti–TRAIL-R1 plus anti-CD4 mAbs (Figure6A). At flow cytometry analysis of control uninfected SupT1 cells, as expected, all the cells coexpressed TRAIL-R1 and CD4, whereas in the HHV-7–infected cultures, 2 distinct cell populations (TRAIL-R1+/CD4+ and TRAIL-R1−/CD4−) were observed (Figure 6A). This suggested that the 2 surface molecules were simultaneously down-regulated in the same cell population, represented by those cells expressing HHV-7 antigens. On the other hand, the surface expression of TRAIL-R2 was unchanged in both the CD4+ and the CD4− subsets of the HHV-7–infected cultures.
Surface TRAIL-R1 and TRAIL-R2 expression analyzed in combination with surface CD4 in SupT1 either uninfected or infected with HHV-7.
(A) At 8 days after infection, relative surface CD4 expression was detected by PE-Cy5 fluorescence intensity (x-axis), whereas TRAIL-R1 and TRAIL-R2 expression was detected by PE fluorescence intensity (y-axis). Representative negative controls, composed of cells stained with irrelevant isotype-matched Ab (Irr Abs) are shown in the top panels. (B) Evaluation of surface CD4 expression in HHV-7–infected cultures after 20 hours of treatment with rTRAIL or rHis6-tag control (cont) peptide. X-axis, relative surface CD4 expression detected by PE-Cy5 fluorescence intensity. Y-axis, relative cell number. Data shown are from a single experiment representative of 5 independent experiments with similar results.
Surface TRAIL-R1 and TRAIL-R2 expression analyzed in combination with surface CD4 in SupT1 either uninfected or infected with HHV-7.
(A) At 8 days after infection, relative surface CD4 expression was detected by PE-Cy5 fluorescence intensity (x-axis), whereas TRAIL-R1 and TRAIL-R2 expression was detected by PE fluorescence intensity (y-axis). Representative negative controls, composed of cells stained with irrelevant isotype-matched Ab (Irr Abs) are shown in the top panels. (B) Evaluation of surface CD4 expression in HHV-7–infected cultures after 20 hours of treatment with rTRAIL or rHis6-tag control (cont) peptide. X-axis, relative surface CD4 expression detected by PE-Cy5 fluorescence intensity. Y-axis, relative cell number. Data shown are from a single experiment representative of 5 independent experiments with similar results.
In additional experiments, we investigated whether the HHV-7–mediated down-modulation of TRAIL-R1 protects the HHV-7–infected/CD4− cell population from TRAIL-induced cytotoxicity. Indeed, when HHV-7–infected SupT1 cultures were challenged with soluble rTRAIL for 16 hours, specific depletion of the CD4+ (TRAIL-R1+) SupT1 cell subpopulation was observed (Figure 6B). On the other hand, the addition of an agonistic anti-CD95 IgM mAb induced cytotoxic effects equally manifest on both the CD4+ and the CD4− subpopulations of the HHV-7–infected cultures (data not shown).
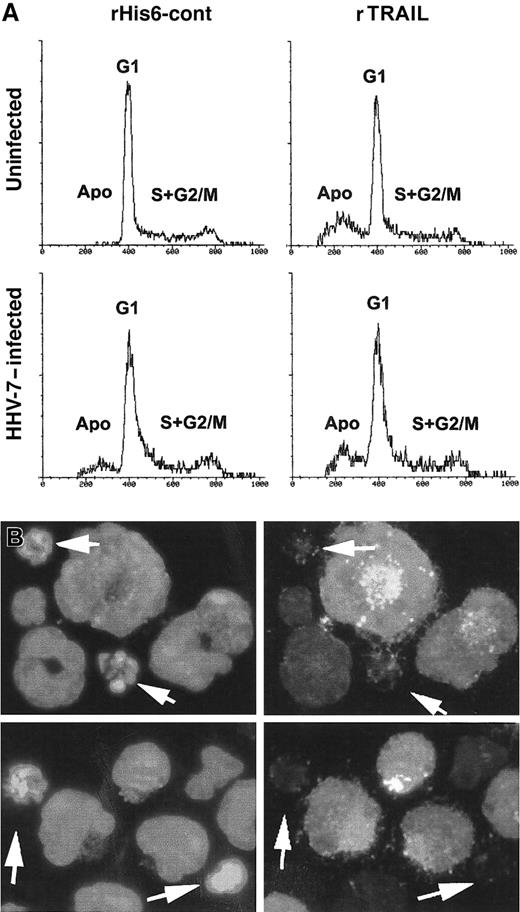
The occurrence of apoptosis was next investigated by PI staining and flow cytometry analysis (Figure 7A). As expected on the basis of our previous studies,21 26HHV-7–infected cultures showed a significant (P < .01) percentage of apoptotic cells (12% ± 4%; mean ± SD of 4 separate experiments) with respect to uninfected SupT1 cultures, which only displayed background levels of apoptosis (2% ± 1.5%, mean ± SD of 4 separate experiments). On the other hand, the addition of rTRAIL induced a marked (P < .01) increase in the percentage of apoptosis in uninfected cells (29% ± 7%; mean ± SD of 4 separate experiments) and a much lower increase in HHV-7–infected cells (22% ± 5%; mean ± SD of 4 separate experiments) (Figure 7A). PI staining and flow cytometry analysis do not allow discrimination between HHV-7–infected and uninfected cells, which coexist in HHV-7– infected cultures. Therefore, the occurrence of TRAIL-induced apoptosis was next analyzed in combination with the expression of viral antigens by using dual-label staining for cell nuclei (DAPI) and HHV-7 antigens (Figure 7B; Table1). After 20 hours of TRAIL challenge, the presence of apoptotic nuclei, evaluated by DAPI staining, was clearly detected in uninfected SupT1 cell cultures at fluorescence microscopy analysis (Table 1). In HHV-7–infected cultures, on TRAIL challenge, few cells staining positively for the expression of viral antigens exhibited an apoptotic morphology, resulting in a significantly (P < .01) lower number compared with apoptotic cells among the HHV-7 antigen–negative cells (Table 1). These findings clearly suggest that the cells expressing HHV-7 antigens are protected from TRAIL-induced apoptosis.
Evaluation of apoptosis in uninfected and HHV-7–infected SupT1 cells treated with rTRAIL or rHis6-tag control peptide.
(A) Flow cytometric evaluation of apoptosis and cell cycle profile in uninfected and HHV-7–infected SupT1cells after 20 hours of treatment with rHis6-tag control peptide (left panels) or rTRAIL (1 μg/mL) (right panels). The x-axis shows the DNA content, determined based on fluorescence resulting from PI staining, and the y-axis reflects the relative number of cells. Cells in apoptosis (Apo) or in the gap1 (G1), synthesis (S), gap2 (G2), and mitosis (M) phases of the cell cycle are indicated. These results are representative of 4 experiments performed in duplicate. (B) Combined immunofluorescence analysis of HHV-7 antigen expression and apoptotic nuclei after TRAIL-challenge in HHV-7–infected SupT1 cells. Cells are scored by fluorescence microscopy. Note that enlarged cells, characteristic of HHV-7–infected cultures, are positively stained for viral antigens, whereas apoptotic nuclei (arrows) are more frequent among small, uninfected cells. Original magnification is × 400. Representative fields of 5 separate experiments are shown.
Evaluation of apoptosis in uninfected and HHV-7–infected SupT1 cells treated with rTRAIL or rHis6-tag control peptide.
(A) Flow cytometric evaluation of apoptosis and cell cycle profile in uninfected and HHV-7–infected SupT1cells after 20 hours of treatment with rHis6-tag control peptide (left panels) or rTRAIL (1 μg/mL) (right panels). The x-axis shows the DNA content, determined based on fluorescence resulting from PI staining, and the y-axis reflects the relative number of cells. Cells in apoptosis (Apo) or in the gap1 (G1), synthesis (S), gap2 (G2), and mitosis (M) phases of the cell cycle are indicated. These results are representative of 4 experiments performed in duplicate. (B) Combined immunofluorescence analysis of HHV-7 antigen expression and apoptotic nuclei after TRAIL-challenge in HHV-7–infected SupT1 cells. Cells are scored by fluorescence microscopy. Note that enlarged cells, characteristic of HHV-7–infected cultures, are positively stained for viral antigens, whereas apoptotic nuclei (arrows) are more frequent among small, uninfected cells. Original magnification is × 400. Representative fields of 5 separate experiments are shown.
TRAIL-induced apoptosis in uninfected and HHV-7–infected SupT1 cultures
| . | Cells scored . | Apoptosis, no. . | Apoptosis, % . |
|---|---|---|---|
| Uninfected SupT1 cultures | 475 | 151 | 31.7 |
| HHV-7–infected SupT1 cultures | |||
| HHV-7 antigen–positive cells | 518 | 23 | 4.4 |
| HHV-7 antigen–negative cells | 507 | 149 | 29.3 |
| . | Cells scored . | Apoptosis, no. . | Apoptosis, % . |
|---|---|---|---|
| Uninfected SupT1 cultures | 475 | 151 | 31.7 |
| HHV-7–infected SupT1 cultures | |||
| HHV-7 antigen–positive cells | 518 | 23 | 4.4 |
| HHV-7 antigen–negative cells | 507 | 149 | 29.3 |
After treatment with recombinant TRAIL, cells were scored by fluorescence microscopy to detect apoptotic nuclei and HHV-7 antigen expression (in HHV-7–infected cultures) by using dual-label staining for cell nuclei (DAPI) and HHV-7 antigens, as described in “Materials and methods” (see also legend to Figure 7B). Data represent the results of cells scored from 4 independent experiments.
Discussion
The presence of at least 5 TRAIL receptors indicates that TRAIL is involved in multiple processes, but the precise roles of TRAIL in health and disease are still largely unknown. Although TRAIL and TRAIL receptors are expressed in various tissues,27,41,42 TRAIL does not induce apoptosis of most nontransformed cells.41,42 It has been shown that TRAIL is up-regulated on T-cell activation,29,39,40 and one of the functions of TRAIL in vivo is to maintain immune homeostasis by inhibiting the cell cycle progression of T lymphocytes.43
We have here demonstrated for the first time that HHV-7 infection induces the simultaneous up-regulation of TRAIL and down-regulation of TRAIL-R1 on the surfaces of CD4+ T cells. Among the different DIL (TRAIL, CD95L, TNF-α) and DIL receptors (TRAIL-R1, TRAIL-R2, CD95, TNF-R1, and TNF-R2) investigated, significant changes in HHV-7–infected cells selectively involved TRAIL and TRAIL-R1. Moreover, HHV-7 infection was able to induce the modulation of TRAIL in both SupT1 and primary CD4+ T cells. More important, these HHV-7–induced modifications of TRAIL expression were functional, as demonstrated by the ability of HHV-7–infected SupT1 and primary CD4+ T cells to efficiently kill uninfected SupT1 and autologous CD4+ T cells, respectively, in a TRAIL-dependent manner. Although we cannot rule out the possibility that soluble cytokines released by infected cells may contribute to the induction of HHV-7–mediated cell death in bystander uninfected cells, our findings indicate that cell-to-cell contact is required for TRAIL-mediated cytotoxicity. Conversely, HHV-7–infected cells were almost completely resistant to TRAIL-mediated apoptosis, likely as a consequence of the down-regulation of surface TRAIL-R1, which represents a major determinant of TRAIL sensitivity.44 An apparent discrepancy was noticed between the number of HHV-7–infected SupT1 cells (approximately 50%-60% at day 8 after infection, as evaluated by indirect immunofluorescence), and the modulation of surface TRAIL and TRAIL-R1, which involved most cells. A possible explanation for these findings is that the proportion of HHV-7–infected cells is higher than that detected by using an antibody directed against a protein of the HHV-7 tegument35 or by the down-regulation of surface CD4.9 Alternatively, indirect mechanisms, such as the release of cytokines (eg, interferon), which have been shown to up-regulate TRAIL expression,40 may account for our results. Concerning the ability of HHV-7–infected CD4+ T cells to specifically kill uninfected CD4+ T cells in a TRAIL-dependent manner, it should be emphasized that membrane-associated TRAIL is more efficient than soluble TRAIL in inducing cell death, probably because its trimeric structure is more stable.39 We have also shown that HHV-7–infected CD4+ T cells kill autologous uninfected CD8+ T cells, but anti-TRAIL neutralizing Ab fails to revert this cytotoxic effect, suggesting that, in addition to TRAIL, other factors are involved in HHV-7–mediated cytotoxicity.
Many viruses have their own antiapoptotic genes or are able to up-regulate antiapoptotic cellular genes, which can block premature death of infected cells.45 As do other large DNA-containing herpesviruses, HHV-7 probably uses multiple viral defense mechanisms that cooperate to prevent premature death of the host infected cell. Our findings strongly suggest that one of these mechanisms is the up-regulation of surface TRAIL, which efficiently kills uninfected T lymphocytes, coupled to the down-regulation of TRAIL-R1 in the productively HHV-7–infected cells. Because HHV-7 is dependent on CD4+ T cells for the production of mature virions, these biologic effects may represent part of a strategy to facilitate a persistent infection or to prolong the survival of the infected cells to maximize the production of viral progeny. Of note, this represents a unique feature of HHV-7 with respect to other herpesviruses. In fact, it has been shown that cytomegalovirus induces the up-regulation of TRAIL-R1 and TRAIL-R2 in infected fibroblasts, which are therefore more susceptible to TRAIL-mediated cytotoxicity,46 whereas HSV-1 selectively up-regulates CD95L but not TRAIL in infected lymphocytes.47
Although the pathogenicity of HHV-7 remains largely to be determined mainly because of the relatively recent discovery of this herpesvirus, compelling evidence indicates that latent HHV-7 infection is reactivated in immunocompromised patients, such as transplant recipients.10,13-16,18-20 Moreover, it has been shown that the expression of HHV-7 is significantly increased in peripheral lymphoid tissues of patients with acquired immune deficiency syndrome,48 strongly indicating that HHV-7 acts as an opportunistic agent in these patients. In this respect, other authors have shown that the TRAIL system is up-regulated during human immunodeficiency virus-1 infection and likely contributes to the pathogenesis of human immunodeficiency virus-1 disease.49-51
Our data demonstrate that the TRAIL-mediated induction of T-cell death may represent an important immune evasion mechanism of HHV-7, helping the virus to persist in the host organism throughout its lifetime. The functional up-regulation of TRAIL may also contribute to the role of HHV-7 as an opportunistic agent in transplantation patients and in patients with acquired immune deficiency syndrome.18-20 51
Supported by AIDS grant and local funds of the Universities of Ferrara and Chieti; A.G. is supported by an FIRC fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Giorgio Zauli, Department of Biomorphology, G. D'Annunzio University of Chieti, Via dei Vestini 6, 66100 Chieti Scalo (CH), Italy; e-mail: g.zauli@morpho.unich.it.


![Fig. 3. Role of TRAIL in the cytotoxic activity of HHV-7–infected cells. / SupT1 cells were either mock-treated (control) or HHV-7–infected (effector cells) and, at 8 days after infection, cultured with [3H] TdR-labeled SupT1 (target) cells at the indicated ratios. (A) After 16 hours, cells were harvested and [3H] TdR was counted. The x-axis gives the degree of fragmentation of labeled target cell DNA (expressed as percentage DNA loss) in response to the cytotoxic attack, represented either by HHV-7–infected cells or by the supernatant (Supern) of the HHV-7–infected cultures. (B) SupT1 cells were either mock-treated (control) or HHV-7 infected and, at 8 days after infection, cultured with [3H] TdR-labeled SupT1 cells at the E/T ratio of 4:1 either in the absence (nil) or in the presence of neutralizing anti-TRAIL (1 μg/mL), anti–TNF-α (1 μg/mL), or anti-CD95 Fab′ (0.1 μ/mL) antibodies. Data are expressed as mean ± SD of 6 separate experiments performed in duplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/8/10.1182_blood.v98.8.2474/6/m_h82011646003.jpeg?Expires=1766049069&Signature=YqpuQtozlS2jSKXEZdclx-kxVbzOqfEc91HrpUl4~3uDLK8JDU8RBgNUe3myNkChe0KgNwKP2eFJV3Td-dbY2cu17-g9ZRxXCHF7h85lrEjzkEX-oUmQentakEXCFsWgRUDu0kWFbUZFKDqeNwT9P6bV1sqiCj0bn8CgJQsMmz1TsgPa0ViBVBP9x5nglf-UMKXkgdhhIzsN82ayxYgbZSITF0aP~tc0HlCsUMkdmXnIyrS3fcUmmZcZUsBxCnOjEq1rqaGDMHk1zBm8oMUvNkbRUKTilJW7Qfh4WXY-Oa6J0cjfsbWhAqsfWQq~6QViLDxLF0P9u551K9Dsc9NiXg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Expression of TRAIL in uninfected and HHV-7–infected activated primary CD4+ T cells. / (A) Virus growth was detected by indirect immunofluorescence assay with the HHV-7–specific 5E mAbs. Representative photographs taken 13 days after infection are shown. (left) HHV-7–specific immunofluorescence. (right) DAPI staining of the nuclei of the same field. Original magnification is × 250. (B) Equivalent amounts of protein lysates obtained from HHV-7–infected and uninfected CD4+ T cells were analyzed by Western blot with an anti-TRAIL mAb. Equal loading of protein in each lane was confirmed by staining with the antibody to β-actin. Molecular size markers are indicated on the right in kilodaltons. Relative intensities of the bands were densitometrically quantified and expressed in arbitrary units. (C, D) CD4+ T cells were either mock-treated (control) or HHV-7 infected and, at 10 to 12 days after infection, cultured with [3H]TdR-labeled CD4+ (C) or CD8+ (D) T cells at the E/T ratio of 4:1, either in the absence (nil) or the presence of neutralizing anti-TRAIL (1 μg/mL), anti–TNF-α (1 μg/mL), or anti-CD95 Fab′ (0.1 μg/mL) Ab. These results are representative of 5 independent experiments performed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/8/10.1182_blood.v98.8.2474/6/m_h82011646004.jpeg?Expires=1766049069&Signature=c4eSfe6FcsCB0c-~caAvgQ5GR7miy0giTVsP~b6pwTLfVwZh2S5CJQlXN7marFp01ilBjrd1BStVhEExpC3OWShTdz07H-nBop5T-fP2UZgcOQr5~GGZt05SnCGflGgWlRIG22t8Tz8gU32J4E8JoyH1VF6N3uPvPodfs0adGcAD3-ZVG3Y7Y0GjEr2vmqx0uuumhbEJiO89jn42j9~gMBrDNS5Swxba8V-gH8sIPgNj3aRiTz85TrwhfsA0o2osuGBTnB6MVRns4KoH3drPVd3tMlE6iBzFnyI-i0wG3MzIMQJMt76Kww~7HJV5mvjr9kMhtZEE-TEJm2mIS4k78Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal