Abstract
Autoimmune lymphoproliferative syndrome (ALPS) type Ia is caused by inherited defects in apoptosis and is characterized by nonmalignant lymphoaccumulation, autoimmunity, and increased α/β+ double-negative T cells (α/β+-DNT cells). This study reports immunophenotypic findings in 166 members of 31 families with ALPS type Ia, associated with genetic mutations in theTNFRSF6 gene encoding Fas. The ALPS type Ia probands (n = 31) and relatives having both a Fas mutation and clinically proven ALPS (n = 28) showed significant expansion of CD8+T cells, α/β+-DNT cells, γ/δ+-DNT cells, CD3+/ HLA-DR+ T cells, CD8+/CD57+ T cells, and CD5+ B cells. Relatives with Fas mutations, but without all the required criteria for ALPS (n = 42), had expansions of CD8+ T cells, α/β+-DNT cells, and γ/δ+-DNT cells. Interestingly, relatives without a Fas mutation and with no features of ALPS (n = 65) demonstrated a small but significant expansion of CD8+ T cells, both DNT cell subsets, and CD5+ B cells. As compared to unrelated healthy controls, lymphocyte subset alterations were greatest in the probands, followed by the relatives with mutations and ALPS. Probands and relatives with mutations and ALPS also showed a lower number of CD4+/CD25+ T cells that, in combination with an independent increase in HLA-DR+ T cells, provided a profile predictive of the presence of clinical ALPS. Because quantitative defects in apoptosis were similar in mutation-positive relatives regardless of the presence of clinical ALPS, factors, other than modifiers of the Fas apoptosis pathway, leading to these distinctive immunophenotypic profiles most likely contribute to disease penetrance in ALPS.
Introduction
Apoptosis plays an important role in the homeostasis of mature lymphocytes. T-cell apoptosis is achieved by both active (antigen-driven) and passive pathways. An integral feature of the active pathway is the expression of the cell surface receptor Fas (CD95/APO-1), which on interaction with its ligand, FasL, induces lymphocyte apoptosis.1
The importance of apoptosis in the maintenance of lymphocyte homeostasis is illustrated by genetic defects of Fas and FasL in both mice and humans. In mice, genetic defects in the TNFRSF6gene encoding Fas and TNFSF6 gene encoding FasL genes cause the lpr and gld phenotypes, respectively, which are characterized by lymphoproliferation, autoantibody formation with autoimmune manifestations, and increased T-cell receptor (TcR)-α/β+ CD4−CD8− T cells (α/β+ double-negative T cells [α/β+-DNT cells]).2,3 The equivalent human disorder is known as autoimmune lymphoproliferative syndrome (ALPS), or, alternatively, as Canale-Smith syndrome or lymphoproliferative syndrome with autoimmunity.4-6 ALPS is associated with diffuse nonmalignant lymphadenopathy, hepatosplenomegaly, autoimmune hemolytic anemia, thrombocytopenia, and less frequently with neutropenia and other autoimmune manifestations, as well as with hypergammaglobulinemia and accumulation of α/β+-DNT cells.4,6-10 Criteria currently used by the National Institutes of Health (NIH) ALPS group to identify patients with ALPS include the case-defining triad of chronic accumulation of nonmalignant lymphoid cells, increased α/β+-DNT cells, and defective in vitro receptor-mediated lymphocyte apoptosis.11 In addition, these findings are associated with characteristic histopathologic findings in lymph nodes and spleen.12
The majority of patients with ALPS have inherited heterozygous mutations in the TNFRSF6 gene (formerly known asAPT1), which encodes Fas. These patients are designated as ALPS type Ia. Among the patients with ALPS who lack TNFRSF6mutations are patients with mutations in the TNFSF6gene, encoding FasL (ALPS type Ib), or in the CASP10 gene, encoding caspase 10 (ALPS type II).13-15 The remainder of patients have defects that are yet to be identified (provisionally referred to as ALPS type III).16,17 Although every person carrying a TNFRSF6 mutation has defective lymphocyte apoptosis in vitro, the variable penetrance of other ALPS features has indicated that factors other than a mutation in a component of the Fas pathway are required for the full clinical expression of ALPS, or, alternatively, that protective factors are present preventing expression of ALPS manifestations.10 18
In this study we report the immunophenotypic features of 166 members of 31 families with ALPS type Ia, and of 12 patients with ALPS type II or III. The results provide a comprehensive immunophenotypic picture of ALPS and support the conclusion that certain alterations in lymphocyte subsets or their causal factors or both are associated with clinical disease.
Materials and methods
Study population
The immunophenotypic studies reported here were performed on 166 members of 31 families with ALPS type Ia studied between 1995 and 2000. Participating patients and their families provided written informed consent for evaluation and follow-up under approved clinical protocols at the NIH. Within the 31 families with ALPS type Ia, the following population groups were created. Proband Ia group: one TNFRSF6mutation-positive proband representing the individual in each family responsible for referral to the NIH and fulfilling the criteria for the diagnosis of ALPS (chronic accumulation of nonmalignant lymphoid cells, defective receptor-mediated lymphocyte apoptosis in vivo and 1% or more α/β+-DNT cells in peripheral blood and/or presence of DNT cells in histologic specimens). Mutation+/ALPS+ (M+/A+) group: family members, other than the first evaluated proband, with mutations in TNFRSF6 and who fulfilled the diagnostic criteria of ALPS. M+/A− group: relatives with TNFRSF6mutations, with and without ALPS features, but not meeting the full diagnostic criteria of ALPS. M−/A− group: relatives withoutTNFRSF6 mutations, none of whom met the criteria for ALPS. Earlier observations that within families, TNFRSF6 mutations were not always associated with one or more of the clinical features of ALPS, provided the rationale to assign the family members to these specific groups.7 In addition, 2 probands with ALPS type II (CASP10 mutations) and 9 probands with ALPS type III (defects currently unknown), who fulfilled the criteria for the diagnosis of ALPS, were studied (combined into the proband II/III group). Individuals were studied while not receiving immune-modulating medications, with the exception of 2 subjects in the proband Ia group, who were taking low doses of daily oral prednisone. Their results were comparable to other subjects in this group. Lymphocyte markers were compared to values established by a control group, consisting of 20 male and 20 female healthy, nonsmoking, adult volunteers (designated the HC group). Immunophenotypic descriptions of several of the ALPS patients and their family members have been published previously.4,7,12,15,17 18
Flow cytometry
Peripheral blood specimens were obtained by phlebotomy on site or were mailed in and studied the next day. Anticoagulated (EDTA) samples were stained using the whole blood lysis method and analyzed with a FACScan or dual-laser FACSort (Becton Dickinson, San Jose, CA) using Cell Quest software (Becton Dickinson), as previously described.17 Control samples from healthy volunteers were analyzed concurrently with experimental samples. T cells and T-cell subsets were identified with the directly conjugated monoclonal antibodies (mAbs): anti-CD3, anti-CD4, anti-CD8, anti-CD57, anti-TcR-α/β, and anti-TcR-γ/δ; T-cell activation markers by anti-HLA-DR and anti-CD25 (interleukin-2 [IL-2] receptor α chain); B cells by anti-CD20 and anti-CD5; and natural killer (NK) cells by a combination of anti-CD16 and anti-CD56, evaluated on CD3−lymphocytes. Irrelevant, directly conjugated, mAbs of the IgG1, IgG2a, and IgG2b subclasses were used to define background staining. All mAbs were obtained from Becton Dickinson Biosciences (San Jose, CA), with the exception of anti-TcR-γ/δ (Endogen, Woburn, ΜΑ). To calculate the absolute numbers of each lymphocyte subset, the percentage of cells staining positive was multiplied by the absolute peripheral blood lymphocyte count.
Detection of Fas-mediated apoptosis in vitro
Apoptosis assays were performed on Epstein-Barr virus (EBV)–transformed B lymphocytes as previously described.7Apoptosis was induced with 500 to 1000 mg/mL APO-1 anti-Fas antibody with protein A added for maximal receptor cross-linking. Family members were analyzed on the same day with the same concentrations of APO-1, and maximally normalized percent cell loss was calculated by dividing the actual cell loss from the most effective dose of APO-1 by the percent cell loss of normal control EBV cell lines (a minimum of 2/experiment). Averages and SDs were calculated using Graphpad Prism software (Graphpad, San Diego, CA).
Statistical analysis
For unpaired comparisons of lymphocyte subsets, the nonparametric Mann-Whitney U test was used. All Pvalues are 2-tailed and are regarded as statistically significant ifP < .05. In addition, Spearman rank correlation coefficients were calculated for correlation analysis of age and lymphocyte markers. For the apoptosis experiments, pairwiset tests were used, and P values <.05 were regarded significant.
Results
Demographic features
Demographic features of the study population are shown in Table1. The male-to-female ratio was 1.8:1 for the proband Ia group, 1:1 for the M+/A+ group, 0.8:1 for the M+/A− group, 1:1 for the M−/A− group, and 1.8:1 for the proband II/III group. As is clear from Table 1, the age of the individuals in the proband Ia and proband II/III group was lower than the other 3 groups. Not shown, all subjects with TNFRSF6 mutations (M+) had defective in vitro lymphocyte apoptosis.
Demographic characteristics and distribution of ALPS features
| Group . | No. . | Age at study . | Gender . | Race . | Number of ALPS features* . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 . | 1 . | 2 . | 3 . | 4 . | 5 . | |||||
| Proband Ia | 31 | 9/8† (1-20) | 20m/11f (1.8:1) | 27W/3B/1Bi | 0 | 0 | 0 | 2 | 12 | 17 |
| M+/A+ | 28 | 28/28 (9-62) | 14m/14f (1:1) | 26W/2B | 0 | 0 | 0 | 9 | 9 | 10 |
| M+/A− | 42 | 41/42 (1-86) | 18m/24f (0.8:1) | 41W/1Bi | 17 | 20 | 4 | 1 | 0 | 0 |
| M−/A− | 65 | 35/33 (4-69) | 33m/32f (1:1) | 59W/6B | 50 | 13 | 2 | 0 | 0 | 0 |
| Proband II/III | 11 | 10/10 (1-20) | 7m/4f (1.8:1) | 9W/2B | 0 | 0 | 0 | 1 | 3 | 7 |
| Group . | No. . | Age at study . | Gender . | Race . | Number of ALPS features* . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 . | 1 . | 2 . | 3 . | 4 . | 5 . | |||||
| Proband Ia | 31 | 9/8† (1-20) | 20m/11f (1.8:1) | 27W/3B/1Bi | 0 | 0 | 0 | 2 | 12 | 17 |
| M+/A+ | 28 | 28/28 (9-62) | 14m/14f (1:1) | 26W/2B | 0 | 0 | 0 | 9 | 9 | 10 |
| M+/A− | 42 | 41/42 (1-86) | 18m/24f (0.8:1) | 41W/1Bi | 17 | 20 | 4 | 1 | 0 | 0 |
| M−/A− | 65 | 35/33 (4-69) | 33m/32f (1:1) | 59W/6B | 50 | 13 | 2 | 0 | 0 | 0 |
| Proband II/III | 11 | 10/10 (1-20) | 7m/4f (1.8:1) | 9W/2B | 0 | 0 | 0 | 1 | 3 | 7 |
W indicates white; B, black; Bi, biracial; m, male; f, female.
Features in addition to impaired lymphocyte apoptosis seen in all M+ subjects: lymphadenopathy, splenomegaly, ≥ 1% α/β+-DNT cells, autoantibodies, autoimmune disease (only features documented in medical records were considered).
Mean/median age in years; (range).
Immunophenotypic profiles of lymphocyte subsets
Absolute numbers of lymphocyte subsets and the percentage expression of the lymphocyte markers are presented in Table2. To determine if specific immunophenotypic profiles distinguished each group, absolute numbers of lymphocyte subsets were compared between the proband Ia, M+/A+, M+/A−, and M−/A− groups and the HC group (Table 2), and between the family groups with or without a diagnosis of ALPS and with different Fas mutation status (Table 3). When compared to unrelated controls, the members of the proband Ia and M+/A+ groups demonstrated significant expansions of CD3+ T cells, CD8+ T cells, DNT cells expressing TcR-α/β, DNT cells expressing TcR-γ/δ, CD8+/CD57+ T cells, and total B cells, as well as B cells coexpressing CD5. However, these 2 groups showed no expansion of CD4+ T cells or NK cells. When the group of TNFRSF6 mutation-positive, ALPS-negative family members (M+/A−) was compared to the HC group, statistically significant alterations were confined to an expansion of CD8+ T cells and both DNT cell subsets. The M−/A− group revealed a small increase in the number of CD3+ T cells, CD8+ T cells, both DNT cell subsets, and CD5+ B cells, all of which were statistically significant. The increase in the absolute number of α/β+-DNT cells in this group was reflective of the entire group because 70% of subjects in the M−/A− group had an absolute number of α/β+-DNT cells that was greater than the median number of α/β+-DNT cells in the HC group (and in one third of the M−/A− group, this was greater than the 90th percentile of the HC group).
Lymphocyte subsets in ALPS Ia families, probands with ALPS II/III, and HCs
| Lymphocyte subset . | Proband Ia (n = 31) . | M+/A+ (n = 28) . | M+/A− (n = 42) . | M−/A− (n = 65) . | Proband II/III (n = 11) . | HC (n = 40) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T cells | ||||||||||||
| CD3+ | 2239*↑ | [70.9†] | 1768↑ | [74.1] | 1159 | [76.7] | 1487↑ | [76.1] | 2138↑ | [71.5] | 1105 | [72.2] |
| CD4+ | 824 | [27.5] | 712 | [34.3] | 654 | [43.2] | 841 | [44.6] | 800 | [27.2] | 723 | [47.1] |
| CD8+ | 1067↑ | [26.1] | 708↑ | [29.8] | 538↑ | [34.1] | 621↑ | [32.0] | 831↑ | [30.7] | 488 | [29.8] |
| α/β+-DNT | 198↑ | [7.3] | 117↑ | [5.9] | 17↑ | [0.8] | 13↑ | [0.5] | 66↑ | [3.5] | 7 | [0.5] |
| γ/δ+-DNT | 93↑ | [2.6] | 47↑ | [1.5] | 33↑ | [1.9] | 32↑ | [1.7] | 53↑ | [1.9] | 18 | [1.1] |
| CD3+/HLA-DR+ | 705↑ | [21.7] | 522↑ | [20.2] | 201 | [11.6] | 187 | [9.3] | 374↑ | [15.2] | 146 | [8.8] |
| CD3+/CD25+ | 270↓ | [7.4] | 378 | [17.3] | 411 | [24.8] | 447 | [25.4] | 211↓ | [9.2] | 425 | [27.6] |
| CD8+/CD57+ | 444↑ | [11.3] | 291↑ | [11.9] | 206 | [12.4] | 246 | [12.0] | 297↑ | [11.3] | 206 | [12.5] |
| B cells | ||||||||||||
| CD20+ | 656↑ | [20.8] | 295↑ | [13.3] | 148 | [9.2] | 235↑ | [11.6] | 436↑ | [14.5] | 148 | [9.8] |
| CD5+/CD20+ | 526↑ | [13.0] | 190↑ | [6.5] | 70 | [4.3] | 114↑ | [5.6] | 339↑ | [10.6] | 56 | [3.3] |
| NK cells | ||||||||||||
| CD3−CD16+ and/or 56+ | 225 | [7.6] | 224 | [10.1] | 209 | [11.2] | 219 | [9.7] | 229 | [10.5] | 212 | [15] |
| Lymphocyte subset . | Proband Ia (n = 31) . | M+/A+ (n = 28) . | M+/A− (n = 42) . | M−/A− (n = 65) . | Proband II/III (n = 11) . | HC (n = 40) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T cells | ||||||||||||
| CD3+ | 2239*↑ | [70.9†] | 1768↑ | [74.1] | 1159 | [76.7] | 1487↑ | [76.1] | 2138↑ | [71.5] | 1105 | [72.2] |
| CD4+ | 824 | [27.5] | 712 | [34.3] | 654 | [43.2] | 841 | [44.6] | 800 | [27.2] | 723 | [47.1] |
| CD8+ | 1067↑ | [26.1] | 708↑ | [29.8] | 538↑ | [34.1] | 621↑ | [32.0] | 831↑ | [30.7] | 488 | [29.8] |
| α/β+-DNT | 198↑ | [7.3] | 117↑ | [5.9] | 17↑ | [0.8] | 13↑ | [0.5] | 66↑ | [3.5] | 7 | [0.5] |
| γ/δ+-DNT | 93↑ | [2.6] | 47↑ | [1.5] | 33↑ | [1.9] | 32↑ | [1.7] | 53↑ | [1.9] | 18 | [1.1] |
| CD3+/HLA-DR+ | 705↑ | [21.7] | 522↑ | [20.2] | 201 | [11.6] | 187 | [9.3] | 374↑ | [15.2] | 146 | [8.8] |
| CD3+/CD25+ | 270↓ | [7.4] | 378 | [17.3] | 411 | [24.8] | 447 | [25.4] | 211↓ | [9.2] | 425 | [27.6] |
| CD8+/CD57+ | 444↑ | [11.3] | 291↑ | [11.9] | 206 | [12.4] | 246 | [12.0] | 297↑ | [11.3] | 206 | [12.5] |
| B cells | ||||||||||||
| CD20+ | 656↑ | [20.8] | 295↑ | [13.3] | 148 | [9.2] | 235↑ | [11.6] | 436↑ | [14.5] | 148 | [9.8] |
| CD5+/CD20+ | 526↑ | [13.0] | 190↑ | [6.5] | 70 | [4.3] | 114↑ | [5.6] | 339↑ | [10.6] | 56 | [3.3] |
| NK cells | ||||||||||||
| CD3−CD16+ and/or 56+ | 225 | [7.6] | 224 | [10.1] | 209 | [11.2] | 219 | [9.7] | 229 | [10.5] | 212 | [15] |
Bold indicates statistically significant change compared to the HC group.
↑Absolute number higher than HC group.
↓Absolute number lower than HC group.
Median number of cells/μL.
Median percentage of lymphocytes.
Comparison of the number of lymphocytes between the ALPS Ia family groups and between the proband Ia group and the proband II/III group
| Lymphocyte subset . | Proband Ia vs M+/A+ . | Proband Ia vs M+/A− . | Proband Ia vs M−/A− . | M+/A+ vs M+/A− . | M+/A+ vs M−/A− . | M+/A− vs M−/A− . | Proband Ia vs proband II/III . |
|---|---|---|---|---|---|---|---|
| T cells | |||||||
| CD3+ | NS | P= .001↑ | P = .006↑ | P = .003↑ | NS | P = .06↓ | NS |
| CD4+ | NS | NS | NS | NS | NS | P= .005↓ | NS |
| CD8+ | NS | P = .001↑ | P = .003↑ | P = .04↑ | NS | NS | NS |
| α/β+-DNT | NS | P < .0001↑ | P < .0001↑ | P < .0001↑ | P < .0001↑ | NS | NS |
| γ/δ+-DNT | P = .01↑ | P < .0001↑ | P < .0001↑ | NS | NS | NS | NS |
| CD3+/HLA-DR+ | NS | P < .0001↑ | P < .0001↑ | P < .0001↑ | P < .0001↑ | NS | NS |
| CD3+/CD25+ | NS | P = .01↓ | P = .002↓ | NS | NS | NS | NS |
| CD8+/CD57+ | NS | P = .004↑ | P = .01↑ | P = .004↑ | P = .04↑ | NS | NS |
| B cells | |||||||
| CD20+ | P = .01↑ | P = .009↑ | P < .0001↑ | P = .003↑ | NS | P = .01↓ | NS |
| CD5+/CD20+ | P = .003↑ | P < .0001↑ | P < .0001↑ | P = .006↑ | NS | P = .007↓ | NS |
| NK cells | |||||||
| CD3−CD16+and/or 56+ | NS | NS | NS | NS | NS | NS | NS |
| Lymphocyte subset . | Proband Ia vs M+/A+ . | Proband Ia vs M+/A− . | Proband Ia vs M−/A− . | M+/A+ vs M+/A− . | M+/A+ vs M−/A− . | M+/A− vs M−/A− . | Proband Ia vs proband II/III . |
|---|---|---|---|---|---|---|---|
| T cells | |||||||
| CD3+ | NS | P= .001↑ | P = .006↑ | P = .003↑ | NS | P = .06↓ | NS |
| CD4+ | NS | NS | NS | NS | NS | P= .005↓ | NS |
| CD8+ | NS | P = .001↑ | P = .003↑ | P = .04↑ | NS | NS | NS |
| α/β+-DNT | NS | P < .0001↑ | P < .0001↑ | P < .0001↑ | P < .0001↑ | NS | NS |
| γ/δ+-DNT | P = .01↑ | P < .0001↑ | P < .0001↑ | NS | NS | NS | NS |
| CD3+/HLA-DR+ | NS | P < .0001↑ | P < .0001↑ | P < .0001↑ | P < .0001↑ | NS | NS |
| CD3+/CD25+ | NS | P = .01↓ | P = .002↓ | NS | NS | NS | NS |
| CD8+/CD57+ | NS | P = .004↑ | P = .01↑ | P = .004↑ | P = .04↑ | NS | NS |
| B cells | |||||||
| CD20+ | P = .01↑ | P = .009↑ | P < .0001↑ | P = .003↑ | NS | P = .01↓ | NS |
| CD5+/CD20+ | P = .003↑ | P < .0001↑ | P < .0001↑ | P = .006↑ | NS | P = .007↓ | NS |
| NK cells | |||||||
| CD3−CD16+and/or 56+ | NS | NS | NS | NS | NS | NS | NS |
NS indicates not significant.
↑Absolute number first group higher than second group.
↓Absolute number first group lower than second group.
Next, absolute numbers of lymphocyte subsets were compared between the ALPS Ia family groups (Table 3). ALPS Ia probands had significantly higher numbers of γ/δ+-DNT cells, total B cells, and CD5+ B cells when compared to the M+/A+ group, and significantly higher numbers of all lymphocyte subsets than the M+/A− and M−/A− groups, except CD4+ T cells and NK cells, whereas CD3+/CD25+ T cells were significantly lower in the proband Ia group, compared to the M+/A− and M−/A− groups. Absolute numbers of all lymphocyte subsets, with the exception of CD4+ T cells, γ/δ+-DNT cells, CD3+/CD25+ T cells, and NK cells, were significantly higher in the M+/A+ group compared to the M+/A− group, whereas only α/β+-DNT cells, CD3+/HLA-DR+ T cells, and CD8+/CD57+ T cells were significantly higher in the M+/A+ group, compared to the M−/A− group. Lastly, the M−/A− group had a significantly higher number of CD3+ and CD4+ T cells, as well as (CD5+) B cells than the M+/A− group.
Immunophenotypic profiles of T-cell activation markers
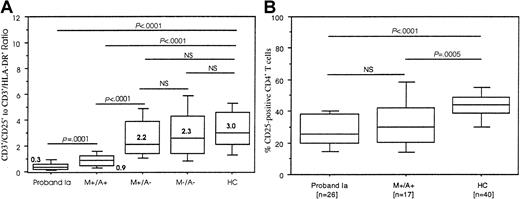
Absolute numbers and the percentage of T cells expressing the activation markers HLA-DR and CD25 were compared between the various groups (Tables 2 and 3). There was a significant increase in the expression of HLA-DR on T cells in both the proband Ia and M+/A+ groups. Both groups also displayed a decrease in CD25+ T cells, but only in the proband Ia group did this reach statistical significance. These findings were not observed in either the M+/A− or M−/A− groups. There was no statistical difference between the proband Ia and M+/A+ groups when comparing T-cell HLA-DR and CD25 expression on T cells. Spearman rank correlation coefficients revealed no correlation between the absolute numbers of CD3+/HLA-DR+and CD3+/CD25+ T cells in members of the proband Ia and M+/A+ groups (data not shown), indicating that increased HLA-DR expression occurred independently of decreased CD25 expression. To further illustrate these opposite and independent immunophenotypic findings, a CD3+/CD25+-to-CD3+/HLA-DR+ratio was generated, the results of which are shown in Figure1A. In healthy controls the median value of this ratio was 3.0 and this was not significantly different in the M+/A− (2.2) and M−/A− (2.3) groups. However, in the proband Ia and M+/A+ groups the ratio was significantly lower than the HC group, having a median value of 0.3 (P < .0001) and 0.9 (P < .0001), respectively. The ratio was significantly lower in the proband Ia group as compared to the M+/A+ group, and the ratio in the M+/A+ group was significantly lower than the ratio in the M+/A− group. The difference between the ratios in the M+/A− and M−/A− groups was not statistically significant.
CD3+/CD25+-to-CD3+/HLA-DR+ratios and selective reduction in CD4+/CD25+ T cells.
(A) Boxplots depict the 10th, 25th, 50th (median), 75th, and 90th percentiles of the CD3+/CD25+-to-CD3+/HLA-DR+ratios. Ratios were obtained by dividing the absolute number of CD3+/CD25+ T cells by the absolute number of CD3+/HLA-DR+ T cells. Bold numbers in or next to the boxes denote median values of the ratios in each group. The ratios of the groups were compared by Mann-Whitney U test. NS indicates not significant. (B) Boxplots depict the 10th, 25th, 50th (median), 75th, and 90th percentiles of CD25+CD4+ T cells in 26 individuals of the proband Ia, 17 subjects from M+/A+, and the HC groups. The groups were compared by Mann-Whitney U test. NS indicates not significant.
CD3+/CD25+-to-CD3+/HLA-DR+ratios and selective reduction in CD4+/CD25+ T cells.
(A) Boxplots depict the 10th, 25th, 50th (median), 75th, and 90th percentiles of the CD3+/CD25+-to-CD3+/HLA-DR+ratios. Ratios were obtained by dividing the absolute number of CD3+/CD25+ T cells by the absolute number of CD3+/HLA-DR+ T cells. Bold numbers in or next to the boxes denote median values of the ratios in each group. The ratios of the groups were compared by Mann-Whitney U test. NS indicates not significant. (B) Boxplots depict the 10th, 25th, 50th (median), 75th, and 90th percentiles of CD25+CD4+ T cells in 26 individuals of the proband Ia, 17 subjects from M+/A+, and the HC groups. The groups were compared by Mann-Whitney U test. NS indicates not significant.
The decrease in CD3+/CD25+ was further explored in the proband Ia group, the group with a significantly lower number of CD3+/CD25+ cells and the lowest CD3+/CD25+-to-CD3+/HLA-DR+ratio, and in the M+/A+ group. Results regarding CD25 expression on CD4+ T cells and CD8+ T cells were available in 26 of 31 members of the proband Ia and 17 of 28 members of the M+/A+ group. It revealed that, although there was no difference in the percentage of CD25−CD4+ T cells, the percentage of CD25+CD4+ T cells was significantly lower in the proband Ia and M+/A+ groups than the HC group (Figure 1B). There was no significant difference in CD8+/CD25+ T cells (data not shown). In addition, CD25 expression on CD4+ T cells and CD8+ T cells was roughly equal to CD25 expression on total T cells, indirectly indicating that DNT cells did not express CD25. In corroboration with the lack of a significant Spearman rank correlation between changes in CD3/HLA-DR+ versus CD3/CD25+T cells in the proband Ia and M+/A+ groups, HLA-DR up-regulation was confined to CD8+ T cells and α/β+-DNT cells (data not shown), and the loss of CD25+ cells was confined to CD4+ T cells, confirming the independent nature of these events.
Comparisons between ALPS type Ia and ALPS type II/III probands
In addition to the 31 probands with ALPS type Ia, lymphocyte subsets were also evaluated in 2 probands with ALPS type II and 9 probands with ALPS type III (proband II/III group). Median cell numbers and percentages are shown in Table 2, and absolute numbers of lymphocyte subsets were compared between the proband II/III group and the HC group (Table 2). This showed a significant increase in CD3+ T cells, CD8+ T cells, both DNT cell subsets, CD8+/CD57+ T cells, and total B cells, including B cells expressing CD5 in the proband II/III group (Table 2). There was a significant increase in CD3+/HLA-DR+ T cells and a decrease in CD3+/CD25+ T cells. Although the number of most lymphocyte subsets was higher in the proband Ia group than in the proband II/III group, this difference did not reach statistical significance for any subset (Table 3). The CD3+/CD25+-to-CD3+/HLA-DR+ratio in the proband II/III group (median of 0.4) was, similar to the proband Ia group, also significantly lower than the HC group (P < .0001).
The influence of age on immunophenotypic findings
It is well known that distribution of certain lymphocyte populations depend on the individual's age.20 Table 1shows that the mean and median age of the individuals in the proband Ia group (and the proband II/III group) was lower than that of the other groups (including the HC group). Two approaches were used to ascertain if immunophenotypic alterations were influenced by age. First, the proband Ia group was divided in 2 groups, based on age. Comparing the absolute numbers of the lymphocyte subsets did not reveal significant changes between younger probands (n = 16; median age = 7) versus older probands (n = 15; median age = 16), with the exception of increased γ/δ+-DNT cells in the younger probands. This was also the only T-cell subset that was significantly increased compared to the M+/A+ group (median age = 28; Table 3). In addition, Spearman rank correlation coefficients were calculated. This analysis revealed no correlation between age and the major T-cell changes including the increase in α/β+-DNT cells and B-cell alterations in the proband group or in the M+/A+ group, which also consisted of younger individuals.
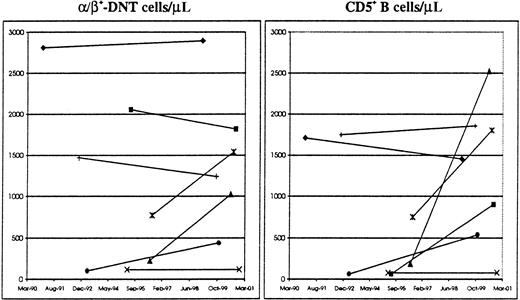
Second, because it has been appreciated that certain ALPS manifestations tend to decrease over time, the possibility that the magnitude of immunophenotypic findings reflects age-related changes stemming from the “natural history” of ALPS was evaluated. To this effect, 7 probands who were studied at least 4 times over a 3- to 8-year period were identified. First and last available numbers of α/β+-DNT cells and CD5+ B cells were used, α/β+-DNT cells because it represents the signature immunophenotypic abnormality in ALPS, and CD5+ B cells because it distinguished between subjects of the proband Ia and M+/A+ groups (Table 3), and because of the known inverse relationship with age.20 With respect to the number of α/β+-DNT cells, the results showed that in 4 of 7 probands, there was an increase over time, including a proband who has been followed for more than 8 years. In 3 of 7 probands, including a proband receiving low-dose daily prednisone, there was a decrease in α/β+-DNT cells varying between 4% and 16% (schematically illustrated in Figure 2). With respect to the number of CD5+ B cells, only the proband studied for 8 years showed a 15% reduction, whereas the other 6 probands experienced an increase in CD5+ B cells that varied between 6% and 1453% (Figure 2).
Longitudinal follow-up of ALPS Ia probands.
Schematic representation of α/β+-DNT cells (left) and CD5+ B cells (right) in 7 subjects from the proband Ia group followed between March 1990 and January 2001. First and last available immunophenotypic studies were used.
Longitudinal follow-up of ALPS Ia probands.
Schematic representation of α/β+-DNT cells (left) and CD5+ B cells (right) in 7 subjects from the proband Ia group followed between March 1990 and January 2001. First and last available immunophenotypic studies were used.
Relationship between immunophenotype and ALPS features
Although the family members in the M+/A− group did not fulfill all ALPS case criteria, specific ALPS features, in addition to defective apoptosis, that are part of those criteria were present in 25 of 42 individuals in this group. To determine if there was a relationship between the presence of more ALPS features and abnormal immunophenotypic findings, the individuals in the M+/A− group were assigned to groups based on the number of ALPS features documented in their medical records. Features included lymphadenopathy/splenomegaly, 1% or more α/β+-DNT cells, autoantibodies, and autoimmunity. Twenty subjects in the M+/A− group showed one ALPS feature, 16 of 20 having 1% or more α/β+-DNT cells and 4 of 20 demonstrating autoantibodies. An additional 4 subjects in this group had 1% or more α/β+-DNT cells together with the presence of circulating autoantibodies. One individual had 3 features, 1% or more α/β+-DNT cells, circulating autoantibodies, and the presence of autoimmunity. In this group, there was no relationship between alterations in lymphocyte subsets (other than the α/β+-DNT cell subset) and the number and nature of ALPS features.
Genotype-immunophenotype analysis
As has been reported, the location of the mutation within theTNFRSF6 gene has an influence on the penetrance of the ALPS phenotype.18,21 To determine if a similar relationship exists between genotype and the immunophenotypic characteristics, the individuals in the M+/A− group were assigned to a subgroup of extracellular TNFRSF6 mutations (exons 1-6, n = 15) or a subgroup of intracellular mutations in TNFRSF6 (exons 7-9, n = 27). This revealed that there were no associations between the localization of the mutation and the absolute number of any of the lymphocyte subsets (data not shown). Likewise, in the proband Ia group, probands with intracellular mutations (n = 20) had similar immunophenotypic results as probands with extracellular mutations (n = 11) (data not shown). Similar ranking was not performed in the M+/A+ group because all but one in this group belonged to families with intracellular TNFRSF6 mutations, confirming the reported increased disease penetrance associated with the intracellular genotypes.18
In vitro Fas-mediated apoptosis in ALPS patients versus relatives
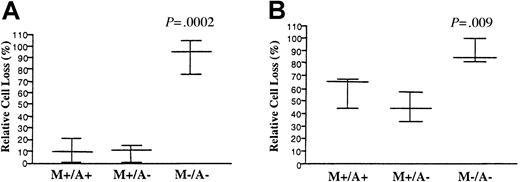
To determine if some of the immunophenotypic and clinical differences between M+/A+ patients and their relatives may stem from differences in the apoptotic defects, EBV-transformed cell lines from a subgroup of 6 ALPS type Ia families, with family members belonging to all of the defined population groups, were analyzed for quantitative defects in Fas-mediated apoptosis. As expected, cells from probands (data not shown) and M+/A+ individuals all had significant apoptotic defects, with intracellular mutations leading to more pronounced defects (Figure 3A) than extracellular mutations (Figure 3B). However, analysis of M+/A− relatives from both groups showed that their apoptotic defects were not significantly different than cells from the M+/A+ family members and probands (data not shown). M−/A− family members were not significantly different than normal controls. Flow cytometric analysis of surface Fas levels from patients versus controls did also not yield any significant differences between the M+/A+ and M+/A− individuals within a family (data not shown). Thus, the immunophenotypic differences found between ALPS patients and mutation-positive relatives without clinical ALPS is not due to any genetic or epigenetic influence on the apoptosis defect itself, but more likely stems from other genetic or environmental factors.
Fas-mediated apoptosis in ALPS patients versus relatives.
The EBV-transformed B cells from a subgroup of 3 ALPS type Ia families (n = 22) with intracellular TNFRSF6 mutations (A) and 3 families (n = 12) harboring extracellular mutations (B) were stimulated with APO-1 and apoptosis was measured as described in “Materials and methods.” The average percentage of cell loss after 18 to 24 hours of culture is shown by the horizontal lines and the SDs by the brackets. Pairwise t tests were done between all groups, and the P values for significant differences between each group and the M+/A+ group are shown.
Fas-mediated apoptosis in ALPS patients versus relatives.
The EBV-transformed B cells from a subgroup of 3 ALPS type Ia families (n = 22) with intracellular TNFRSF6 mutations (A) and 3 families (n = 12) harboring extracellular mutations (B) were stimulated with APO-1 and apoptosis was measured as described in “Materials and methods.” The average percentage of cell loss after 18 to 24 hours of culture is shown by the horizontal lines and the SDs by the brackets. Pairwise t tests were done between all groups, and the P values for significant differences between each group and the M+/A+ group are shown.
Discussion
From an immunophenotypic standpoint, ALPS is characterized by expansion of CD3+ T cells, CD8+ T cells, CD3+/HLA-DR+ T cells, CD8+/CD57+ T cells, both DNT cell subsets, total B cells, and CD5+ B cells, as well as low numbers of CD3+/CD25+ T cells, due to a reduction in CD4+/CD25+ T cells. Immunophenotypic profiles of persons in families with ALPS are distinctive for each family group. When the groups are ranked, based on median numbers of cells positive for specific lymphocyte markers, the proband Ia group and the M+/A+ group are clearly distinguishable from the other groups.
Although no age-matched controls were used for the proband Ia group, several findings validate the age-independent significance of the results. First, comparing younger probands with older probands, as well as Spearman rank correlation analysis, established the lack of age-related influences, with the exception of the number of γ/δ+-DNT cells. Second, long-term immunophenotypic follow-up of ALPS probands does not substantiate the theoretical possibility that older ALPS patients may have less significant immunophenotypic findings than younger patients, as part of the “natural” history of ALPS. Similar findings were also reported by Rieux-Laucat and coworkers in 1999.22 Third, CD5+ B cells showed a tendency to increase over time, which is opposite to the known inverse relationship between this subset and age in normals.20 Finally, and most importantly, the overall profile of the M+/A+ group that consists mainly of adult subjects is similar to that of the proband Ia group, with a smaller expansion of γ/δ+-DNT cells, total B cells, and CD5+ B cells.
In an initial survey (data not shown), subjects older than 14 years from the proband Ia and M+/A+ groups were combined in one group and the results were comparable to the current results from the M+/A+ group. This is a further indication that any age-related influence in the proband Ia group does not have an impact on the overall significance of the immunophenotypic findings and profiles in ALPS patients, compared to the profiles of the HC group, M+/A−, and M−/A− groups.
The expansion of HLA-DR+ T cells and CD57+ T cells in the groups with clinical disease was not observed in the M+/A− and M−/A− groups, indicating that these findings are specific for the presence of ALPS.
The M−/A− group immunophenotypic profile illustrates that a small, but significant, expansion of double-negative T cells bearing the TCR-α/β can occur in the absence of TNFRSF6 mutations. This is not influenced by age and is compatible with the contribution of an additional factor (or factors) to the pathogenesis of ALPS that may exacerbate the consequences of the Fas mutation in the proband Ia and M+/A+ groups, but not in the M+/A− group. The profiles of the proband Ia and M+/A+ groups therefore appear to be a consequence of both the TNFRSF6 mutation and another factor, found in the ALPS type Ia families, including mutation-negative members, but not in HCs. The statistically significant expansion of α/β+-DNT cells, γ/δ+-DNT cells, CD8+ T cells, and CD5+ B cells signifies a unique immunophenotypic profile for this group and argues against simply a coincidental increase in DNT cells. Rather, it suggests the presence of a distinct pathogenic process of lymphocyte expansion involving multiple subsets of lymphocytes. In addition, identification of similar immunophenotypic findings in patients with ALPS type II (and ALPS type III) suggests that a defective Fas pathway associated with decreased lymphocyte apoptosis in vitro (despite normalTNFRSF6) is sufficient for the expansion of lymphocyte subsets.15 The individuals in the M−/A− group included parents and siblings, who might share similar environmental factors (eg, exposures to infectious agents), but also relatives who did not share a common environment. Thus, the composition of this group is more suggestive of inherited modifiers of defective apoptosis, which is the main pathogenetic mechanism of ALPS.
An increase in γ/δ+-DNT cells in ALPS has been noted before.7 In the majority of family members, expansion of γ/δ+-DNT cells accompanied the expansion of α/β+-DNT cells. Interestingly, in 21% of family members with ALPS, the expansion of γ/δ+-DNT cells actually exceeded the expansion of the α/β+-DNT cells. The significance of these cells in the context of ALPS is unclear. The clinical picture of these individuals was not particularly distinctive. In the lpr model, in the absence of α/β+ T cells, the development of isotype-specific hypergammaglobulinemia and autoantibody production, and mild immune complex renal disease was linked to the presence of γ/δ+ T cells.23 On the other hand, mice lacking γ/δ+ T cells had a more severe disease phenotype, as reflected in augmented hypergammaglobulinemia and autoantibody production, more severe renal disease, and increased mortality.23
CD5+ B cells were significantly increased in the proband Ia group, the proband II/III group, the M+/A+ group, and the M−/A− group, and this constitutes an interesting deviation from thelpr model. In this murine model, B-cell expansion is attributed to CD5−/CD23low B cells and not CD5+ B cells.24,25 Furthermore, it has been shown that CD5− B cells are responsible for the production of autoantibodies in lpr mice.26CD5+ B cells are involved in another murine model of lupus erythematosus, the New Zealand black– (NZB) and New Zealand white (NZW)–derived strain.27 Interestingly, the NZB parental strain develops autoimmune hemolytic anemia, rather than the lupus-like nephritis seen in the F1 hybrids. In this regard, the majority of ALPS patients develop autoimmune hemolytic anemia, whereas very few have renal involvement, the reverse of the pattern seen in thelpr model.10,27 28 The B-cell immunophenotypic changes and autoimmune findings in ALPS patients are similar to the NZB model and reinforce the possibility of another genetic factor, distinct from the Fas defect that contributes to disease development.
The CD5+ B-cell expansion in the proband Ia and M+/A+ groups may be related to the elevated levels of circulating IL-10.19 In support of this hypothesis, there was a significant correlation between the number of CD5+ B cells and the level of IL-10 in individuals in the proband Ia group (rho = .6; P = .02, data not shown). In mice, IL-10 is an essential growth factor for CD5+ B-1 cells, and a factor capable of preventing apoptosis of B cells, including CD5+B cells.29-31 The normal levels of IL-10 found in the M−/A− group, despite increased CD5+ B cells, would indicate that increased IL-10 is not the only factor involved in human CD5+ B-cell expansion.30 With respect to the CD5− B cells, they were not significantly elevated in any of the family groups, indicating that the expansion of B cells in the aforementioned groups was entirely due to an increase in CD5+ B cells.
Not all immunophenotypic features of ALPS reflect lymphoaccumulation. The profile of the proband Ia group reveals an absolute decrease in CD25+ T cells, a finding also present in the proband II/III group, but not in the other ALPS Ia family groups. In combination with the increased HLA-DR expression on T cells, these changes are reflected in abnormal CD3+/CD25+-to-CD3+/HLA-DR+ratios for the proband Ia group, M+/A+ group, and proband II/III group. This measurement is reflective of 2 independent changes, specific for clinical ALPS, regardless of the type, and thus may serve as an immunophenotypic marker of ALPS. The ratio demonstrated a sensitivity and specificity of 83% and 90%, respectively, when using a ratio of 1 or less to discriminate for clinical ALPS, and 95% and 71%, respectively, at a ratio of 1.4 or less (the median ratio of the 166 subjects in the 4 family groups). At these ratios, the specificity of the marker for the HC group was 98% and 88%, respectively.
Additional data in subjects of the proband Ia and M+/A+ groups revealed that the reduction in CD25+ T cells was due to a loss of CD4+/CD25+ T cells (Figure 2B). In this regard, it is of interest that the CD4+ T-cell subset is the only T-cell subset not significantly expanded in any of the groups. The loss of CD4+/CD25+ T cells is not the result of accumulation of CD25+ T cells within the DNT pool because CD25+ T cells were accounted for by the CD4+and CD8+ T-cell subsets. It is also unlikely to be secondary to a redistribution to lymphoid tissue because there is also a paucity of CD25+ T cells in lymph nodes and spleen.12 The in vitro apoptosis data in several ALPS families demonstrate that relatives with TNFRSF6 mutations have comparable levels of defective Fas-mediated apoptosis, regardless of alterations in CD4+/CD25+ T cells, and suggest a contribution of reduced CD4+/CD25+ T cells to the ALPS pathogenesis, through a mechanism independent of the Fas pathway of cellular suicide.
A growing body of evidence indicates the importance of CD25 in the life and death of lymphocytes. CD25-deficient mice develop significant polyclonal lymphoproliferation and autoimmunity, including autoimmune hemolytic anemia, which may have both Fas-independent and Fas-dependent causes.32,33 A naturally occurring defect in human CD25 caused lymphadenopathy and hepatosplenomegaly as well as severe immunodeficiency.34 Corroboration of the importance of CD25 has been provided by recent studies in mice, in which CD25+ CD4+ T cells were implicated as immunoregulatory cells, capable of maintaining self-tolerance and preventing autoimmunity by suppressing CD25− T cells.35-42 Thus, if a similar subset of immunoregulatory cells exists in humans, the finding of decreased CD4+/CD25+ T cells could have consequences for the integrity of the IL-2/IL-2 receptor system and represent a marker of disturbed lymphocyte immunoregulation in ALPS.
This comprehensive immunophenotypic study of a human disorder of defective lymphocyte apoptosis has provided several new insights. Changes in lymphocyte subset distribution in relatives withoutTNFRSF6 mutations offer support for the concept of additional contributing factors to the complex pathogenesis of ALPS. The changes in particular (immunoregulatory) subsets, and the fact that the apoptotic defects are not more severe in probands and M+/A+ patients than M+/A− relatives, points to factors outside of the Fas pathway as critical elements in the pathogenesis of ALPS in susceptible individuals with TNFRSF6 mutations. Defining the additional factors that contribute to this syndrome may help to elucidate mechanisms or risk factors involved in the evolution of autoimmunity in general.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Note added in proof
CD4+/CD25+ (immunoregulatory) T cells have recently been identified in humans.43-45
Current members of the National Institutes of Health ALPS group are Stephen E. Straus, Jennifer M. Puck, Michael J. Lenardo, Jack J. H. Bleesing, Margaret R. Brown, Hyung Chun, Janet K. Dale, Joie Davis, Faith Dugan, Roxanne E. Fisher, Thomas A. Fleisher, Fred Gill, Amy P. Hsu, John Hurley, Christine E. Jackson, Elaine S. Jaffe, Frances Ka-Ming, Lilia Lei Bi, Adrian Lobito, Julie Niemela, Richard M. Siegel, Michael C. Sneller, Warren Strober, David Stroncek, Jin Wang, and Lixin Zheng.
Author notes
Jack J. H. Bleesing, Arkansas Children's Hospital Research Institute, 1120 Marshall St, Little Rock, AR 72202; e-mail: bleesingjacobh@uams.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal