Abstract
The interaction of human plasma fibrinogen with leukocyte integrins αMβ2 (CD11b/CD18, Mac-1) and αXβ2 (CD11c/CD18, p150,95) is an important component of the inflammatory response. Previously, it was demonstrated that binding of fibrinogen to these integrins is mediated by γC, the globular C-terminal domain of the γ chain. In this study, evidence was found of another fibrinogen domain that can serve as a ligand for the 2 leukocyte integrins: αEC, a homologous domain that extends the α chains in a recently discovered subclass of fibrinogen known as fibrinogen-420. Recombinant αEC supported strong adhesion and migration of cells expressing αMβ2 and αXβ2, including nonactivated and activated U937 and THP-1 monocytoid cells, and neutrophils. Cells transfected with complementary DNA for these integrins also bound αEC. The specificity of interaction was substantiated by inhibition of cell adhesion with antibodies against αM, αX, and β2subunits. Also, neutrophil inhibitory factor, a specific inhibitor of αMβ2 and αXβ2function, efficiently blocked cell adhesion to αEC. In αMβ2 and αXβ2, the I domain is the binding site for αEC, since αEC bound to recombinant αM I and αXI domains in a dose-dependent and saturable manner. Synthetic peptides that duplicated sequences γ190 to 202 and γ377 to 395, previously considered putative binding sites in γC, effectively inhibited αMβ2- and αXβ2-mediated adhesion to αEC, suggesting that recognition of αEC by the I domain involves structural features in common with those of γC. These findings identify αEC as a second domain in fibrinogen-420 that binds αMβ2 and αXβ2 and can mediate leukocyte adhesion and migration.
Introduction
In addition to its function in the coagulation and hemostatic systems, plasma protein fibrinogen (Fg) also participates in inflammatory responses. This function of Fg depends on its ability to interact with leukocyte receptors. On inflammatory challenge, Fg mediates adhesive and migratory reactions of leukocytes, such as leukocyte attachment to the vessel wall and subsequent transmigration through the endothelium into a subendothelial matrix.1-3 In addition, fibrinogen and fibrin deposited at sites of vascular injury and within tissues4,5 promote accumulation of inflammatory cells.6,7 The accessory role of Fg in inflammation was documented by in vivo studies in which congenital afibrinogenemia in patients or experimental depletion of Fg from the circulation in animals altered the manifestation of inflammatory responses.8-12
The interaction of leukocytes with Fg is mediated by 2 transmembrane receptors that belong to the integrin gene superfamily: αMβ2 (CD11b/CD18, Mac-1) and αXβ2 (CD11c/CD18, p150,95). Abundantly expressed on monocytes and neutrophils and induced on lymphocytes during their activation, integrin αMβ2 is primarily responsible for leukocyte adhesion to Fg.13Studies in mice deficient in αMβ2demonstrated that fibrinogen-dependent inflammatory reactions were significantly curtailed in these animals.14 Specifically, the mice failed to accumulate phagocytes at the site of implantation of biomaterials, a process that depends entirely on Fg that is spontaneously adsorbed on the implant surfaces.10 The role of αXβ2, which is enriched on macrophages and dendritic cells, in binding of Fg is poorly characterized.15 16
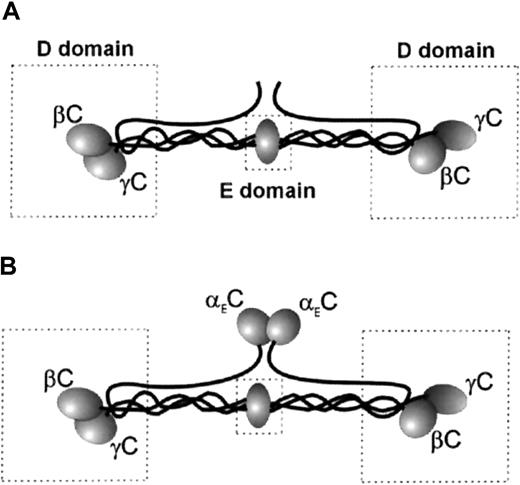
Plasma Fg is a dimer composed of 2 copies of 3 nonidentical polypeptide chains (Aα, Bβ, and γ) linked by disulfide bonds.17Structurally, the molecule is organized into a central E domain and 2 peripheral D domains (Figure 1A). The COOH-terminal parts of the Bβ and γ chains in the D domain are folded into the globular βC and γC domains. Previous studies found that the binding site for αMβ2 and αXβ2 in Fg resides in γC.18-20 Two sequences in γC, corresponding to γ190 to 202 and γ377 to 395, were suggested as the putative recognition sites for leukocyte β2 integrins.21,22Analyses of synthetic peptides duplicating γ190 to 202 and γ377 to 395, designated P1 and P2, respectively, showed that the peptides inhibited adhesion of cells expressing αMβ2and αXβ2, directly supported cell adhesion20,22 and migration,23 and promoted accumulation of leukocytes on implanted biomaterials.24 It was also shown that the sequence γ383 to 395 (P2-C) is the major active site in P2.22 The complementary binding site for P1 and P2 peptides in the heterodimeric αMβ2receptor was localized in the I domain, a region of about 200 amino acid residues inserted in the αMsubunit.22 25
Schematic representation of the domain structure of the 2 species of human Fg.
(A) Fg-340. (B) Fg-420. The D and E domains are boxed. The locations of αEC (in Fg-420 only), γC, and βC are shown.
Schematic representation of the domain structure of the 2 species of human Fg.
(A) Fg-340. (B) Fg-420. The D and E domains are boxed. The locations of αEC (in Fg-420 only), γC, and βC are shown.
In the past decade, it was discovered that normal plasma Fg consists of 2 species differentiated by the length of their Aα chains—a more abundant form with a molecular weight of 340 kd (Fg-340) and a minor form with extended α chains (αE) and hence a higher molecular weight of 420 kd (Fg-420; Figure 1B).26-28 The globular C-terminal domain of αE (αEC), which closely resembles βC and γC, consists of 236 amino acid residues that are missing in the common α chains of Fg-340. In vivo, αEC originates by alternative splicing of the α gene transcript to include the exon VI sequence.29,30 The properties that the presence of αEC confers on Fg-420, which is structurally identical to Fg-340 in all other respects, are only beginning to be explored. The properties of the 2 Fg species that are related to classic functions of Fg (ie, clotting, cross-linking by factor XIII, and fibrinolysis) are similar, except that degradation of Fg-420 by plasmin releases a stable product containing the αEC domain in addition to the conventional degradation products X, Y, D, and E.31 However, little is known about how the presence of αEC contributes to recognition of Fg-420 by leukocyte integrins. It is known that αEC and γC share about 40% of amino acid identity,30 and analyses of x-ray structures revealed that folding of the αEC domain closely resembles that of γC.32-34 In this study, we analyzed the interaction of αEC with leukocyte integrins. We found that the αEC domain of Fg-420 is a ligand for αMβ2 and αXβ2capable of mediating strong leukocyte adhesion and promoting directed migration of leukocytes.
Materials and methods
Proteins, peptides, and monoclonal antibodies
Fraction I-2 Fg was purified from umbilical cord plasma, and separation of human Fg-340 and Fg-420 was achieved by ion-exchange chromatography on Mono Q as described previously.31 Fragment D100 (Mr100 000) was prepared by digestion of human Fg with plasmin. Fg and plasmin were obtained from Enzyme Research Laboratories (South Bend, IN). Fragment D100 was purified by ion-exchange chromatography on CM-Sephadex followed by gel filtration on Sephacryl S-200.35 A recombinant wild-type αEC domain (αEC 610-847) was expressed in Pichia pastorisand purified as described previously.36 A recombinant wild-type γC domain was expressed in P pastoris by using an Invitrogen protocol (San Diego, CA). Briefly, a complementary DNA (cDNA) fragment corresponding to γ143 to 411 was generated by polymerase chain reaction from a template consisting of full-length cDNA encoding the human Fg γ chain that was provided by Dr S. Lord (University of North Carolina).37 The amplified cDNA fragment was ligated into pPIC9 expression vector by means ofSnabI and NotI sites and cloned in DH5α cells. The plasmid was linearized by using SalI and transformed into P pastoris (strain GS115) by electroporation. Expression was induced by transferring the yeast into medium containing 0.5% methanol. The recombinant γC was purified by chromatography on Reactive Red-120 CL-6B agarose (Sigma-Aldrich, St Louis, MO). The yeast supernatant was applied to the column, equilibrated with 20 mM Tris-HCl buffer (pH 7.4) containing 80 mM NaCl. Bound protein was eluted with 50 mM Tris-HCl buffer (pH 7.4) containing 0.5 M NaCl. The protein was dialyzed against 50 mM Tris-HCl (pH 7.4) with 0.15 M NaCl, and 45% glycerol was added to prevent aggregation. The purified γC was homogeneous, as revealed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and contained an intact COOH terminus, as demonstrated by Western blot analysis using mAb 4A5, which recognizes γ406 to 411.38 The recombinant αEC and γC domains were labeled with iodine 125 (125I) by using Iodobeads according to the manufacturer's protocol (Pierce, Rockford, IL).
The recombinant αM I domain corresponding to the human αM sequence D132 to A318 and the αX I domain (residues E148-A335) were expressed in Escherichia coli as fusion proteins with glutathione S transferase (GST) by using the expression vector pGEX-4T-1 (Amersham Pharmacia Biotech, Piscataway, NJ) as described previously.39 The GST–I domain fusion proteins from the cell lysate were purified by affinity chromatography using glutathione-agarose (Sigma-Aldrich). To obtain the αM I domain, the fusion partner was removed by digestion with thrombin and the mixture of GST and αM I domain was separated by cycling through glutathione-agarose. The Fg peptides P1 and P2-C corresponding to the γ-chain sequences 190 to 202 (Gly-Trp-Thr-Val-Phe-Gln-Lys-Arg-Leu-Asp-Gly-Ser-Val) and 383 to 395 (Thr-Met-Lys-Ile-Ile-Pro-Phe-Asn-Arg-Leu-Thr-Ile-Gly), respectively, were described previously.22
The mAb 4A5 directed against γ406 to 41138 was provided by Dr G. Matsueda (Bristol-Meyers Squibb, Princeton, NJ). The monoclonal antibodies (mAbs) 44a40 and IB441directed against the αM and β2 subunits, respectively, and mAb w6/32 (antihuman HLA) were obtained from the American Type Culture Collection (ATCC; Rockville, MD). Serotec (Raleigh, NC) provided mAb 3.9 directed against the αXsubunit. Neutrophil inhibitory factor (NIF) was a gift from Corvas International (San Diego, CA).
Cells
Human embryonic kidney (HEK) 293 cells expressing αMβ2 were described previously.42 Chinese hamster ovary (CHO) cells expressing αXβ2 were provided by Dr D. Golenbock (Boston University). The cell lines were maintained in Dulbecco modified Eagle medium (DMEM)–F-12 medium (BioWhittaker, Walkersville, MD) or Ham medium (Life Technologies, Rockville, MD) for αMβ2 and αXβ2transfectants, respectively, and supplemented with 10% fetal-calf serum (FCS) and 25 mM HEPES. The U937 and THP-1 monocytoid cells were obtained from the ATCC and cultured in RPMI 1640 medium supplemented with 10% FCS.
Granulocytes were isolated from peripheral blood obtained from consenting volunteers and anticoagulated with acid-citrate-dextrose essentially as described previously.43 The cells in the preparation were 98% granulocytes, of which at least 96% were neutrophils and 2% eosinophils.
Cell-adhesion assays
The wells of polystyrene microtiter plates (Costar, Cambridge, MA; or Immulon 4HBX, Dynex Technologies, Chantilly, VA) were coated with various concentrations of protein ligands for 3 hours at 37°C or overnight at 4°C. The wells were postcoated with 1% polyvinylpyrrolidone (PVP) for 1 hour at 37°C. The cells expressing αMβ2 and αXβ2were harvested from the flasks with a cell-dissociating buffer (Gibco, Grand Island, NY) and washed twice in Hanks balanced salt solution (HBSS). The U937 cells, THP-1 cells, and isolated neutrophils were harvested by centrifugation. The cells were labeled with CalceinAM (Molecular Probes, Eugene, OR) for 30 minutes at 37°C, washed with HBSS and resuspended in the same medium at a concentration of 2.5 × 105 cells/mL. Aliquots (100 μL) of the labeled cells were added to each well. For inhibition experiments, cells were mixed with either antibodies, function-blocking peptides, or NIF and incubated for 20 minutes at 22°C before they were added to the coated wells. After 25 minutes of incubation at 37°C in a 3% CO2 humidified atmosphere, the nonadherent cells were removed and the plates were washed once with HBSS. Fluorescence was measured in a fluorescence plate reader (Perseptive Biosystems, Framingham, MA), and the number of adherent cells was determined from a standard curve constructed by using the fluorescence of 100-μL aliquots with a known number of labeled cells.
Cell-migration assays
Chemotactic motility experiments were done under sterile conditions by using transwell chambers with a pore size 8 μm in diameter (Costar; Corning, Corning, NY). Here, 600 μL DMEM–F-12 medium containing either the D100 fragment or the αEC domain was placed in the lower chamber. The αMβ2-expressing cells or U937 cells harvested as described above were resuspended at a concentration of 2 × 106/mL in DMEM–F-12 and RPMI 1640, respectively. Cells (150 μL) with or without inhibitory antibodies were placed in the upper chamber and incubated for 5 to 15 hours at 37°C in a 3% CO2 humidified atmosphere. Cells on the upper surface of the polycarbonate membrane were then removed by wiping the surface twice with a cotton-tipped applicator. The membranes were fixed with formaldehyde and stained with hematoxylin 7211 (Richard-Allan Scientific, Kalamazoo, MI). The migrated cells on the lower surface of the filter in 6 randomly chosen low-power fields (×20 magnification) were counted and the values obtained were averaged. No migration was observed in chambers with no added proteins.
Solid-phase binding assays
Twelve-well microtiter strips (Immulon 2HB; Dynex Technologies) were coated with 100 μL of 10 μg/mL recombinant αM I domain in Tris-buffered saline (TBS) containing 1 mM CaCl2and 1 mM MgCl2 overnight at 4°C and postcoated with 1% bovine serum albumin (BSA) for 1 hour at 22°C. Increasing concentrations of 125I-labeled recombinant αEC or γC in TBS and 0.05% Tween 20 containing 1 mM CaCl2 and 1 mM MgCl2 were added to the wells, which were incubated for 3 hours at 37°C. After washing with TBS and 0.05% Tween 20, bound radioactivity was measured and the amount of bound proteins was calculated, with correction for nonspecific binding to BSA-coated wells.
To test the interaction of the αX I domain, 96-well plates (Immulon 4BX; Dynex Technologies) were coated with 50 μg/mL αEC and γC overnight at 4°C and postcoated with 3% BSA. Different concentrations of the GST–αX I domain in 20 mM TBS (containing 1 mM MgCl2, 1 mM CaCl2, 0.05% Tween 20, and 5% glycerol) were added to the wells and incubated for 3 hours at 22°C. After washing, bound I domain was detected with an anti-GST mAb (Upstate Biotechnology, Lake Placid, NY) at a 1:5000 dilution. After another washing, goat anti–mouse IgG conjugated to alkaline phosphatase was added for 1 hour, and binding of the I domain was measured by reaction with p-nitrophenyl phosphate. The control binding of GST to immobilized αEC and γC was typically about 5% to 10% that of the αM I domain. Background binding to BSA was subtracted.
Analytical procedures
To determine the amounts of recombinant αEC and γC immobilized on the wells of the microtiter plates, each domain was adsorbed on the plastic for 3 hours at 37°C at a concentration of 10 or 20 μg/mL and then washed with phosphate-buffered saline. The concentration of bound proteins was determined by the bicinchoninic acid method, according to the manufacturer's protocol (Pierce).
Results
Adhesion of cells expressing αMβ2 and αXβ2 to the αEC domain of Fg-420
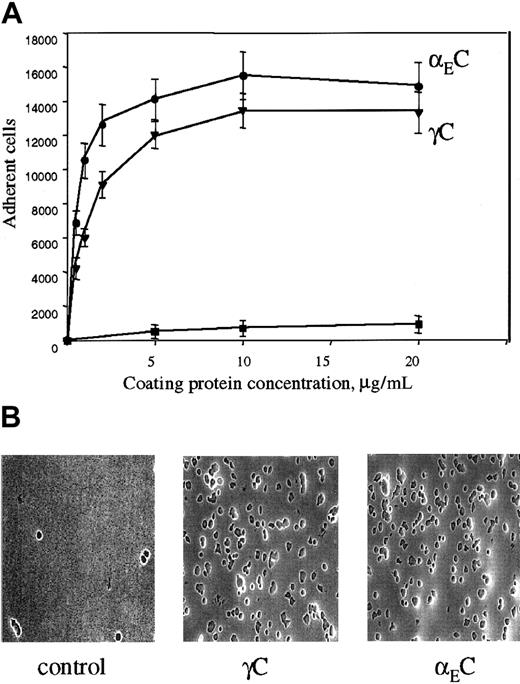
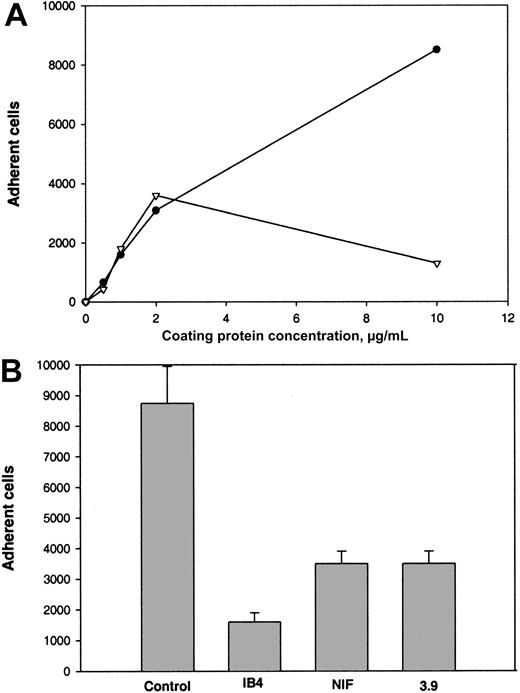
The binding site for αMβ2 in Fg-340 was shown previously to reside in γC, a constituent subdomain of the D domain (Figure 1).18,21,22 Because γC shares about 40% amino acid identity with the αEC domain of Fg-420,30 and the 2 domains are folded into almost identical structures,33 we tested adhesion of αMβ2-expressing cells to recombinant αEC. As shown in Figure 2A, when recombinant proteins were immobilized on tissue culture–treated polystyrene plates, both proteins supported efficient adhesion of αMβ2-transfected HEK 293 cells. Adhesion depended on the concentration used, and similar numbers of cells adhered to each protein. The concentrations of αEC and γC required for half-maximal adhesion were 0.6 μM and 2.1 μM, respectively. The appearance of the cells adherent to the 2 substrates was similar (Figure 2B).
Adhesion of cells transfected with αMβ2 to recombinant αEC and γC.
(A) αMβ2-transfected HEK 293 cells (5 × 104) labeled with Calcein AM in HBSS and HEPES were added to the wells of 48-well, tissue culture–treated microtiter plates coated with different concentrations of the recombinant αEC (●) and γC (▾) domains and postcoated with 1% PVP. After 25 minutes at 37°C, nonadherent cells were removed by 3 washes with phosphate-buffered saline. Fluorescence of adherent cells was measured in a fluorescence plate reader and converted to cell number. Adhesion of mock-transfected cells (▪) to αEC is shown for comparison. Results are the mean ± SE values from 3 individual experiments done in triplicate. (B) Microscopical study (original magnification, × 20) of the cells adherent to the control (PVP), γC, and αEC.
Adhesion of cells transfected with αMβ2 to recombinant αEC and γC.
(A) αMβ2-transfected HEK 293 cells (5 × 104) labeled with Calcein AM in HBSS and HEPES were added to the wells of 48-well, tissue culture–treated microtiter plates coated with different concentrations of the recombinant αEC (●) and γC (▾) domains and postcoated with 1% PVP. After 25 minutes at 37°C, nonadherent cells were removed by 3 washes with phosphate-buffered saline. Fluorescence of adherent cells was measured in a fluorescence plate reader and converted to cell number. Adhesion of mock-transfected cells (▪) to αEC is shown for comparison. Results are the mean ± SE values from 3 individual experiments done in triplicate. (B) Microscopical study (original magnification, × 20) of the cells adherent to the control (PVP), γC, and αEC.
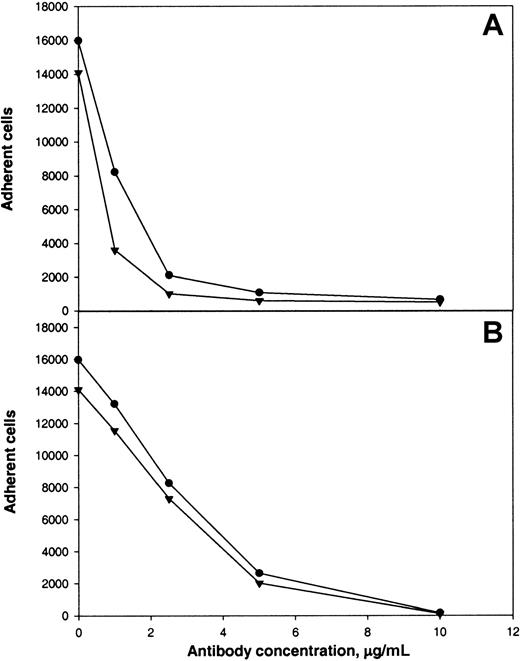
The following results supported the idea that integrin αMβ2 on the surface of αMβ2-transfected cells is responsible for recognition of the αEC domain. First, mock-transfected cells adhered poorly to αEC (Figure 2A). Second, mAb 44a and mAb IB4 directed to the αM and β2subunits, respectively, inhibited adhesion to αEC (Figure3A and 3B). Both antibodies inhibited the αMβ2-mediated adhesion to both αEC and γC almost completely (at 2.5 μg/mL mAb 44A and 10 μg/mL mAb IB4). A control mAb (w6/32) against the class I major histocompatibility complex did not produce inhibition (data not shown). Third, specificity of the interaction between αMβ2 and αEC was confirmed by the finding that NIF, a specific inhibitor of αMβ2,44 45 abolished adhesion to αEC completely (data not shown).
Effect of mAbs on adhesion of αMβ2-transfected cells to αEC and γC.
Calcein-labeled cells were preincubated with different concentrations of mAb 44a (A) or mAb IB4 (B) for 20 minutes at 22°C with constant agitation. Then, aliquots (0.1 mL) containing 5 × 104cells were added to the wells of microtiter plates coated with 5 μg/mL recombinant αEC (●) or γC (▾) domains. After 25 minutes at 37°C, nonadherent cells were removed and cell fluorescence was measured.
Effect of mAbs on adhesion of αMβ2-transfected cells to αEC and γC.
Calcein-labeled cells were preincubated with different concentrations of mAb 44a (A) or mAb IB4 (B) for 20 minutes at 22°C with constant agitation. Then, aliquots (0.1 mL) containing 5 × 104cells were added to the wells of microtiter plates coated with 5 μg/mL recombinant αEC (●) or γC (▾) domains. After 25 minutes at 37°C, nonadherent cells were removed and cell fluorescence was measured.
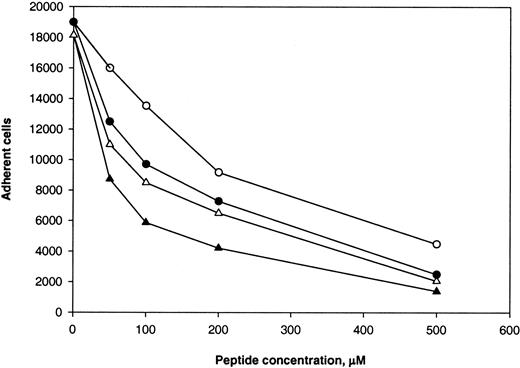
We found previously that P1 and P2-C peptides from γC blocked adhesion of αMβ2-bearing cells to the D100 fragment, with P2-C being a stronger inhibitor of adhesion.22 Because P1 (γ190-202) and P2-C (γ383-395) sequences are similar to corresponding regions 652 to 664 and 838 to 847 in αEC, we tested the effect of the P1 and P2-C peptides on adhesion of αMβ2-transfected cells to immobilized αEC. As shown in Figure4, both P1 and P2-C blocked adhesion to αEC and D100 in a dose-dependent manner. On a molar basis, P2-C was more effective than P1 peptide. For D100, 50% inhibition (IC50) was attained with 210 μM P2-C and 350 μM P1. The peptides were less potent inhibitors of adhesion of the cells to αEC than to immobilized D100 (IC50, 65 μM for P2-C and 195 μM for P1). The competence of both P1 and P2-C to inhibit cell adhesion to αEC seems to be consistent with their ability to cross-inhibit each other's activity22; ie, P2 could inhibit adhesion of the αMβ2-expressing cells to immobilized P1 and vice versa. The ability of P1 and P2-C to inhibit cell adhesion to αEC and γC suggests that these Fg domains may share common molecular or structural determinants required for recognition by αMβ2. Alternatively, the peptides may inhibit cell adhesion to αEC and γC by a steric or allosteric mechanism.
Inhibition of cell adhesion to the immobilized αEC and D100 by P1 and P2-C peptides.
Calcein-labeled αMβ2-transfected cells were preincubated with increasing concentrations of P1 (open symbols) and P2-C (solid symbols) peptides for 20 minutes at 22°C. Aliquots (0.1 mL; 2.5 × 105 cells/mL) were added to wells coated with 10 μg/mL αEC (circles) or 2 μg/mL D100(triangles), and adhesion was quantitated. Values are the numbers of adherent cells. Data from a representative experiment are shown.
Inhibition of cell adhesion to the immobilized αEC and D100 by P1 and P2-C peptides.
Calcein-labeled αMβ2-transfected cells were preincubated with increasing concentrations of P1 (open symbols) and P2-C (solid symbols) peptides for 20 minutes at 22°C. Aliquots (0.1 mL; 2.5 × 105 cells/mL) were added to wells coated with 10 μg/mL αEC (circles) or 2 μg/mL D100(triangles), and adhesion was quantitated. Values are the numbers of adherent cells. Data from a representative experiment are shown.
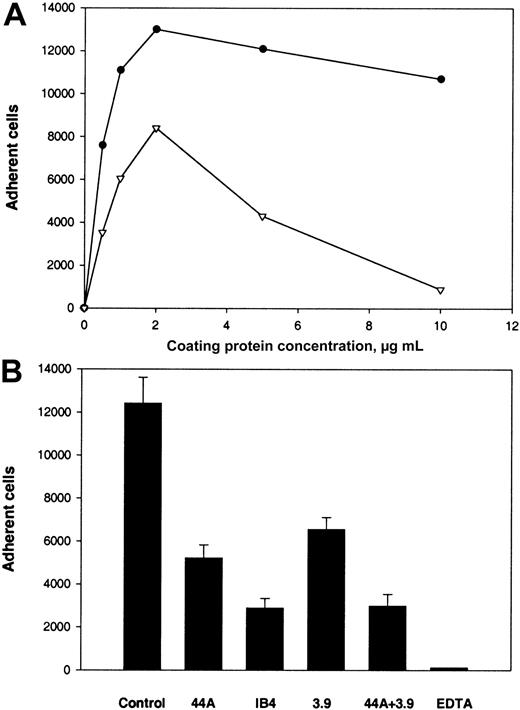
The second β2 integrin, αXβ2, can recognize γC in the D domain of fibrinogen.20 To test the capacity of αEC to support αXβ2-mediated adhesion, we used CHO cells expressing αXβ2. Adhesion of these cells to several fibrinogen derivatives, including D100, was characterized previously.20 Although D100includes a second homologous subdomain, βC, earlier studies found that βC does not contribute significantly to the adhesion-promoting activity of D100 (Merkulov S. and Ugarova T., unpublished data, 2000). As shown in Figure5A, this study found that αXβ2-expressing CHO cells adhered to αEC in a dose-dependent manner. Cell adhesion to αEC was similar to that to D100 when the coating concentrations used were in the range of 0 to 2 μg/mL. However, at concentrations greater than 2 μg/mL, the cells continued to show dose-dependent adhesion to αEC but adhesion to D100 declined. Wild-type CHO cells adhered poorly to all 3 substances (αEC, γC, and D100 ligands; 5% adhesion; data not shown). The anti-β2 mAb IB4, anti-αX mAb 3.9, and NIF produced 80%, 62%, and 62% inhibition, respectively, of the αXβ2-expressing cells (Figure 5B).
Adhesion of αXβ2-expressing CHO cells to αEC and D100.
(A) Calcein-labeled cells (0.1 mL; 5 × 105cells/mL) were added to the wells of 96-well microtiter plates coated with various concentrations of αEC (●) and D100 (▿) and postcoated with 1% PVP. After the cells were allowed to adhere for 25 minutes at 37°C, nonadherent cells were removed by 3 washes with phosphate-buffered saline and the number of adherent cells was determined by measuring the fluorescence. (B) Effect of mAbs and NIF on adhesion of cells to wells coated with 10 μg/mL αEC. The cells were preincubated with 10 μg/mL mAb IB4, 10 μg/mL mAb 3.9, or 2 μg/mL NIF for 15 minutes at 22°C. Aliquots of cells were then added to wells coated with αEC. Results are the total numbers of adherent cells and the mean ± SE values from 2 individual experiments.
Adhesion of αXβ2-expressing CHO cells to αEC and D100.
(A) Calcein-labeled cells (0.1 mL; 5 × 105cells/mL) were added to the wells of 96-well microtiter plates coated with various concentrations of αEC (●) and D100 (▿) and postcoated with 1% PVP. After the cells were allowed to adhere for 25 minutes at 37°C, nonadherent cells were removed by 3 washes with phosphate-buffered saline and the number of adherent cells was determined by measuring the fluorescence. (B) Effect of mAbs and NIF on adhesion of cells to wells coated with 10 μg/mL αEC. The cells were preincubated with 10 μg/mL mAb IB4, 10 μg/mL mAb 3.9, or 2 μg/mL NIF for 15 minutes at 22°C. Aliquots of cells were then added to wells coated with αEC. Results are the total numbers of adherent cells and the mean ± SE values from 2 individual experiments.
Because of the findings showing that αEC contains the binding site for αMβ2 and αXβ2, we investigated whether the presence of additional recognition sites in Fg-420 confers greater adhesive potency than that observed with Fg-340. Various concentrations of purified Fg-340 and Fg-420 were deposited on plastic and adhesion of αMβ2-transfected cells was tested. As expected, cells adhered to both Fg forms; however, adhesion to Fg-420 was not significantly greater than that to Fg-340 (data not shown). It is not known whether adhesion to Fg-420, with its multiple binding sites, differs from that of Fg-340 with respect to the cellular activities it triggers.
We next studied the ability of αEC to support adhesion of U937 and THP-1 monocytoid cells. As shown in Figure6A, U937 cells stimulated with phorbol 12-myristate 12-acetate (PMA) recognized and attached to αEC strongly, and the adhesion was more extensive than that to D100. Similar results were obtained for adhesion of THP-1 (data not shown). Adhesion of both cell lines in the absence of PMA to both immobilized ligands was only slightly less than with PMA stimulation. Adhesion of U937 and THP-1 to αEC was inhibited by anti-αM mAb 44a and anti-β2mAb IB4 (Figure 6B). However, in contrast to the results with αMβ2-transfected cells, inhibition was not complete. The maximal inhibition level achieved at a concentration of 20 μg/mL was 58% with mAb 44a and 77% with mAb IB4.
Adhesion of U937 cells to αEC and D100.
(A) U937 cells in HBSS were labeled with Calcein and activated with 10 nM PMA. Aliquots of 5 × 105cells/mL were then added to the wells coated with αEC (●) and D100(▿). (B) Effect of mAbs on adhesion to αEC. Activated U937 cells were preincubated with either mAb 44a, mAb 3.9, or mAb IB4 alone (20 μg/mL), with a combination of 44a and 3.9 or with 4 mM EDTA for 15 minutes at 22°C. Then, aliquots of 5 × 104cells were added to microtiter plates coated with 10 μg/mL αEC.
Adhesion of U937 cells to αEC and D100.
(A) U937 cells in HBSS were labeled with Calcein and activated with 10 nM PMA. Aliquots of 5 × 105cells/mL were then added to the wells coated with αEC (●) and D100(▿). (B) Effect of mAbs on adhesion to αEC. Activated U937 cells were preincubated with either mAb 44a, mAb 3.9, or mAb IB4 alone (20 μg/mL), with a combination of 44a and 3.9 or with 4 mM EDTA for 15 minutes at 22°C. Then, aliquots of 5 × 104cells were added to microtiter plates coated with 10 μg/mL αEC.
Because U937 and THP-1 cells express both αMβ2 and αXβ2, the effect of mAb 3.9 against the αX subunit was tested alone and in combination with mAb 44a. We found that mAb 3.9 inhibited adhesion of U937 cells by 48% and that adding mAb 3.9 to mAb 44a resulted in 75% inhibition. Similar results were obtained with THP-1 cells. Thus, an additional interaction of αEC with other receptors on the surface of U937 and THP-1 cells may account for the incomplete inhibition of adhesion. It is notable that EDTA, a specific inhibitor of integrin-mediated interaction, completely inhibited adhesion of monocytoid cells to immobilized αEC, whereas mAb w6/32 against HLA did not produce inhibition (data not shown).
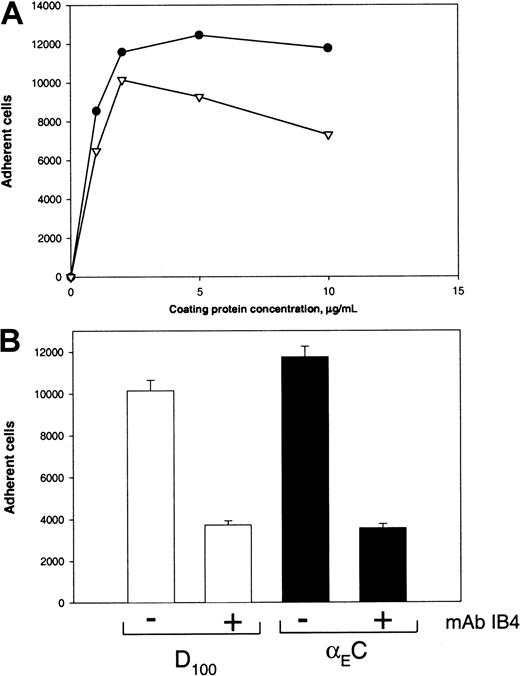
To extend our findings obtained with transfected cells and monocytoid cells, we examined the ability of neutrophils, which are known to express high levels of αMβ2, to bind to αEC. We found that nonstimulated neutrophils isolated from fresh human blood attached readily to immobilized αEC and D100 in a dose-dependent manner (Figure 7A) and that mAb IB4, which recognizes the common β2 subunit of αMβ2 and αXβ2on the surface of neutrophils, effectively inhibited adhesion (Figure7B). In addition, mAbs against αM (44a) and αX (3.9) inhibited neutrophil adhesion to αEC by 48% and 33%, respectively (data not shown). The combination of both anti-α–subunit mAbs decreased adhesion by about 70%, similar to the mAb IB4 inhibition. Taken together, these results clearly show that αEC can support strong adhesion of leukocytes that depends to a large extent on integrins αMβ2 and αXβ2.
Adhesion of activated neutrophils to surfaces coated with αEC and D100.
(A) Neutrophils were isolated from human blood, resuspended in HBSS, and labeled with Calcein. Cells (0.1 mL; 2 × 105cells/mL) were activated with 10 nM PMA and then distributed into wells of plates coated with various concentrations of αEC (●) and D100 (▿). Adhesion was done as described in the legends for Figures 2 to 5. (B) Adhesion of activated neutrophils to wells coated with 10 μg/mL αEC (solid bars) and 10 μg/mL D100 (open bars) in the absence or presence of 10 μg/mL mAb IB4.
Adhesion of activated neutrophils to surfaces coated with αEC and D100.
(A) Neutrophils were isolated from human blood, resuspended in HBSS, and labeled with Calcein. Cells (0.1 mL; 2 × 105cells/mL) were activated with 10 nM PMA and then distributed into wells of plates coated with various concentrations of αEC (●) and D100 (▿). Adhesion was done as described in the legends for Figures 2 to 5. (B) Adhesion of activated neutrophils to wells coated with 10 μg/mL αEC (solid bars) and 10 μg/mL D100 (open bars) in the absence or presence of 10 μg/mL mAb IB4.
Binding of αEC to αMβ2and αXβ2 is mediated by the corresponding I domains
In the heterodimeric αMβ2 receptor, the I domain, a region of about 200 amino acid residues inserted in the αM subunit, contributes importantly to recognition of several ligands, including Fg.46 It was shown previously that synthetic peptides P1 and P2, which duplicate recognition sequences in the γC domain, specifically bound to the recombinant I domain.22,25 Also, in earlier studies, we found that the αX I domain of αXβ2 is responsible for the binding of P2-C.39 Therefore, in this study, we examined whether αEC can directly interact with the αM I and αX I domains. The recombinant αM I domain was immobilized on the wells of microtiter plates, and the binding of 125I-labeled αEC and γC was measured. To examine the interaction between αEC and the αX I domain, binding of the αX I domain as a fusion protein with GST was assessed. Figure 8 shows that αEC bound to the αM I and αX I domains in a dose-dependent and saturable manner. The interaction of αEC and γC with the corresponding I domains seemed to be similar in affinity because the molar concentrations of each ligand required for half-maximal binding were about the same.
Binding of αEC and γC to the αM I and αX I domains.
(A) Different concentrations of 125I-labeled ligands αEC (●) and γC (▾) in TBS, 1 mM CaCl2, and 1 mM MgCl2 were added to wells coated with 10 μg/mL of the recombinant αM I domain and incubated for 3 hours at 37°C. After washing with TBS and 0.05% Tween 20, bound radioactivity was measured and the amounts of bound ligands were calculated, with correction for nonspecific binding to BSA-coated wells. (B) Different concentrations of recombinant αX I domain produced as a fusion protein with GST in TBS containing 1 mM MgCl2, 1 mM CaCl2, 0.05% Tween 20, and 5% glycerol were added to microtiter plates coated with 50 μg/mL αEC (●) and γC (▾) and postcoated with 3% BSA. After incubation for 3 hours at 22°C, anti-GST mAb (1:5000) was added to the wells for an additional 1.5 hours. Binding of the I domains was then detected with a secondary goat anti–mouse IgG conjugated to alkaline phosphatase, with subsequent development of the reaction with p-nitrophenyl phosphate.
Binding of αEC and γC to the αM I and αX I domains.
(A) Different concentrations of 125I-labeled ligands αEC (●) and γC (▾) in TBS, 1 mM CaCl2, and 1 mM MgCl2 were added to wells coated with 10 μg/mL of the recombinant αM I domain and incubated for 3 hours at 37°C. After washing with TBS and 0.05% Tween 20, bound radioactivity was measured and the amounts of bound ligands were calculated, with correction for nonspecific binding to BSA-coated wells. (B) Different concentrations of recombinant αX I domain produced as a fusion protein with GST in TBS containing 1 mM MgCl2, 1 mM CaCl2, 0.05% Tween 20, and 5% glycerol were added to microtiter plates coated with 50 μg/mL αEC (●) and γC (▾) and postcoated with 3% BSA. After incubation for 3 hours at 22°C, anti-GST mAb (1:5000) was added to the wells for an additional 1.5 hours. Binding of the I domains was then detected with a secondary goat anti–mouse IgG conjugated to alkaline phosphatase, with subsequent development of the reaction with p-nitrophenyl phosphate.
αEC supports migration of cells expressing αMβ2
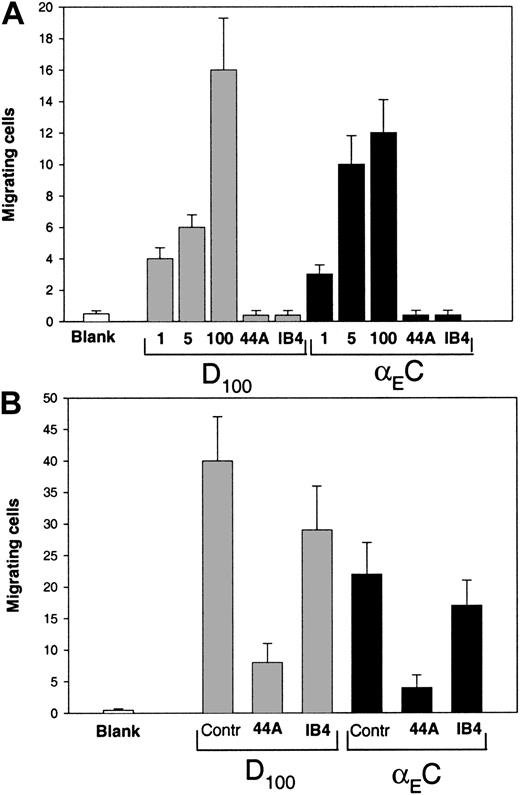
Fg was shown previously to promote αMβ2-mediated migration of leukocytes in an in vivo animal model,3 and D100 and Fg recognition peptides P1 and P2 supported a chemotactic response of leukocytes in vitro.2,23 47 Therefore, we tested the ability of αEC to mediate migration of αMβ2-transfected HEK 293 cells and monocytoid cells. The αMβ2-transfected cells were allowed to migrate in a transwell toward a gradient of αEC or D100 at 37°C for 15 hours, after which the cells that migrated through and attached to the underside of the membrane were fixed, stained, and counted. Different concentrations of each protein (range, 1-100 μg/mL) placed in the lower chamber of the transwell system supported efficient migration of the cells, and on a molar basis, D100 was a somewhat more efficient inducer of migration than was αEC (Figure9A). The migration toward αEC depended on αMβ2, since mAb 44a against the αM subunit and mAb IB4 against the β2 subunit blocked the response completely (Figure 9A). Also, αEC and D100 promoted efficient migration of nonstimulated U937 cells (Figure 9B). As with the αMβ2-transfected cells, D100was a more potent migratory agent: at a concentration of 50 μg/mL of each protein in the lower chamber, 40 ± 8 cells/field migrated to D100, whereas 22 ± 5 cells/field migrated to αEC. Migration of U937 cells to both ligands was strongly inhibited by mAb 44a, but mAb IB4, the anti-β2–specific mAb, impeded migration by only 30%. The ability of αEC to mimic γC in vivo in promoting αMβ2-mediated migration of leukocytes remains to be tested and the advantage of such apparent redundancy explored.
Migration of the αMβ2-bearing cells to αEC and D100.
(A) Cells expressing αMβ2 (150 μL; 3 × 105) in DMEM–F-12 medium were added to the upper chambers of transwells and allowed to migrate to different concentrations of αEC or D100 (1, 5, and 100 μg/mL) placed in the lower chamber. Some cells were preincubated with 40 μg/mL mAb 44a or mAb IB4 and allowed to migrate to 100 μg/mL of each domain. Cell migration was assessed after 15 hours at 37°C in a humidified CO2 atmosphere. Cells from the upper chamber were removed, and migrating cells on the lower surface of the filter were fixed, stained, and counted. Results are the mean ± SE values per field for 6 random fields counted. (B) Migration of U937 cells to αEC or D100. The U937 cells grown in RPMI 1640 were washed twice in the same medium and added to the upper chambers of transwells at a concentration of 2 × 106cells/mL. The lower chambers contained 50 μg/mL αEC or D100. In some experiments, the cells were preincubated with 40 μg/mL mAb 44a and mAb IB4, and migration to 100 μg/mL of each D100 or αEC sample was measured. After 5 hours at 37°C, cells migrating through the 5-μm membrane were fixed, stained, and counted as described above.
Migration of the αMβ2-bearing cells to αEC and D100.
(A) Cells expressing αMβ2 (150 μL; 3 × 105) in DMEM–F-12 medium were added to the upper chambers of transwells and allowed to migrate to different concentrations of αEC or D100 (1, 5, and 100 μg/mL) placed in the lower chamber. Some cells were preincubated with 40 μg/mL mAb 44a or mAb IB4 and allowed to migrate to 100 μg/mL of each domain. Cell migration was assessed after 15 hours at 37°C in a humidified CO2 atmosphere. Cells from the upper chamber were removed, and migrating cells on the lower surface of the filter were fixed, stained, and counted. Results are the mean ± SE values per field for 6 random fields counted. (B) Migration of U937 cells to αEC or D100. The U937 cells grown in RPMI 1640 were washed twice in the same medium and added to the upper chambers of transwells at a concentration of 2 × 106cells/mL. The lower chambers contained 50 μg/mL αEC or D100. In some experiments, the cells were preincubated with 40 μg/mL mAb 44a and mAb IB4, and migration to 100 μg/mL of each D100 or αEC sample was measured. After 5 hours at 37°C, cells migrating through the 5-μm membrane were fixed, stained, and counted as described above.
Discussion
In this study, we showed that leukocytes can bind to αEC, the domain that distinguishes Fg-420 from Fg-340, by means of the integrins αMβ2 and αXβ2. The recombinant αEC domain supported adhesion and migration of cells expressing αMβ2 and αXβ2, including cultured monocytoid cells U937 and THP-1 and freshly isolated neutrophils. In addition, cells transfected with cDNA for the 2 integrins bound αEC. Identification of αMβ2 and αXβ2as receptors for αEC was based on inhibition of cell adhesion and migration by antibodies against the αM, αX, and β2 subunits; specific interaction of αEC with the recombinant αM I domain of αMβ2 and the recombinant αX I domain of αXβ2; and inhibition of the αMβ2- and αXβ2-mediated adhesion to αEC by NIF, a specific inhibitor of these integrins. These findings suggest that αEC could function as an independent recognition site for leukocyte β2 integrins in Fg-420.
The binding site for αMβ2 and αXβ2 was localized previously to 2 sequences in γC of the D domain of Fg-34020-22: γ190 to 202 (P1) and γ383 to 395 (P2-C).20-22 Although the γC and αEC domains are structurally very similar,33,34 alignment of amino acid sequences indicates that the P1 and P2-C regions in γC contain only 54% and 31%, respectively, of residues identical to those in corresponding segments of αEC. Notably, the residues in the C-terminal part of P2-C, (392)Leu-Thr-Ile-Gly(395), responsible for its high cell-adhesive activity22 are not conserved in αEC. Nevertheless, we found that both γC peptides inhibited adhesion of αMβ2-expressing cells to αEC, a result consistent with the idea that interaction between αEC and integrin may involve structural features in common with those of γC.
The data obtained with αEC and the γC-containing D100 fragment indicate that engagement of binding sites on both domains by the αMβ2 integrin on leukocytes can elicit cell migration. Yet the relative availability of those sites in vivo may be a critical differentiating factor permitting initiation of an efficient chemotactic response. During fibrin(ogen)olysis in vitro, the αEC domains are released early as stable fragments.31 Such αEC-containing fragments were also detected in plasma obtained from patients with myocardial infarction shortly after initiation of thrombolytic therapy.31 Each fragment is released from Fg-420 by cleavage of a single bond in the proteolytically susceptible region that tethers αEC to the core of the fibrinogen molecule. Hence, the first degradation products with chemotactic activity released during fibrinolysis would set up a gradient of soluble monomeric αEC-containing fragments, while γC remained anchored in the D or DD domains incorporated in the fibrin clot. The meaning of these findings may be that αEC serves as the initial attractant for leukocytes to a thrombus.
The presence in Fg-420 of 2 different interactive regions for leukocyte β2 integrins, residing in αEC and γC, raises questions regarding how leukocyte adhesion is regulated and the functional consequences of integrin engagement by these domains together or alone (as in Fg-340, which bears only the γC sites). It is well documented that integrin-mediated cellular adhesion triggers a series of complex signaling events leading to phenotypic changes in cells and that it also initiates gene expression.48Moreover, adhesion to Fg was shown to stimulate synthesis of products relevant to the inflammatory response, including interleukin 1β and oxidative molecules.49,50 Several studies using various integrin-ligand systems demonstrated that differential recognition of various domains in the same or related ligands by a single receptor transduces distinct signals.51-53 For example, occupancy of α5β1 on the surface of fibroblasts by different domains of fibronectin induced different cellular responses.52 Fibroblast adhesion to the N-terminal domain of fibronectin induced assembly of novel focal contacts different from those observed after adhesion to the central Arg-Gly-Asp–containing domain. In addition, ligation of α5β1 by different fibronectin domains triggered different patterns of tyrosine phosphorylation of signaling molecules. We previously showed that α4β1-mediated adhesion of leukocytes to 2 related ligands, vascular cell adhesion molecule 1 (VCAM-1) and the alternatively spliced fragment of fibronectin, IIICS-1, can transduce different signals leading to up-regulation of different sets of matrix metalloproteinases.53 These studies suggest the possibility that engagement of different domains of fibrinogen by means of αMβ2 or αXβ2may also induce distinct patterns of activation of intracellular signaling.
We thank Dr S. E. Plow and L. Zhang for providing the αMβ2-expressing cells, Dr D. Golenbock for providing αXβ2-expressing cells, Dr S. Lord for the γ-chain cDNA, Corvas International for NIF, Dr D. Solovjov for help in preparing the recombinant γC, Peter Baker for assisting with purification of recombinant αEC, and Timothy Burke for help with neutrophil isolation.
Supported by grants from the National Institutes of Health (HL-63199 to T.P.U. and HL-51050 to G.G.), the American Heart Association, and the Abby R. Mauze Charitable Trust (to G.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tatiana P. Ugarova, Cleveland Clinic Foundation, Mail Code NB 50, Cleveland, OH 44195; e-mail: ugarovt@ccf.org; or Gerd Grieninger, Lindsley F. Kimball Research Institute of the New York Blood Center, New York, NY 10021; e-mail: ggrien@nybc.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal