Tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL) induced both cytotoxic (apoptosis) and cytostatic (cell cycle perturbation) effects on the human myeloid K562 cell line. TRAIL stimulated caspase 3 and nitric oxide synthase (NOS) activities, and both pathways cooperate in mediating inhibition of K562 survival/growth. This was demonstrated by the ability of z-VAD-fmk, a broad inhibitor of effector caspases, and N-nitro-l-arginine methyl ester (L-NAME), an NOS pharmacologic inhibitor, to completely (z-VAD-fmk) or partially (L-NAME) suppress the TRAIL-mediated inhibitory activity. Moreover, z-VAD-fmk was able to block TRAIL-mediated apoptosis and cell cycle abnormalities and increase of NOS activity. The addition of the NO donor sodium nitroprusside (SNP) to K562 cells reproduced the cytostatic effect of TRAIL without inducing apoptosis. When TRAIL was associated to SNP, a synergistic increase of apoptosis and inhibition of clonogenic activity was observed in K562 cells as well as in other myeloblastic (HEL, HL-60), lymphoblastic (Jurkat, SupT1), and multiple myeloma (RPMI 8226) cell lines. Although SNP greatly augmented TRAIL-mediated antileukemic activity also on primary leukemic blasts, normal erythroid and granulocytic cells were less sensitive to the cytotoxicity mediated by TRAIL with or without SNP. These data indicate that TRAIL promotes cytotoxicity in leukemic cells by activating effector caspases, which directly lead to apoptosis and stimulate NO production, which mediates cell cycle abnormalities. Both mechanisms seem to be essential for TRAIL-mediated cytotoxicity.
Introduction
Tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL),1 also known as Apo-2 ligand,2 is one of the new members of the TNF family of cytokines, which are structurally related proteins playing a key role in the regulation of cell death, immune response, and inflammation (reviewed in Gruss and Dower3 and Baker and Reddy4). TRAIL is a type II membrane protein, having an intracellular amino-terminal portion, an internal transmembrane domain, and a carboxyl terminus external to the cell.1,2 In addition, a soluble form of TRAIL has been identified.5Among the TNF family members, TRAIL shares the highest amino acid identity with CD95L.1,5 The unique feature of TRAIL with respect to CD95L and TNF-α is considered its ability to induce apoptosis of various continuous cell lines and primary tumor cells, including several malignancies of hematopoietic origin,6,7displaying minimal or no toxicity on normal cells and tissues (reviewed in Ashkenazi and Dixit8). Although a role for TRAIL in physiologic conditions has not been clearly envisioned yet, TRAIL shows inhibitory effects on normal immature erythroblasts,9,10 T lymphocytes,11 and hepatocytes.12 13
Via recruitment of the adapter molecules FADD,14-16 TRAIL induces a hierarchical cascade of cysteine proteases, which are termed caspases according to their lytic specificity for aspartic acid residues.17 Caspases are synthesized as proenzymes and activated by cleavage through upstream caspases or by intermolecular autoproteolysis. They can be grouped into 3 main families: (1) the ICE subgroup (caspases 1, 4, and 5) corresponds to proteases involved in the process of cytokine maturation; (2) the CPP32 subgroup (caspases 3, 6, and 7) includes caspases that mediate downstream steps of proteolysis with preferential cleavage of the DEVD sequence; (3) caspases 2, 8, 9, and 10, which are characterized by the presence of a long prodomain and are often related to events of receptor-dependent initiation of apoptosis.17
The nitric oxide (NO) synthase (NOS) enzyme synthesizes NO from the amino acid l-arginine.18,19 Three isoforms of NOS have been identified, which conform to 2 biochemical profiles. The neuronal (nNOS or NOS-1) and the endothelial (eNOS or NOS-3) isoforms are calcium/calmodulin-dependent and result in physiologic low output of NO. A low level of NO synthesized by constitutive nNOS and eNOS for short periods of time acts as (1) neurotransmitter, (2) regulator of blood pressure, (3) platelet aggregation, and possibly as (4) antiapoptotic agent. The calcium-independent inducible isoform (iNOS or NOS-2) is expressed after transcriptional induction by several stimuli and contributes to the pathologic high output of NO.18,19 Of note, it has been demonstrated that iNOS can be expressed by normal hematopoietic progenitors following the induction of interferon-γ (IFN-γ) or TNF-α. NO produced in the course of such activation inhibits proliferation of target cells.20,21 Moreover, it has been shown that NO donors display a cytostatic activity on leukemic cell lines.22 23
On these bases, the present experiments were designed to investigate the molecular mechanisms underlining the cytotoxic/cytostatic activity of TRAIL on leukemic cells, by investigating the activation of NOS and caspases following incubation with TRAIL, and to ascertain whether these mechanisms could be potentiated to improve the antileukemic therapeutic potential of TRAIL.
Materials and methods
Reagents
Both recombinant Histidine6-tagged (His) TRAIL and recombinant His control peptide were produced in bacteria, purified by affinity chromatography on Ni++ affinity resin, and tested for biologic activity on the TRAIL-sensitive Jurkat cell line, as previously described.24
The broad caspase inhibitor Cbz-Val-Ala-Asp (Ome)-fluoromethyl ketone (z-VAD-fmk), the peptide control Cbz-Phe-Ala-fluoromethyl ketone (z-FA-fmk), the selective caspase 8 Cbz-Ile-Glu(Ome)-Thr-Asp(Ome)-CH2F (z-IETD-fmk), and caspase 9 Cbz-Leu-Glu(Ome)-His-Asp(Ome)-CH2F (z-LEHD-fmk) inhibitors were from Calbiochem (La Jolla, CA). The activity of most effector caspases (3, 4, and 7) as well as of caspase 1, is blocked by z-VAD-fmk, whereas z-IETD-fmk and z-LEHD-fmk specifically block the activity of caspase 8 and 9, respectively. All caspase inhibitors were dissolved in dimethyl sulfoxide (DMSO), stocked in aliquots at −20°C, and used at the final concentration of 10 to 100 μM.
Inhibitors of iNOS N-nitro-l-arginine methyl ester (L-NAME) or NG-monomethyl-l-arginine (Calbiochem) were added to the culture medium at a concentration of 0.2 mM. The NO donor sodium nitroprusside (SNP; Sigma Chemicals, St Louis, MO) was dissolved in water immediately before use and added to the culture at a concentration of 1 mM.
Neoplastic cells
Human myeloid K562, HEL and HL-60, T lymphoblastoid Jurkat and SupT1, and multiple myeloma RPMI 8226 cell lines were grown in RPMI (Gibco Laboratories Life Technologies, Grand Island, NY) supplemented with 10% fetal calf serum (FCS, Gibco) at an optimal cell density of 3 × 105 to 1 × 106 cells/mL.
Peripheral blood (PB) specimens were obtained from 2 men (45 and 56 years old) and 1 woman (38 years old) with acute myelogenous leukemia (M2 and M3 of FAB classification of AML), who gave their informed consent to the study according to the Helsinki Declaration of 1975. PB specimens showing blast counts more than 80% were collected at diagnosis before any therapy. Mononuclear cells were separated by density gradient centrifugation (Ficoll/Hystopaque 1077 g/mL, Sigma) and immediately frozen. Aliquots containing 15 to 53 × 106 mononuclear cells were thawed at the time of the study, cultured for 24 hours in RPMI plus 10% FCS, subjected to density gradient centrifugation (Ficoll/Histopaque 1077 g/mL) to eliminate dead cells, and then seeded in semisolid cultures as described below.
Purification of primary normal CD34+ cells and liquid cultures of primary erythroid and granulocytic cells
Cord blood (CB) specimens, collected according to institutional guidelines, were obtained during normal full-term deliveries. CB mononuclear cells were isolated by density gradient centrifugation (Ficoll/Histopaque 1077 g/mL). CB CD34+ cells were then isolated by using a magnetic cell sorting program Mini-MACS and the CD34 isolation kit (Miltenyi Biotech, Auburn, CA) in accordance with the manufacturer's instructions. The purity of CD34+ranged between 90% and 98%. CD34+ cells were cultured in X-vivo (Biowittaker, Walkersville, MD) serum-free medium, supplemented with nucleosides (10 μg/mL each), 0.5% bovine serum albumin (BSA, fraction V of Chon), 10−4 M BSA-adsorbed cholesterol, 10 μg/mL insulin, 200 μg/mL iron-saturated transferrin, and 5 × 10−5 M 2-β-mercaptoethanol (all purchased from Sigma). Erythroid and granulocytic cultures were obtained by seeding CD34+ cells in the presence of stem cell factor (SCF, 50 ng/mL) plus interleukin-3 (IL-3) (0.5 ng/mL) plus erythropoietin (EPO, 4 U/mL) or SCF plus IL-3 plus granulocyte colony-stimulating factor (G-CSF, 10 ng/mL), respectively, as previously described.10,25 All cytokines were purchased from Genzyme (Cambridge, MA). Every 3 to 4 days, cultures were demipopulated by removing half volume of the medium, which was substituted with fresh medium supplemented with cytokines. The cells removed were analyzed for the degree of maturation by FACScan, after staining with lineage-specific monoclonal antibodies, performed as previously described.25 Liquid cultures were then supplemented with His control peptide, TRAIL, SNP, either alone or in combination. After 24 hours, cells were collected, fixed with cold 70% ethanol, and analyzed for cell cycle and apoptosis as described below.
Cell treatment and evaluation of TRAIL cytotoxicity
All experiments were performed on cells showing a viability higher than 95% at trypan blue dye exclusion. Cells were treated with control His peptide or TRAIL, used alone or in combination with SNP. For the experiments with pharmacologic inhibitors, cells were preincubated with the inhibitors for 45 minutes, before addition of TRAIL with or without SNP.
The cytostatic and cytotoxic effects of TRAIL and SNP, used either alone or in combination, were evaluated by: (1) counting the total number of viable cells by trypan blue dye exclusion; (2) 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, performed according to Tada and colleagues26; and (3) apoptosis detection and quantification, performed by 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) staining followed by fluorescence microscopy examination, and by propidium iodide (PI) staining followed by flow cytometry analysis, as described below.
Evaluation of cell cycle and apoptosis
Samples containing 2 to 5 × 105 cells were harvested by centrifugation at 200g for 10 minutes at 4°C, fixed with cold 70% ethanol for at least 1 hour at 4°C, and treated as previously detailed.27 Analysis of PI fluorescence was performed by FACScan flow cytometer with the FL2 detector in a linear mode using the Lysis II software (Becton Dickinson, San José, CA). For cell cycle analysis, only the inferred gap 1 (G1), synthesis (S), and gap 2 plus mitosis (G2/M) peaks were considered. For quantitative evaluation of apoptosis, the subdiploid (< 2n) DNA content was calculated as described,27 and expressed as percentage of apoptotic versus nonapoptotic cells, regardless of the specific cell cycle phase. It should be emphasized that the percentage of apoptosis was calculated using very strict criteria, which have been previously described,27 to score only apoptotic cells and exclude cell debris.
The S-phase labeling was performed by evaluating 5-bromo-2′-deoxyuridine (BrdU) uptake, followed by flow cytometric analysis as described previously.28 Briefly, cells were supplemented for 16 hours with 10 μM BrdU (Sigma) and simultaneously treated with His control peptide, TRAIL, or SNP. BrdU detection was performed by using an anti-BrdU–fluorescein isothiocyanate (FITC) monoclonal antibody (Boehringer Mannheim, Mannheim, Germany) followed by FACScan analysis.
For DAPI staining of nuclei, cells were washed with phosphate-buffered saline (PBS), fixed in cold methanol (−20°C) for 5 minutes, washed again with PBS, and incubated with 500 ng/mL DAPI (Sigma) in PBS for 15 minutes at 37°C in a dark, humidified chamber. After several washes in PBS, the coverslips were mounted on PBS/glycerin. The intercalation of DAPI was visualized by means of an Axyophot Zeiss fluorescence microscope.
Evaluation of NOS activity and nitrite/nitrate production
The NOS enzyme activity was evaluated by determination of (14C)- l-citrulline, generated from (14C)- l-arginine (Amersham, Braunschweig, Germany). The assay was performed using the NOSdetect assay kit (Stratagene, Heidelberg, Germany) according to the manufacturer's instructions. Radioactivity was counted in a β-scintillation counter (Beckmann, Münich, Germany).
The NO production was determined by assaying for nitrite and nitrate accumulation in the culture media. For these experiments, cells were cultured in serum-free medium (X-vivo, Biowittaker). Briefly, following cell treatment with TRAIL, culture media were filtered with 0.2-μm filters, and 80 μL of each sample was treated with nitrate reductase and its cofactors to convert all of nitrate to nitrite before applying 100 μL of the Griess reagent (0.5% naphthylethylenediamine dihydrochloride, 1% sulfanylamidein, 2.5% phosphoric acid). Absorbance was measured at 543 nm, and nitrite concentration was determined using a standard curve of sodium nitrite concentrations ranging from 0 to 50 nmol/L.
Caspase assay
The activity of caspases was measured in K562 cell extracts by using the fluorometric CaspACE assay system (Promega, Madison, WI). To determine ICE (caspase 1) and CPP32 (caspase 3) protease activities, assays were performed in triplicate in 96-well flat-bottom plates by incubating 10 μg cell proteins/sample with the specific fluorogenic substrates according to the manufacturer's instructions. After 1 hour of incubation, the products of reaction were measured by using a plate reader (Victor 1420, Wallac, Freiburg, Germany). Increase in fluorescence was linear over time and extract concentration.
Phenotypic analysis of cell surface TRAIL-R1, TRAIL-R2, TRAIL-R3, and TRAIL-R4
All leukemic cell lines were analyzed for the surface expression of TRAIL-R1, TRAIL-R2, TRAIL-R3, and TRAIL-R4 by indirect staining with primary goat antihuman TRAIL-R1, TRAIL-R2, TRAIL-R3, and TRAIL-R4 antibodies (R & D Systems, Oxon, United Kingdom) followed by phycoerythrin (PE)-conjugated rabbit antigoat secondary antibody (Sigma). Briefly, staining was performed by using 0.6 μg of each primary goat antibody followed by incubation with 3 μL PE-conjugated rabbit antigoat IgG. Nonspecific fluorescence was assessed by using normal goat IgG followed by second layer as above. Flow cytometry analyses were performed by FACScan.
Clonogenic assays
K562 cells, primary AML blasts, and normal erythroid burst-forming unit (BFU-E) and granulocyte-macrophage colony-forming unit (GM-CFU) progenitors were assayed in plasmaclot cultures. Briefly, 5 × 103 K562 cells, primary AML blasts, or primary normal CB CD34+ cells were cultured in 1 mL Iscoves modified Dulbecco medium (Gibco), containing 10% detoxified BSA, 10% heat-inactivated pooled human AB sera, 10% citrated bovine plasma (Gibco), 20 μg l-asparagine (Sigma), and 3.4 mg/mL CaCl2. The standard sources of growth factors were the same as used in liquid cultures: SCF plus IL-3 plus EPO for the growth of BFU-E; SCF plus IL-3 plus G-CSF for the growth of GM-CFU; and SCF plus IL-3 for the growth of primary AML blasts.
At day 14, for BFU-E identification, the clots were fixed in situ and stained with 3,3′-dimethoxybenzidine and hematoxylin. Colonies of more than 50 red hemoglobin-containing cells were scored as BFU-Es. GM-CFU, K562, and AML blast colonies were scored at day 14 (for GM-GFUs) or day 8 to 10 (for K562 and AML) without prior fixation.
Statistical analysis
Data were analyzed using the 2-tailed, 2-sample ttest (Minitab statistical analysis software, State College, PA). Values of P < .05 were considered significant. Interactions between TRAIL and SNP were classified by the fractional inhibition method as follows. When expressed as the fractional inhibition of cell viability, additive inhibition produced by both inhibitors occurred when i1,2 = i1 + i2; synergism when i1,2 > i1 + i2; and antagonism when i1,2 < i1 + i2.29
Results
Characterization of the TRAIL-induced inhibitory effects on K562 cells
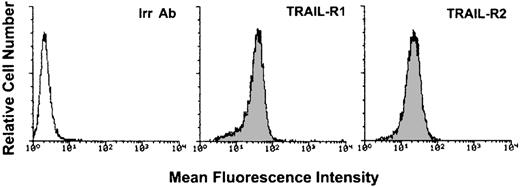
To characterize the biologic activity of TRAIL on AML, we have used, as a model system, the K562 cell line, which derives from a blastic crisis of a chronic myeloid leukemia.30 K562 cells expressed high surface levels of TRAIL-R1 and TRAIL-R2 (Figure1), whereas surface expression of TRAIL-R3 and TRAIL-R4 was not detectable (data not shown). Following treatment of K562 cells with recombinant His-tagged TRAIL, containing the TRAIL protein fused to a tag of 6 histidine residues,24 or with a control His peptide, cell viability was determined by trypan blue dye exclusion and MTT assay; the extent of apoptosis was determined by PI and DAPI staining followed by flow cytometry and fluorescence microscopy analyses, respectively. Addition of recombinant TRAIL, but not of the control His peptide, reduced the total number of viable cells, as determined by trypan blue dye exclusion, in a dose-dependent manner (Figure2A). This effect was associated with a concomitant increase of apoptosis, quantitatively evaluated by flow cytometry after PI staining (Figure 2B). Analysis of K562 nuclei after DAPI staining confirmed that TRAIL-treated cells exhibited morphologic changes characteristic of apoptosis, such as chromatin condensation and formation of micronuclei. Some cells also showed necrotic features, such as a vacuolized cytoplasm and discontinuation of the plasma membrane without chromatin condensation (data not shown).
Flow cytometry evaluation of the surface expression of TRAIL-R1 and TRAIL-R2 in K562 cells.
Shadowed histograms represent cells stained with anti–TRAIL-R1 or anti–TRAIL-R2 antibodies, whereas unshadowed histogram represents control cells, stained with control goat antibodies (Irr Ab). A representative of 4 separate experiments is shown.
Flow cytometry evaluation of the surface expression of TRAIL-R1 and TRAIL-R2 in K562 cells.
Shadowed histograms represent cells stained with anti–TRAIL-R1 or anti–TRAIL-R2 antibodies, whereas unshadowed histogram represents control cells, stained with control goat antibodies (Irr Ab). A representative of 4 separate experiments is shown.
Evaluation of the cytotoxic activity of human recombinant TRAIL and control His peptide on K562.
Dose-dependent effect of TRAIL on cell viability evaluated by trypan blue dye exclusion (A) and on apoptosis quantitatively evaluated by flow cytometry after PI staining (B) are shown. Cells were cultured for 24 hours with the indicated concentrations of TRAIL or with His control peptide (1 μg/mL). Data represent the means ± SDs of 5 independent experiments performed in duplicate. In panel A, data are expressed as percentage of untreated control cells. In panel C, the cell cycle was evaluated by PI DNA staining and flow cytometry. The insets show the percentage of cells with a G1(2n), S, G2/M(4n) DNA content, calculated excluding apoptotic cells (Apo). A representative analysis of 4 separate experiments is shown. Panel D shows the percentage of cells incorporating BrdU, evaluated by flow cytometry, in samples left untreated or treated for 16 hours with TRAIL or His peptide. Data represent the means ± SDs of 3 separate experiments performed in duplicate.
Evaluation of the cytotoxic activity of human recombinant TRAIL and control His peptide on K562.
Dose-dependent effect of TRAIL on cell viability evaluated by trypan blue dye exclusion (A) and on apoptosis quantitatively evaluated by flow cytometry after PI staining (B) are shown. Cells were cultured for 24 hours with the indicated concentrations of TRAIL or with His control peptide (1 μg/mL). Data represent the means ± SDs of 5 independent experiments performed in duplicate. In panel A, data are expressed as percentage of untreated control cells. In panel C, the cell cycle was evaluated by PI DNA staining and flow cytometry. The insets show the percentage of cells with a G1(2n), S, G2/M(4n) DNA content, calculated excluding apoptotic cells (Apo). A representative analysis of 4 separate experiments is shown. Panel D shows the percentage of cells incorporating BrdU, evaluated by flow cytometry, in samples left untreated or treated for 16 hours with TRAIL or His peptide. Data represent the means ± SDs of 3 separate experiments performed in duplicate.
The fact that the drop in total cell number was greater than the increase of apoptosis (43% drop in cell number versus 20% of apoptosis) in TRAIL-treated cultures suggested that the TRAIL-induced cytotoxicity included a cytostatic effect in addition to the cytocidal/apoptotic effect. In fact, cell cycle analysis, performed by PI staining, showed an increase of S-G2/M phases in TRAIL-treated K562 cells (Figure 2C) compared to His peptide–treated control cells. Although small, the TRAIL-mediated increase of the S-G2/M phases of the cell cycle was reproducibly observed in different experiments. To independently confirm that TRAIL affected the S phase of K562 cell cycle, we have performed a BrdU incorporation assay revealed by flow cytometry. As shown in Figure 2D, TRAIL induced a statistically significant (P < .05) increase of BrdU uptake in K562 cells. In time-course experiments, maximal inhibition of cell survival/proliferation and increase of apoptosis occurred at 24 hours of TRAIL treatment (data not shown). Therefore, the next experiments were performed using 1 μg/mL recombinant TRAIL for 24 hours.
Because it has been previously shown that the cytotoxic/cytostatic activity of other death-inducing ligands (DILs), and in particular TNF-α, is mediated, at least in part, by the production of NO,31-34 we next investigated whether recombinant TRAIL was able to induce NO production in K562 cells. The NOS activity was assessed in cell lysates after treatment with TRAIL at different time points. A significant (P < .01) increase in NOS activity was observed starting at 6 hours of TRAIL treatment (Figure3A). On the other hand, neither His peptide nor lipopolysaccharide (LPS, used at concentration up to 1 μg/mL) affected NOS activity, demonstrating that stimulation of NOS was specifically due to TRAIL, and it could not be ascribed to the presence of potential contaminating LPS in the TRAIL preparations. In addition, the supernatant of TRAIL-treated K562 cells contained increasing levels of the NO oxidation products nitrite and nitrate, which represent the stable end-products of NO and accumulate in the cell culture media (Figure 3B).
Stimulation of NOS activity.
Evaluation of the NOS activity (A), nitrite/nitrate production (B), and caspase activity (C) in K562. Cells were treated with TRAIL (1 μg/mL) or with control His peptide for the indicated times. In panels A and C, data are expressed as percentage of His peptide–treated control cells. Data represent the means ± SDs of 4 independent experiments performed in duplicate.
Stimulation of NOS activity.
Evaluation of the NOS activity (A), nitrite/nitrate production (B), and caspase activity (C) in K562. Cells were treated with TRAIL (1 μg/mL) or with control His peptide for the indicated times. In panels A and C, data are expressed as percentage of His peptide–treated control cells. Data represent the means ± SDs of 4 independent experiments performed in duplicate.
In parallel experiments, the activity of the effector caspase subgroup (including caspase 3) and the ICE subgroup (including caspase 1) were assessed after treatment with TRAIL at different time points. In agreement with previous data of other authors (reviewed in Walczak and Krammer16), we found that TRAIL did not activate caspase 1, which cleaves the ICE substrate. On the other hand, TRAIL induced a significant increase (P < .01) of caspase 3 (peak between 3 and 6 hours), an executioner of apoptosis, which cleaves the CPP32 substrate (reviewed in Cohen17) (Figure 3C).
Differential effect of NOS and broad caspase inhibitors on TRAIL-mediated cytotoxic and cytostatic activity on K562 cells
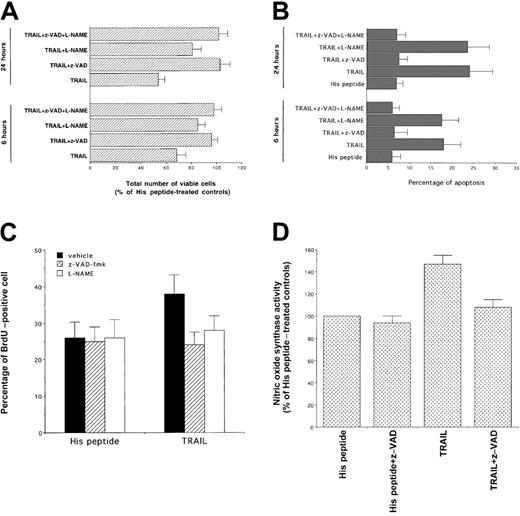
To clarify the role of NO generation and caspase 3 activation in the signaling cascade and cytotoxicity triggered by TRAIL treatment in human leukemic cells, we have used pharmacologic inhibitors that affect specific intracellular pathways. In this set of experiments, cells were preincubated with each inhibitor for 45 minutes, followed by the coincubation of TRAIL for an additional 3 to 24 hours. After addition of TRAIL, the total number of viable cells and the degree of apoptosis were examined. Cell viability was not affected by any of the inhibitors, used at indicated concentrations, in control K562 cells. To elucidate whether the induction of NOS activity plays a role in TRAIL-mediated cytotoxicity, NO production was inhibited by using 200 μM of the NOS inhibitors, L-NAME (Figure4A,B) and NG-monomethyl-l-arginine (data not show). Of note, the presence of NOS inhibitors in TRAIL-treated cells significantly (P < .01) increased the total number of viable cells (Figure 4A), but it did not affect (P > .1) the degree of apoptosis (Figure 4B). The inactive d-isomers of these compounds did not interfere with the cytotoxic/cytostatic action of TRAIL (data not shown). On the other hand, incubation of K562 cells with the broad inhibitor of effector caspases, z-VAD-fmk, totally prevented both TRAIL-mediated reduction in cell number (P < .01) and apoptosis (P < .01) (Figure4A,B). Moreover, both z-VAD-fmk and L-NAME were able to block the cell cycle perturbations induced by TRAIL (Table1) and to significantly (P < .05) revert the TRAIL-mediated increase of BrdU uptake (Figure 4C). These data indicate that the TRAIL-mediated induction of NO has a more prominent cytostatic than cytocidal effect. This effect of NO has been previously demonstrated in primary AML cells.22 23 Moreover, the ability of z-VAD-fmk to completely block both cytostatic and cytotoxic effects of TRAIL suggested that the activation of effector caspases was upstream to the activation of NOS. This hypothesis was confirmed by the ability of z-VAD-fmk to completely (P < .01) abrogate NOS induction by TRAIL (Figure 4D).
Evaluation of the effect of z-VAD-fmk, inhibitor of effector caspases, and of L-NAME, inhibitor of NOS, on the TRAIL-mediated cytotoxicity.
At 6 and 24 hours after TRAIL treatment, viable cells were counted by trypan blue dye exclusion (A, data are expressed as percentage of His peptide–treated controls) and the percentage of apoptosis was quantitatively evaluated by flow cytometry after PI staining (B). Data represent the means ± SDs of 5 independent experiments performed in duplicate. Panel C shows the percentage of cells incorporating BrdU, evaluated by flow cytometry, in samples treated for 16 hours with TRAIL or His peptide in the presence or absence (vehicle) of the indicated inhibitors. In panel D, NOS activity was evaluated in cells treated with TRAIL or His peptide in the absence or presence of z-VAD-fmk. In panels C and D, data represent the means ± SDs of 3 separate experiments performed in duplicate.
Evaluation of the effect of z-VAD-fmk, inhibitor of effector caspases, and of L-NAME, inhibitor of NOS, on the TRAIL-mediated cytotoxicity.
At 6 and 24 hours after TRAIL treatment, viable cells were counted by trypan blue dye exclusion (A, data are expressed as percentage of His peptide–treated controls) and the percentage of apoptosis was quantitatively evaluated by flow cytometry after PI staining (B). Data represent the means ± SDs of 5 independent experiments performed in duplicate. Panel C shows the percentage of cells incorporating BrdU, evaluated by flow cytometry, in samples treated for 16 hours with TRAIL or His peptide in the presence or absence (vehicle) of the indicated inhibitors. In panel D, NOS activity was evaluated in cells treated with TRAIL or His peptide in the absence or presence of z-VAD-fmk. In panels C and D, data represent the means ± SDs of 3 separate experiments performed in duplicate.
Cell cycle analysis performed by flow cytometry after 24 hours in cells treated with TRAIL or His peptide in the absence or presence of inhibitors
| Treatment . | Percentage of cells . | ||
|---|---|---|---|
| G1 . | S . | G2/M . | |
| His peptide | 50 | 29 | 21 |
| His peptide + z-VAD | 49 | 30 | 21 |
| His peptide + L-NAME | 51 | 29 | 20 |
| TRAIL | 41 | 41 | 18 |
| TRAIL + z-VAD | 51 | 29 | 20 |
| TRAIL + L-NAME | 47 | 32 | 21 |
| Treatment . | Percentage of cells . | ||
|---|---|---|---|
| G1 . | S . | G2/M . | |
| His peptide | 50 | 29 | 21 |
| His peptide + z-VAD | 49 | 30 | 21 |
| His peptide + L-NAME | 51 | 29 | 20 |
| TRAIL | 41 | 41 | 18 |
| TRAIL + z-VAD | 51 | 29 | 20 |
| TRAIL + L-NAME | 47 | 32 | 21 |
The percentage of cells with a G1(2n), S, G2/M(4n) DNA content were calculated excluding apoptotic cells. Data represent the means of 3 separate experiments. SDs were comprised within 10% of the means and are omitted for clarity.
Sensitization of K562 cells to TRAIL-mediated cytotoxicity by the NO donor SNP
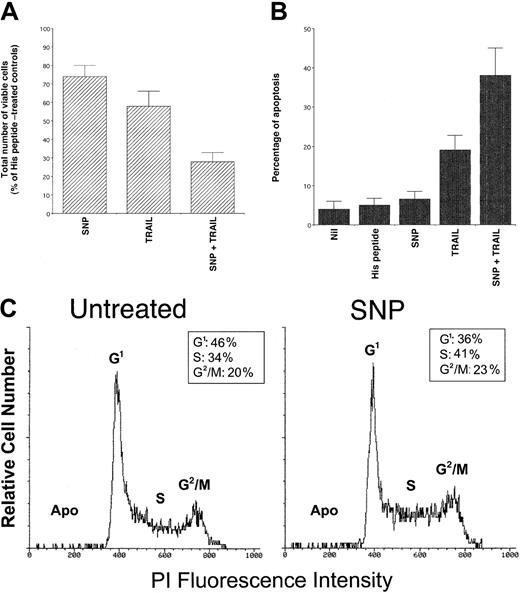
To further clarify the role of NO on TRAIL-induced cytotoxic and cytostatic activity, K562 cells were treated with the NO donor SNP, either alone or in combination with TRAIL. Treatment with SNP (1 mM) alone induced a moderate but significant (P < .01) decrease in the total number of viable cells (Figure5A) and showed no induction of apoptosis above background levels (Figure 5B). Analysis of K562 cell cycle revealed an increased percentage of cells in S-G2/M phases on SNP treatment compared to control cells (Figure 5C). Similarly, SNP induced a significant (P < .05) increase of BrdU uptake with respect to K562 cells left untreated (38 ± 6 versus 26 ± 4, respectively, means ± SDs of 3 separate experiments). These alterations of the cell cycle mirrored those previously observed in TRAIL-treated cells (Figure 2C,D), further indicating that the TRAIL-mediated induction of NO was responsible for the cytostatic activity of TRAIL on leukemic cells.
Evaluation of the cytotoxic/cytostatic activity of SNP (1 mM), used alone or in combination with TRAIL (1 μg/mL) on K562.
After 24 hours of culture, viable cells were counted by trypan blue dye exclusion (A; data are expressed as percentage of His peptide–treated controls) and the percentage of apoptosis was quantitatively evaluated by flow cytometry after PI staining (B). In panels A and B, data represent the means ± SD of 5 independent experiments performed in duplicate. In panel C, the cell cycle was evaluated by PI DNA staining and flow cytometry. The insets show the percentage of cells with a G1(2n), S, G2/M(4n) DNA content, calculated excluding apoptotic cells (Apo). A representative analysis of 4 separate experiments is shown.
Evaluation of the cytotoxic/cytostatic activity of SNP (1 mM), used alone or in combination with TRAIL (1 μg/mL) on K562.
After 24 hours of culture, viable cells were counted by trypan blue dye exclusion (A; data are expressed as percentage of His peptide–treated controls) and the percentage of apoptosis was quantitatively evaluated by flow cytometry after PI staining (B). In panels A and B, data represent the means ± SD of 5 independent experiments performed in duplicate. In panel C, the cell cycle was evaluated by PI DNA staining and flow cytometry. The insets show the percentage of cells with a G1(2n), S, G2/M(4n) DNA content, calculated excluding apoptotic cells (Apo). A representative analysis of 4 separate experiments is shown.
It is also remarkable that, when used in combination with TRAIL, SNP significantly (P < .01) potentiated the TRAIL-mediated cytotoxicity, both in terms of inhibition of total viable cell counts (Figure 5A) and induction of apoptosis (Figure 5B). This indicates that the perturbations of the cell cycle induced by high levels of exogenous NO sensitize K562 cells to the cytotoxic effect of TRAIL. Because it has previously been demonstrated that genotoxic stresses, such as ionizing radiation and chemotherapeutic drugs,35-40increase the cytotoxic activity of TRAIL by up-regulating TRAIL-R1 or TRAIL-R2, the surface expression of TRAIL receptors in K562 cells was examined before and after treatment with SNP. No modifications in the TRAIL-R1 (mean fluorescence intensity 43 versus 45) and TRAIL-R2 (mean fluorescence intensity 24 versus 22) surface expression were noticed between cultures left untreated and those supplemented with SNP, which clearly demonstrates that the ability of SNP to enhance TRAIL cytotoxicity was not due to changes in the expression of surface TRAIL receptors.
Selective block of the cytotoxic activity of TRAIL with or without SNP by caspase 8 but not by caspase 9 inhibitors in K562 cells
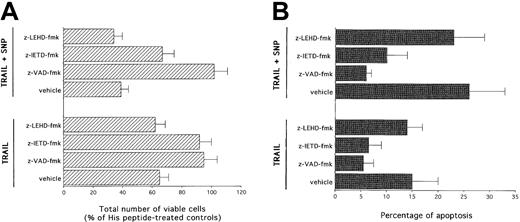
Two main pathways have been described in the induction of apoptosis mediated by other DILs, such as CD95L.41 The type I pathway is mediated by high levels of activation of caspase 8, which directly activates effector caspases. On the other hand, the type II or intrinsic pathway is mediated by mitochondrial dysfunctions, which precede activation of caspase 9 (reviewed in Walczak and Krammer16). Therefore, in the next group of experiments, the cytotoxic activity of TRAIL with or without SNP was evaluated in the presence of selective inhibitors for caspase 8 and caspase 9. As shown in panels A and B of Figure 6, z-VAD-fmk completely abrogated (P < .01) the cytotoxicity mediated by TRAIL used alone or in combination with SNP. z-IETD-fmk, a selective caspase 8 inhibitor, was as efficient as z-VAD-fmk in blocking (P < .01) the TRAIL-mediated cytotoxicity. z-IETD-fmk was also able to significantly (P < .01) counteract the cytotoxicity induced by SNP plus TRAIL, although less efficiently than z-VAD-fmk. On the other hand, z-LEHD-fmk, a selective caspase 9 inhibitor, was unable to prevent the cytotoxicity mediated by either TRAIL or TRAIL plus SNP.
Evaluation of the effect of z-VAD-fmk, z-LEHD-fmk (specific caspase 8 inhibitor), and z-IETD-fmk (specific caspase 9 inhibitor) on TRAIL- and SNP plus TRAIL–mediated cytotoxicity.
After 24 hours of culture, viable cells were counted by trypan blue dye exclusion (A; data are expressed as percentage of His peptide controls) and the percentage of apoptosis was quantitatively evaluated by flow cytometry after PI staining (B). Data represent the means ± SDs of 4 independent experiments performed in duplicate.
Evaluation of the effect of z-VAD-fmk, z-LEHD-fmk (specific caspase 8 inhibitor), and z-IETD-fmk (specific caspase 9 inhibitor) on TRAIL- and SNP plus TRAIL–mediated cytotoxicity.
After 24 hours of culture, viable cells were counted by trypan blue dye exclusion (A; data are expressed as percentage of His peptide controls) and the percentage of apoptosis was quantitatively evaluated by flow cytometry after PI staining (B). Data represent the means ± SDs of 4 independent experiments performed in duplicate.
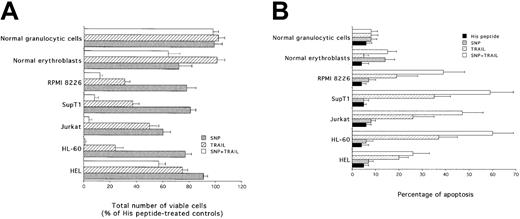
Broad synergistic cytotoxicity of SNP plus TRAIL in leukemic and multiple myeloma cell lines
To ascertain whether the synergistic cytotoxic activity of SNP plus TRAIL was an event confined to K562 cells or whether it represented a phenomenon widespread to different hematologic malignancies, the combination of SNP plus TRAIL was evaluated on additional myeloid (HEL, HL-60), lymphoblastoid (Jurkat, SupT1), and multiple myeloma (RPMI 8226) cell lines, as well as on primary normal CD34-derived erythroblasts and granulocytic cells. SNP plus TRAIL induced a variable but statistically significant (P < .01) synergistic inhibition in all examined leukemic cell lines, both in terms of total number of viable cells (Figure7A) and in terms of apoptosis (Figure7B). In agreement with previous findings of our group and others,9 10 TRAIL alone did not show any cytotoxicity on primary normal mature erythroblasts and granulocytic cells, whereas SNP alone showed toxicity (P < .01) on erythroblasts, but not on granulocytic cells (Figure 7A,B). However, no synergistic cytotoxicity was observed on either normal erythroblasts or granulocytic cells in the presence of SNP plus TRAIL (Figure 7A,B). Notably, the degree of apoptosis observed in mature erythroblasts was significantly (P < .01) lower than that observed in all leukemic cell lines examined (Figure 7B). Cell cycle analysis showed that both TRAIL and SNP induced an accumulation in the S-G2/M phases of the cell cycle in all the cell lines examined (Table 2). The combination of TRAIL plus SNP did not further induce significant cell cycle modifications with respect to the 2 agonists used alone, probably as a consequence of the strong induction of apoptosis by the combination of the 2 agonists (Figure 7B). Among primary normal cells, SNP, but not TRAIL, promoted the accumulation in S-G2/M phases only in normal erythroblasts.
Evaluation of the cytotoxicity of TRAIL, SNP, and SNP plus TRAIL on HEL, HL-60, Jurkat, SupT1, RPMI 8226, normal erythroblasts, and normal granulocytic cells.
After 24 hours of culture, viable cells were counted by trypan blue dye exclusion (A; data are expressed as percentage of His peptide controls) and the percentage of apoptosis was quantitatively evaluated by flow cytometry after PI staining (B). Data represent the means ± SDs of 4 independent experiments performed in duplicate.
Evaluation of the cytotoxicity of TRAIL, SNP, and SNP plus TRAIL on HEL, HL-60, Jurkat, SupT1, RPMI 8226, normal erythroblasts, and normal granulocytic cells.
After 24 hours of culture, viable cells were counted by trypan blue dye exclusion (A; data are expressed as percentage of His peptide controls) and the percentage of apoptosis was quantitatively evaluated by flow cytometry after PI staining (B). Data represent the means ± SDs of 4 independent experiments performed in duplicate.
Cell cycle analysis performed by flow cytometry after 24 hours of treatment with SNP, TRAIL, and SNP plus TRAIL
| Cells and treatment . | Percentage of cells . | ||
|---|---|---|---|
| G1 . | S . | G2/M . | |
| HEL | |||
| Control | 41 | 31 | 28 |
| SNP | 33 | 39 | 28 |
| TRAIL | 36 | 37 | 26 |
| TRAIL + SNP | 40 | 40 | 20 |
| HL-60 | |||
| Control | 66 | 21 | 13 |
| SNP | 45 | 36 | 19 |
| TRAIL | 50 | 39 | 11 |
| TRAIL + SNP | 56 | 32 | 12 |
| Jurkat | |||
| Control | 63 | 27 | 10 |
| SNP | 50 | 32 | 18 |
| TRAIL | 54 | 33 | 13 |
| TRAIL + SNP | 50 | 41 | 9 |
| SupT1 | |||
| Control | 73 | 16 | 11 |
| SNP | 60 | 23 | 17 |
| TRAIL | 54 | 33 | 13 |
| TRAIL + SNP | 58 | 33 | 9 |
| Normal erythroblast | |||
| Control | 38 | 41 | 21 |
| SNP | 30 | 50 | 20 |
| TRAIL | 37 | 43 | 20 |
| TRAIL + SNP | 33 | 48 | 19 |
| Normal granulocytic cells | |||
| Control | 73 | 21 | 6 |
| SNP | 72 | 22 | 6 |
| TRAIL | 72 | 23 | 5 |
| TRAIL + SNP | 71 | 23 | 6 |
| Cells and treatment . | Percentage of cells . | ||
|---|---|---|---|
| G1 . | S . | G2/M . | |
| HEL | |||
| Control | 41 | 31 | 28 |
| SNP | 33 | 39 | 28 |
| TRAIL | 36 | 37 | 26 |
| TRAIL + SNP | 40 | 40 | 20 |
| HL-60 | |||
| Control | 66 | 21 | 13 |
| SNP | 45 | 36 | 19 |
| TRAIL | 50 | 39 | 11 |
| TRAIL + SNP | 56 | 32 | 12 |
| Jurkat | |||
| Control | 63 | 27 | 10 |
| SNP | 50 | 32 | 18 |
| TRAIL | 54 | 33 | 13 |
| TRAIL + SNP | 50 | 41 | 9 |
| SupT1 | |||
| Control | 73 | 16 | 11 |
| SNP | 60 | 23 | 17 |
| TRAIL | 54 | 33 | 13 |
| TRAIL + SNP | 58 | 33 | 9 |
| Normal erythroblast | |||
| Control | 38 | 41 | 21 |
| SNP | 30 | 50 | 20 |
| TRAIL | 37 | 43 | 20 |
| TRAIL + SNP | 33 | 48 | 19 |
| Normal granulocytic cells | |||
| Control | 73 | 21 | 6 |
| SNP | 72 | 22 | 6 |
| TRAIL | 72 | 23 | 5 |
| TRAIL + SNP | 71 | 23 | 6 |
The percentage of cells with a G1(2n), S, G2/M(4n) DNA content, were calculated after removal of the apoptotic cells. Data represent the means of 3 separate experiments. SDs were comprised within 10% of the means and are omitted for clarity.
Profound inhibition of the colony-forming ability of K562 and primary AML blasts by TRAIL with or without SNP
In the last group of experiments, we sought to investigate whether TRAIL, used alone or in combination with SNP, affected the colony-forming ability of the K562 cell line, primary leukemic blasts purified from the peripheral blood of 3 patients diagnosed with AML, and primary normal hematopoietic progenitor cells. As shown in Table3, TRAIL alone induced a strong (P < .01) inhibitory activity on the colony-forming ability of K562 cells. Of note, the inhibitory activity of TRAIL alone on the clonogenic activity of K562 cells (80% inhibition) was significantly (P < .01) greater than that previously observed in the short-term assay performed in liquid culture (46% inhibition, Figure 2). This suggests that the stem/progenitor leukemic cells are more susceptible to the inhibitory activity of TRAIL with respect to more differentiated leukemic blasts, which represent the bulk population of K562 cell line. Interestingly, SNP also significantly (P < .05) reduced the colony-forming ability of both leukemic and normal cells, clearly indicating that the cytostatic effect of either TRAIL or SNP was a major mechanism for the inhibition of the clonogenic activity. Moreover, the combination of TRAIL plus SNP almost completely suppressed the ability of K562 to generate colonies in semisolid cultures. Remarkably, primary AML blasts were (P < .01) inhibited by TRAIL, and SNP significantly (P < .01) potentiated the cytotoxic activity of TRAIL also on these primary leukemic blasts (Table 3), which might be of clinical relevance. On the other hand, normal hematopoietic progenitors showed a partial sensitivity to the inhibitory activity of SNP and TRAIL, used alone, but they were significantly (P < .01) less susceptible than leukemic cells to the cytotoxicity of the combination of TRAIL with or without SNP. As previously observed for mature cells generated in liquid culture (Figure 7A,B), granulocytic progenitors were less affected than BFU-Es by the combination of TRAIL plus SNP.
Effect of SNP, TRAIL, and SNP plus TRAIL on the colony-forming ability of K562 cells, primary AML blasts, and primary normal BFU-Es and GM-CFUs
| . | Control . | SNP . | TRAIL . | SNP + TRAIL . |
|---|---|---|---|---|
| K562 | 103 ± 13 | 53 ± 7 | 20 ± 5 | 5 ± 3 |
| Sample 1 (AML M2) | 55 | 43 | 25 | 2 |
| Sample 2 (AML M3) | 38 | 29 | 18 | 0 |
| Sample 3 (AML M3) | 49 | 36 | 24 | 11 |
| Normal BFU-E | 107 ± 19 | 66 ± 8 | 75 ± 16 | 41 ± 8 |
| Normal GM-CFU | 146 ± 28 | 78 ± 13 | 139 ± 24 | 79 ± 13 |
| . | Control . | SNP . | TRAIL . | SNP + TRAIL . |
|---|---|---|---|---|
| K562 | 103 ± 13 | 53 ± 7 | 20 ± 5 | 5 ± 3 |
| Sample 1 (AML M2) | 55 | 43 | 25 | 2 |
| Sample 2 (AML M3) | 38 | 29 | 18 | 0 |
| Sample 3 (AML M3) | 49 | 36 | 24 | 11 |
| Normal BFU-E | 107 ± 19 | 66 ± 8 | 75 ± 16 | 41 ± 8 |
| Normal GM-CFU | 146 ± 28 | 78 ± 13 | 139 ± 24 | 79 ± 13 |
Data are expressed as number of colonies, and represent the results of individual experiments (for the primary AML samples) or the means ± SDs of 3 to 5 separate experiments (for K562, BFUE-E, and GM-CFU). In each experiment cells were seeded in triplicate.
Discussion
We have here demonstrated for the first time that the antileukemic activity of TRAIL comprises the induction of both cytostatic and cytotoxic effects. More importantly, our data indicate that the simultaneous activation of both NOS and effector caspases is required for induction of TRAIL-mediated effects and suggest a synergism between caspases and NOS in the antileukemic activity of TRAIL. Interestingly, a distinction was noticed between the TRAIL-mediated induction of apoptosis, which was blocked only by z-VAD-fmk, a broad pharmacologic inhibitor of effector caspases and the TRAIL-mediated cytostatic activity, which was blocked by both z-VAD-fmk and L-NAME, a pharmacologic inhibitor of NOS. In experiments performed with the NO donor SNP, we could also demonstrate that NO not only mediated the cell cycle abnormalities, but also sensitized leukemic cells to the TRAIL-mediated apoptosis.
Previous studies have shown that TNF-α, the prototype of the TNF family of cytokines, activates iNOS (NOS-2) and induces the generation of NO-radical (NO·) froml-arginine.31-34 Induction of iNOS depends on activation of the death domain of TNF-R1 and it has been shown to require additional stimulation of an adjacent part of the intracellular receptor, the so-called NO domain.31 Although TNF-α is known to interact with 2 high-affinity receptors (TNF-R1 and TNF-R2),3 an extreme complexity characterizes the expression and function of TRAIL receptors (reviewed in Ashkenazi and Dixit8). In fact, at least 5 TRAIL receptors have been described so far. TRAIL-R1 (DR4) and TRAIL-R2 (DR5) transduce apoptotic signals following binding of TRAIL, whereas TRAIL-R3 (DcR1) and TRAIL-R4 (DcR2) as well as osteoprotegerin are homologous to DR4 and DR5 in their cysteine-rich extracellular domain but lack intracellular death domain and apoptosis-inducing capability. TRAIL-R1 and TRAIL-R2 show a comparable expression in normal and malignant cells, whereas TRAIL-R3 and TRAIL-R4 often show a higher expression in normal tissues.8 Consistently, we found that K562 leukemic cells express TRAIL-R1 and TRAIL-R2, but not TRAIL-R3 and TRAIL-R4. Although we have not addressed the intracellular signal transduction pathway by which TRAIL activates NOS, it has been previously shown that binding of TRAIL to TRAIL-R1, TRAIL-R2, and TRAIL-R4 induces the activation of the transcription factor NF-kB,42 which in turn is a potent activator of iNOS.43
The role of NOS activation as a mediator of cytostatic/cytotoxic effects is still a topic of discussion. Although several studies describe a proapoptotic action of high levels of NO,33,34,44,45 lower levels of NO have even been demonstrated to exert a protective function in leukemic46or melanoma47 cells and to inhibit effector caspases by S-nitrosylation.48 It has been demonstrated that the cytotoxic effect of high levels of NO depends on (1) the cell type considered, (2) the interaction with other reactive oxygen species,49,50 (3) the nitrosylation of thiol groups and iron-sulfur clusters followed by inactivation of critical cellular enzymes,50 and (4) the inhibition of mitochondrial respiration and DNA synthesis.32,51 Moreover, consistent with our data showing that z-VAD-fmk caspase inhibitor blocked both the inhibitory activity of TRAIL with or without SNP and completely blocked the TRAIL-mediated induction of NOS, it has been demonstrated that caspases mediate NO cytotoxicity in lymphoblastoid leukemic cells.44
In analogy to CD95L,41 the response to TRAIL is specific for cell type and might be characterized by 2 distinct cell death pathways. It has been shown that the induction of apoptosis in the type I pathway is accompanied by the activation of large amounts of caspase 8 followed by the rapid cleavage of caspase 3 prior to loss of mitochondria transmembrane potential (Δψm). In contrast, in the type II intrinsic pathway of apoptosis, low concentrations of caspase 8, insufficient to allow an effective downstream activation of caspase 3, induce cleavage of the Bcl-2 family member Bid and loss of Δψm. This, in turn, induces the release of cytochrome C and procaspase 9 from mitochondria, its activation in the cytosol, and the activation of caspase 3.52 We could demonstrate that, in the leukemic cell lines examined, z-IETD-fmk, a selective pharmacologic inhibitor of caspase 8, reproduced the ability of z-VAD-fmk, a broad inhibitor of effector caspases, to counteract the cytotoxicity mediated by TRAIL with or without SNP. On the other hand, z-LEHD-fmk, a selective pharmacologic inhibitor of caspase 9, was unable to counteract the cytotoxicity mediated by TRAIL with or without SNP. These findings clearly indicate that the type II intrinsic death pathway does not appear to play a prominent role in either TRAIL or TRAIL plus SNP cytotoxicity on the examined leukemic cell lines. Our findings are consistent with previous data, showing that TRAIL-induced apoptosis was not blocked by Bcl-2, an inhibitor of the intrinsic death pathway,16,41,52 in lymphoblastoid and multiple myeloma cell lines.53-55 On the other hand, in epithelial cancer cells56-59 or in normal hepatocytes,60 TRAIL preferentially induces the activation of the intrinsic pathway through the caspase 8–mediated cleavage of Bid with subsequent loss of the mitochondrial Δψm and release and activation of caspase 9. In this context, it is noteworthy that most chemotherapeutic drugs trigger the intrinsic, type II, apoptosis pathway by inducing loss of Δψm (reviewed in Walczak and Krammer16).
Because we have here shown that TRAIL with or without SNP preferentially triggers the type I pathway of apoptosis in leukemic cells, TRAIL with or without SNP may substantially enhance the antileukemic activity of conventional radiotherapy and chemotherapy also in Bcl-2 overexpressing hematologic malignancies.61In this respect, the observation of a lack of cytotoxicity of TRAIL toward normal cells and tissues has been extended successfully to mice62 and nonhuman primates.63 Although other authors have shown that TRAIL alone does not exert a high cytotoxic activity against primary leukemic cells in short-term assays,6,7 we could here demonstrate that clonogenic leukemic stem/progenitor cells are significantly more susceptible to TRAIL cytotoxicity with respect to more differentiated leukemic blasts. Moreover, the combination of SNP with TRAIL almost completely abolished the colony-forming ability of both K562 cells and primary AML blasts. These findings suggest that TRAIL might have a stronger antileukemic activity than previously thought,6 7especially if used in combination with NO donors.
In conclusion, the presented results suggest that TRAIL stimulates a 2-sided effector mechanism, both parts of which are required for execution of the apoptotic death program and the cell cycle arrest in both myeloid and lymphoblastoid cell lines. These data also suggest the possibility of combining TRAIL with conventional or new drugs (ie, NO donors) in a regimen that would optimize the antileukemic activity against neoplastic cells that lack a functional p53 wild-type64 or overexpressBcl-253-55 orMDR6 genes.
Supported by local funds from the Universities of Ferrara and Trieste, by Associazione Italiana per la Ricera sul Cancro funds (G.Z.) and by the “Excellence project” of the University of Chieti. A.G. is supported by a Fondazione Italiana per la Ricera sul Cancro fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paola Secchiero, Department of Morphology and Embryology, Human Anatomy Section, University of Ferrara, Via Fossato di Mortara 66, 44100 Ferrara, Italy; e-mail:secchier@mail.umbi.umd.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal