Rapid proliferation of atypical megakaryoblasts is a characteristic of megakaryoblastic leukemia. Cells from patients with this disorder and cell lines established from this type of leukemia showed the presence of gelsolin but the absence of scinderin expression, 2 filamentous actin-severing proteins present in normal megakaryocytes and platelets. Vector-mediated expression of scinderin in the megakaryoblastic cell line MEG-01 induced a decrease in both F-actin and gelsolin. This was accompanied by increased Rac2 expression and by activation of the PAK/MEKK.SEK/JNK/c-jun, c-fos transduction pathway. The Raf/MEK/ERK pathway was also activated in these cells. Transduction pathway activation was followed by cell differentiation, polyploidization, maturation, and apoptosis with release of platelet-like particles. Particles expressed surface CD41a antigen (glycoprotein IIb/IIIa or fibrinogen receptor), had dense bodies, high-affinity serotonin transport, and circular array of microtubules. Treatment of particles with thrombin induced serotonin release and aggregation that was blocked by CD41a antibodies. PAC-1 antibodies also blocked aggregation. Exposure of cells to PD98059, a blocker of MEK, inhibited antigen CD41a expression, increases in cell volume, and number of protoplasmic extensions. Cell proliferation and cell ability to form tumors in nude mice were also inhibited by the expression of scinderin. MEG-01 cells expressing scinderin had the same fate in vivo as in culture. Thus, when injected into nude mice, they entered apoptosis and released platelet-like particles. The lack of scinderin expression in megakaryoblastic leukemia cells seems to be responsible for their inability to enter into differentiation and maturation pathways characteristic of their normal counterparts.
Introduction
Acute megakaryoblastic leukemia is a recognized disorder characterized by rapid proliferation of atypical megakaryocytes and their precursor cells. This disease is often associated with myelofibrosis.1 Cell lines have been established with cells from patients with this disease,2,3and these cells have shown some degree of differentiation with phorbol ester treatment.4 Megakaryopoiesis is a complex process that involves the proliferation of committed precursor cells and their differentiation with nuclear polyploidization, leading to platelet formation.5-7 This process is thought to be regulated by a lineage-specific humoral factor called thrombopoietin.8After differentiation, the fate of megakaryocytes is apoptosis, with cell fragmentation resulting in cytoplasmic areas released as newly formed platelets.9
It has been suggested that cytoskeleton elements play an important role in polyploidization and platelet formation.10 Indeed, increasing F-actin depolymerization increases the number of cells entering endomitosis.11Actin microfilament dynamics is controlled by several proteins able either to sequester actin monomers or to control actin filament length. Among the last category, gelsolin and scinderin are 2 Ca++-dependent, filamentous actin-severing proteins found in normal megakaryocytes and platelets.12,13 Scinderin was discovered in chromaffin cells, and its gene was cloned in our laboratory.14-16 This protein, which is present in all secretory cells,17 controls dynamic changes observed in cortical F-actin during secretion.18-20 Megakaryoblastic leukemia cells express gelsolin, but they do not express scinderin. Therefore, the lack of expression of scinderin in these cells and, consequently, the lack of proper F-actin dynamics might be related to the cells' inability to enter into differentiation and maturation pathways leading to platelet formation and release.
Experiments described here involve transfection of megakaryoblastic cell lines with vectors carrying a scinderin cDNA insert. The expression of scinderin resulted in remarkable changes in morphology, showing cells with the appearance of mature megakaryocytes. This was accompanied by differentiation, maturation, polyploidization, and apoptosis with the release of platelet-like particles. Moreover—and most important—scinderin expression inhibited cell proliferation and tumorigenesis. Changes brought about by scinderin expression were mediated through the activation of Rac/PAK/MEKK.SEK/JNK/c-jun, c-fos, and Raf/MEK/ERK pathways.
Materials and methods
Cell cultures
Suspension cultures.
Cell lines (K-562, HEL, HL-60, and MEG-01) obtained from the American Tissue Culture Collection (Manassas, VA) and cell line NS-MEG (a gift from Dr R. Tsuyuoka, Kyoto University, Japan) were transfected with plasmids and cultured in RPMI 1640 medium containing 10% fetal calf serum (FCS) in the presence of 0.8 nM G-418 (geneticin) at 37°C in a 5% CO2 atmosphere. Half the media was replaced either every 3 days or weekly according to the protocol used. Cells were then maintained in culture for up to 4 weeks after the removal of G-418. The day in culture was always assigned from the day of G-418 withdrawal.
Semisolid cultures.
Whole fresh human bone marrow from healthy donors was purchased from All Cells (Foster City, CA). Bone marrow was treated with ice-cold 0.8% NH4Cl and 10 μM EDTA and was washed twice in phosphate-buffered saline (PBS) containing 2% FCS. An enriched population of CD34+/CD38+ cells was obtained by immunomagnetic labeling the bone marrow with human progenitor enrichment cocktail (Stem Cell Technologies, Vancouver, BC, Canada). This was followed by magnetic cell separation by gravity using a Stem Sep System (Stem Cell Technologies). Twenty thousand CD34+/CD38+ cells per chamber were seeded on a collagen-based semisolid culture substrate21 in serum-free Iscove modified Dulbecco medium provided with a Megacult-C kit for human megakaryocytic progenitor assays (Stem Cell Technologies). The medium also contained recombinant human (rh) thrombopoietin (TPO) (50 ng/mL), rh interleukin (IL)-6 (10 ng/mL), and rhIL-3 (10 ng/mL) (Stem Cell Technologies). Cells were incubated at 37°C in a 5% CO2 atmosphere. Megakaryocyte colony-forming units (CFU-MK) were characterized, and their number was determined as previously described.22
Preparation of vectors
A pGEX-4T2 plasmid containing scinderin cDNA, previously prepared in our laboratory, was digested with restriction enzymesBamHI and NotI to yield a 2.9-kb fragment corresponding to the entire scinderin sequence (2145 bp). The fragment was subcloned in a pcDNA3 vector (Invitrogen, San Diego, CA). Orientation and insertion sites of scinderin cDNA (Sc-cDNA) in the pcDNA3 vector were checked by sequencing both ends of the constructs. A preparative batch of high-quality cDNA was obtained from 250 mL bacterial culture (Escherichia coli JM105 strain) and was purified through QIAgen columns (QIAgen Canada, Mississauga, ON).
Generation of clones
Cell lines (K-562, HEL, NS-MEG, and MEG-01) were transfected with control (no insert) vectors (pc-DNA3) or Sc-cDNA containing vectors (pc-DNA3-Sc) using Lipofectamine following the manufacturer's protocol (Life Technologies, Burlington, ON, Canada). After transfection, cells were incubated in T75 flasks and were grown in selection medium (RPMI 1640, 10% FCS, 0.8 nM G-418 geneticin) until cells in mock transfections died. Clones were analyzed for scinderin expression by immunoblotting and immunocytochemistry. The MEG-01 cell line produced the best results. After dilution cloning and 3 passes, 16 MEG-01 cell clones showing 100% scinderin-positive cells were kept and were used in the experiments.
Immunocytochemistry and fluorescence microscopy
Cells were cytospun onto glass slides, fixed with 3.7% formaldehyde, and permeabilized with acetone.23 F-actin was detected with rhodamine-phalloidin (Molecular Probes, Eugene, OR), a probe for filamentous actin,18 and scinderin was detected with a polyclonal antibody (1:500 dilution) previously raised in our laboratory.18 In some experiments, monoclonal antibodies against α-tubulin (1:200 dilution) (Invitrogen, Carlsbad, CA) and CD41a (1:100 dilution) (Biodesign, Kennebunk, ME) were used. Secondary antibodies labeled with either fluorescein isothiocyanate or rhodamine were used, and preparations were washed and mounted as previously described.23 Slides were observed under incident light in a Leitz Ortholux fluorescence microscope (Leitz Canada, Montreal, Quebec), photographs were taken with a Sony digital camera (Sony Canada, Toronto, Ontario), and images were saved using a Northern Eclipse software (Empix, Mississauga, ON, Canada). Images were digitally imported into Adobe Photoshop software for further analysis and then printed on an Epson Stylus Photo printer (Epson American, Long Beach, CA). Quantitative analysis of rhodamine phalloidin fluorescence (F-actin) was performed using a Hamamatsu Photonic Argus 50/CL image processor coupled to a TV3M Zeiss video camera as previously described.24 Cell apoptosis was measured counting the number of fluorescent nuclei after the TUNEL reaction according to the manufacturer's guidelines (Boehringer Mannheim, Indianapolis, IN), and dead cell numbers were determined with 1% trypan blue. Cell volumes were calculated from cell diameters measured from Wright-Giemsa–stained preparations.
Electrophoresis and immunoblotting
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed as previously described,25 and proteins were electrotransferred onto nitrocellulose membranes. These were blocked with 5% low-fat milk in PBS and then incubated with antibodies according to the protocols. The following antibodies were used: mouse anti–α tubulin, mouse anti–gelsolin, and rabbit anti–actin (Sigma Canada, Oakville, ON); mouse anti c-fosand mouse anti–ERK1 (Pharmingen Canada, Mississauga, ON); rabbit anti–c-jun, rabbit anti–JNK, and rabbit anti–pJNK (New England Biolabs, Beverly, MA); rabbit anti–Rac2, rabbit anti–Cdc42, and mouse anti–RhoA (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti–Ras (Oncogene Research Products, Cambridge, MA), and rabbit anti–PAK (Upstate Biotechnology, Lake Placid, NY). Membranes were washed and incubated with the corresponding secondary antibody labeled with horseradish peroxidase and then with enhanced chemiluminescence (ECL) detection cocktail (Amersham, Oakville, ON, Canada) for 60 seconds. Membranes were exposed to Hyperfilm-ECL for different periods of time, and the intensity of fluorogram bands was measured using Scion Image Beta-3b software (Scion, Frederick, MD). Areas under peaks were integrated using the same program, and results were expressed in arbitrary units as ratios to tubulin band intensity (gel-loading control).
Isolation of platelet-like particles and techniques used for their characterization
Scinderin expressing MEG-01 clones were cultured until cells entered apoptosis and released cytoplasmic particles (19- to 22-day cultures). Preparations were centrifuged at 150g for 15 minutes, and sediments were discarded and centrifuged again at 750g for 15 minutes. Supernatants thus obtained were centrifuged at 1600g for 15 minutes, and sediments containing platelet-like particles were resuspended in culture medium. For serotonin uptake and release studies, particles were labeled by incubation at 37°C with 0.6 nmol [3H]5-HT/mL as described elsewhere.26 [3H]5-HT uptake, its inhibition by 6 nM fluoxetine, or its release in response to 1 U thrombin/mL was measured as previously described.26 Uptake of serotonin into MEG-01 clones was similarly measured. Platelet-like particles were washed once in whole plasma, and aggregation in response to thrombin either in the absence or the presence of CD41a antibodies was measured in a dual-chamber aggregometer (Chronolog, Havertown, PA) as previously described.26 PAC-1 antibody was tested by the pre-incubation of particles for 5 minutes in the presence of 1 U thrombin/mL and 40 μg PAC-1/mL. Aggregation was started by the addition of fibrinogen (100 μg/mL) and CaCl2 (100 μM) as described elsewhere.27 Electron microscopy of the particles was performed by the incubation of particles for 120 minutes with 1 mM serotonin, followed by fixation for 3 hours in 4% glutaraldehyde.
Flow cytometry
Cultured cells fixed in 80% ethanol were incubated for 1 hour at 4°C with 1 μg propidium iodide and 200 μg RNase/mL PBS containing 1% Tween 20. Samples were analyzed on a Coulter Epics-Atra flow cytometer (Coulter, Miami, FL) using Expo 2 software.
Incorporation of thymidine
Cells cultured for 8 days were incubated for 60 minutes with [3H]-thymidine (0.74 MBq/mL), and the incorporation of thymidine was measured as described elsewhere.28
Bone marrow of patients
Bone marrow samples were kindly provided by Dr A. Zipursky (Hospital for Sick Children Research Institute, University of Toronto). These were from 3 patients (patients 1, 2, and 3) with M7 megakaryoblastic leukemia. Two patients (patients 2 and 3) had the diagnosis of Down syndrome.
Animals
Balb/c nude mice were obtained from Charles River Canada (St Constant, Quebec City), housed at 26°C to 28°C in sterile polycarbonate micro-isolators, and fed with 18% Charles River autoclavable Agway rodent chow and acidified–autoclaved water ad libitum. After acclimatization for 5 days, each mouse was injected once in the abdominal flank subcutaneously with 100 μL saline containing 107 cells. Tumor growth was determined by measuring the smallest and the largest tumor diameters with a caliber, and volumes were calculated according to standard procedures. Animals with large tumors were killed according to institutional animal care policies.
Statistical analysis
Data were analyzed by t test using Slide Write Software (Advanced Graphics Software, Carlsbad, CA).
Results
Expression of scinderin in cells of the megakaryocyte lineage and its absence from leukemia cells and leukemia cell lines
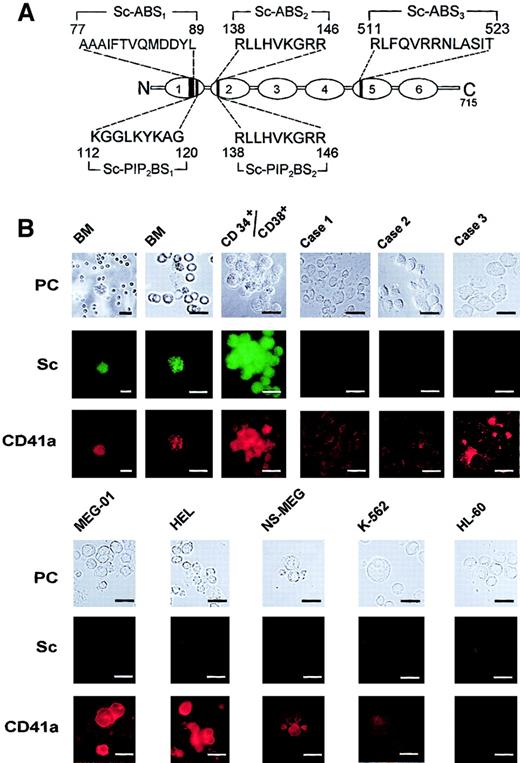
Scinderin, a Ca++-dependent actin-severing protein (Figure 1A), was found to be expressed in human bone marrow cells that also expressed glycoprotein IIb/IIIa3,29 or antigen CD41a3,30 (Figure1B), a platelet marker expressed in cells of megakaryocyte lineage.30 CD34+ cells were also isolated from human bone marrow and were cultured in the presence of TPO, IL-3, and IL-6 to induce the development of megakaryocytic lineage progenitors. Under these conditions, cells formed colonies (CFU-MK) that were tested every other day for scinderin and CD41a expression with corresponding antibodies. Cells in the CFU-MK were found to express both antigens after 11 to 12 days in culture (Figure 1B). Moreover, blasts in bone marrow samples from 3 patients (patients 1, 2, and 3) with acute megakaryoblastic leukemia (M7) expressed low levels of antigen CD41a, but scinderin was undetected (Figure 1B). However, a few cells in these M7 bone marrow preparations showed hyperlobulated nuclei, CD41a staining stronger that that observed in the blasts, and low levels of scinderin staining. These could be either normal megakaryocyte lineage cells or atypical leukemia megakaryoblasts showing some degree of differentiation. The lack of expression of scinderin was also observed in cell lines MEG-01, NS-MEG, HEL, K562, and HL-60 (Figures1B,2B). All cells lines expressed antigen CD41a with the exception of cell line HL-60 (Figure 1B), which was established from a patient with acute promyelocytic leukemia.31
Expression of antigen CD41a and scinderin in normal human bone marrow cells, cell lines, and cells from patients with acute megakaryoblastic leukemia.
(A) Schematic representation of scinderin (Sc) domains 1 to 6 shows amino acid sequences corresponding to the 3 actin-binding sites (Sc-ABS1, Sc-ABS2, and Sc-ABS3) and to the 2 PIP2-binding sites (Sc-PIP2BS1 and Sc-PIP2BS2). (B) Two normal human bone marrow (BM) preparations were treated with NH4Cl and EDTA, as indicated in “Materials and methods,” and they were cytospun onto glass slides. An enriched preparation of CD34+/CD38+ cells was isolated from human bone marrow and cultured in the presence of TPO, IL-3, and IL-6, as indicated in “Materials and methods.” A colony (CFU-MK) formed after 12 days in culture is shown. BM samples from 3 patients (patients 1, 2, and 3) with M7 acute megakaryoblastic leukemia were cytospun onto glass slides. Two of the samples were from patients with Down syndrome (patients 2 and 3). Patients 1 and 2 had low levels of CD41a staining. Patient 3 had much higher levels of CD41a staining and cells that appeared to be more mature than those in the other 2 patients. Cell lines MEG-01, HEL, NS-MEG, K562, and HL-60 were also grown in culture as described in “Materials and methods,” and cell samples from the cultures were cytospun onto glass slides. All preparations were fixed and double stained with antibodies against scinderin and CD41a, as indicated in “Materials and methods.” PC, phase-contrast microscopy. Horizontal bars, 15 μm.
Expression of antigen CD41a and scinderin in normal human bone marrow cells, cell lines, and cells from patients with acute megakaryoblastic leukemia.
(A) Schematic representation of scinderin (Sc) domains 1 to 6 shows amino acid sequences corresponding to the 3 actin-binding sites (Sc-ABS1, Sc-ABS2, and Sc-ABS3) and to the 2 PIP2-binding sites (Sc-PIP2BS1 and Sc-PIP2BS2). (B) Two normal human bone marrow (BM) preparations were treated with NH4Cl and EDTA, as indicated in “Materials and methods,” and they were cytospun onto glass slides. An enriched preparation of CD34+/CD38+ cells was isolated from human bone marrow and cultured in the presence of TPO, IL-3, and IL-6, as indicated in “Materials and methods.” A colony (CFU-MK) formed after 12 days in culture is shown. BM samples from 3 patients (patients 1, 2, and 3) with M7 acute megakaryoblastic leukemia were cytospun onto glass slides. Two of the samples were from patients with Down syndrome (patients 2 and 3). Patients 1 and 2 had low levels of CD41a staining. Patient 3 had much higher levels of CD41a staining and cells that appeared to be more mature than those in the other 2 patients. Cell lines MEG-01, HEL, NS-MEG, K562, and HL-60 were also grown in culture as described in “Materials and methods,” and cell samples from the cultures were cytospun onto glass slides. All preparations were fixed and double stained with antibodies against scinderin and CD41a, as indicated in “Materials and methods.” PC, phase-contrast microscopy. Horizontal bars, 15 μm.
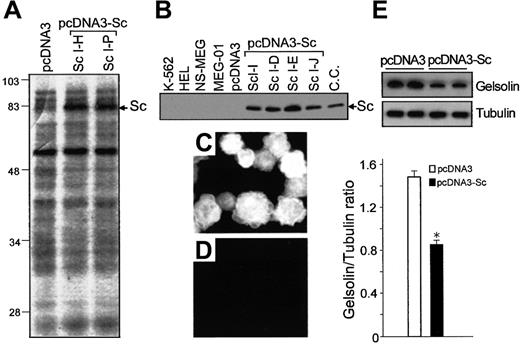
Expression of scinderin and gelsolin in cell lines.
MEG-01 cells were transfected with either pcDNA3 vectors (control) or the vectors carrying full-length scinderin (Sc) cDNA (pcDNA3-Sc) inserts, as indicated in “Materials and methods.” (A) Coomassie blue-stained SDS-PAGE gel shows Sc protein bands (Sc) for 2 Sc-expressing clones. The band is not seen in the gel corresponding to cells transfected with the pcDNA3 vector (left lane). (B) Western blot performed with a polyclonal antibody raised against recombinant Sc. Lanes from left to right: cell lines K562, HEL, NS-MEG, MEG-01 (wild-type) cells, cells transfected with pcDNA3, 4 Sc-expressing clones, and chromaffin cells (positive control). Immunostaining with Sc antibody of cells transfected with pcDNA3-Sc (C) and pcDNA3 (D). (E) Western blot with a monoclonal gelsolin antibody (top) and levels of gelsolin expression (bottom) in MEG-01 cells transfected with either pcDNA3 or pcDNA3-Sc. Tubulin levels detected by a polyclonal antibody were used as control for gel loading. Bars represent mean ± SEM from 4 different experiments (*P < .001).
Expression of scinderin and gelsolin in cell lines.
MEG-01 cells were transfected with either pcDNA3 vectors (control) or the vectors carrying full-length scinderin (Sc) cDNA (pcDNA3-Sc) inserts, as indicated in “Materials and methods.” (A) Coomassie blue-stained SDS-PAGE gel shows Sc protein bands (Sc) for 2 Sc-expressing clones. The band is not seen in the gel corresponding to cells transfected with the pcDNA3 vector (left lane). (B) Western blot performed with a polyclonal antibody raised against recombinant Sc. Lanes from left to right: cell lines K562, HEL, NS-MEG, MEG-01 (wild-type) cells, cells transfected with pcDNA3, 4 Sc-expressing clones, and chromaffin cells (positive control). Immunostaining with Sc antibody of cells transfected with pcDNA3-Sc (C) and pcDNA3 (D). (E) Western blot with a monoclonal gelsolin antibody (top) and levels of gelsolin expression (bottom) in MEG-01 cells transfected with either pcDNA3 or pcDNA3-Sc. Tubulin levels detected by a polyclonal antibody were used as control for gel loading. Bars represent mean ± SEM from 4 different experiments (*P < .001).
Vector-mediated expression of scinderin in megakaryocytic cell lines
Cell lines were transfected with pcDNA3 vector alone (control) or the same vector carrying full-length scinderin cDNA (Figure 1A), as indicated in “Materials and methods.” Good results with transfections were obtained with K562 and MEG-01 cell lines. However, because cell line K562 can also be induced to show characteristics of erythroid lineage,32 33 whereas line MEG-01 can show properties corresponding only to the megakaryocytic lineage, the latter was selected for experiments. After dilution cloning and 3 passes, 16 MEG-01 clones expressing different levels of scinderin were obtained as demonstrated by SDS-PAGE (Figure 2A) and immunoblotting (Figure 2B). In those clones, 100% of the cells expressed scinderin, as revealed by immunocytochemistry with scinderin antibodies (Figure 2C,D). The expression of gelsolin, another F-actin–severing protein normally expressed in MEG-01 cells, was significantly reduced (Figure2E).
Characteristics of cells expressing scinderin
Cells expressing scinderin had larger volumes (10 250 ± 340 μm3; n = 720) when compared to those cells transfected with the pcDNA3 vector alone (4700 ± 100 μm3; n = 690) (Figure 3A). Scinderin-positive cells were not only bigger, but they also entered into endomitosis showing either hyperlobulated nuclei or several nuclei. Polyploidization was observed in all cells (Figure 3B), and this was accompanied by a significant decrease in [3H]-thymidine incorporation (Figure 3C). However, decreased thymidine use in cells undergoing endomitosis (DNA replication without late phase of mitosis) is not a good indication of decreased cell proliferation; a better method is to measure cell number. Indeed, there was a marked reduction in the number of cells in all cell clones expressing scinderin, with levels that were 21% and 9% of those transfected with the vector alone after 12 and 24 days in culture, respectively (Figure 3D). Cells transfected with the vector alone had the same proliferation rate as the wild type (Figure 3D). In all cases, proliferation was exponential. In addition to large volumes, cells expressing scinderin displayed surface morphologic changes consisting of protoplasmic extensions with the appearance of beads (Figure 3F,G). Although some small protoplasmic extensions were observed in some wild-type cells and in cells transfected with the vector alone (10.5% ± 3.1%; n = 500) (Figure 3E), most of the scinderin-positive cells displayed extensions (85.6% ± 1.2%; n = 500). The number of extensions per cell was several times greater in cells expressing scinderin (Figure 3G). Protoplasmic extensions also contained scinderin and filamentous actin (Figure 3H,I). Changes observed in volume, endomitosis, proliferation, and morphology were similar for clones ScI-E and ScI-J, clones with the highest and lowest levels of scinderin expression, respectively.
Effect of scinderin expression on MEG-01 cell morphology, volume, nucleus number, and rate of proliferation.
Cells transfected with either pcDNA3 or pcDNA3-Sc were cultured for 8 days after removal of the antibiotic G 418. (A, B, E-G) Cells were then fixed and stained with Wright-Giemsa, and (A) volumes of pcDNA3 (n = 484) and pcDNA3-Sc (n = 600) transfected cells were measured (*P < .001). (B) Comparison of gated DNA distribution of 35 000 cells transfected with pcDNA3 and the same number of cells transfected with pcDNA3-Sc (clone ScI-E). Similar results were obtained with 5 other Sc expressing clones. (C) Cells cultured as indicated in (A) were tested for [3H]-thymidine incorporation and for their ability to take up [3H]serotonin. Bars represent mean ± SEM from 4 different experiments (*P < .001; **P < .01). (D) Number of cells present in cultures of MEG-01 cells (wild type) and cells transfected with either pcDNA3 or pcDNA3-Sc after 12 and 24 days in culture. Bars represent mean ± SEM from 4 experiments (*P < .001). Cells, cultured for 12 days, were also fixed and stained with Wright-Giemsa (E-G), rhodamine phalloidin, or a probe for F-actin (I), or they were immunostained with an Sc antibody (H). Cells transfected with pcDNA3 (E) showed a large single nucleus surrounded by a thin layer of cytoplasm (×400). (F) Cells expressing Sc (pcDNA3-Sc) were much larger and were multinucleated or had multilobulated nuclei, abundant cytoplasm, and numerous cytoplasmic extensions (×400). (G) Same cells at a large magnification (×1000). Distribution of Sc (H) and filamentous actin (I) in a double-stained cell (×1200). There was some degree of correlation between the distribution of the 2 markers, especially in the cytoplasmic extensions (arrowheads).
Effect of scinderin expression on MEG-01 cell morphology, volume, nucleus number, and rate of proliferation.
Cells transfected with either pcDNA3 or pcDNA3-Sc were cultured for 8 days after removal of the antibiotic G 418. (A, B, E-G) Cells were then fixed and stained with Wright-Giemsa, and (A) volumes of pcDNA3 (n = 484) and pcDNA3-Sc (n = 600) transfected cells were measured (*P < .001). (B) Comparison of gated DNA distribution of 35 000 cells transfected with pcDNA3 and the same number of cells transfected with pcDNA3-Sc (clone ScI-E). Similar results were obtained with 5 other Sc expressing clones. (C) Cells cultured as indicated in (A) were tested for [3H]-thymidine incorporation and for their ability to take up [3H]serotonin. Bars represent mean ± SEM from 4 different experiments (*P < .001; **P < .01). (D) Number of cells present in cultures of MEG-01 cells (wild type) and cells transfected with either pcDNA3 or pcDNA3-Sc after 12 and 24 days in culture. Bars represent mean ± SEM from 4 experiments (*P < .001). Cells, cultured for 12 days, were also fixed and stained with Wright-Giemsa (E-G), rhodamine phalloidin, or a probe for F-actin (I), or they were immunostained with an Sc antibody (H). Cells transfected with pcDNA3 (E) showed a large single nucleus surrounded by a thin layer of cytoplasm (×400). (F) Cells expressing Sc (pcDNA3-Sc) were much larger and were multinucleated or had multilobulated nuclei, abundant cytoplasm, and numerous cytoplasmic extensions (×400). (G) Same cells at a large magnification (×1000). Distribution of Sc (H) and filamentous actin (I) in a double-stained cell (×1200). There was some degree of correlation between the distribution of the 2 markers, especially in the cytoplasmic extensions (arrowheads).
F-actin cytoskeleton in cells expressing scinderin
Scinderin is a Ca++-dependent filamentous actin severing and capping protein.15 34 Therefore, its expression may produce changes in the content and distribution of F-actin. Immunocytochemistry studies with scinderin antibodies and rhodamine-phalloidin (a probe for filamentous actin) showed some degree of co-localization for both proteins in scinderin-positive cells (Figures 3H,I, 4A). It was evident from these experiments that the intensity of fluorescence of F-actin in vector-transfected cells was greater than in scinderin-positive cells, suggesting, in this case, a decrease in filamentous actin, as indicated by image and Western blot analysis (Figure 4B,C). Differences in fluorescence were apparent when intensity was expressed either per cell or per surface square micron (Figure 4B). F-actin fluorescence was further and significantly reduced in scinderin-positive cells on treatment for 2 minutes with 2 μM Ca++ ionophore A23187 (Figure 4D,E). Cells transfected with vector alone were refractory to this treatment (Figure 4D), and, though all cells expressed gelsolin, the concentration of ionophore used was probably high enough to stimulate the disassembly of F-actin only in cells expressing scinderin.
Effect of scinderin expression and its activation by ionophore A-23187 on filamentous actin content in MEG-01 cells.
(A) Fluorescence microscope images of cells stained with scinderin (Sc) antibodies and rhodamine phalloidin (a probe for F-actin) after transfection either with vector pcDNA3 (2 left panels) or with Sc cDNA carrying vector pcDNA3-Sc (2 right panels). (B) Image analysis data of the fluorescent cells described in panel A. Twelve preparations of pcDNA3-transfected cells were analyzed, and 18 preparations from 3 different clones (6 from each clone) were measured for pcDNA3-Sc–transfected cells (*P < .001). (C) Western blot analysis carried out with an actin antibody on supernatants (S) and sediments of Triton X-100 extracts prepared from wild-type MEG-01 cells (W.T.) and from cells transfected with either pcDNA3 or pcDNA3-Sc. A decrease in the Triton X-100–insoluble (I) fraction (filamentous actin) was observed in pcDNA3-Sc–transfected cells. (D) Two-minute treatment with ionophore A23187 further decreased levels of F-actin in pcDNA3-Sc–transfected cells. F-actin was measured as described in panel B. Bars represent mean ± SEM from 4 to 5 different preparations, each containing 20 to 35 cells (**P < .05). (E) Fluorescence microscope images of a sample of cells described in panel D after rhodamine phalloidin staining.
Effect of scinderin expression and its activation by ionophore A-23187 on filamentous actin content in MEG-01 cells.
(A) Fluorescence microscope images of cells stained with scinderin (Sc) antibodies and rhodamine phalloidin (a probe for F-actin) after transfection either with vector pcDNA3 (2 left panels) or with Sc cDNA carrying vector pcDNA3-Sc (2 right panels). (B) Image analysis data of the fluorescent cells described in panel A. Twelve preparations of pcDNA3-transfected cells were analyzed, and 18 preparations from 3 different clones (6 from each clone) were measured for pcDNA3-Sc–transfected cells (*P < .001). (C) Western blot analysis carried out with an actin antibody on supernatants (S) and sediments of Triton X-100 extracts prepared from wild-type MEG-01 cells (W.T.) and from cells transfected with either pcDNA3 or pcDNA3-Sc. A decrease in the Triton X-100–insoluble (I) fraction (filamentous actin) was observed in pcDNA3-Sc–transfected cells. (D) Two-minute treatment with ionophore A23187 further decreased levels of F-actin in pcDNA3-Sc–transfected cells. F-actin was measured as described in panel B. Bars represent mean ± SEM from 4 to 5 different preparations, each containing 20 to 35 cells (**P < .05). (E) Fluorescence microscope images of a sample of cells described in panel D after rhodamine phalloidin staining.
Expression of platelet markers
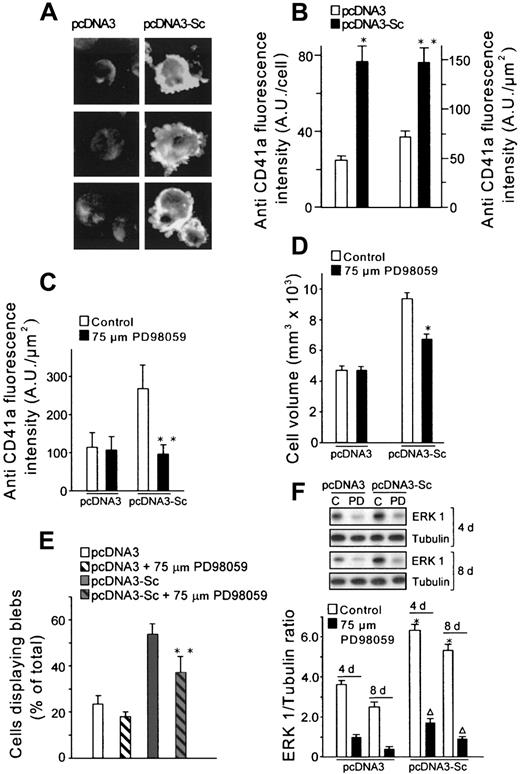
It has been demonstrated that MEG-01 cells display a serotonin uptake system.35,36 Indeed, cells transfected with the vector alone showed serotonin uptake, but the capacity to take up serotonin was significantly increased in scinderin-positive cells (Figure 3C). Moreover, uptake was inhibited 44% by fluoxetine, a specific inhibitor of serotonin uptake (data not shown), suggesting the presence of a high-affinity uptake system. Antigen CD41a,3,30 also known as fibrinogen receptor,37 is a platelet marker expressed in normal megakaryocytes30 and MEG-01 cells.3Scinderin-expressing cells also showed an increased expression of this receptor (Figure 5A,B). The expression of CD41a seems to be mediated through activation of the mitogen-activated protein kinase (MAPK)/ERK pathway.38 Compound PD98059 prevents activation of the MAPK/ERK kinase (MEK), which results in inhibition of this pathway.39 Treatment with 75 μM PD98059 for 4 days produced a significant inhibition in the expression of CD41a in scinderin-positive cells. Levels of CD41a expression in these cells were reduced by the compound to the levels found in cells transfected with vector alone (Figure 5C). PD98059 also produced a significant reduction in cell volume and number of protoplasmic extensions (Figure 5D,E). All this was accompanied by a reduction in the expression of ERK1 (Figure 5F). In view of the changes observed in morphology and expression of platelet antigens, it can be concluded that cells expressing scinderin entered the megakaryocytic differentiation pathway.
Cells expressing scinderin showed high levels of antigen CD41a (fibrinogen receptor) as a result of activation of the MEK-ERK1 pathway.
(A) Fluorescence microscope images of cells stained with a CD41a antibody. (B) Image analysis of the fluorescence intensity (A.U., arbitrary units) of the cells described in panel A. Bars represent mean ± SEM of 6 preparations (10-20 cells each) of pcDNA3-transfected cells and 19 preparations (5-10 cells each) from 6 different clones of pcDNA3-Sc–transfected cells. Fluorescence intensity was expressed per cell (*P < .01) and per μm2 of cell surface (**P < .05). (C) pcDNA3-Sc–transfected cells treated with 75 μM PD98059 for 4 days showed a decrease in the expression of CD41a (**P < .05) and (D) in cell volume (*P < .01). (E) The number of pcDNA3-Sc–transfected cells displaying surface blebs was also decreased by PD98059 treatment (**P < .05). (F) Western blots with an ERK1 antibody show the expression of the protein in untreated pcDNA3 and pcDNA3-Sc–transfected cells (C) and in the cells after treatment with 75 μM PD98059 (PD) for 4 and 8 days. Tubulin was used as gel-loading control. Bars represent the average from 6 different experiments (*P < .01; **P < .001).
Cells expressing scinderin showed high levels of antigen CD41a (fibrinogen receptor) as a result of activation of the MEK-ERK1 pathway.
(A) Fluorescence microscope images of cells stained with a CD41a antibody. (B) Image analysis of the fluorescence intensity (A.U., arbitrary units) of the cells described in panel A. Bars represent mean ± SEM of 6 preparations (10-20 cells each) of pcDNA3-transfected cells and 19 preparations (5-10 cells each) from 6 different clones of pcDNA3-Sc–transfected cells. Fluorescence intensity was expressed per cell (*P < .01) and per μm2 of cell surface (**P < .05). (C) pcDNA3-Sc–transfected cells treated with 75 μM PD98059 for 4 days showed a decrease in the expression of CD41a (**P < .05) and (D) in cell volume (*P < .01). (E) The number of pcDNA3-Sc–transfected cells displaying surface blebs was also decreased by PD98059 treatment (**P < .05). (F) Western blots with an ERK1 antibody show the expression of the protein in untreated pcDNA3 and pcDNA3-Sc–transfected cells (C) and in the cells after treatment with 75 μM PD98059 (PD) for 4 and 8 days. Tubulin was used as gel-loading control. Bars represent the average from 6 different experiments (*P < .01; **P < .001).
Apoptosis and production of platelet-like particles is the ultimate fate of megakaryoblastic leukemia cell clones expressing scinderin
At the end of the normal process of platelet production, megakaryocytes show a large nucleus enveloped by a thin layer of cytoplasm (denuded megakaryocyte).9 At the time of producing platelets, megakaryocytes enter into apoptosis, a mechanism that can be delayed, but only to certain degree, by thrombopoietin.9,40 The fact that numerous scinderin-expressing MEG-01 cells die in culture, with numbers increasing with time, prompted us to determine their ultimate fate. Percentages of dead cells at day 14 in culture was double for scinderin-positive cells when compared to cells transfected with vector; by day 23 in culture, the number of scinderin-positive dead cells reached 35% of the total population. This, of course, was accompanied by increasing numbers of cells entering apoptosis, as revealed by the TUNEL assay (Figure6E,F). Between days 18 and 25 in culture, only scinderin-positive cells released large numbers of cytoplasmic fragments of an average size of 1.63 ± 0.04 μm (n = 45). These particles showed CD41a antigen fluorescence (Figure 6M). They had a high-affinity serotonin transport system sensitive to fluoxetine (Figure 6Q), dense bodies, as demonstrated by electron microscopy (Figure 6P), and, similar to platelets,41 a circular array of microtubules, as demonstrated by immunocytochemistry with antibodies against α-tubulin (Figure 6N,O). Moreover, treatment of particles with 1 U thrombin/mL induced serotonin release (Figure 6R) and aggregation (Figure 6G-J), an effect also blocked by 40% in the presence of an antibody against the fibrinogen receptor (Figure 6L). Platelet-like particles were pre-incubated with 1 U thrombin/mL for 5 minutes, and their aggregation in response to fibrinogen plus CaCl2 was inhibited when PAC-1 antibody27 was added with thrombin (Figure 6K).
Expression of scinderin in the MEG-01 cell line induced formation and release of platelet-like particles.
Cells transfected with either vector pcDNA3 (A,C) or vector (pcDNA3-Sc) carrying a full-length scinderin (Sc) cDNA insert (B, D) were cultured for 11 (A,B) and 23 (C,D) days and then were fixed and stained with Wright-Giemsa. After 23 days in culture, preparations of pcDNA3-Sc cells showed cytoplasmic areas smaller than those observed in panel B and many relatively uniform particles of dimensions similar to those of platelets. (E) Numerous cells in these cultures entered apoptosis as determined by the TUNEL assay. Bright green fluorescence indicates apoptotic nuclei and apoptotic bodies. Percentages of apoptosis after 4 and 23 days in culture are shown in panel F. Bars represent mean ± SEM of 5 different experiments (*P < .01). Platelet-like particles were purified as described in “Materials and methods” and were treated with 1 U thrombin/mL. This induced aggregation, as shown, after fixation and staining with Wright-Giemsa, before (G) and 6 minutes after (H, I) the addition of thrombin. (I) At-large magnification of the particle aggregate contained within the box shown in panel H. (J) Decrease in light absorbance of the same preparation. (K) Fibrinogen plus CaCl2 induced the aggregation of platelet-like particles pre-incubated for 5 minutes with 1 U thrombin/mL in the absence or presence of 40 μg PAC-1 antibody/mL. (L) Thrombin-induced aggregation was significantly inhibited in the presence of a CD41a (fibrinogen receptor) antibody, and (M) all particles present in the preparation showed intense fluorescence when stained with the same antibody. Tubulin antibody staining showing a similar array of microtubules in normal human platelets (N) and platelet-like particles (O). (P) Platelet-like particles were incubated with 1 μM serotonin for 60 minutes, fixed in glutaraldehyde, and processed for electron microscopy. The micrograph shows dense bodies within the cytoplasm. Platelet-like particles were also incubated with 10-8 M [3H]5-HT as indicated in “Materials and methods,” and serotonin uptake was measured in the absence or presence of 6 nM fluoxetine (Q). Bars represent mean ± SEM from 8 preparations (**P < .05). Treatment of [3H]5-HT-labeled particles with 1 U thrombin/mL for 2 minutes also induced the release of amines (R). Bars represent mean ± SEM of 8 preparations (*P < .001).
Expression of scinderin in the MEG-01 cell line induced formation and release of platelet-like particles.
Cells transfected with either vector pcDNA3 (A,C) or vector (pcDNA3-Sc) carrying a full-length scinderin (Sc) cDNA insert (B, D) were cultured for 11 (A,B) and 23 (C,D) days and then were fixed and stained with Wright-Giemsa. After 23 days in culture, preparations of pcDNA3-Sc cells showed cytoplasmic areas smaller than those observed in panel B and many relatively uniform particles of dimensions similar to those of platelets. (E) Numerous cells in these cultures entered apoptosis as determined by the TUNEL assay. Bright green fluorescence indicates apoptotic nuclei and apoptotic bodies. Percentages of apoptosis after 4 and 23 days in culture are shown in panel F. Bars represent mean ± SEM of 5 different experiments (*P < .01). Platelet-like particles were purified as described in “Materials and methods” and were treated with 1 U thrombin/mL. This induced aggregation, as shown, after fixation and staining with Wright-Giemsa, before (G) and 6 minutes after (H, I) the addition of thrombin. (I) At-large magnification of the particle aggregate contained within the box shown in panel H. (J) Decrease in light absorbance of the same preparation. (K) Fibrinogen plus CaCl2 induced the aggregation of platelet-like particles pre-incubated for 5 minutes with 1 U thrombin/mL in the absence or presence of 40 μg PAC-1 antibody/mL. (L) Thrombin-induced aggregation was significantly inhibited in the presence of a CD41a (fibrinogen receptor) antibody, and (M) all particles present in the preparation showed intense fluorescence when stained with the same antibody. Tubulin antibody staining showing a similar array of microtubules in normal human platelets (N) and platelet-like particles (O). (P) Platelet-like particles were incubated with 1 μM serotonin for 60 minutes, fixed in glutaraldehyde, and processed for electron microscopy. The micrograph shows dense bodies within the cytoplasm. Platelet-like particles were also incubated with 10-8 M [3H]5-HT as indicated in “Materials and methods,” and serotonin uptake was measured in the absence or presence of 6 nM fluoxetine (Q). Bars represent mean ± SEM from 8 preparations (**P < .05). Treatment of [3H]5-HT-labeled particles with 1 U thrombin/mL for 2 minutes also induced the release of amines (R). Bars represent mean ± SEM of 8 preparations (*P < .001).
Transduction pathways involved in changes observed in scinderin-expressing cells
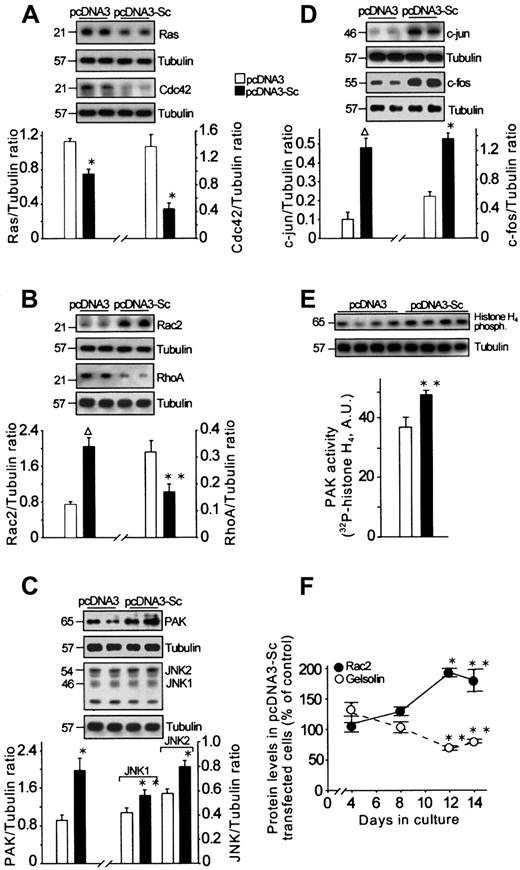
Stimulation of different types of cytokine receptors, including thrombopoietin receptors, activates different transduction pathways leading to cell proliferation, differentiation, and apoptosis.8,42,43 We have examined some of the numerous components of these pathways to understand how scinderin expression triggers directly or, most probably, indirectly through F-actin disassembly the activity of these pathways. We have described above that enhanced expression of CD41a was accompanied by an increased expression of ERK1 and that both were blocked by PD98059. This suggests involvement of the Raf/MEK/ERK pathway in the differentiation process triggered by scinderin expression. On the other hand, Ras, a G protein known to participate in this pathway, was found to be decreased in scinderin-positive cells at 12 and 14 days in culture (Figure7A). It is known that Ras activation is necessary for a proliferative response and that differentiation requires either an additional or perhaps a separate pathway.43 Therefore, an observed decrease in Ras levels would agree with low levels of proliferation found in these clones.
Effect of scinderin expression on cellular levels and activity of different transduction and transcription factors.
Extracts were prepared from cells transfected with either pcDNA3 or pcDNA3-Sc and cultured for 14 days. SDS-PAGE of the extracts was performed in quadruplicate and was followed by immunoblotting. Western blots with the corresponding antibodies are shown for Ras and Cdc42 (A); Rac2 and RhoA (B); PAK, JNK1, and JNK2 (C); c-jun and c-fos (D); and PAK activity using histone H4 as substrate (E). Tubulin was used as gel-loading control and to express results as a ratio between each protein and tubulin after imaging bands from the autoradiographs. (F) Time course of Rac2 and gelsolin expression in cells transfected with pcDNA3-Sc. Bars and circles represent mean ± SEM for those experiments (**P < .05; *P < .01; ΔP < .001).
Effect of scinderin expression on cellular levels and activity of different transduction and transcription factors.
Extracts were prepared from cells transfected with either pcDNA3 or pcDNA3-Sc and cultured for 14 days. SDS-PAGE of the extracts was performed in quadruplicate and was followed by immunoblotting. Western blots with the corresponding antibodies are shown for Ras and Cdc42 (A); Rac2 and RhoA (B); PAK, JNK1, and JNK2 (C); c-jun and c-fos (D); and PAK activity using histone H4 as substrate (E). Tubulin was used as gel-loading control and to express results as a ratio between each protein and tubulin after imaging bands from the autoradiographs. (F) Time course of Rac2 and gelsolin expression in cells transfected with pcDNA3-Sc. Bars and circles represent mean ± SEM for those experiments (**P < .05; *P < .01; ΔP < .001).
The Rac/Cdc42/PAK/MEKK.SEK/JNK transduction pathway has been found to be responsible for triggering a decrease in proliferation and apoptosis through an increased expression of c-jun and c-fos.42,44 Cells in scinderin-positive clones showed a significant increase in c-jun and c-foslevels (Figure 7D), and a proof for the activation of this pathway was the observation of increases in expressions of Rac2, JNK 1, JNK 2, and PAK in these cells (Figure 7B,C). Normally, PAK is activated by Rac2; in cells expressing scinderin, there was a significant increase in PAK levels and activity (Figure 7C,D). Furthermore, it has been shown that Rac, together with Cdc42 and RhoA—also small-molecular–weight G proteins—is involved in the control of cytoskeleton organization.45 However, Cdc42 and RhoA levels were decreased in scinderin-positive cells (Figure 7A,B). Moreover, as indicated above, the expression of gelsolin, an F-actin cytoskeleton regulatory protein, was decreased with a time course that was a mirror image of that followed by Rac2 expression increase (Figure 7F). This observation suggests a relationship in the regulation of expression of these 2 proteins.
Effect of scinderin expression on tumor formation
Nine Balb/c mice were injected subcutaneously in their abdominal flanks with 1 × 107 MEG-01 cells previously transfected with vector pcDNA3 (controls), and a similar group of mice received injections of the same number of cells, also previously transfected with vectors carrying a full-length scinderin cDNA insert (pcDNA3-Sc). All cells were cultured for 14 days before injections. Seven mice of those that received pcDNA3-transfected cells developed large tumors and were killed 3 weeks after injection in accordance with institutional animal care policies (Figure 8A). On the other hand, only 2 small tumors were observed in the group of 9 mice injected with clones expressing scinderin (pcDNA3-Sc). Remaining animals in this group were free of tumors (Figure 8A). Histology of these 2 small tumors was then compared with that of large tumors found in animals of the control (pcDNA3) group. The latter set consisted of solid tumors of well-packed cells showing single nuclei surrounded by small cytoplasmic areas (Figure 8B). Conversely, the 2 small tumors formed by scinderin expressing cells showed large areas of cells with apoptotic nuclei surrounded by large numbers of platelet-like particles (Figure 8C), a situation similar to that observed with these cells in culture (see above Figure 6D). Therefore, it seems that in vitro as well as in vivo, apoptosis with platelet-like particles release is the fate of cells transfected with pcDNA3-Sc.
Expression of scinderin inhibits tumor growth in nude mice.
Balb/c nude mice were injected with cells cultured for 14 days after transfection with either vector (pcDNA3) alone or the same vector carrying a full-length insert of Scinderin cDNA (pcDNA3-Sc). (A) Tumor volumes after subcutaneous injection (inj) with 107 cells in 100 μL saline for each condition. Open and closed circles represent mean ± SEM from 2 groups of 9 mice each (*P < .05). †All pcDNA3-transfected mice were killed at 3 weeks after injection in accordance with institutional animal care policies. (B) Hematoxylin-eosin staining of a section from a large tumor produced by pcDNA3-transfected cells. Histology of 8 remaining tumors in this group was similar, and so was that of tumors formed by wild-type cells (data not shown). (C) Similar staining of a section from 1 of the 2 small tumors produced by pcDNA3-Sc–transfected cells showing apoptotic nuclei surrounded by numerous platelet-like particles. The second small tumor in this group had a similar histology.
Expression of scinderin inhibits tumor growth in nude mice.
Balb/c nude mice were injected with cells cultured for 14 days after transfection with either vector (pcDNA3) alone or the same vector carrying a full-length insert of Scinderin cDNA (pcDNA3-Sc). (A) Tumor volumes after subcutaneous injection (inj) with 107 cells in 100 μL saline for each condition. Open and closed circles represent mean ± SEM from 2 groups of 9 mice each (*P < .05). †All pcDNA3-transfected mice were killed at 3 weeks after injection in accordance with institutional animal care policies. (B) Hematoxylin-eosin staining of a section from a large tumor produced by pcDNA3-transfected cells. Histology of 8 remaining tumors in this group was similar, and so was that of tumors formed by wild-type cells (data not shown). (C) Similar staining of a section from 1 of the 2 small tumors produced by pcDNA3-Sc–transfected cells showing apoptotic nuclei surrounded by numerous platelet-like particles. The second small tumor in this group had a similar histology.
Discussion
Scinderin is a Ca++-dependent filamentous actin-severing protein14 present in platelets and megakaryocytes but, as demonstrated here, absent from megakaryoblastic leukemic cells and the cell lines derived from them. The scinderin gene has been cloned,15 and one scinderin function has been shown to be the control of F-actin networks during secretion from cells such as chromaffin cells16,18,19 and platelets.20 However, scinderin may participate in the control of other dynamic changes of actin cytoskeleton networks such as extensive cytoskeletal reorganization and morphologic changes occurring in megakaryocytes during proplatelet formation and platelet release.5-7,10,46 47
The current experiments show that the expression of scinderin cDNA in the MEG-01 cell line decreases cell proliferation and induces polyploidization, differentiation, and apoptosis with the release of platelet-like particles. Moreover, unlike cells transfected with vector alone, cells expressing scinderin were unable to induce the formation of large tumors in nude mice.
The initial observation in MEG-01 cells expressing scinderin was a decrease in filamentous actin as a result of the severing activity of fully active scinderin; full activity was demonstrated by a further decrease in F-actin with ionophore A23187 treatment. Under these conditions, there was no decrease in F-actin in cells transfected only with vector. This occurred in spite of the presence of gelsolin in these cells. One possibility for the difference in response to the ionophore between both groups of cells was that cellular concentrations of Ca++ reached on ionophore treatment were only high enough to stimulate the overexpressed scinderin. It has also been shown that cancer cells express low levels of gelsolin,48-50 but little is known about the properties of this gelsolin except that it has been implicated in apoptosis.51 The significant decrease in gelsolin levels in cells expressing scinderin could be the result of scinderin overexpression or, more likely, an effect of low levels of F-actin, a condition that might regulate the expression of gelsolin. The dramatic decrease in the proliferation of cells observed in MEG-01 clones expressing scinderin could also be attributed, at least in part, to low levels of F-actin because it is known that filamentous actin plays an important role in cell division.52 However, the decrease in proliferation might have been caused by the induction of endomitosis and polyploidization as part of the differentiation process that these cells have entered. Actin networks might also play a role in polyploidization—it has been demonstrated that treatment with cytochalasin B or D, substances that depolymerize F-actin, induces polyploidization.10,11,53However, this effect of cytochalasins is not accompanied by increased expression of platelet antigens11 as are the cells that express scinderin described here. In the DAMI cell line, phorbol 12-myristate 13-acetate treatment increases polyploidization, a response accompanied by a several-fold increase in cyclin D1.4 Whether a similar increase in cyclin D1 takes place in scinderin-positive cells remains to be established.
The decrease in proliferation observed in scinderin-positive clones was also accompanied by an increase in apoptosis as revealed by the TUNEL assay. Apoptosis is the physiological fate of normal megakaryocytes9; these cells enter into programmed cell death at the end of their maturation and differentiation and release platelets. Similarly, cells expressing scinderin, after polyploidization and expression of platelet-specific antigens (ie, glycoprotein IIb/IIIa), release cytoplasmic particles by day 18 to 24 in culture. It is known that MEG-01 cells can spontaneously release a small number of cytoplasmic particles.54 However, cells expressing scinderin released cytoplasmic particles in numbers 2 orders of magnitude greater. These particles had characteristics and behavior very much like those of platelets. They expressed the fibrinogen receptor, high-affinity serotonin uptake system, and dense bodies, and they responded to thrombin with secretion and aggregation. This last effect was inhibited in the presence of PAC-1 monoclonal antibodies and antibodies against antigen CD41a. As did platelets,41these particles showed a circular array of microtubules. The stimulus responsible for the assembly of microtubules in a coil is unknown and has been a subject of interest for a long time.41,47,55 In this regard, it has been suggested that microtubule rings may play a role in the control of the intervals during which proplatelets break into platelets.56 As mentioned above, the F-actin network seems to be involved in platelet formation and release,6,7,10 and, in the presence of cytochalasin B, platelet formation not only proceeds but is accelerated.6It seems, therefore, that conditions that decrease filamentous actin, as in scinderin-positive MEG-01 cells, favor the formation and release of platelets. However, the expression of scinderin in MEG-01 cells induced more cellular changes than the simple treatment of cells with cytochalasins. Therefore, in scinderin-expressing cells, additional mechanisms, such as the activation of specific transduction pathways, might be responsible for the maturation and differentiation changes observed. Transduction pathways involved in cell proliferation, differentiation, and apoptosis have been described.42,43,57-60 These pathways are not completely understood because several of the components can stimulate effectors through more than one pathway (known as cross talk). Nevertheless, we have made attempts to understand the transduction mechanism involved in scinderin-expressing cells by measuring levels and activities of several of these transduction factors. The Ras-Raf-MEK-ERK cascade (where ERK is extracellular-signal regulated kinase and MEK is mitogen-activated protein kinase [MAPK]/ERK) is a pathway stimulated by growth factors and mitogens. Two other pathways (PAK-MEKK-SEK-JNK and MKK3-p38-MAPK-ALK2) that are activated mainly by cytokines, hormones, and various forms of stress, used p21 proteins of the Rho family (Rho, Rac, Cdc42, and so on).43,44 Ras can also participate in these pathways.43,44,61 However, these pathways can be largely activated (ie, stress) in a Ras-Raf-MEK-ERK–independent manner, though Ras is found in all cell types and shows high levels in proliferating cells.43,44The disruption of F-actin cytoskeleton, as observed in MEG-01 cells expressing scinderin, could indeed induce cellular stress with activation of the PAK-MEKK-SEK-JNK pathway. It is known that the Rho family of small-molecular–weight G proteins, which includes RhoA, B, C, Rac1 and 2, and Cdc42, has an important role in the regulation of the actin cytoskeleton and focal contacts mediating the formation of lamellipodia and filopodia.62 The current experiments show that at day 14 in culture, scinderin-expressing MEG-01 cells have high levels of Rac2 and low levels of RhoA and Cdc42. It has also been shown that Rac2 levels increased drastically during the differentiation of MEG-01 cells in response to treatment with phorbol esters.63 It is known that increases in Rac evoke rapid synthesis of PIP2, which results in an increase in the uncapping of filamentous actin followed by actin polymerization.64 The increase in Rac2 expression observed in scinderin-positive cells may be a cellular response to increase actin polymerization as the result of decreased F-actin cellular levels. Alternatively, an increase in Rac might be the result of the decreased expression of gelsolin observed in these cells because it has been demonstrated that there is a reciprocal correlation between gelsolin and Rac expressions.65 Indeed, in the current experiments, a good reciprocal correlation between the levels of these 2 proteins was also observed (Figure 7F). In gelsolin-null mice, Rac is overexpressed.65 Re-expression of gelsolin in these animals restores normal levels of Rac.65 Gelsolin-null animals did not show changes in cellular levels of either Rho or Cdc42.65 Decreases in RhoA and Cdc42 expression observed in scinderin-positive cells has no explanation except that changes in the cytoskeleton are such that cells do not require high levels of these proteins any longer. Additional experiments are needed to clarify this point. PAK (p21-activated kinase) has been found to be an effector of Rac, and scinderin-positive cells showed increases in levels and activity of PAK and in JNK2, a factor downstream of PAK in the cascade PAK-MEEK-SEK-JNK. Activation of JNK is also involved in the activation of c-jun in cells entering apoptosis42,43 and in hematopoietic precursor cells during their development into mature cells.42 In this regard, it has been shown that c-jun/c-fos (also known as AP-1 factor) are highly expressed in terminally differentiated megakaryocytic lineages.66,67 The increases in JNK, c-jun, c-fos, and apoptosis in cells expressing scinderin are clear indications of the activation of this pathway, which was observed at the time Ras levels were decreased. This is, as suggested earlier, evidence that this pathway can operate with no dependency on Ras levels. Although Ras expression was decreased, there was evidence of early activation of the Raf-MEK-ERK pathway because of the increased expression of platelet antigen CD41a between days 4 and 8 in culture. Expression of this antigen in K562 cells has been found to be the result of the activation of this pathway.38 The fact that in the current experiments compound PD98059, a known inhibitor of MEK, inhibited CD41a expression in vector-transfected and in scinderin-positive cells is an indication of the involvement of this cascade in the expression of platelet antigens.
An important observation was the fact that cells expressing scinderin either did not form tumors in nude mice or that the 2 small tumors observed have histologic characteristics different from those large, solid, and vascularized tumors observed in mice injected with cells previously transfected with vectors. The small tumors produced by pcDNA3-Sc–transfected cells showed cells in apoptosis surrounded by large numbers of platelet-like particles, a situation similar to that observed in vitro. Therefore, it seems that the restitution of scinderin expression in human megakaryoblastic leukemia cells activates specific transduction pathways leading to cell differentiation and maturation, together with the inhibition of proliferation and tumor formation. Whether these cells had acquired all characteristics of normal cells, including lack of tumorigenesis, should be determined in future experiments.
Supported by a Canadian Institutes of Health Research grant (J.M.T.) and CIHR-CONICET exchange program (J.M.T., N.C.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
J.-M. Trifaró, Dept of Cellular and Molecular Medicine, Faculty of Medicine, University of Ottawa, 451 Smyth Rd, Ottawa, ON, K1H 8M5, Canada; e-mail: jtrifaro@uottawa.ca.

![Fig. 3. Effect of scinderin expression on MEG-01 cell morphology, volume, nucleus number, and rate of proliferation. / Cells transfected with either pcDNA3 or pcDNA3-Sc were cultured for 8 days after removal of the antibiotic G 418. (A, B, E-G) Cells were then fixed and stained with Wright-Giemsa, and (A) volumes of pcDNA3 (n = 484) and pcDNA3-Sc (n = 600) transfected cells were measured (*P < .001). (B) Comparison of gated DNA distribution of 35 000 cells transfected with pcDNA3 and the same number of cells transfected with pcDNA3-Sc (clone ScI-E). Similar results were obtained with 5 other Sc expressing clones. (C) Cells cultured as indicated in (A) were tested for [3H]-thymidine incorporation and for their ability to take up [3H]serotonin. Bars represent mean ± SEM from 4 different experiments (*P < .001; **P < .01). (D) Number of cells present in cultures of MEG-01 cells (wild type) and cells transfected with either pcDNA3 or pcDNA3-Sc after 12 and 24 days in culture. Bars represent mean ± SEM from 4 experiments (*P < .001). Cells, cultured for 12 days, were also fixed and stained with Wright-Giemsa (E-G), rhodamine phalloidin, or a probe for F-actin (I), or they were immunostained with an Sc antibody (H). Cells transfected with pcDNA3 (E) showed a large single nucleus surrounded by a thin layer of cytoplasm (×400). (F) Cells expressing Sc (pcDNA3-Sc) were much larger and were multinucleated or had multilobulated nuclei, abundant cytoplasm, and numerous cytoplasmic extensions (×400). (G) Same cells at a large magnification (×1000). Distribution of Sc (H) and filamentous actin (I) in a double-stained cell (×1200). There was some degree of correlation between the distribution of the 2 markers, especially in the cytoplasmic extensions (arrowheads).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2210/5/m_h81911604003.jpeg?Expires=1769132277&Signature=NFNLMQfIAmVzJppqHfUaab-y6RSbl8-EiLvA4mP1q9D~uFTdKRJ4M~JY4QbbGvGwStHJ3zQ2gnmbEQXXjPcOg5Ur26FDVdsmZaYDQ-0ZmlxhByDm0adVplHad0TeLHdOa8naL8K5td~AsGmtahoeOZzrp1ylvpRrP7UrOIxdH8h467~1-11idU82IAxYBSLOsvYqUlMUz5SltiVbkyXqfbBI1Pa-EK~PTc0TrVT0izt~1zr6lLdlvi9fT8nUZT2KY9G07OocR3KBadHRMC717nQHHR98D1SQoWmVOjFY1nOSOc~N5Wf04V988XFQpM4cUqhWK445pfvccVS8z4jtkg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 6. Expression of scinderin in the MEG-01 cell line induced formation and release of platelet-like particles. / Cells transfected with either vector pcDNA3 (A,C) or vector (pcDNA3-Sc) carrying a full-length scinderin (Sc) cDNA insert (B, D) were cultured for 11 (A,B) and 23 (C,D) days and then were fixed and stained with Wright-Giemsa. After 23 days in culture, preparations of pcDNA3-Sc cells showed cytoplasmic areas smaller than those observed in panel B and many relatively uniform particles of dimensions similar to those of platelets. (E) Numerous cells in these cultures entered apoptosis as determined by the TUNEL assay. Bright green fluorescence indicates apoptotic nuclei and apoptotic bodies. Percentages of apoptosis after 4 and 23 days in culture are shown in panel F. Bars represent mean ± SEM of 5 different experiments (*P < .01). Platelet-like particles were purified as described in “Materials and methods” and were treated with 1 U thrombin/mL. This induced aggregation, as shown, after fixation and staining with Wright-Giemsa, before (G) and 6 minutes after (H, I) the addition of thrombin. (I) At-large magnification of the particle aggregate contained within the box shown in panel H. (J) Decrease in light absorbance of the same preparation. (K) Fibrinogen plus CaCl2 induced the aggregation of platelet-like particles pre-incubated for 5 minutes with 1 U thrombin/mL in the absence or presence of 40 μg PAC-1 antibody/mL. (L) Thrombin-induced aggregation was significantly inhibited in the presence of a CD41a (fibrinogen receptor) antibody, and (M) all particles present in the preparation showed intense fluorescence when stained with the same antibody. Tubulin antibody staining showing a similar array of microtubules in normal human platelets (N) and platelet-like particles (O). (P) Platelet-like particles were incubated with 1 μM serotonin for 60 minutes, fixed in glutaraldehyde, and processed for electron microscopy. The micrograph shows dense bodies within the cytoplasm. Platelet-like particles were also incubated with 10-8 M [3H]5-HT as indicated in “Materials and methods,” and serotonin uptake was measured in the absence or presence of 6 nM fluoxetine (Q). Bars represent mean ± SEM from 8 preparations (**P < .05). Treatment of [3H]5-HT-labeled particles with 1 U thrombin/mL for 2 minutes also induced the release of amines (R). Bars represent mean ± SEM of 8 preparations (*P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2210/5/m_h81911604006.jpeg?Expires=1769132277&Signature=rMKxrlYxzOlLG0Dv-5pdJ6wYO7DaLbDWzV0QMfQ0ijDSfE~noWhXxdwh2OQG2qbRQ6f3fKcC~gXPYlEFCN0vlyavY7GS2ph~84Cjksyr-Sy1LrK7UTz~eFVRDnkZbIA0mzIJ6bVUUn6iVY~mbin5ZALHTUNqdU5S3qewMsNJC6eMFAnINmUqf973FJucneiAjUYpJsdLf-nQQB-sNiiGYQ3iV4wqGd4sZG3IbHGr3ihD0u~DNdHKcp6BIIi8KJSVT8cYn-v8iEbZ18GRBBY6lvj9ZCGEvehmKrwDJnrr7yqOBeRLFlJ6tRZ-dRi23fTgdtFoY-jTdseYNDbwyi1uOg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal