Abstract
We previously showed that adhesion of myeloma cells to fibronectin (FN) by means of β1 integrins causes resistance to certain cytotoxic drugs. The study described here found that adhesion of U937 human histiocytic lymphoma cells to FN provides a survival advantage with respect to damage induced by the topoisomerase (topo) II inhibitors mitoxantrone, doxorubicin, and etoposide. Apoptosis induced by a topo II inhibitor is thought to be initiated by DNA damage. The neutral comet assay was used to determine whether initial drug-induced DNA damage correlated with cellular-adhesion–mediated drug resistance. Cellular adhesion by means of β1 integrins resulted in a 40% to 60% reduction in mitoxantrone- and etoposide-induced DNA double-strand breaks. When the mechanisms regulating the initial drug-induced DNA damage were examined, a β1 integrin–mediated reduction in drug-induced DNA double-strand breaks was found to correlate with reduced topo II activity and decreased salt-extractable nuclear topo IIβ protein levels. Confocal studies showed changes in the nuclear localization of topo IIβ; however, alterations in the nuclear-to-cytoplasmic ratio of topo IIβ in FN-adhered cells were not significantly different. Furthermore, after a high level of salt extraction of nuclear proteins, higher levels of topo IIβ–associated DNA binding were observed in FN-adhered cells than in cells in suspension. Together, these data suggest that topo IIβ is more tightly bound to the nucleus of FN-adhered cells. Thus, FN adhesion by means of β1 integrins appears to protect U937 cells from initial drug-induced DNA damage by reducing topo II activity secondarily to alterations in the nuclear distribution of topo IIβ.
Introduction
Studies have found that cellular adhesion by means of β1 integrins inhibits cell death induced by DNA cross-linking agents and topoisomerase (topo) II inhibitors.1 2 The mechanisms of resistance associated with β1-mediated adhesion are unknown. It is thought that DNA cross-linking agents and topo II inhibitors initiate cellular death by inducing DNA damage. Thus, cellular adhesion by means of β1 integrins could confer resistance by either decreasing drug-induced DNA damage or increasing cellular tolerance to such damage. To address this issue, we examined the effects of β1-mediated cellular adhesion to fibronectin (FN) on DNA damage induced by pharmacologic inhibitors of topo II. If β1 integrin–mediated adhesion reduces DNA damage induced by this class of drugs, then alterations in the putative target (topo II) may represent one mechanism of cellular-adhesion–mediated drug resistance (CAM-DR).
Topo II is an adenosine triphosphate (ATP)–dependent enzyme that reversibly cuts double-stranded DNA and is transiently linked to the 5′ end of the break site by phosphotyrosyl bonds. Mammalian cells contain 2 isoforms of topo II (topo IIα and topo IIβ, which are 170 kd and 180 kd, respectively). These 2 isoforms are encoded by separate human genes and differ with respect to molecular mass, sequence specificity for DNA cleavage, regulation of expression, and tissue distribution.3,4 Many topo II inhibitors stabilize this normally transiently bound DNA-protein complex and form what is referred to as the cleavable complex.5 Stabilization of this complex is thought to initiate apoptotic cell-death pathways. Qualitative and quantitative changes in topo IIα and topo IIβ are associated with drug resistance and decreased drug-induced DNA damage.6-8 The amount of drug-induced cleavable complex can be detected indirectly by measuring DNA double-strand breaks.9 In this study, we found that β1-mediated adhesion of U937 cells to FN reduced the number of mitoxantrone- and etoposide-induced DNA double-strand breaks as assessed by the neutral single-cell gel-electrophoresis (comet) assay. Furthermore, the β1-adhesion–mediated reduction in DNA double-strand breaks correlated with decreased salt-extractable topo IIβ protein levels and alterations in the nuclear localization of topo IIβ.
Materials and methods
Cell culture
The U937 human histiocytic lymphoma cell line was obtained from the American Type Culture Collection (Rockville, MD). The cells were grown in suspension in RPMI 1640 medium (Cellgro; Fischer Scientific, Pittsburgh, PA) supplemented with 10% fetal-calf serum (FCS; Omega Scientific, Tarzana, CA), penicillin (100 μg/mL), streptomycin (100 μg/mL), and 2 mM L-glutamine (Gibco, Grand Island, NY). Cells were maintained at 37°C in an atmosphere of 5% carbon dioxide and 95% air and underwent passage twice weekly.
Drugs and antibodies
Mitoxantrone and doxorubicin were obtained from Sigma (St Louis, MO) and dissolved in sterile double-distilled water. Etoposide was obtained from Sigma and dissolved in dimethyl sulfoxide (DMSO). The integrin-blocking antibodies included α4 blocking antibody (P4G9; Dako, Carpinteria, CA), α5 blocking antibody (P1D6; Dako), and β1 blocking antibody (P4C10; Gibco). MAR4 antibody obtained from Pharmingen (San Diego, CA) was used to detect expression of β1 integrin on the cell surface. Topo IIβ and topoIIα polyclonal antibodies were generated in the laboratory of Dr D. Sullivan (Moffitt Cancer Center, Tampa, FL),10 and topo I monoclonal antibody was a generous gift from Dr Y.-C. Cheng (Yale University Medical School, New Haven, CT).11
Cell-surface expression and functional adhesion assay
Cell-surface expression of integrins was measured by incubating 1 million cells with primary or isotype control antibody for 30 minutes on ice.1 After 2 washes with phosphate-buffered saline (PBS), cells were incubated with a secondary fluorescein isothiocyanate (FITC)–conjugated goat antimouse antibody (Dako). After incubation with the secondary antibody, the samples were washed twice with PBS. Fluorescence was analyzed by flow cytometry using a fluorescence-activated cell-sorter scanner (Becton Dickinson, Mountain View, CA) to record 10 000 events. Mean fluorescence values for the isotype control were subtracted from the mean fluorescence values for integrin staining. Mean and SD values were calculated from the results of 3 independent experiments.
The adhesion assay was done as described previously.1Briefly, 96-well Immunosorp (Nunc, Denmark) plates were coated with either 50 μL (40 μg/mL) of soluble FN (Gibco) or bovine serum albumin (BSA) and allowed to evaporate overnight at room temperature. Cells were washed once in serum-free RPMI medium and resuspended at a density of 1 × 106 cells/mL. Before cell adhesion, cells were incubated for 30 minutes with a 1:100 dilution of either isotype control, α4 blocking antibody (P4G9), α5 blocking antibody (P1D6), or β1 blocking antibody (P4C10). After incubation with antibodies, 1 × 105 cells were added to each well. After 2 hours of adhesion, unattached cells were removed by 3 washes with RPMI medium, and adherent cells were fixed with 70% methanol for 10 minutes, dried, and subsequently stained with a solution of 0.02% crystal violet and 0.2% ethanol. The stained cells were solubilized in 100 μL Sorenson solution, and absorbance was read at 540 nm with an automated 96-well plate reader (Dynex, Chantilly, VA). Mean and SE values were calculated from the results in 4 independent wells. Experiments were repeated at least twice, and results of representative experiments are shown (Figure 1).
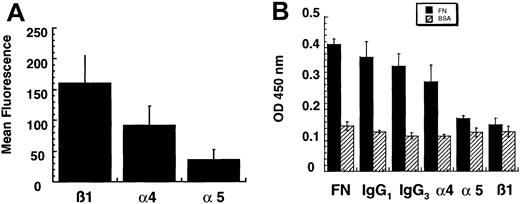
U937 cells expressed both very late antigen (VLA) 4 (α4β1 heterodimer) and VLA-5 (α5β1 heterodimer) integrin but adhered to FN primarily by means of VLA-5 integrin.
(A) Cell-surface expression of integrin subunits was measured using flow cytometry. The mean fluorescence of 10 000 events for each subunit (3 independent experiments) is shown. (B) Blocking antibodies were used to determine the specificity of binding of U937 cells to FN. The presence of the α5 or the β1 blocking antibody reduced adhesion of U937 cells to levels comparable to those with BSA. In contrast, the α4 blocking antibody did not reduce cellular adhesion to FN.
U937 cells expressed both very late antigen (VLA) 4 (α4β1 heterodimer) and VLA-5 (α5β1 heterodimer) integrin but adhered to FN primarily by means of VLA-5 integrin.
(A) Cell-surface expression of integrin subunits was measured using flow cytometry. The mean fluorescence of 10 000 events for each subunit (3 independent experiments) is shown. (B) Blocking antibodies were used to determine the specificity of binding of U937 cells to FN. The presence of the α5 or the β1 blocking antibody reduced adhesion of U937 cells to levels comparable to those with BSA. In contrast, the α4 blocking antibody did not reduce cellular adhesion to FN.
Inhibition of cell growth
Inhibition of cell growth was determined by using a modified monotetrazolium (MTT) dye assay with the following modifications.1 A 96-well Immunosorp plate was coated with FN as described previously.1 Briefly, cells were washed once in serum-free RPMI medium, and FN samples were plated at a density of 150 000 cells/mL. Cells in suspension were incubated in serum-free RPMI medium in a conical tube at the same density as cells attached to FN. After 2 hours of cellular adhesion, wells were aspirated and 180 μL RPMI medium containing 10% FCS was added to each well. Cells maintained in suspension were centrifuged and resuspended in RPMI medium containing 10% FCS at a concentration of 100 000 cells/mL. Plates were treated with various concentrations of drug for 1 hour. After 1 hour of drug exposure, plates were washed 3 times with RPMI containing 10% FCS. After a 72-hour incubation at 37°C, 50 μL MTT dye (2 mg/mL) was added to each well, and the cells were incubated for an additional 4 hours. Plates were centrifuged once at 500g, medium was aspirated, the water-insoluble product was dissolved in DMSO, and absorbance was read at 490 nm on an automatic plate reader. The concentration of drug that produced 50% inhibition of growth (IC50) was calculated by using linear regression analysis.
Apoptosis
A flow cytometric assay assessing annexin V staining was used to count apoptotic cells after drug exposure, as described previously.1 After 2 hours of adhesion to FN, cells were exposed to drug for 1 hour and extracellular drug was removed by 3 washes with RPMI medium containing 10% FCS. For experiments examining etoposide-induced apoptosis, cells were exposed continuously to various doses of etoposide. Apoptotic cells were detected 20 hours after initial drug exposure by using annexin V staining and flow cytometric analysis. Ten thousand events were analyzed by flow cytometry (Becton Dickinson, San Jose, CA). Mean and SD values were calculated from the results of at least 3 independent experiments done in duplicate. Statistical comparisons used the Student t test.
Comet assay
Cells were placed in serum-free RPMI medium (750 000 cells/mL) and either adhered to FN-coated, 35-mm plates (Nunc) or placed in suspension for 2 hours. After 2 hours of adhesion, cells were exposed to various concentrations of drug for 1 hour. Subsequently, 5000 cells were placed in a microcentrifuge tube containing 1 mL cold PBS, and the neutral comet assay was done as described by Kent et al.12Briefly, cells were centrifuged and resuspended in 500 μL cold PBS, and 1.5 mL 1% agarose was added to each sample. The agarose-cell suspension was gently layered on a frosted-glass microscope slide, allowed to solidify for 5 minutes, and then placed immediately in ice-cold lysis buffer containing 30 mM disodium ethylenediamine tetraacetic acid (EDTA, pH 8.0), 0.5% sodium dodecyl sulfate (SDS), and 0.25 mg/mL proteinase K (Fisher Scientific, Norcross, GA). The samples were lysed for 1 hour at 4°C and then kept at 37°C for 12 to 16 hours. After cell lysis and digestion of protein-DNA complexes with proteinase K, the agar slides were re-equilibrated in TBE (90 mM Tris-hydrochloric acid, 90 mM boric acid, and 2 mM EDTA [pH 8.0]) for 2 hours, with a change of buffer every 15 minutes. The samples were electrophoresed with TBE buffer for 20 minutes at 25 V. The DNA was then stained with a 1:10 000 dilution of Sybr Green (Molecular Probes, Eugene, OR) for 20 minutes and slides were washed twice for 5 minutes in TBE. To ensure random sampling, 50 images/slide were captured and, in some experiments, the observer was blinded to the conditions. The images were captured on a fluorescent microscope (Vysis, Downers Grove, IL) and quantified by using Imagequant software (Molecular Dynamics, Sunnyvale, Ca). The comet moment was calculated by using the following equation described by Kent et al12: comet moment = Σ0−n ((intensity of DNA at distance X) × (distance))/intensity of total DNA.
The mean comet-moment value obtained from vehicle-control samples was subtracted from the mean comet-moment value for each drug dosage. Data shown are the mean and SD values from 3 independent experiments (50 images for each dose of each independent experiment). An analysis of variance (ANOVA) model was used to quantify the relation between the response variable and the 2 independent variables. The response variable in the analysis was the difference between the mean comet-moment values for the control and drug-treated samples. Independent variables were the dosage of mitoxantrone (0.1 μM, 1 μM, and 10 μM) and the treatment type (FN versus suspension). The variance estimate for the test statistic was calculated by pooling the variances from each of the 2 groups (control and treated).
Accumulation of carbon 14 (14C)–mitoxantrone
Cellular accumulation of 14C-mitoxantrone in FN-adhered cells and cells in suspension (1 × 106) was compared after a 1-hour exposure to 2.5 μM14C-mitoxantrone (specific activity, 0.3 GBq/mM). After exposure to drug at 37°C for 1 hour, FN-adhered and suspension cells were washed 3 times in cold PBS. The cells were counted before cell lysis with 10% SDS, and the data were normalized to counts per minute of 14C-mitoxantrone/1 million cells.
Topo II activity and expression
Cells in log-phase growth were washed once in serum-free RPMI medium, resuspended at density of 1 × 106 cells/mL in serum-free RPMI medium, and adhered to FN or placed in suspension as described previously.1 Nuclear extracts were prepared as described by Sullivan et al.7 All the following procedures were done at 4°C, and 1 mM phenylmethylsulfonyl fluoride, 5 μg/mL leupeptin, and 5 μg/mL aprotinin were added to buffers B to F. For FN samples, cells remained on FN-coated plates until placed in a dounce homogenizer. Approximately 25 million cells were washed once in PBS and then washed once with 25 mL buffer A (0.15 M sodium chloride [NaCl] and 10 mM potassium phosphate, monobasic [KH2PO4]). Samples were incubated for 30 minutes on ice with 4 mL buffer B (5 mM KH2PO4, 2 mM magnesium chloride [MgCl2], 4 mM dithiothreitol [DTT], and 0.1 mM sodium (NA2) EDTA). Cells were then dounce homogenized for 15 strokes; the release of nuclei was followed microscopically before proceeding to the next step. Nuclei were collected at 2500g for 15 minutes, further purified by resuspension in 2 mL buffer C (buffer B and 0.25 M sucrose), and layered over 1 mL buffer D (buffer B and 0.6 M sucrose). The sucrose gradient was centrifuged in a swinging-bucket rotor for 20 minutes at 2000g. The nuclear pellet was resuspended in 100 μL buffer E (5 mM KH2PO4, 4 mM DTT, and 1 mM Na2 EDTA), and the total volume was measured. An equal volume of buffer F (40 mM Tris [pH 7.5], 2 M NaCl, and 4 mM DTT) was added to the solution, which was incubated for an additional 30 minutes on ice. The lysate was centrifuged at 100 000g for 1 hour, and the supernatant was adjusted to 10% glycerol (vol/vol). To decrease the chance of topo II degradation, all topo activity assays were done on the same day the nuclear extracts were obtained.
For immunoblotting, 30 μg fresh nuclear extract from suspension and FN-adhered samples were separated on a 7.5% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. The blot was probed with either a topo IIα or topo IIβ polyclonal antibody or a topo I monoclonal antibody. The band of interest was detected by chemiluminescence (NEN, Boston, MA) and quantified by using Imagequant (Molecular Dynamics).
The catalytic activity of topo II was measured as the decatenation of networks of kinetoplast DNA (kDNA). The kDNA was labeled with tritium-thymidine and isolated from Crithidia fasciculata as described previously.7 One microgram nuclear protein extract and 0.40 μg kDNA was incubated in a total volume of 40 μL at 30°C for selected times. The reaction buffer consisted of the following: 50 mM Tris (pH 7.5), 100 mM NaCl, 10 mM MgCl2,1.0 mM ATP, 0.5 mM DTT, and 30 μg/mL BSA. The reaction was terminated by the addition of 5 μL 2.5% SDS. The samples were then microcentrifuged for 10 minutes at 12 000 rpm at room temperature. After centrifugation, 30 μL of the supernatant, which contained the released kDNA minicircles, was removed, liquid scintillation fluid was added, and radioactivity was measured with a scintillation counter (Beckman, Palo Alto, CA).
Immunohistochemical analysis
Confocal microscopy was used to determine whether cellular adhesion changed the intracellular localization of topo II. U937 cells were adhered to FN for 2 hours. After 2 hours of cellular adhesion, cells maintained in suspension or cells adhered to FN were fixed with 4% paraformaldehyde for 10 minutes. The cells were then cytospinned and subsequently permeabilized with 0.5% Triton-X, 1% glycine, and PBS for 1 hour as described previously.10 Briefly, after permeabilization, slides were incubated with either a 1:100 dilution of topo IIα or topo IIβ polyclonal antibody for 1 hour (0.1% NP-40 and 1% BSA in PBS). After several washes in PBS for 2 hours, slides were incubated with a goat anti–rabbit immunoglobulin G (IgG)–tetrarhodamine isothiocyanate–labeled antibody (Sigma) at a 1:80 dilution in 0.1% NP-40 and 1% BSA in PBS for 35 minutes in the dark. After incubation with the secondary antibody, slides were washed several times in PBS for 2 hours. Immunofluorescence was observed with a scanning confocal microscope (LSM 510; Zeiss, Göttingen, Germany). To obtain nuclear-to-cytoplasmic ratios of FN-adhered cells and cells in suspension, 100 individual cells were analyzed for pixel density of the nucleus and cytosol. The mean pixel density of the background was subtracted from all values before calculation of the nuclear-to-cytoplasmic ratio.
Results
Adhesion to FN is mediated by α5β1 integrin for U937 cells
Because surface expression of integrin subunits may not reflect functional adhesion, both cell-surface expression and functional adhesion with blocking antibodies were measured in U937 cells. Surface expression of α4, α5, and β1 integrin subunits is shown in Figure1A. The U937 cells expressed more α4 (mean fluorescence, 91.32 ± 31.74) than α5 (mean fluorescence, 36.11 ± 15.97); however, the use of blocking antibodies showed that adhesion of U937 cells is mediated primarily by α5β1 integrin (Figure 1B).
Adhesion of U937 cells to FN for 2 hours causes resistance to topo II inhibitors
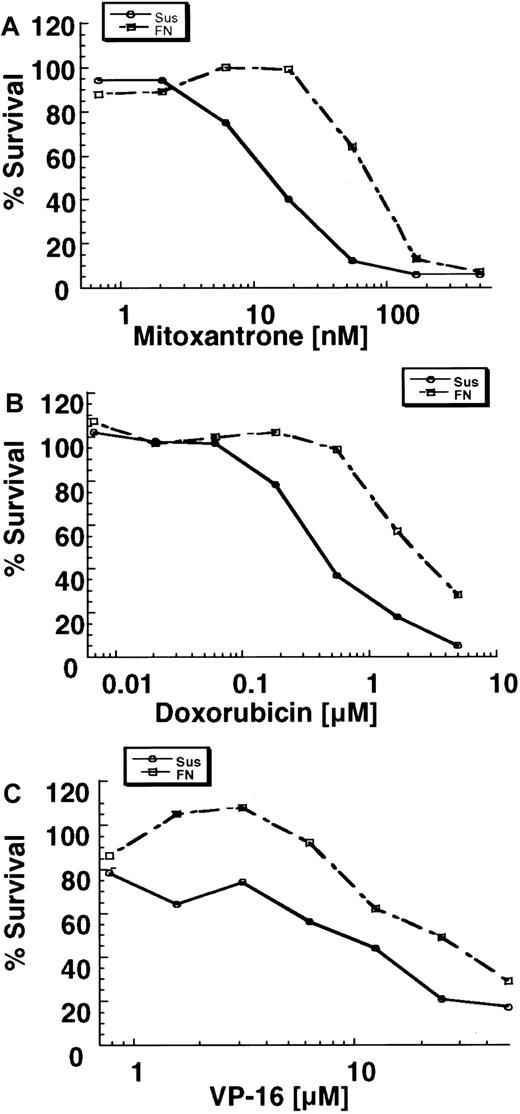
The MTT assay was used to determine whether the adhesion of U937 cells to FN protects cells from drug-induced cytotoxicity. As shown in Figure 2, adhesion of U937 cells to FN for 2 hours before drug exposure increased the IC50 value of mitoxantrone approximately 10 fold (range, 5-17 fold). The IC50 value of doxorubicin was increased approximately 3 fold (range, 2-5 fold). The degree of resistance for the nonintercalating topo II inhibitor etoposide, as measured by MTT assay, was less than that for mitoxantrone and doxorubicin, being approximately 2 fold (range, 1.3-4 fold).
U937 cells adhered to FN had increased survival (MTT analysis) after exposure to mitoxantrone (A), doxorubicin (B), or etoposide (C).
Shown are the mean results from a representative experiment done in quadruplicate wells. Three to six independent experiments were done, and similar results were obtained.
U937 cells adhered to FN had increased survival (MTT analysis) after exposure to mitoxantrone (A), doxorubicin (B), or etoposide (C).
Shown are the mean results from a representative experiment done in quadruplicate wells. Three to six independent experiments were done, and similar results were obtained.
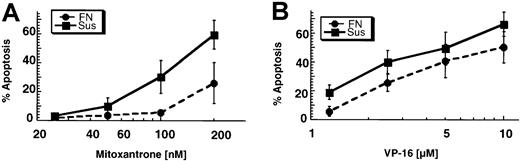
In addition to measuring cytotoxicity with the MTT assay, we used an apoptosis assay to assess the effects of FN adhesion on drug-induced apoptosis. FITC-conjugated annexin V, which binds to inverted phosphatidylserine on the surface of the plasma membrane, was used to identify apoptotic cells. As shown in Figure3A, cells adhered to FN before a 1-hour exposure to various doses of mitoxantrone had reduced apoptosis on annexin V staining. Cells treated while adhered to FN were also protected from etoposide-induced apoptosis (Figure 3B). These data indicate that adhesion of U937 cells to FN protects against mitoxantrone-induced apoptosis and, to a lesser extent, etoposide-induced apoptosis. Studies using doxorubicin were not done because the drug interfered with this fluorescence assay.
U937 cells adhered to FN for 2 hours were resistant to apoptosis induced by mitoxantrone and etoposide.
(A) Adhesion to FN significantly reduced mitoxantrone-induced apoptosis (P < .05 on ANOVA). Shown are the mean results and 95% confidence intervals (CIs) from 3 independent experiments done in duplicate (the percentage of apoptosis is equal to the percentage of apoptosis in drug-treated samples minus the percentage of apoptosis in the vehicle control). (B) Adhesion to FN significantly decreased etopside-induced apoptosis (P < .05 by ANOVA). Shown are the mean results and 95% CIs from 4 independent experiments done in duplicate (the percentage of apoptosis is equal to the percentage of apoptosis in drug-treated samples minus the percentage of apoptosis in the vehicle control).
U937 cells adhered to FN for 2 hours were resistant to apoptosis induced by mitoxantrone and etoposide.
(A) Adhesion to FN significantly reduced mitoxantrone-induced apoptosis (P < .05 on ANOVA). Shown are the mean results and 95% confidence intervals (CIs) from 3 independent experiments done in duplicate (the percentage of apoptosis is equal to the percentage of apoptosis in drug-treated samples minus the percentage of apoptosis in the vehicle control). (B) Adhesion to FN significantly decreased etopside-induced apoptosis (P < .05 by ANOVA). Shown are the mean results and 95% CIs from 4 independent experiments done in duplicate (the percentage of apoptosis is equal to the percentage of apoptosis in drug-treated samples minus the percentage of apoptosis in the vehicle control).
Adhesion of U937 cells to FN reduces mitoxantrone- and etoposide-induced DNA double-strand breaks as measured by neutral comet assay
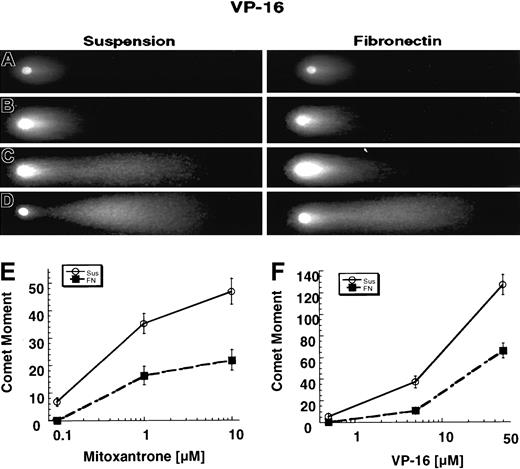
Mitoxantrone and etoposide are known to stabilize topo II–DNA complexes, resulting in DNA double-strand breaks.13-15 We used the neutral comet assay to compare the amount of mitoxantrone- or etoposide-induced DNA double-strand breaks in U937 cells that were either exposed to drug in suspension or adhered to FN. The comet moment is a function of both the distance and the amount (measured in pixel density) of DNA that migrates from the center of the head of the comet. As shown in Figure 4, the tail length, tail intensity, and tail shape differed according to whether the cells were treated with drug in suspension or while adhered to FN. After 2 hours of adhesion to FN, both mitoxantrone- and etoposide-induced comet-moment values were decreased by approximately 40% to 60% compared with results in cells treated in suspension (Figure 4E-F). The ANOVA showed a significant difference between cells treated with drug in suspension and adhered to FN (P < .01 for all doses tested).
The comet tail shape, intensity, and length differed according to whether the cells were treated in suspension or adhered to FN.
The comet moment is a function of the distance and intensity of DNA from the center of the comet head. (A) Results with 0.1% DMSO. (B) Results with 0.5 μM etoposide. (C) Results with 5.0 μM etoposide. (D) Results with 50 μM etoposide. (D-E) Cells adhered to FN for 2 hours showed a significant (P < .01) reduction in mitoxantrone- and etoposide-induced DNA double-strand breaks. The neutral comet assay was used to compare drug-induced DNA double-strand breaks in cells adhered to FN and those cultured in suspension. Cells were exposed to various concentrations of either mitoxantrone (E) or etoposide (F) for 1 hour. The comet moment was then calculated. Fifty images were captured for each dose, and 3 independent experiments were done. The graph represents the mean values and 95% CIs from 3 independent experiments.
The comet tail shape, intensity, and length differed according to whether the cells were treated in suspension or adhered to FN.
The comet moment is a function of the distance and intensity of DNA from the center of the comet head. (A) Results with 0.1% DMSO. (B) Results with 0.5 μM etoposide. (C) Results with 5.0 μM etoposide. (D) Results with 50 μM etoposide. (D-E) Cells adhered to FN for 2 hours showed a significant (P < .01) reduction in mitoxantrone- and etoposide-induced DNA double-strand breaks. The neutral comet assay was used to compare drug-induced DNA double-strand breaks in cells adhered to FN and those cultured in suspension. Cells were exposed to various concentrations of either mitoxantrone (E) or etoposide (F) for 1 hour. The comet moment was then calculated. Fifty images were captured for each dose, and 3 independent experiments were done. The graph represents the mean values and 95% CIs from 3 independent experiments.
A drug-accumulation assay was done to determine whether the reduction in drug-induced DNA double-strand breaks correlated with reduced intracellular drug accumulation. Total intracellular14C-mitoxantrone was measured after a drug exposure of 1 hour, a time consistent with the measurement of drug-induced DNA damage. The adhesion-dependent decrease in DNA double-strand breaks could not be attributed to decreased intracellular drug accumulation (counts per minute for 14C-mitoxantrone, 8853 ± 1329 in cells in suspension and 10 266 ± 1052 in FN-adhered cells; no significant difference at the P < .05 level). These data indicate that the reduction in cytotoxicity and DNA damage that occurs when cells are adhered to FN is not due to a decrease in intracellular concentration of the drug. These findings are consistent with our previous study showing that adhesion of the multiple myeloma 8226 cell line to FN did not alter the intracellular concentration of doxorubicin.1
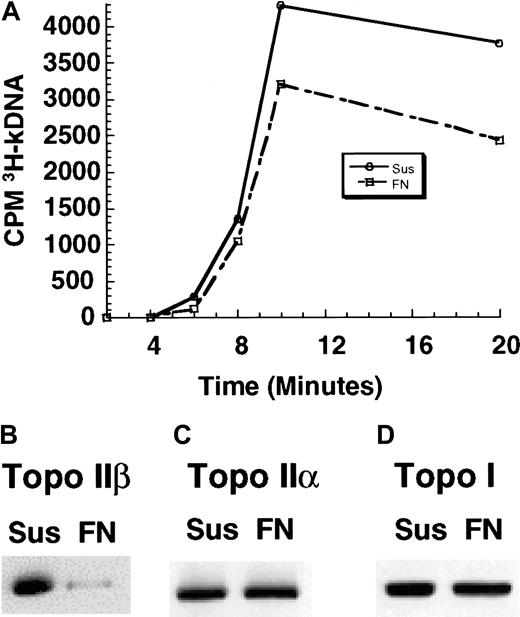
Adhesion of U937 cells to FN reduces salt-extractable topo II activity and topo IIβ protein levels and alters the nuclear localization of topo IIβ but does not change the total levels or nuclear-to-cytoplasmic ratio of topo IIβ
Mitoxantrone and etoposide were previously reported to stabilize topo II–DNA complexes.8,13,14 Furthermore, cell lines selected in vitro for resistance to mitoxantrone and etoposide often have qualitative or quantitative changes in topo II.8,16 17 To determine whether the decrease in drug-induced DNA double-strand breaks correlated with alterations in topo II, we examined nuclear topo II activity by measuring the decatenation activity of the enzyme. After 2 hours of cellular adhesion to FN, the amount of cleaved kDNA was approximately 20% to 30% lower (Figure 5A) than that in cells grown in suspension (2 independent nuclear extracts). Western blotting was done to determine whether the adhesion-dependent decrease in topo II catalytic activity correlated with decreased nuclear protein levels. As shown in Figure 5B, Western blot analysis of nuclear extracts found that topo IIβ levels were decreased by 66% (mean value from 3 independent experiments) in cells adhered to FN compared with cells grown in suspension. In contrast, adhesion of cells to FN did not alter the nuclear levels of topo IIα (Figure 5C). Furthermore, topo I levels remained constant in nuclear extracts prepared from either cells in suspension or FN-adhered cells (Figure 5D).
Adhesion of U937 cells to FN decreased topo II activity and nuclear topo IIβ protein levels.
(A) Nuclear extracts were obtained, and 1 μg nuclear extract from cells in suspension and FN-adhered cells were incubated with 0.4 μg tritium-kDNA for the times indicated. The amount of cleaved kDNA was reduced by approximately 20% to 30% in FN-adhered cells compared with cells grown in suspension (results were identical for 2 independent experiments, and the figure represents results from one experiment). (B) Western blot analysis of 1.0-M NaCl nuclear extracts showed a 66% reduction (mean value from 3 independent experiments) in nuclear topo IIβ protein levels when cells were adhered to FN. (C) Topo IIα protein levels were unchanged when cells were adhered to FN. (D) Topo I levels were unchanged when cells were adhered to FN.
Adhesion of U937 cells to FN decreased topo II activity and nuclear topo IIβ protein levels.
(A) Nuclear extracts were obtained, and 1 μg nuclear extract from cells in suspension and FN-adhered cells were incubated with 0.4 μg tritium-kDNA for the times indicated. The amount of cleaved kDNA was reduced by approximately 20% to 30% in FN-adhered cells compared with cells grown in suspension (results were identical for 2 independent experiments, and the figure represents results from one experiment). (B) Western blot analysis of 1.0-M NaCl nuclear extracts showed a 66% reduction (mean value from 3 independent experiments) in nuclear topo IIβ protein levels when cells were adhered to FN. (C) Topo IIα protein levels were unchanged when cells were adhered to FN. (D) Topo I levels were unchanged when cells were adhered to FN.
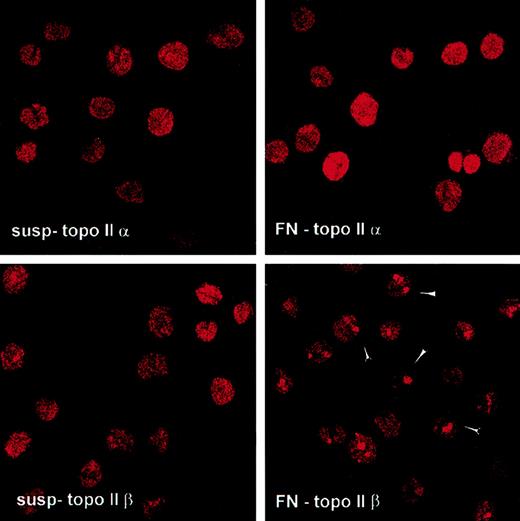
Confocal microscopy was used to determine whether cellular adhesion to FN altered the cellular distribution of topo IIα and topoIIβ. We found that adhesion of U937 cells to FN resulted in distinct punctate clusters of topo IIβ in the nuclei of adhered cells (Figure6), suggesting that adhesion alters the nuclear distribution of topo IIβ. After cellular adhesion to FN, most topo II remained nuclear (Figure 6; nuclear-to-cytoplasmic ratio for topo IIα, 7.56 ± 0.51 in cells in suspension and 5.39 ± 0.37 in FN-adhered cells; ratio for topo IIβ, 6.01 ± 1.58 in cells in suspension and 4.87 ± 0.33 in FN-adhered cells). Furthermore, the mean total fluorescence/cell was not altered in FN-adhered cells (pixel density for topo IIα/cell, 174 537 ± 34 112 in cells in suspension and 134 275 ± 24 173 in FN-adhered cells; density for topo IIβ/cell, 77 534 ± 10 263 in cells in suspension and 83 732 ± 7845 in FN-adhered cells).
Cellular adhesion to FN altered the nuclear distribution of topo IIβ.
After 2 hours of cellular adhesion to FN, cells were fixed and stained for either topo IIα or topo IIβ. One hundred cells were analyzed for pixel density of the cytoplasm and nucleus. The nuclear-to-cytoplasmic ratio for topo IIα for cells grown in suspension was 7.56 ± 0.51, whereas that for FN-adhered cells was 5.39 ± 0.37. For topo IIβ, the nuclear-to-cytoplasmic ratio was 6.01 ± 1.58 for cells grown in suspension and 4.87 ± 0.33 for cells adhered to FN.
Cellular adhesion to FN altered the nuclear distribution of topo IIβ.
After 2 hours of cellular adhesion to FN, cells were fixed and stained for either topo IIα or topo IIβ. One hundred cells were analyzed for pixel density of the cytoplasm and nucleus. The nuclear-to-cytoplasmic ratio for topo IIα for cells grown in suspension was 7.56 ± 0.51, whereas that for FN-adhered cells was 5.39 ± 0.37. For topo IIβ, the nuclear-to-cytoplasmic ratio was 6.01 ± 1.58 for cells grown in suspension and 4.87 ± 0.33 for cells adhered to FN.
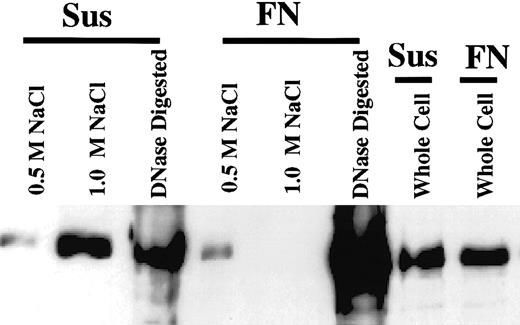
To assess whether cellular adhesion to FN altered the nuclear binding properties of topo IIβ, we measured the amount of topo IIβ remaining in the nuclear fraction after 2 sequential salt extracts of 0.5 M and 1.0 M NaCl. After the last salt extract, the remaining pellet was digested with DNase, and proteins were separated by SDS–polyacrylamide gel electrophoresis (PAGE). We found that salt extracts from FN-adhered samples contained less topo IIβ than cells grown in suspension (Figure 7). In contrast, the remaining DNase-digested pellet contained more topo IIβ than cells grown in suspension. These results are consistent with increased nuclear binding of topo IIβ in cells adhered to FN.
Adhesion of cells to FN increased the amount of topo IIβ bound to the nucleus after a 1.0-M NaCl extract.
Nuclei isolated from FN-adhered cells or cells in suspension were sequentially extracted in 0.5 M and 1 M NaCl. After the 1.0-M salt extract, the remaining pellet was sonicated and digested with DNase. Ten micrograms of the 0.5-M and 1.0-M NaCl extracts and 100 μg of the DNase-digested extracts were separated by SDS-PAGE and immunoblotted for topo IIβ.
Adhesion of cells to FN increased the amount of topo IIβ bound to the nucleus after a 1.0-M NaCl extract.
Nuclei isolated from FN-adhered cells or cells in suspension were sequentially extracted in 0.5 M and 1 M NaCl. After the 1.0-M salt extract, the remaining pellet was sonicated and digested with DNase. Ten micrograms of the 0.5-M and 1.0-M NaCl extracts and 100 μg of the DNase-digested extracts were separated by SDS-PAGE and immunoblotted for topo IIβ.
Discussion
Durand and Sutherland18 were among the first investigators to show that changes in the microenvironment can alter the response to radiation. In their model, cells grown as a spheroid were more resistant to drugs and radiation than cells grown as a monolayer. The increase in drug resistance associated with spheroid cultures compared with monolayer cultures was shown to correlate with decreased drug-induced DNA damage.19 These results suggest that cell-cell contact or cell-matrix contact modulates the cellular response to drug-induced DNA damage. Moreover, the decrease in drug-induced DNA damage was found to be correlated with the redistribution of topo IIα from the nucleus to the cytosol.20 However, studies using a cell-spheroid model have not identified a cell-surface receptor responsible for drug resistance. We chose to focus on the role of β1 integrin–mediated adhesion and the contribution of this specific receptor-ligand interaction to mediating drug resistance and drug-induced DNA damage.
We previously showed that β1 integrin–mediated adhesion inhibits drug-induced apoptosis in myeloma cell lines.1 Similarly, Sethi et al2 reported that β1–mediated adhesion confers resistance to chemotherapeutic drugs in small-cell lung cancer cell lines. Similar to the results in the spheroid model was the finding by Hoyt et al21 that adhesion of murine tumor–derived endothelial cells by means of β1 integrins resulted in diminished etoposide-induced DNA damage, indicating that β1 integrins modulate drug-induced DNA damage in nontransformed cells. We previously determined that adhesion of myeloma cells to FN resulted in inhibition of cell-cycle progression and increased p27kip1 protein levels. Furthermore, the increase in p27kip1 protein was casually related to the CAM-DR phenotype.22 However, it is not known how the FN-induced increase in p27kip1 confers drug resistance.
In this study, we found that adhesion of U937 cells to FN by means of β1 integrin for 2 hours is sufficient to inhibit initial DNA double-strand breaks induced by the topo II inhibitors mitoxantrone and etoposide. After a 1-hour exposure to mitoxantrone, the decrease in drug-induced DNA double-strand breaks did not correlate with decreased total intracellular drug concentration. However, these results do not exclude the possibility that changes in drug efflux over time or alterations in the intracellular localization of drug contribute to a decrease in initial DNA damage and drug resistance.
Topo II is the putative target of mitoxantrone and etoposide that causes DNA double-strand breaks. Thus, qualitative or quantitative changes in topo II can decrease such drug-induced breaks. We observed that adhesion of U937 cells to FN resulted in diminished topo II catalytic activity as measured by the release of minicircles from kDNA. The decrease in enzymatic activity correlated with a decrease in salt-extractable nuclear topo IIβ protein levels. However, the nuclear-to-cytoplasmic ratio of topo IIβ was only marginally decreased (by approximately 20%), and no significant changes in total pixel density were observed. The discrepancy between the confocal data and Western blot results regarding salt-extractable topo IIβ suggests that cellular adhesion to FN results in changes in the affinity of topo IIβ for the nucleus. The confocal studies showed a redistribution of topo IIβ in the nucleus, suggesting the presence of qualitative differences in topo IIβ in cells in suspension and FN-adhered cells. Furthermore, the decrease in salt-extractable nuclear protein was confirmed by demonstration of an increase in topo IIβ associated with DNA after a 1M NaCl extraction. Additional studies are needed to determine whether these changes in the nuclear localization of Topo IIβ produce the reduction in drug-induced DNA damage.
Topo II has been shown to be posttranscriptionally modified by phosphorylation and ribosylation,23,24 and perhaps posttranscriptional modifications alter the affinity of topo IIβ for DNA. An alternative explanation for our results is that topo IIβ is sequestered by DNA-binding proteins in the nucleus. Topo IIβ was shown to bind specifically to several nuclear binding proteins, including histone deacetylase (HDAC) 1 and HDAC2, and this protein complex was found to contain HDAC activity.25 The investigators also showed that topo IIβ coprecipitated with metastasis-associated protein 2, a component of the nucleosome remodeling and deacetylating (NuRD) complex. The NuRD complex contains both chromatin remodeling activity and HDAC activity.26 27It is possible that recruitment of topo IIβ into the NuRD complex decreases the ability to extract topo IIβ from the nucleus with 1.0 M NaCl. Additional studies are needed to determine whether FN adhesion results in alterations in the binding or activity of protein complexes associated with topo IIβ.
There is evidence that etoposide and mitoxantrone inhibit both topo IIα and topo IIβ. However, cells derived from a murine topo IIβ knockout were more resistant to mitoxantrone and amsacrine than to etoposide.28 We found that reduced extractable topo IIβ protein levels correlated with decreased mitoxantrone- and etoposide-induced DNA double-strand breaks; however, the protection of mitoxantrone was greater than that of etoposide. A possible explanation for the greater protection observed for mitoxantrone is that mitoxantrone-induced topo IIβ complexes are more lethal than etoposide-stabilized topo IIβ–DNA complexes. Alternatively, the increased resistance observed with mitoxantrone compared with etoposide may have resulted from activation of separate signal transduction pathways initiated by binding to FN that are unrelated to DNA double-strand breaks.
Little is known about extracellular signals that regulate expression and activity of topo IIβ. Activation of β1 integrin signaling is known to activate several signal transduction pathways, including phosphatidylinositol 3 kinase,29 protein kinase B,30,31 mitogen-activated protein kinase,32and focal adhesion kinase.33 We are currently investigating whether activation of these known β1–mediated signaling pathways contributes to changes in the nuclear distribution of topo IIβ or diminished drug-induced DNA double-strand breaks.
In summary, we found that adhesion of U937 cells to FN attenuates DNA double-strand breaks induced by mitoxantrone and etoposide. The decrease in these breaks correlated with a decrease in topo II catalytic activity and salt-extractable topo IIβ protein levels. Collectively, these data suggest that cell-adhesion–mediated protection from drug-induced apoptosis mediated by topo II inhibitors is the result of decreased drug-induced DNA damage. Furthermore, the decrease in drug-induced DNA damage may be related to the changes in the nuclear localization or binding properties of the nuclear pool of topo IIβ protein.
We thank the H. Lee Moffitt Analytical Microscopy Core Facility, the H. Lee Moffitt Flow Cytometry Core Facility, the H. Lee Moffitt Biostatistics Core Facility, all of which are supported by National Institutes of Health grant P30-CA76292-04-08; and Peggy Farrell for careful editing of the manuscript.
Supported in part by National Cancer Institute grants CA77859 (W.S.D.) and CA82533 (W.S.D.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
William S. Dalton, H. Lee Moffitt Cancer Center, 12902 Magnolia Drive, Tampa, FL 33612; e-mail: dalton@moffitt.usf.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal