Abstract
Autoreactive CD4+ T cells to β2-glycoprotein I (β2GPI) that promote antiphospholipid antibody production were recently identified in patients with antiphospholipid syndrome (APS). To further examine antigen recognition profiles and T-cell helper activity in β2GPI-reactive T cells, 14 CD4+ T-cell clones specific to β2GPI were generated from 3 patients with APS by repeated stimulation of peripheral blood T cells with recombinant β2GPI. At least 4 distinct T-cell epitopes were identified, but the majority of the β2GPI-specific T-cell clones responded to a peptide encompassing amino acid residues 276 to 290 of β2GPI (KVSFFCKNKEKKCSY; single-letter amino acid codes) that contains the major phospholipid-binding site in the context of the DRB4*0103 allele. Ten of 12 β2GPI-specific T-cell clones were able to stimulate autologous peripheral blood B cells to promote anti-β2GPI antibody production in the presence of recombinant β2GPI. T-cell helper activity was exclusively found in T-cell clones capable of producing interleukin 6 (IL-6). In vitro anti-β2GPI antibody production induced by T-cell clones was inhibited by anti-IL-6 or anti-CD40 ligand monoclonal antibody. In addition, exogenous IL-6 augmented anti-β2GPI antibody production in cultures of the T-cell clone lacking IL-6 expression. These results indicate that β2GPI-specific CD4+ T cells in patients with APS preferentially recognize the antigenic peptide containing the major phospholipid-binding site and have the capacity to stimulate B cells to produce anti-β2GPI antibodies through IL-6 expression and CD40-CD40 ligand engagement. These findings are potentially useful for clarifying the pathogenesis of APS and for developing therapeutic strategies that suppress pathogenic antiphospholipid antibody production in these patients.
Introduction
Antiphospholipid syndrome (APS) is characterized by arterial and venous thrombosis as well as recurrent intrauterine fetal loss in association with antiphospholipid antibodies.1 It is now widely accepted that β2-glycoprotein I (β2GPI) is the most common antigenic target for the antiphospholipid antibodies associated with the clinical features of APS.2,3 Beta2-GPI is a plasma glycoprotein that binds various kinds of negatively charged substances, including phospholipids (PLs), lipoproteins, activated platelets, and endothelial cells.4-6 It possesses 5 complement control protein repeats (domains I-V), called “sushi” domains.7 These domains have a homologous 60-amino acid repeating sequence with both inter- and intraregional disulfide bridges, and domain V has a 6-residue insertion and a 19-residue C-terminal extension. The major PL-binding site on β2GPI has been identified as a highly positively charged amino acid sequence located at positions 281 to 288 in domain V.8-10 Recent crystal structure analysis has revealed that β2GPI adheres to anionic PLs via a large positively charged area formed by 14 basic amino acid residues in domain V, including the major PL-binding site.11
Autoantibodies to β2GPI were shown to be pathogenic, based on the following observations in patients with APS and experimental animal models. First, the presence of serum anti-β2GPI antibody is a major risk factor for arterial and venous thrombosis in patients with systemic lupus erythematosus (SLE).12 Second, animals immunized with foreign β2GPI develop a clinical manifestation of APS, including intrauterine fetal death and thrombocytopenia, along with the induction of anti-β2GPI antibody production.13,14Finally, normal mice infused with anti-β2GPI monoclonal antibodies (mAbs) or the IgG fraction of sera from patients with APS develop fetal resorption.15,16 However, some reports failed to induce fetal death or resorption in similar immunization or passive transfer experiments.17,18 These variable effects of anti-β2GPI antibodies on murine pregnancy outcome could be explained by the antibody recognition of heterogeneous epitopes relevant or irrelevant to APS symptoms. Precise mechanisms for the thrombophilia caused by anti-β2GPI antibodies remain unclear, but it is proposed that anti-β2GPI antibodies bind to endothelial cell surfaces by recognizing the adhered β2GPI and induce endothelial cell activation, resulting in the up-regulation of procoagulant and inflammatory processes.19 20
We have recently identified β2GPI-reactive CD4+ T cells in the peripheral blood of patients with APS.21 These β2GPI-reactive T cells possess helper activity that induces the production of antibodies that specifically bind to β2GPI immobilized on cardiolipin. Autoreactive T cells to β2GPI have been presumed to recognize cryptic determinants, because they react with chemically reduced β2GPI and recombinant β2GPI fragments produced in bacteria, but not with β2GPI in its native form. However, we were unable to analyze the T-cell epitopes on β2GPI or the cytokines involved in the helper activity in bulk T-cell cultures. In this study, we have established β2GPI-specific CD4+ T-cell clones from the peripheral blood of patients with APS and investigated their antigen recognition profiles and helper activity involved in promoting anti-β2GPI antibody production from B cells.
Patients, materials, and methods
Patients
Peripheral blood T cells from 3 Japanese patients with APS (O.M., E.Y., and K.S.) were analyzed in this study. All patients fulfilled the preliminary classification criteria for APS proposed by the International Workshop in Sapporo, Japan.22 Primary APS was diagnosed in patients O.M. and K.S., and secondary APS accompanied by SLE was diagnosed in patient E.Y. At the time of blood examination, all patients were taking low-dose corticosteroids (< 10 mg/d) and aspirin. All samples were obtained after the patients gave their written informed consent, following Keio University Institutional Review Board guidelines.
Genotyping for HLA class II alleles and β2GPI
Genomic DNA was isolated from peripheral blood leukocytes using a standard phenol extraction procedure. The HLA-DRB1, DRB4, DRB5, DQB1, and DPB1 alleles were determined using polymerase-chain reaction (PCR) followed by analysis of restriction fragment length polymorphisms.23,24 The polymorphism at position 247 of β2GPI (247V-247L; single-letter amino acid codes) was determined by RsaI digestion of the PCR-amplified DNA.25
Detection of anti-β2GPI antibody
The IgG anti-β2GPI antibody levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Yamasa, Choshi, Japan), in which cardiolipin-coated plates were incubated with purified human β2GPI as a cofactor.26 A cutoff value for serum samples was 3.5 U/mL, according to the manufacturer's recommendation.
β2GPI preparations and synthetic peptides
Recombinant maltose-binding protein (MalBP) fusion proteins encoding β2GPI amino acid sequences were prepared and used as antigens for T-cell stimulation.21 These included GP-F encoding the entire amino acid sequence of β2GPI (amino acid residues 1-326, reported by Matsuura et al27); GP1, encoding domains I and II (amino acid residues 1-133); GP2, encoding domains III and IV (amino acid residues 119-254); and GP3, encoding domains IV and V (amino acid residues 182-326). MalBP was also prepared and used as a control antigen. Human β2GPI purified from pooled human plasma was provided by Yamasa, and native and reduced β2GPI were prepared as described elsewhere.21
Fifteen 15-mer peptides with 9- or 10-residue overlaps encompassing the entire domain V of β2GPI were synthesized using a solid-phase multiple synthesizer (Advanced Chemtech, Louisville, KY). The purity of all peptide preparations was more than 50%, as determined by high-performance liquid chromatography.
All β2GPI preparations at a concentration of 20 μg/mL were confirmed not to interfere with T-cell proliferation induced by phytohemagglutinin (PHA).
Establishment of β2GPI-specific T-cell clones
The β2GPI-specific T-cell clones were generated according to a previously described method28 with some modifications. Briefly, peripheral blood mononuclear cells (PBMNCs) were isolated from heparinized venous blood by Lymphoprep (Nycomed Pharma, Oslo, Norway) density-gradient centrifugation. The cells (2 × 106/well) were cultured in 24-well plates with GP-F (10 μg/mL) in RPMI 1640 supplemented with 8% autologous heat-inactivated plasma, 2 mM l-glutamine, 10 mMN-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 50 U/mL penicillin, and 50 μg/mL streptomycin in a humidified atmosphere of 5% CO2 at 37°C. On day 3, 30 U/mL interleukin (IL) 2 was added to the culture. Cells were restimulated with GP-F (10 μg/mL), IL-2 (100 U/mL), and 106 irradiated (40 Gy) autologous PBMNCs in fresh medium at day 10. Seven days after the second stimulation, T-cell blasts were cloned by limiting dilution at 0.3 cells/well using round-bottomed 96-well plates in the presence of GP-F, IL-2, and irradiated PBMNCs. After an additional stimulation, growth-positive wells were selected and the cells in these wells were expanded by a repeated stimulation with GP-F, IL-2, and autologous Epstein-Barr virus–transformed lymphoblastoid B-cell line cells irradiated at 100 Gy. The specificity of each T-cell clone was assessed by antigen-induced T-cell proliferation, and T-cell clones that proliferated in response to GP-F, but not to MalBP, were selected as β2GPI-specific T-cell clones. T-cell clones were maintained in cultures by stimulation with GP-F, IL-2, and irradiated autologous B-cell line cells at 7- to 10-day intervals. The cell surface expression of CD4 and CD8 was examined by flow cytometry using fluorescein isothiocyanate–conjugated mAbs to CD4 and CD8 (Becton Dickinson, San Jose, CA), respectively.
T-cell proliferation assay
The antigen-specific proliferation of T-cell clones was determined principally as described previously.28 The T-cell clones (2 × 104/well) were cultured with irradiated autologous B-cell line cells (2 × 104/well) and antigen, including GP-F, GP1, GP2, GP3, MalBP, native β2GPI, and reduced β2GPI (10 μg/mL) as well as tetanus toxoid (TT; List Biological Laboratories, Campbell, CA; 5 μg/mL). PHA (1 μg/mL) was also used to exclude nonspecific unresponsiveness. L cells transfected with the DRA gene and one of the following DRB genes, DRB1*1501 (LDR2B), *1502 (LARB1-1212), *0403 (B19), *0901 (L/KR1-3-A10), DRB4*0103 (L17.8), DRB5*0101 (LDR2A), and *0102 (AB5), were also used as antigen-presenting cells (APCs). These cell lines were distributed by the 11th International Histocompatibility Workshop.29 L cells transfected with the Neor gene alone were used as a control. All transfectants were confirmed to express human HLA-DR molecules by flow cytometry before use in the assay. L cells were irradiated at 40 Gy and incubated with synthetic peptides (5 μg/mL unless indicated otherwise) for 2 hours before mixing with T-cell clones. After 60 hours of incubation with antigen, 0.5 μCi/well 3H-thymidine was added to the cultures for 16 hours. The cells were then harvested and 3H-thymidine incorporation was determined in a Top-Count microplate scintillation counter (Packard, Meriden, CT). All cultures were carried out in duplicate or triplicate, and all values represent the mean of duplicate or triplicate determinations.
The HLA class II restriction of individual T-cell clones was determined based on the inhibitory effects of anti-HLA-DR (L243; IgG2a), anti-HLA-DQ (1a3; IgG2a), and anti-HLA-DP (B7/21; IgG3) mAbs (1 μg/mL; Leinco Technologies, Ballwin, MO) on GP-F–induced T-cell proliferation.
Analysis of in vitro anti-β2GPI antibody production
The helper activity of β2GPI-specific T-cell clones was evaluated using an in vitro culture system in which β2GPI-specific T-cell clones (3 × 105cells) were cultured with autologous peripheral blood B cells (3 × 105 cells) in the presence or absence of antigen (GP-F, MalBP, or TT; 10 μg/mL) and pokeweed mitogen (1 μg/mL) for 10 days.30 B cells were obtained from nonadherent PBMNCs by 2 purifications using nylon wool columns, followed by the depletion of contaminating T cells by incubation with anti-CD4 and anti-CD8 mAb-coupled magnetic beads (Dynal, Oslo, Norway).30Culture supernatants were then harvested and the anti-β2GPI antibody levels were measured using an anti-β2GPI ELISA. All samples were tested in duplicate, and results were expressed as the mean of the duplicate values minus the mean of the reference blank values.
The effects of blocking T- and B-cell interactions on in vitro anti-β2GPI antibody production were determined by the addition of anti-HLA-DR, anti-HLA-DQ, anti-HLA-DP (1 μg/mL), anti-CD40 ligand (CD40L; 1 μg/mL; IgG1) (Ancell, Bayport, MN), anti-IL-6 (25 μg/mL); IgG1 or anti-interferon-γ (IFN-γ) mAb (25 μg/mL unless indicated otherwise; IgG2a) (Genzyme, Cambridge, MA). In some experiments, exogenous IL-4 or IL-6 (Life Technologies, Grand Island, NY) was added to the cultures. All mAbs and exogenous cytokines were added at the initiation of the cultures.
Cytokine production assay
The β2GPI-specific T-cell clones were stimulated with PHA (1 μg/mL) and anti-CD3 mAb (OKT3; 30 ng/mL) for 48 hours. Culture supernatants were collected, and amounts of human IFN-γ, IL-4, IL-6, and IL-10 were measured in duplicate using ELISA kits (Biosource International, Camarillo, CA) according to the manufacturer's instruction.
Statistical analysis
Proliferation of T cells, anti-β2GPI antibody, and cytokine data were summarized with means ± SDs. Statistical comparisons were performed using Student t tests for independent samples. A correlation between anti-β2GPI antibody levels produced in vitro and the amounts of individual cytokines in supernatants was examined using a single regression model.P less than.05 was regarded as a significant difference.
Results
Establishment of β2GPI-specific T-cell clones
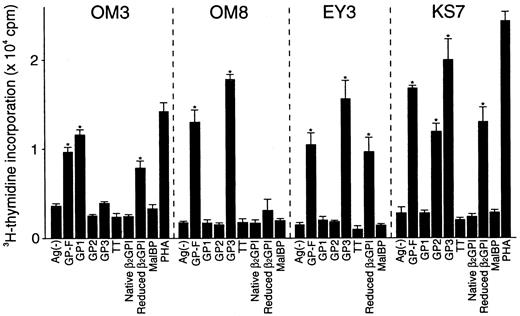
A total of 14 T-cell clones specific to β2GPI were generated from 3 patients who had typical clinical features of APS and a high level of serum anti-β2GPI antibodies (Table1). The clonality was confirmed in 7 lines based on the observation that each had only one type of functionally rearranged T-cell receptor β chain (data not shown). As shown in Figure 1, representative T-cell clones OM3, OM8, EY3, and KS7 proliferated in response to GP-F, but not to MalBP. Reduced β2GPI induced a proliferative response in OM3, EY3, and KS7, but not in OM8. In addition, OM3 responded to GP1, OM8 and EY3 responded to GP3, and KS7 responded to both GP2 and GP3. Table 2 summarizes the surface phenotype, T-cell responses to various β2GPI preparations, and HLA class II restriction for the 14 β2GPI-specific T-cell clones. All T-cell clones had a CD4+CD8− helper phenotype. None of the clones responded to native β2GPI, but all except OM8 responded to reduced β2GPI. Eleven T-cell clones generated from the 3 patients responded to GP3 (domains IV and V), but not to GP2 (domains III and IV). The β2GPI-specific T-cell clones with this antigenic specificity recognized epitope(s) present within domain V in the context of HLA-DR. OM3 responded to GP1 (domains I and II) in an HLA-DP–restricted manner. KS7 and KS10 proliferated in response to both GP2 and GP3 in an HLA-DR–restricted manner and these T- cell clones appeared to recognize domain IV.
Clinical and laboratory findings, HLA class II alleles, β2GPI genotypes, and the number of β2GPI-reactive CD4+ T cell clones generated in patients with APS
| Patient . | Age/sex . | Clinical findings . | Anti-β2GPI antibody*(U/mL) . | HLA class II alleles . | β2GPI genotype at position 247† . | Number of β2GPI-specific T-cell clones . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Thrombosis . | Pregnancy loss . | DRB1 . | DRB4 . | DRB5 . | DQB1 . | DPB1 . | |||||
| O.M. | 39/F | Stroke | + | > 125.0 | *1501/*0403 | *0103 | *0101 | *0602/*0302 | *0201 | 247L/247L | 4 |
| E.Y. | 38/F | DVT, PE | N/A | 92.9 | *0901 | *0103 | — | *0303 | *0201 | 247V/247L | 4 |
| K.S. | 38/F | Stroke | + | > 125.0 | *1502/*0901 | *0103 | *0102 | *0601/*0303 | *0501/*0901 | 247V/247L | 6 |
| Patient . | Age/sex . | Clinical findings . | Anti-β2GPI antibody*(U/mL) . | HLA class II alleles . | β2GPI genotype at position 247† . | Number of β2GPI-specific T-cell clones . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Thrombosis . | Pregnancy loss . | DRB1 . | DRB4 . | DRB5 . | DQB1 . | DPB1 . | |||||
| O.M. | 39/F | Stroke | + | > 125.0 | *1501/*0403 | *0103 | *0101 | *0602/*0302 | *0201 | 247L/247L | 4 |
| E.Y. | 38/F | DVT, PE | N/A | 92.9 | *0901 | *0103 | — | *0303 | *0201 | 247V/247L | 4 |
| K.S. | 38/F | Stroke | + | > 125.0 | *1502/*0901 | *0103 | *0102 | *0601/*0303 | *0501/*0901 | 247V/247L | 6 |
DVT indicates deep venous thrombosis of lower extremity; PE, pulmonary embolism; N/A, not applicable.
Normal range < 3.5 U/mL.
A valine (247V) to leucine (247L) amino acid dimorphism was determined by RsaI digestion of the PCR-amplified DNA.
Proliferative responses of β2GPI-specific CD4+ T cell clones to various β2GPI preparations.
T-cell clones were cultured with autologous APCs for 3 days in medium alone or in medium supplemented with GP-F, GP1, GP2, GP3, native β2GPI, reduced β2GPI, MalBP (10 μg/mL), TT (5 μg/mL), or PHA (1 μg/mL), and antigen-induced T-cell proliferation was measured by 3H-thymidine incorporation. Significant T-cell proliferation to recombinant β2GPI or reduced β2GPI in comparison with the respective controls is shown as an asterisk. A representative result of 3 independent experiments is shown.
Proliferative responses of β2GPI-specific CD4+ T cell clones to various β2GPI preparations.
T-cell clones were cultured with autologous APCs for 3 days in medium alone or in medium supplemented with GP-F, GP1, GP2, GP3, native β2GPI, reduced β2GPI, MalBP (10 μg/mL), TT (5 μg/mL), or PHA (1 μg/mL), and antigen-induced T-cell proliferation was measured by 3H-thymidine incorporation. Significant T-cell proliferation to recombinant β2GPI or reduced β2GPI in comparison with the respective controls is shown as an asterisk. A representative result of 3 independent experiments is shown.
Surface phenotype, T-cell proliferative responses to various β2GPI preparations, predicted β2GPI domain containing the epitope, and HLA class II restriction in 14 β2GPI-specific CD4+ T-cell clones
| T-cell clones . | Surface phenotype . | Antigen-induced T-cell proliferative response . | Predicted β2GPI domain containing epitope . | HLA class II restriction* . | ||||
|---|---|---|---|---|---|---|---|---|
| Native β2GPI . | Reduced β2GPI . | GP1 . | GP2 . | GP3 . | ||||
| Patient O.M. | ||||||||
| OM2 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| OM3 | CD4+CD8− | − | + | + | − | − | I/II | HLA-DP |
| OM7 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| OM8 | CD4+CD8− | − | − | − | − | + | V | HLA-DR |
| Patient E.Y. | ||||||||
| EY3 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| EY8 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| EY9 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| EY12 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| Patient K.S. | ||||||||
| KS3 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| KS4 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| KS5 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| KS7 | CD4+CD8− | − | + | − | + | + | IV | HLA-DR |
| KS8 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| KS10 | CD4+CD8− | − | + | − | + | + | IV | HLA-DR |
| T-cell clones . | Surface phenotype . | Antigen-induced T-cell proliferative response . | Predicted β2GPI domain containing epitope . | HLA class II restriction* . | ||||
|---|---|---|---|---|---|---|---|---|
| Native β2GPI . | Reduced β2GPI . | GP1 . | GP2 . | GP3 . | ||||
| Patient O.M. | ||||||||
| OM2 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| OM3 | CD4+CD8− | − | + | + | − | − | I/II | HLA-DP |
| OM7 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| OM8 | CD4+CD8− | − | − | − | − | + | V | HLA-DR |
| Patient E.Y. | ||||||||
| EY3 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| EY8 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| EY9 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| EY12 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| Patient K.S. | ||||||||
| KS3 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| KS4 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| KS5 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| KS7 | CD4+CD8− | − | + | − | + | + | IV | HLA-DR |
| KS8 | CD4+CD8− | − | + | − | − | + | V | HLA-DR |
| KS10 | CD4+CD8− | − | + | − | + | + | IV | HLA-DR |
Determined based on blocking experiments using anti-HLA-DR, anti-HLA-DQ, and anti-HLA-DP mAbs.
Determination of T-cell epitopes within domain V
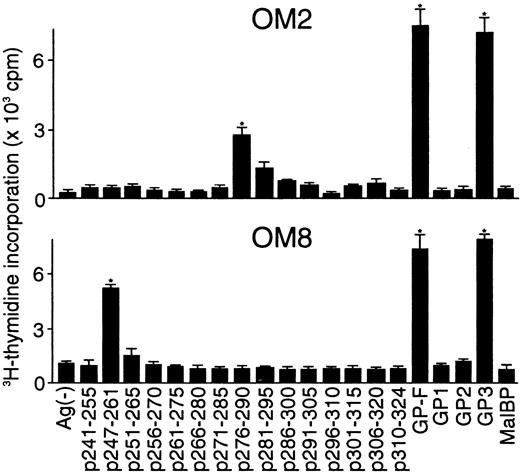
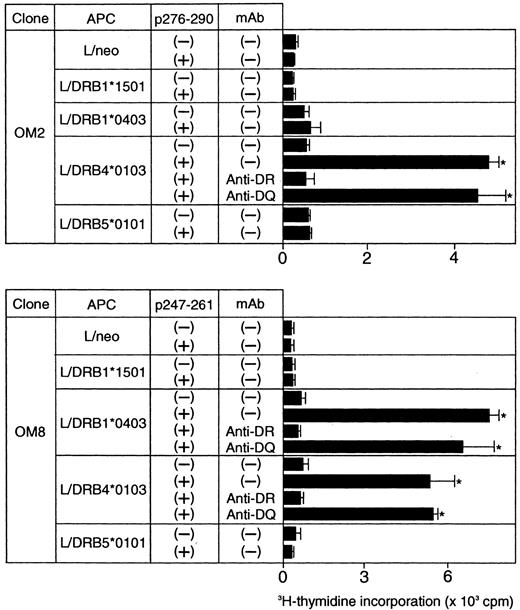
Because the majority of the β2GPI-specific CD4+ T-cell clones recognized domain V, T-cell epitopes within domain V were further analyzed using 15 synthetic peptides spanning the entire domain. As shown in Figure2, OM2 responded to p276 to 290 (KVSFFCKNKEKKCSY), which contains the major PL-binding site. T-cell proliferation induced by p276 to 290 at 5 μg/mL was weak, but was enhanced by higher peptide concentrations in a dose-dependent fashion (data not shown). In contrast, OM8 recognized another peptide, p247 to 261 (VPVKKATVVYQGERV). To identify HLA-DR molecules that present antigenic peptides to OM2 and OM8, a panel of L-cell transfectants expressing single HLA-DR molecules was used as APCs in the presence or absence of antigenic peptides (Figure 3). Patient O.M. possessed 4 different HLA-DRB alleles, including DRB1*1501, *0403, DRB4*0103, and DRB5*0101 (Table 1). OM2 responded to p276 to 290 presented by DRB4*0103+ L cells, but not to p276 to 290 in the presence of L cells expressing DRB1*1501, *0403, or DRB5*0101. In contrast, OM8 responded to p247 to 261 presented by both DRB1*0403+ L cells and DRB4*0103+ L cells, indicating that OM8 was able to respond to p247 to 261 in the context of 2 different HLA-DR molecules. Because the clonality of OM8 was not confirmed, the possibility that OM8 consisted of 2 or more clones was not excluded. The specificity of these responses was confirmed by the specific inhibition of the peptide-induced T-cell proliferation by anti-HLA-DR mAb. The T-cell epitope peptide and HLA restriction were further examined in 5 additional domain V-reactive T-cell clones, OM7, EY3, EY8, KS3, and KS5, all of which were found to recognize p276 to 290 in the context of the DRB4*0103 allele. Four T-cell clones responsive to domain V were not available because they died out before these assays were performed.
Proliferative responses of β2GPI-specific CD4+ T-cell clones to 15 synthetic peptides covering the entire domain V.
The β2GPI-specific CD4+ T-cell clones OM2 and OM8 were cultured with autologous APCs for 3 days in medium alone or in medium supplemented with individual synthetic peptides (5 μg/mL), and peptide-induced T-cell proliferation was measured by3H-thymidine incorporation. GP-F, GP1, GP2, GP3, and MalBP (10 μg/mL) were also used as antigens for T-cell proliferation. Significant T-cell proliferation to domain V peptide or recombinant β2GPI in comparison with the respective controls is shown as an asterisk. Similar results were obtained in 4 independent experiments.
Proliferative responses of β2GPI-specific CD4+ T-cell clones to 15 synthetic peptides covering the entire domain V.
The β2GPI-specific CD4+ T-cell clones OM2 and OM8 were cultured with autologous APCs for 3 days in medium alone or in medium supplemented with individual synthetic peptides (5 μg/mL), and peptide-induced T-cell proliferation was measured by3H-thymidine incorporation. GP-F, GP1, GP2, GP3, and MalBP (10 μg/mL) were also used as antigens for T-cell proliferation. Significant T-cell proliferation to domain V peptide or recombinant β2GPI in comparison with the respective controls is shown as an asterisk. Similar results were obtained in 4 independent experiments.
Peptide-induced proliferative responses of β2GPI-specific CD4+ T-cell clones incubated with L-cell transfectants expressing human HLA class II molecules.
The β2GPI-specific CD4+ T-cell clones OM2 and OM8 were incubated with a series of L-cell transfectants in the presence or absence of p276 to 290 (10 μg/mL) and p247 to 261 (5 μg/mL), respectively. The peptide-induced T-cell proliferation was measured by 3H-thymidine incorporation. In some experiments, T-cell clones were cultured with peptide-pulsed L cells in the presence of anti-HLA-DR or anti-HLA-DQ mAb (1 μg/mL). Significant T-cell proliferation in comparison with the control culture without antigenic peptide is shown as an asterisk. The results were similar in 2 independent experiments.
Peptide-induced proliferative responses of β2GPI-specific CD4+ T-cell clones incubated with L-cell transfectants expressing human HLA class II molecules.
The β2GPI-specific CD4+ T-cell clones OM2 and OM8 were incubated with a series of L-cell transfectants in the presence or absence of p276 to 290 (10 μg/mL) and p247 to 261 (5 μg/mL), respectively. The peptide-induced T-cell proliferation was measured by 3H-thymidine incorporation. In some experiments, T-cell clones were cultured with peptide-pulsed L cells in the presence of anti-HLA-DR or anti-HLA-DQ mAb (1 μg/mL). Significant T-cell proliferation in comparison with the control culture without antigenic peptide is shown as an asterisk. The results were similar in 2 independent experiments.
Helper activity and cytokine profiles in β2GPI-specific CD4+ T-cell clones
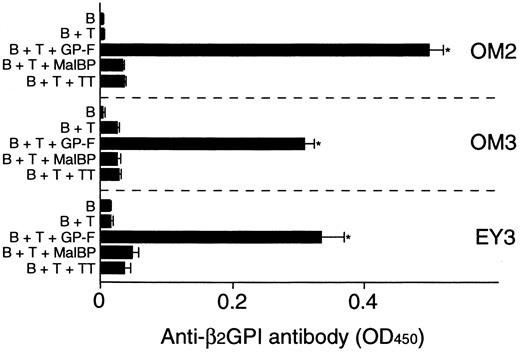
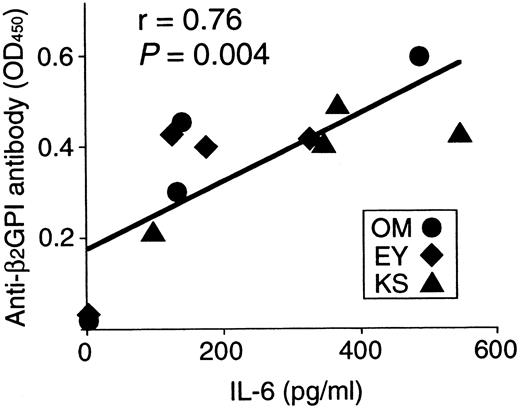
Anti-β2GPI antibody levels were measured in the supernatants of cultures containing individual β2GPI-specific CD4+ T-cell clones and autologous B cells stimulated with GP-F, MalBP, or TT (Figure4). The β2GPI-specific T-cell clones OM2, OM3, and EY3 induced the production of anti-β2GPI antibodies in response to GP-F, but anti-β2GPI antibody synthesis was not observed in cultures without antigen or in cultures with irrelevant antigen. As summarized in Table 3, 10 of 12 β2GPI-specific T-cell clones were able to promote anti-β2GPI antibody production, whereas the remaining 2 (OM8 and EY9) lacked the helper activity. When the expression of cytokines, including IFN-γ, IL-4, IL-6, and IL-10, was examined in the 12 β2GPI-specific T-cell clones, individual T-cell clones were found to express different sets of cytokines. Six β2GPI-specific T-cell clones (OM8, EY3, EY8, EY9, KS4, and KS7) expressed IFN-γ, but expressed no or minimal amounts of IL-4. This pattern of cytokine expression is consistent with that of a Th1 subset. The remaining 6 clones expressed both IFN-γ and IL-4, a pattern of cytokine expression consistent with a Th0 phenotype. When the cytokine profiles of β2GPI-specific T-cell clones were compared according to their ability to drive anti-β2GPI antibody production, there was no correlation between the helper activity and the Th0/Th1 phenotype. Instead, it was evident that the 10 T-cell clones that provided help to B cells expressed IL-6, whereas the 2 clones lacking the helper activity did not express IL-6. Furthermore, the amounts of anti-β2GPI antibody produced in the in vitro cultures were positively correlated with the IL-6 expression levels in β2GPI-specific T-cell clones (Figure 5). In contrast, the levels of anti-β2GPI antibody produced in vitro were not significantly correlated with the expression levels of IFN-γ, IL-4, or IL-10.
In vitro production of anti-β2GPI antibodies in cultures of β2GPI-specific CD4+T-cell clones and autologous B cells stimulated with GP-F.
Peripheral blood B cells were cultured with β2GPI-specific CD4+ T-cell clones in the presence or absence of antigen (GP-F, MBP, or TT; 10 μg/mL) for 10 days. Anti-β2GPI antibody levels in undiluted culture supernatants were measured by ELISA. Significant anti-β2GPI antibody production in culture with antigen compared with the control culture without antigen is shown as an asterisk. A representative result of 3 independent experiments is shown.
In vitro production of anti-β2GPI antibodies in cultures of β2GPI-specific CD4+T-cell clones and autologous B cells stimulated with GP-F.
Peripheral blood B cells were cultured with β2GPI-specific CD4+ T-cell clones in the presence or absence of antigen (GP-F, MBP, or TT; 10 μg/mL) for 10 days. Anti-β2GPI antibody levels in undiluted culture supernatants were measured by ELISA. Significant anti-β2GPI antibody production in culture with antigen compared with the control culture without antigen is shown as an asterisk. A representative result of 3 independent experiments is shown.
Ability to induce in vitro anti-β2GPI antibody production from autologous B cells and cytokine profiles in 12 β2GPI-specific CD4+ T-cell clones
| T-cell clones . | Anti-β2GPI antibody levels in supernatants (OD450)3-150 . | Cytokine levels (pg/mL)3-151 . | |||
|---|---|---|---|---|---|
| IFN-γ . | IL-4 . | IL-6 . | IL-10 . | ||
| Patient O.M. | |||||
| OM2 | 0.590 ± 0.022 | 880 ± 18 | > 500 | 487 ± 27 | 525 ± 30 |
| OM3 | 0.297 ± 0.014 | 1248 ± 22 | 191 ± 10 | 132 ± 7 | < 1 |
| OM7 | 0.450 ± 0.034 | 280 ± 11 | 305 ± 9 | 138 ± 5 | 720 ± 18 |
| OM83-152 | 0.016 ± 0.002 | 560 ± 15 | < 1 | < 1 | < 1 |
| Patient E.Y. | |||||
| EY3 | 0.422 ± 0.034 | 380 ± 10 | < 1 | 126 ± 16 | 316 ± 24 |
| EY8 | 0.412 ± 0.024 | 468 ± 9 | < 1 | 326 ± 25 | 461 ± 13 |
| EY93-152 | 0.023 ± 0.003 | 746 ± 18 | 7 ± 1 | < 1 | 262 ± 11 |
| EY12 | 0.396 ± 0.031 | 1632 ± 39 | > 500 | 175 ± 10 | 41 ± 5 |
| Patient K.S. | |||||
| KS3 | 0.400 ± 0.028 | 490 ± 7 | > 500 | 343 ± 25 | 371 ± 17 |
| KS4 | 0.418 ± 0.041 | 1214 ± 32 | 13 ± 7 | 546 ± 5 | 74 ± 11 |
| KS5 | 0.486 ± 0.020 | 824 ± 21 | 286 ± 16 | 366 ± 13 | 626 ± 22 |
| KS7 | 0.209 ± 0.012 | 653 ± 29 | < 1 | 97 ± 4 | 759 ± 30 |
| T-cell clones . | Anti-β2GPI antibody levels in supernatants (OD450)3-150 . | Cytokine levels (pg/mL)3-151 . | |||
|---|---|---|---|---|---|
| IFN-γ . | IL-4 . | IL-6 . | IL-10 . | ||
| Patient O.M. | |||||
| OM2 | 0.590 ± 0.022 | 880 ± 18 | > 500 | 487 ± 27 | 525 ± 30 |
| OM3 | 0.297 ± 0.014 | 1248 ± 22 | 191 ± 10 | 132 ± 7 | < 1 |
| OM7 | 0.450 ± 0.034 | 280 ± 11 | 305 ± 9 | 138 ± 5 | 720 ± 18 |
| OM83-152 | 0.016 ± 0.002 | 560 ± 15 | < 1 | < 1 | < 1 |
| Patient E.Y. | |||||
| EY3 | 0.422 ± 0.034 | 380 ± 10 | < 1 | 126 ± 16 | 316 ± 24 |
| EY8 | 0.412 ± 0.024 | 468 ± 9 | < 1 | 326 ± 25 | 461 ± 13 |
| EY93-152 | 0.023 ± 0.003 | 746 ± 18 | 7 ± 1 | < 1 | 262 ± 11 |
| EY12 | 0.396 ± 0.031 | 1632 ± 39 | > 500 | 175 ± 10 | 41 ± 5 |
| Patient K.S. | |||||
| KS3 | 0.400 ± 0.028 | 490 ± 7 | > 500 | 343 ± 25 | 371 ± 17 |
| KS4 | 0.418 ± 0.041 | 1214 ± 32 | 13 ± 7 | 546 ± 5 | 74 ± 11 |
| KS5 | 0.486 ± 0.020 | 824 ± 21 | 286 ± 16 | 366 ± 13 | 626 ± 22 |
| KS7 | 0.209 ± 0.012 | 653 ± 29 | < 1 | 97 ± 4 | 759 ± 30 |
IgG anti-β2GPI antibody levels were measured in supernatants of β2GPI-specific T-cell clones cultured with autologous B cells in the presence of GP-F. Results are shown in mean ± SD. IgG anti-β2GPI antibody levels in cultures of B cells alone were 0.011 ± 0.001 (O.M.), 0.012 ± 0.002 (E.Y.), and 0.001 ± 0.001 (K.S.).
Individual cytokine levels were measured in culture supernatants of β2GPI-specific T-cell clones stimulated with anti-CD3 mAb and PHA. Results are shown in mean ± SD.
T-cell clones lacking helper activity.
Correlation between anti-β2GPI antibody levels produced in cultures and IL-6 expression in 12 β2GPI-specific CD4+ T cells.
Anti-β2GPI antibody levels produced in cultures of individual β2GPI-specific T-cell clones plus autologous B cells are significantly correlated with amounts of IL-6 expressed on stimulation with PHA and anti-CD3 mAb (r = 0.76,P = .004).
Correlation between anti-β2GPI antibody levels produced in cultures and IL-6 expression in 12 β2GPI-specific CD4+ T cells.
Anti-β2GPI antibody levels produced in cultures of individual β2GPI-specific T-cell clones plus autologous B cells are significantly correlated with amounts of IL-6 expressed on stimulation with PHA and anti-CD3 mAb (r = 0.76,P = .004).
Role of IL-6 on in vitro anti-β2GPI antibody production
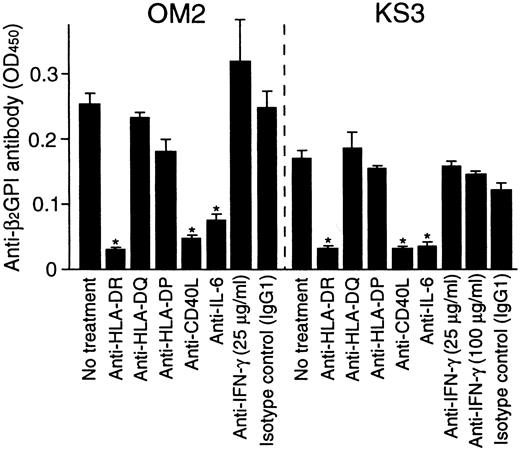
To further examine the role of T-cell–derived IL-6 in the helper activity, the effects of anti-IL-6 and anti-IFN-γ mAbs on in vitro anti-β2GPI antibody production were examined in cultures of β2GPI-specific T-cell clones OM2 or KS3 with autologous B cells and GP-F (Figure 6). The effects of anti-HLA class II and anti-CD40L mAbs on the anti-β2GPI antibody production were also tested. Anti-IL-6 mAb inhibited the anti-β2GPI antibody production, but anti-IFN-γ mAb had no effect. Anti-β2GPI antibody production was also blocked by anti-HLA-DR or anti-CD40L mAb. Similar results were obtained when β2GPI-specific T-cell clones OM7 and EY3 were tested.
Effects of anti-HLA class II, anti-CD40L, anti-IL-6, and anti-IFN-γ mAbs on in vitro anti-β2GPI antibody production.
Autologous peripheral blood B cells were cultured with β2GPI-specific CD4+ T-cell clones (OM2 and KS3) and GP-F (10 μg/mL) in the presence of anti-HLA-DR, anti-HLA-DQ, anti-HLA-DP, anti-CD40L (1 μg/mL), anti-IL-6, or anti-IFN-γ (25 μg/mL unless indicated otherwise) mAbs for 10 days. Anti-β2GPI antibody levels in undiluted culture supernatants were measured by ELISA. Significant inhibition of anti-β2GPI antibody production by anti-HLA class II or anticytokine mAbs in comparison with the control culture with isotype control mAb is shown as an asterisk. Similar results were obtained in 3 independent experiments.
Effects of anti-HLA class II, anti-CD40L, anti-IL-6, and anti-IFN-γ mAbs on in vitro anti-β2GPI antibody production.
Autologous peripheral blood B cells were cultured with β2GPI-specific CD4+ T-cell clones (OM2 and KS3) and GP-F (10 μg/mL) in the presence of anti-HLA-DR, anti-HLA-DQ, anti-HLA-DP, anti-CD40L (1 μg/mL), anti-IL-6, or anti-IFN-γ (25 μg/mL unless indicated otherwise) mAbs for 10 days. Anti-β2GPI antibody levels in undiluted culture supernatants were measured by ELISA. Significant inhibition of anti-β2GPI antibody production by anti-HLA class II or anticytokine mAbs in comparison with the control culture with isotype control mAb is shown as an asterisk. Similar results were obtained in 3 independent experiments.
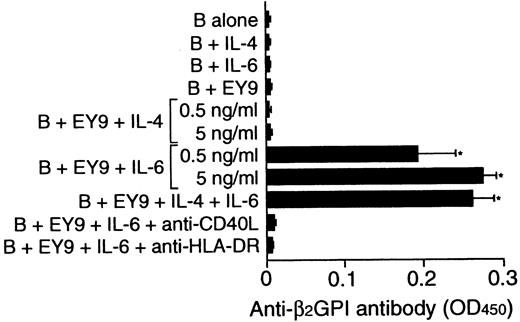
To examine whether the inability of some β2GPI-specific T-cell clones to provide help to B cells could be due to the lack of endogenous IL-6 in cultures, the effect of exogenous IL-6 on in vitro anti-β2GPI antibody production was examined using β2GPI-specific T-cell clone EY9 that lacked the helper activity (Figure 7). Because EY9 lacked the expression of IL-4 in addition to IL-6, the possible involvement of IL-4 in this process was also tested. B cells alone did not produce anti-β2GPI antibody when stimulated with IL-4 or IL-6. IL-6 did induce anti-β2GPI antibody production when it was added to the culture of B cells and EY9, but exogenous IL-4 had no effect. The IL-6–induced anti-β2GPI antibody production was completely abolished when the cells were cultured with anti-HLA-DR or anti-CD40L mAb.
Effect of exogenous IL-4 and IL-6 on in vitro anti-β2GPI antibody production.
Peripheral blood B cells from patient E.Y. were cultured with or without β2GPI-specific CD4+ T-cell clone EY9 in the presence of GP-F (10 μg/mL) plus exogenous IL-4, IL-6, or both IL-4 and IL-6 (5 ng/mL unless indicated otherwise) for 10 days. In some experiments, anti-HLA-DR (1 μg/mL) or anti-CD40L (1 μg/mL) mAb was added to the cultures supplemented with IL-6 as indicated. Anti-β2GPI antibody levels in undiluted culture supernatants were measured by ELISA. Significant anti-β2GPI antibody production in culture with exogenous cytokine compared with the control culture without cytokine is shown as an asterisk. The results were similar in 2 independent experiments.
Effect of exogenous IL-4 and IL-6 on in vitro anti-β2GPI antibody production.
Peripheral blood B cells from patient E.Y. were cultured with or without β2GPI-specific CD4+ T-cell clone EY9 in the presence of GP-F (10 μg/mL) plus exogenous IL-4, IL-6, or both IL-4 and IL-6 (5 ng/mL unless indicated otherwise) for 10 days. In some experiments, anti-HLA-DR (1 μg/mL) or anti-CD40L (1 μg/mL) mAb was added to the cultures supplemented with IL-6 as indicated. Anti-β2GPI antibody levels in undiluted culture supernatants were measured by ELISA. Significant anti-β2GPI antibody production in culture with exogenous cytokine compared with the control culture without cytokine is shown as an asterisk. The results were similar in 2 independent experiments.
Discussion
This is the first report describing T-cell epitopes recognized by β2GPI-specific CD4+ T cells capable of inducing anti-β2GPI antibody production in patients with APS. Our results obtained from 14 β2GPI-specific CD4+ T-cell clones generated from 3 patients with APS can be summarized as follows: (1) an immunodominant epitope on β2GPI is located at p276 to 290, which corresponds to the major PL-binding site, although other minor determinants are also recognized by β2GPI-specific T-cell clones; (2) T-cell recognition of p276 to 290 is restricted by DRB4*0103 (DR53); (3) the majority of β2GPI-specific T-cell clones have the helper activity promoting anti-β2GPI antibody production from B cells; and (4) both T cell-derived IL-6 and CD40-CD40L engagement are necessary for the anti-β2GPI antibody production from B cells.
The β2GPI-specific T-cell clones that recognized p276 to 290 in the context of DRB4*0103 were frequently generated from all 3 patients examined and had strong helper activity inducing anti-β2GPI antibody production. These findings strongly suggest that autoreactive CD4+ T cells carrying this antigenic specificity are primarily involved in anti-β2GPI antibody production in many patients with APS. On the other hand, β2GPI-specific T-cell clones responsive to the epitopes in domains I/II, IV, and p247 to 261 in domain V were also generated from patients with APS, indicating heterogeneous antigenic specificities in β2GPI-reactive T cells in APS. T-cell clones responsive to the epitopes other than p276 to 290 may be of the minor repertoire or present only in a small number of APS patients because they were generated from a single patient in this study.
Recently, Ito and coworkers analyzed the T-cell epitopes on β2GPI using β2GPI-specific CD4+T-cell lines established from patients with APS and healthy individuals, by the repeated stimulation of peripheral blood T cells with a mixture of 40 synthetic peptides covering the entire amino acid sequence of β2GPI.31 They identified 4 distinct epitopes, including p64 to 83, p154 to 174, p226 to 246, and p244 to 264. The majority of the β2GPI-reactive T-cell lines responded to p244 to 264, which was also recognized in this study by β2GPI-specific T-cell clone OM8. However, Ito and coworkers did not generate T-cell lines that were reactive with the peptide containing the major PL-binding site. The reason for this difference in the epitope recognition is unclear, but it is possible that the antigenic peptide containing the major PL-binding site was not efficiently presented to T cells in the context of the HLA-DR molecule in their cultures, because synthetic peptides containing the major PL-binding site have been shown to bind negatively charged PLs,8 32 which are thought to be expressed in abundance on the surfaces of apoptotic cells in cultures. In fact, T-cell clones reactive to p276 to 290 required a much higher peptide concentration to respond in the proliferation assay, compared with the β2GPI-specific T-cell clone OM8 response to p247 to 261. In contrast, in our study, β2GPI-specific T-cell clones were established in response to the peptides naturally processed from recombinant β2GPI.
It is interesting to note that p276 to 290 includes the epitopes recognized by anti-β2GPI antibodies in the sera from some patients with APS33 and by anti-β2GPI mAbs derived from a patient with APS.34 Therefore, both T- and B-cell epitopes are located in the vicinity of and included in p276 to 290, although the major B-cell epitopes on β2GPI are located in domain I35 or IV36 or both. In addition, Gharavi and colleagues reported that the immunization of normal mice with a peptide encompassing amino acid residues 274 to 288, which contains the major PL-binding site of human β2GPI, conjugated to bovine serum albumin induced the production of anti-β2GPI antibodies possessing thrombotic properties.37 38 Because the amino acid sequence of this region is highly conserved between human and mouse, an immunodominant region on β2GPI that induces the production of pathogenic anti-β2GPI antibodies may be shared by patients with APS and experimental mouse models for APS.
This study demonstrates that DR53 is a restricting element in the presentation of the immunodominant p276 to 290 to β2GPI-specific T cells. This finding may explain the previously reported associations of anti-β2GPI antibodies with DR4, DR7, and DR9 haplotypes,39-41 all of which are in linkage disequilibrium with DR53. Moreover, CD4+ T-cell responses to β2GPI in bulk PBMNC cultures are associated with the presence of DR53 in patients with APS and healthy individuals.21 The DR53-specific peptide-binding motif is composed of a positively charged residue at positions 1 and 9, and a hydrophobic residue at positions 4 and 10.42 For the binding of p276 to 290 to the DR53 molecule, 276K,279F, and 284K are likely to be the 1st, 4th, and 9th DR anchors, respectively, whereas the 10th anchor is absent.
At least 4 different allelic polymorphisms have been reported in theβ2GPI gene, and these residues include position 88 in domain II and positions 247, 306, and 316 in domain V.25 43 A valine-leucine dimorphism at position 247 (247V-247L) was included in the epitope peptide recognized by the β2GPI-specific T-cell clone OM8. Analysis of the β2GPI genotype at position 247 revealed that patient OM had a β2GPI gene that was homozygous for 247L. Because the recombinant β2GPI fragments and synthetic peptides used to stimulate T cells had a valine residue at position 247, it is possible that OM8 was generated due to alloreactivity. In this regard, OM8 had a typical Th1 phenotype and lacked helper activity. However, it is also possible that OM8 is an autoreactive T cell to β2GPI with247L and that it cross-reacts with β2GPI containing 247V, because valine and leucine residues have similar aliphatic and hydrophobic characteristics.
All β2GPI-specific CD4+ T-cell clones produced IFN-γ and had a Th1- or Th0-like cytokine expression profile, but our results indicated that IFN-γ was not involved in the B-cell activation leading to anti-β2GPI antibody production. Visvanathan and colleagues have recently reported that Th1 cells responsive to β2GPI activate monocytes to produce tissue factor through IFN-γ synthesis44 45; therefore, the β2GPI-specific T-cell clones generated in this study could mediate the production of tissue factor from monocytes. However, in the reports by Visvanathan and coworkers, T cell-dependent monocyte activation was induced by β2GPI in its native form, which stimulated none of our β2GPI-specific T-cell clones. Although the helper activity inducing anti-β2GPI antibody production in CD4+ T cells reactive with native β2GPI was not examined in their reports, it is possible that anti-β2GPI antibody synthesis from B cells and tissue factor production from monocytes are mediated by a different population of β2GPI-reactive CD4+ T cells.
The mechanisms for the T- and B-cell collaboration regulating the production of anti-β2GPI antibodies were examined using an in vitro assay system consisting of β2GPI-specific CD4+ T-cell clones and autologous B cells. Our results identified IL-6 as a major B cell-activating factor produced by β2GPI-specific CD4+ T cells that promotes anti-β2GPI antibody production, because (1) IL-6 expression was detected exclusively in the β2GPI-specific T-cell clones capable of driving anti-β2GPI antibody production; (2) the levels of anti-β2GPI antibody produced in vitro were positively correlated with the expression levels of IL-6; (3) neutralization of IL-6 by anti-IL-6 mAb inhibited the in vitro anti-β2GPI antibody production; and (4) exogenous IL-6 augmented the helper function of β2GPI-specific T-cell clone lacking IL-6 expression. A primary role of T-cell–derived IL-6 in autoantibody production is analogous to findings in our previous study on the antitopoisomerase I antibody response in patients with scleroderma.30 However, the effect of IL-6 required the CD40-CD40L engagement, because IL-6 alone did not induce anti-β2GPI antibody production and anti-CD40L mAb almost completely blocked anti-β2GPI antibody production induced by IL-6. Therefore, CD4+ T cell-dependent B-cell activation depends on 2 types of stimuli: CD40-CD40L engagement and T-cell–derived IL-6.
The β2GPI-specific CD4+ T-cell clones responded to reduced β2GPI and recombinant β2GPI fragments produced in bacteria, but none of them responded to native β2GPI. Taken together with the fact that β2GPI-reactive T cells are present in some healthy individuals,21,32 it is likely that the epitopes recognized by β2GPI-specific T cells are cryptic determinants that are not produced from native β2GPI under normal circumstances. Lehmann and coworkers proposed that a pathogenic autoreactive T-cell response is induced by the de novo presentation of a previously cryptic self-determinant under special conditions.46 Cryptic self-peptides can be revealed due to factors that affect normal antigen processing such as structural modification of self-antigens due to an unusual cleavage event or the formation of a complex with ligands.47,48 Such modifications are thought to mask or unmask cleavage sites for proteases and reductases in endosomes, resulting in the expression of cryptic self-peptides. The factors that induce the expression of cryptic determinants on β2GPI that result in the activation of β2GPI-reactive T cells in patients with APS are unknown, but several lines of evidence suggest that cryptic epitopes are revealed when β2GPI is complexed with anionic surfaces. For example, PL-bound β2GPI, but not PL or β2GPI alone, induces a high level of anti-β2GPI antibodies and lupus anticoagulant activity in normal mice without adjuvant.49 Moreover, immunization of β2GPI-bound apoptotic cells into normal mice induces the production of pathogenic anti-β2GPI antibodies.50 The major PL-binding site recognized by the majority of β2GPI-specific T-cell clones has a positively charged sequence located on a surface-exposed turn11 and is presumed to be easily accessed by proteases during antigen processing. Therefore, β2GPI binding to anionic surfaces, which protects the major PL-binding site from protease attack, could induce the appearance of the previously cryptic peptide containing the PL-binding site. Further studies examining the conditions that reveal the cryptic epitope within p276 to 290 could provide a clue to the pathogenesis of the induction of the anti-β2GPI antibody response in patients with APS.
In addition, this study provides novel information that is potentially useful in developing therapeutic strategies that suppress pathogenic anti-β2GPI antibody production in patients with APS. It has been shown that manipulation of autoreactive CD4+T-cell responses can be achieved by altered peptide ligands51 or induction of regulatory T cells,52 when immunodominant T-cell epitope and its restricting element are already known. Furthermore, IL-6 and CD40L that mediate the T-cell helper function inducing anti-β2GPI antibody production are candidate targets for biologic agents. In fact, humanized antibodies to IL-6 receptor and those to CD40L are already manufactured and used in clinical trials in several autoimmune diseases.53 54 Further studies should be done to evaluate the effectiveness of these potential therapies for patients with APS who are resistant to anticoagulation.
We thank Yuka Okazaki and Kyoko Kimura for their expert technical assistance, Drs Tomonobu Fujita and Katsuaki Dan for synthesis of the β2GPI peptides, and Noriko Hattori for helpful discussions.
Supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan; the Keio University Medical Science Fund; and the Japan Intractable Diseases Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Masataka Kuwana, Institute for Advanced Medical Research, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan; e-mail:kuwanam@sc.itc.keio.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal