Abstract
Delayed donor red cell engraftment and pure red cell aplasia (PRCA) are well-recognized complications of major ABO-incompatible hematopoietic stem cell transplantation (SCT) performed by means of myeloablative conditioning. To evaluate these events following reduced-intensity nonmyeloablative SCT (NST), consecutive series of patients with major ABO incompatibility undergoing either NST (fludarabine/cyclophosphamide conditioning) or myeloablative SCT (cyclophosphamide/high-dose total body irradiation) were compared. Donor red blood cell (RBC) chimerism (initial detection of donor RBCs in peripheral blood) was markedly delayed following NST versus myeloablative SCT (median, 114 versus 40 days;P < .0001) and strongly correlated with decreasing host antidonor isohemagglutinin levels. Antidonor isohemagglutinins declined to clinically insignificant levels more slowly following NST than myeloablative SCT (median, 83 versus 44 days;P = .03). Donor RBC chimerism was delayed more than 100 days in 9 of 14 (64%) and PRCA occurred in 4 of 14 (29%) patients following NST, while neither event occurred in 12 patients following myeloablative SCT. Conversion to full donor myeloid chimerism following NST occurred significantly sooner in cases with, compared with cases without, PRCA (30 versus 98 days; P = .008). Cyclosporine withdrawal appeared to induce graft-mediated immune effects against recipient isohemagglutinin-producing cells, resulting in decreased antidonor isohemagglutinin levels and resolution of PRCA following NST. These data indicate that significantly delayed donor erythropoiesis is (1) common following major ABO-incompatible NST and (2) associated with prolonged persistence of host antidonor isohemagglutinins. The clinical manifestations of these events are affected by the degree and duration of residual host hematopoiesis.
Introduction
The ABO blood group system is of critical importance in transfusion medicine, but has had less dramatic impact in hematopoietic transplantation.1-4 ABO antigens are potently immunogenic and are expressed on multiple tissues in addition to red blood cells (RBCs).5 Human beings begin to produce isohemagglutinins against non–self-ABO antigens shortly after birth and continue to do so throughout adult life.1 Since ABO and human leukocyte antigens (HLAs) are inherited independently, ABO incompatibility may occur in up to 20% to 40% of HLA-matched allogeneic hematopoietic stem cell transplants (SCTs).4Graft failure does not occur with increased frequency, and most studies indicate that the incidence of graft-versus-host disease (GVHD) is also not increased following ABO-incompatible versus ABO-identical SCT.2,4,6 Although increased transfusion requirements and immunohematologic events occur, the overall impact of ABO incompatibility in SCT is generally considered low, provided that appropriate transfusion practices are followed.2,4 7-10
Major ABO incompatibility in SCTs, defined as incompatibility of donor ABO antigens with the recipient's immune system, has been associated with pure red cell aplasia (PRCA) following conventional myeloablative conditioning.11-17 Proposed risks for PRCA in this setting include the use of cyclosporine (CsA) as GVHD prophylaxis,12,13 high initial or persistently elevated host antidonor isohemagglutinin levels,13,16 and RBC incompatibility involving donor A antigens.16,17 In this setting, host hematopoiesis is eradicated by the conditioning regimen while, at the same time, antibody-mediated inhibition of donor erythropoiesis can be demonstrated to occur by in vitro analysis.13,18 Although the in vivo mechanisms responsible for these effects have not been fully elucidated, they presumably involve interactions of antidonor isohemagglutinins with incompatible ABO antigens expressed on primitive donor erythropoietic cells.10 19-21
Low-intensity, nonmyeloablative hematopoietic stem cell transplants, or NSTs, use potent immunosuppressive conditioning regimens to promote donor engraftment sufficient for the generation of graft-versus-tumor effects against host malignant cells.22,23 By decreasing the toxicity associated with dose-intensive conditioning, NSTs have expanded the application of SCTs to include patients with advanced age or comorbid medical conditions, as well as those with chronic and debilitating RBC-transfusion–dependent disorders.24,25Substantial host hematopoiesis may persist following NST, and hematopoietic and immune function may be both host and donor in origin (mixed chimerism) for prolonged periods.22,23-26 Since nonmyeloablative transplantation approaches rely on allogeneic immune effects, rather than dose-intensive myeloablative conditioning, to eradicate both host hematopoietic and immune function, the kinetics of donor erythropoiesis might differ substantially after major ABO-incompatible NST compared with myeloablative SCT.27 To investigate these events, we compared the clinical and laboratory features of consecutive, concurrent series of patients undergoing major ABO-incompatible NST and myeloablative SCT.
Patients and methods
Patients
Patients participated in Institutional Review Board–approved investigational protocols (97-H-0099, 97-H-0196, 97-H-0202; 98-H-0006; 99-H-0046, 99-H-0050, 99-H-0064) between January 1997 and May 2000 and gave informed consent for allogeneic SCT. The study group consisted of consecutive patients who received major ABO-incompatible NST for hematologic and nonhematologic malignancies or for debilitating RBC-transfusion–dependent nonmalignant diseases. This group was compared with a concurrent series of consecutive patients who received major ABO-incompatible myeloablative SCT for hematologic malignancies. Only patients evaluable for at least 90 days after transplantation were included in this analysis.
Donors and peripheral blood stem cell grafts
All donors were HLA-identical family members, except for 2 with a single-locus mismatch (unique patient number [UPN] 193 and 201). Allele-level HLA testing at major and minor loci was performed by means of polymerase chain reaction (PCR) techniques.27 Donors gave informed consent for peripheral blood stem cell (PBSC) collection by leukapheresis after mobilization with granulocyte-colony stimulating factor administered at 10 μg/kg/d for 5 days. Starch sedimentation of the PBSC product was used to remove incompatible donor RBCs to a residual volume of less than 15 mL, followed by enumeration of the CD34 and CD3 cell content by flow cytometric analysis.28
Transplantation practices
Nonmyeloablative conditioning consisted of 60 mg/kg/d cyclophoshamide for 2 consecutive days followed by 25 mg/m2/d fludarabine for 5 days. Antithymocyte globulin was added to the conditioning regimen in patients with a strong prior transfusion history (n = 2) or those receiving non–HLA-identical PBSC allografts (n = 2), who were considered at high risk for graft rejection. CsA was started on day −4 as host-versus-graft prophylaxis and continued thereafter as GVHD prophylaxis. After patient UPN 215, mycophenolate mofetil was added to CsA as GVHD prophylaxis for NST recipients; it was started on day 0 and discontinued in conjunction with CsA. Decisions regarding the tapering of CsA and the administration of donor lymphocyte infusions (DLIs) following NST were based on the presence or absence of GVHD, the status of the underlying malignancy, and the degree of donor/host T-cell chimerism.27 Briefly, for patients with complete donor T-cell chimerism on day 30, CsA taper was begun on day 60 and discontinued on day 100 if GVHD did not occur. For patients with mixed T-cell chimerism on day 30, CsA was tapered over a 2-week period. Patients not converting to 100% donor T-cell chimerism after CsA withdrawal received monthly escalating doses of DLI with weekly reassessment of chimerism until 100% donor T-cell chimerism, GVHD, disease regression, or graft rejection occurred.27
Myeloablative conditioning consisted of 1360 cGy total body irradiation in 8 fractions over 4 days, followed by 60 mg/kg/d cyclophosphamide for 2 days and infusion of a T-cell–depleted PBSC allograft on day 0, as previously described.29 CsA alone was administered as GVHD prophylaxis, starting on day −4, and was tapered at 100 days after transplantation. Patients without GVHD received DLI at doses of 1 × 107 and 5 × 107 CD3+cells per kilogram on posttransplantation days 45 and 100, respectively, to enhance immune function and promote a graft-versus-leukemia effect.
Transfusion support consisted of host-compatible RBCs and donor-compatible platelets and plasma. Patients with alloantibodies to RBC antigens K (UPN 113) or to K, C, and e (UPN 149) prior to NST received antigen-negative RBC transfusions and PBSCs from donors whose RBCs lacked these antigens. Blood products were irradiated and leukocyte-reduced by filtration. Plasma exchange of 1.5 to 2 plasma volumes per procedure by means of 5% albumin replacement was performed in 2 patients who developed PRCA (UPN 149 and 178). Prophylactic plasma exchange to reduce isohemagglutinin levels prior to PBSC infusion was not performed.
Laboratory analysis
PCR-based analysis of the degree of donor and recipient chimerism in CD3+ T-lymphocyte and CD14+/CD15+ myeloid lineages was performed as clinically indicated at 2 weeks; 1, 2, and 3 months; and other relevant time points following NST by means of informative minisatellite regions as previously described.27 The onset of donor RBC chimerism, defined as the first detection of donor-type RBCs on 2 or more consecutive peripheral blood samples, was determined serologically.30-32 Serologic data (forward and reverse type) following NST were obtained retrospectively from transfusion records and patient charts (n = 4) or prospectively (forward and reverse type, isohemagglutinin titer) in weekly samples beginning after UPN 138 (n = 10). Serologic data were obtained retrospectively for the recipients of myeloablative SCT.
Serologic studies were performed by means of standard agglutination assays on whole blood samples collected in EDTA.31-33 The strength of agglutination was graded as weak, 1+, 2+, 3+, or 4+ by means of monoclonal anti-A and anti-B for RBC tests (forward type) or A1 and B cells for serum tests (reverse type and isohemagglutinin titers). Detection of a minor population of group A or B RBCs by forward type was sensitive to a level of 2% to 5% when tested on prepared concentrations of 1%, 2%, 5%, and 10% group A or B RBCs mixed with group O RBCs.31 32 There was no agglutination observed in A/O RBC mixtures with anti-B, or in B/O RBC mixtures with anti-A.
Isohemagglutinin (anti-A and anti-B) titers were determined by serially diluting plasma samples in saline and incubating at room temperature for 30 minutes. The end point for the titration was the highest dilution giving a 1+ reaction. Reverse group reactions of 1+, 2+ to 3+, and 4+ performed on undiluted samples yielded titration results in the range of 1, 4 to 16, and 16 or more, respectively, in normal donor samples. Isohemagglutinin titers before and after plasma exchange were performed with and without addition of dithiothreitol (DTT), to determine if the antibodies were a mixture of immunoglobulin G (Ig-G) and IgM.34
PRCA was determined to be present when bone marrow biopsy demonstrated adequate myeloid, lymphoid, and megakaryocyte populations in the setting of absent or nearly absent erythroid precursors and profound peripheral blood reticulocytopenia. Reticulocyte and complete blood counts were performed with use of an automated cell analyzer (Cell Dyn 3500) (Abbott, Santa Clara, CA). Other laboratory tests were performed by means of standard assays available in routine clinical practice.
Statistics
Data, which included censored results, such as the time of onset of donor chimerism and the time of decrease of host isohemagglutinin levels following NST or myeloablative SCT, were compared by means of the log-rank test. Other comparisons of data from NST or myeloablative SCT were made with nonpaired 2-tailed t tests for data with unequal variance. The incidence of delayed donor red cell chimerism following NST and myeloablative SCT was compared by means of a 2-tailed Fisher exact test. Data within NST subgroups defined in Table1 were analyzed by means of analysis of variance and post-hoc Games Howell analysis (P = .05) for determination of significance between individual groups. Patient data were censored at the time of death or last follow-up for those not attaining defined serologic or molecular chimerism end points. Values provided are the median (range) unless otherwise stated.
Clinical characteristics of major ABO-incompatible nonmyeloablative stem cell transplant patients
| UPN and diagnosis . | ABO D → R (titer)* . | Cell dose CD34/CD3† . | Anti-GVHD . | Serologic and molecular chimerism data (d)‡ . | Acute GVHD Gr/(d) . | Response,1-153 status1-155 (d) . | |||
|---|---|---|---|---|---|---|---|---|---|
| First donor RBC . | Host isohemagglutinin ≤ 1+ . | 100% donor T cell . | 100% donor myeloid . | ||||||
| First donor RBC chimerism after day 100 (n = 9) | |||||||||
| Pure red cell aplasia (n = 4) | |||||||||
| 113 RCC | A → O (NT) | 5.9/151 | CsA | 174 | 188 | 14 | 60 | II/78 | PR, D(699) |
| 138 CML | A → O (NT) | 15/533 | CsA | 188 | 128 | 14 | 14 | None | CR, RL, D(415) |
| 149 PNH | A → O (1024) | 21/445 | CsA | 134 | 144 | 30 | 30 | None | CR, A(544) |
| 178 SAA | A → O (256) | 3.5/389 | CsA | 218 | 222 | 14 | 30 | I/27 | CR, A(425) |
| Median values | 10/417 | 181 | 166 | 14 | 30 | ||||
| No pure red cell aplasia (n = 5) | |||||||||
| 159 RCC | AB → A (16) | 2.6/476 | CsA | 116 | 53 | 12 | 145 | None | NR, D(193) |
| 192 RCC | B → O (16) | 14/300 | CsA | 113+ | 35 | 47 | 97+ | IV/20 | NR, D(126) |
| 193 RCC1-154 | A → O (128) | 7.4/363 | CsA | 113+ | 113+ | 30 | 97 | None | NR, D(116) |
| 215 Sarc | A → O (64) | 7.3/265 | CsA/MMF | 130 | 183+ | 99 | 220+ | None | NR, D(229) |
| 216 RCC | B → A (64) | 4.3/206 | CsA/MMF | 125 | 119 | 13 | 65 | II/115 | PR, A(246) |
| Median values | 5.9/332 | 116 | 113 | 30 | 97 | ||||
| First donor RBC chimerism before day 100 (n = 5) | |||||||||
| 115 CML | B → O (NT) | 3.7/351 | CsA | 16+ | 16+ | 30 | 90 | II/30 | CR, A(749) |
| 118 MM | A → O (NT) | 6.4/333 | CsA | 47+ | 47+ | 21 | 100 | None | PR, A(711) |
| 198 Sarc | B → O (8) | 18/363 | CsA | 47 | 19 | 14 | 30 | None | NR, D(118) |
| 201 RCC1-159 | B → O (32) | 5.1/290 | CsA | 60 | 17 | 14 | 14 | IV/15 | NR, D(158) |
| 222 CLL | A → O (NT) | 4.0/153 | CsA/MMF | 28 | 28 | 30 | 146+ | None | NR, D(146) |
| Median values | 5.8/312 | 47 | 19 | 30 | 100 | ||||
| ALL NSTs (n = 14)1-160 | 6.2/342 | 114 | 83 | 21 | 81 | 6A, 8D(209) | |||
| UPN and diagnosis . | ABO D → R (titer)* . | Cell dose CD34/CD3† . | Anti-GVHD . | Serologic and molecular chimerism data (d)‡ . | Acute GVHD Gr/(d) . | Response,1-153 status1-155 (d) . | |||
|---|---|---|---|---|---|---|---|---|---|
| First donor RBC . | Host isohemagglutinin ≤ 1+ . | 100% donor T cell . | 100% donor myeloid . | ||||||
| First donor RBC chimerism after day 100 (n = 9) | |||||||||
| Pure red cell aplasia (n = 4) | |||||||||
| 113 RCC | A → O (NT) | 5.9/151 | CsA | 174 | 188 | 14 | 60 | II/78 | PR, D(699) |
| 138 CML | A → O (NT) | 15/533 | CsA | 188 | 128 | 14 | 14 | None | CR, RL, D(415) |
| 149 PNH | A → O (1024) | 21/445 | CsA | 134 | 144 | 30 | 30 | None | CR, A(544) |
| 178 SAA | A → O (256) | 3.5/389 | CsA | 218 | 222 | 14 | 30 | I/27 | CR, A(425) |
| Median values | 10/417 | 181 | 166 | 14 | 30 | ||||
| No pure red cell aplasia (n = 5) | |||||||||
| 159 RCC | AB → A (16) | 2.6/476 | CsA | 116 | 53 | 12 | 145 | None | NR, D(193) |
| 192 RCC | B → O (16) | 14/300 | CsA | 113+ | 35 | 47 | 97+ | IV/20 | NR, D(126) |
| 193 RCC1-154 | A → O (128) | 7.4/363 | CsA | 113+ | 113+ | 30 | 97 | None | NR, D(116) |
| 215 Sarc | A → O (64) | 7.3/265 | CsA/MMF | 130 | 183+ | 99 | 220+ | None | NR, D(229) |
| 216 RCC | B → A (64) | 4.3/206 | CsA/MMF | 125 | 119 | 13 | 65 | II/115 | PR, A(246) |
| Median values | 5.9/332 | 116 | 113 | 30 | 97 | ||||
| First donor RBC chimerism before day 100 (n = 5) | |||||||||
| 115 CML | B → O (NT) | 3.7/351 | CsA | 16+ | 16+ | 30 | 90 | II/30 | CR, A(749) |
| 118 MM | A → O (NT) | 6.4/333 | CsA | 47+ | 47+ | 21 | 100 | None | PR, A(711) |
| 198 Sarc | B → O (8) | 18/363 | CsA | 47 | 19 | 14 | 30 | None | NR, D(118) |
| 201 RCC1-159 | B → O (32) | 5.1/290 | CsA | 60 | 17 | 14 | 14 | IV/15 | NR, D(158) |
| 222 CLL | A → O (NT) | 4.0/153 | CsA/MMF | 28 | 28 | 30 | 146+ | None | NR, D(146) |
| Median values | 5.8/312 | 47 | 19 | 30 | 100 | ||||
| ALL NSTs (n = 14)1-160 | 6.2/342 | 114 | 83 | 21 | 81 | 6A, 8D(209) | |||
Donors were HLA-identical siblings except for a B-locus mismatch, and a DR-locus mismatch, indicated by footnotes below.
UPN indicates unique patient number; GVHD, graft-versus-host disease; d, day of event; RBC, red blood cell; Gr, maximum grade acute GVHD; RCC, renal cell carcinoma; CML, chronic myelogenous leukemia; PNH, paroxysmal nocturnal hemoglobinuria; SAA, aplastic anemia; Sarc, high-grade metastatic soft-tissue sarcoma; CsA, cyclosporine; MM, multiple myeloma; CLL, chronic lymphocytic leukemia; NST, nonmyeloablative stem cell transplant; NT, not tested; MMF, mycophenolate mofetil.
Donor → recipient ABO group (recipient titer against relevant donor ABO antigen).
Cell dose is 106/kg.
A plus sign indicates censored data point.
Response: CR indicates complete response, PR, partial response; RL, relapse; NR, no response.
Status: A indicates alive; D, dead.
Donor was a B-locus mismatch.
Donor was a DR mismatch.
Totals are median values.
Results
Patients
The subjects of this report are patients who received major ABO-incompatible PBSC allografts following NST or myeloablative SCT and who survived at least 90 days. During the study period, 81 consecutive patients underwent NST, of whom 55 had ABO-identical donors, while 16 had major, 9 had minor, and 1 had both major and minor ABO-incompatible donors. Three of the 17 patients with major ABO incompatibility after NST were not evaluable owing to graft rejection (n = 1); death before day 60 due to GVHD (n = 1); or death due to progressive disease (n = 1). Of the 14 evaluable cases, there were 8 group A and 4 group B donors with group O recipients, 1 group AB donor with a group A recipient, and 1 group B donor with a group A recipient (Table 1). A concurrent series of 57 patients underwent myeloablative SCT, of whom 12 received major ABO-incompatible allografts; all 12 were evaluable for study. Of these 12, there were 8 group A and 2 group B donors with group O recipients, 1 group A donor with a group B recipient, and 1 group B donor with a group A recipient. The effect of ABO incompatibility and GVHD on transfusion requirements in these patients following NST and ablative SCT has been previously reported.35
PBSC grafts
The CD34 content of PBSC allografts was slightly higher in the 14 patients receiving NST compared with the 12 receiving myeloablative SCT: 6.2 (range, 2.6-21) versus 4.6 (range, 2.3-8.5) × 106 CD34+ cells/kg, respectively (P = .049). The CD3 content of PBSC allografts was 4 logs higher for patients receiving NST compared with those receiving T-cell–depleted myeloablative SCT: 3.42 (range, 1.5-5.3) × 108 versus 6.0 (range, 4.0-17) × 104CD3+ cells/kg, respectively (P < .00001).
Graft-versus-host disease
Acute GVHD occurred in 5 of 12 patients following myeloablative SCT; 3 had grade II or lower-grade disease at a median of 76 days after transplantation, and 2 had higher than grade II disease at a median of 114 days. The incidence and severity of acute GVHD was similar following NST, with grade II or lower disease in 4 patients at a median of 54 days after transplantation and higher than grade II disease in 2 patients at a median of 17 days.
Erythropoietic recovery and isohemagglutinin levels following SCT
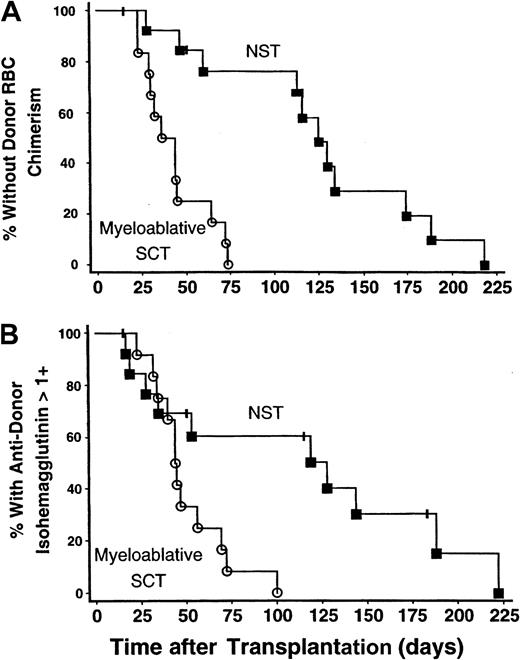
The onset of donor RBC chimerism was markedly delayed following NST compared with myeloablative SCT: 114 (range, 28-218) versus 40 (range, 23-73) days, respectively (P < .0001) (Figure1A). In 9 of 14 patients undergoing NST, donor RBC chimerism was not detected until more than 100 days after transplantation, whereas this delay did not occur in any of 12 subjects undergoing myeloablative SCT (P = .0013). Similarly, the time until host antidonor isohemagglutinins declined to clinically insignificant levels (1+ or lower in strength) was longer following NST than myeloablative SCT: 83 (range, 17-222) versus 44 (range, 23-100) days, respectively (P = .012) (Figure 1B).
The onset of donor RBC chimerism and decline in antidonor isohemagglutinin levels after NST compared with myeloablative SCT.
The onset of donor RBC chimerism and decline of host antidonor isohemagglutinins to clinically insignificant levels were markedly delayed following major ABO-incompatible NST compared with myeloablative SCT. NST data are represented by solid squares; myeloablative SCT data, by open circles. (A) Kaplan-Meier plot of the percentage of patients without detectable donor RBC chimerism as a function of days after transplantation. The time until detection of donor RBC chimerism was significantly prolonged following NST versus myeloablative SCT; P < .0001. (B) Kaplan-Meier plot showing the percentage of patients with persistent host antidonor isohemagglutinins greater than 1+ in strength. Isohemagglutinins decreased significantly faster after myeloablative SCT than after NST;P = .012.
The onset of donor RBC chimerism and decline in antidonor isohemagglutinin levels after NST compared with myeloablative SCT.
The onset of donor RBC chimerism and decline of host antidonor isohemagglutinins to clinically insignificant levels were markedly delayed following major ABO-incompatible NST compared with myeloablative SCT. NST data are represented by solid squares; myeloablative SCT data, by open circles. (A) Kaplan-Meier plot of the percentage of patients without detectable donor RBC chimerism as a function of days after transplantation. The time until detection of donor RBC chimerism was significantly prolonged following NST versus myeloablative SCT; P < .0001. (B) Kaplan-Meier plot showing the percentage of patients with persistent host antidonor isohemagglutinins greater than 1+ in strength. Isohemagglutinins decreased significantly faster after myeloablative SCT than after NST;P = .012.
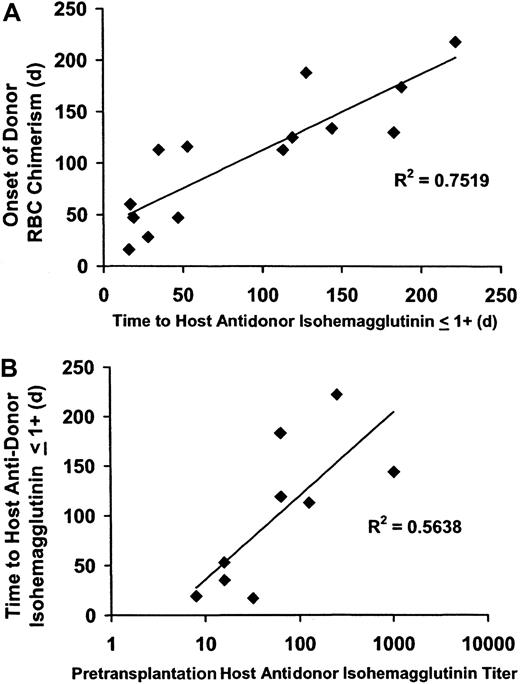
The time until the first appearance of donor RBC chimerism was strongly associated with a decrease in antidonor isohemagglutinins to 1+ or lower following both NST (R2 = .72;P < .0005) (Figure 2A) and myeloablative SCT (R2 = 0.49;P < .02). The posttransplantation time until isohemagglutinins decreased to 1+ or lower was significantly associated with the pretransplantation isohemagglutinin titer in the 9 NST patients in whom this parameter was obtained (R2 = 0.56; P < .02) (Figure2B). Pretransplantation isohemagglutinin titers were not obtained prior to myeloablative SCT; however, initial isohemagglutinin levels prior to SCT were 4+ in strength in both the NST and the myeloablative SCT study groups.
Correlation of the onset of donor RBC chimerism with a decline in host antidonor isohemagglutinins after major ABO-incompatible NST.
Following major ABO-incompatible NST, the onset of donor RBC chimerism was strongly correlated with a decline in host antidonor isohemagglutinins to 1+ or lower, and the time until isohemagglutinins decreased to levels 1+ or lower was related to the pretransplantation isohemagglutinin titer. (A) The onset of donor RBC chimerism after NST strongly correlated with a decline in host isohemagglutinins to clinically insignificant levels (1+ or lower on reverse type;R2 = 0.72; P < .0005). (B) The time until host antidonor isohemagglutinins decreased to levels 1+ or lower correlated with the log value of the pretransplantation isohemagglutinin titer (R2 = 0.56;P = .02).
Correlation of the onset of donor RBC chimerism with a decline in host antidonor isohemagglutinins after major ABO-incompatible NST.
Following major ABO-incompatible NST, the onset of donor RBC chimerism was strongly correlated with a decline in host antidonor isohemagglutinins to 1+ or lower, and the time until isohemagglutinins decreased to levels 1+ or lower was related to the pretransplantation isohemagglutinin titer. (A) The onset of donor RBC chimerism after NST strongly correlated with a decline in host isohemagglutinins to clinically insignificant levels (1+ or lower on reverse type;R2 = 0.72; P < .0005). (B) The time until host antidonor isohemagglutinins decreased to levels 1+ or lower correlated with the log value of the pretransplantation isohemagglutinin titer (R2 = 0.56;P = .02).
The relationships between the onset of donor RBC chimerism, host antidonor isohemagglutinin levels, and reticulocyte counts following SCT are shown in Figure 3A. Reticulocyte counts on days 14 and 21 were significantly higher following NST compared with myeloablative SCT (P < .02 andP < .04, respectively). However, in contrast to the sustained erythroid recovery following myeloablative SCT, reticulocyte counts declined after day 60 following NST and were significantly decreased by day 90 (P < .006), corresponding to the median time of conversion to full donor myeloid chimerism (loss of recipient hematopoiesis). Four cases of PRCA occurred among the 14 major ABO-incompatible NSTs compared with no cases of PRCA among the 12 major ABO-incompatible SCTs performed with myeloablative conditioning.
Erythropoietic function following major ABO-incompatible SCT.
Erythropoietic function following major ABO-incompatible SCT was related to the disappearance of clinically significant host antidonor isohemagglutinins and was further affected after NST by the relationship between the occurrence of this parameter and the time of conversion to full donor (loss of recipient) myeloid chimerism. ▪, host antidonor isohemagglutinin above 1+; ▨ detectable donor RBC chimerism; and ▥, detectable host myeloid chimerism. (A) Reticulocyte counts recovered more slowly after myeloablative SCT (n = 12) compared with NST (n = 14). Reticulocyte counts after NST decreased significantly at the time of conversion to full donor myeloid chimerism, reflecting loss of autologous erythropoiesis at a time when persistent host antidonor isohemagglutinin activity inhibited the onset of donor RBC chimerism. Error bars (± SEM) are shown at points with significant differences between NST and myeloablative SCT (P < .05). Laboratory analysis for the degree of host myeloid chimerism after myeloablative SCT was not performed. (B) Data for patients following NST who had either early donor RBC chimerism, late donor RBC chimerism with PRCA, or late donor RBC chimerism without PRCA. In patients with delayed donor RBC chimerism, full donor myeloid chimerism occurred significantly sooner in those with PRCA compared with those without PRCA. The time intervals between conversion to full donor myeloid chimerism and a decrease in host antidonor isohemagglutinins to 1+ or lower were significantly different among these 3 groups.
Erythropoietic function following major ABO-incompatible SCT.
Erythropoietic function following major ABO-incompatible SCT was related to the disappearance of clinically significant host antidonor isohemagglutinins and was further affected after NST by the relationship between the occurrence of this parameter and the time of conversion to full donor (loss of recipient) myeloid chimerism. ▪, host antidonor isohemagglutinin above 1+; ▨ detectable donor RBC chimerism; and ▥, detectable host myeloid chimerism. (A) Reticulocyte counts recovered more slowly after myeloablative SCT (n = 12) compared with NST (n = 14). Reticulocyte counts after NST decreased significantly at the time of conversion to full donor myeloid chimerism, reflecting loss of autologous erythropoiesis at a time when persistent host antidonor isohemagglutinin activity inhibited the onset of donor RBC chimerism. Error bars (± SEM) are shown at points with significant differences between NST and myeloablative SCT (P < .05). Laboratory analysis for the degree of host myeloid chimerism after myeloablative SCT was not performed. (B) Data for patients following NST who had either early donor RBC chimerism, late donor RBC chimerism with PRCA, or late donor RBC chimerism without PRCA. In patients with delayed donor RBC chimerism, full donor myeloid chimerism occurred significantly sooner in those with PRCA compared with those without PRCA. The time intervals between conversion to full donor myeloid chimerism and a decrease in host antidonor isohemagglutinins to 1+ or lower were significantly different among these 3 groups.
Influence of ABO blood type on serologic observations
All 4 patients who developed PRCA following NST involved group A donors and group O recipients, representing 50% (4 of 8) of such NST pairs. Host antidonor isohemagglutinins more rapidly decreased to 1+ or lower following NST with incompatible donor-B versus incompatible donor-A antigens: 35 (range, 17-119) versus 144 (range, 28-222) days, respectively (P < .02). However, donor RBC chimerism following NST was not detected significantly earlier in cases with incompatible donor-B versus incompatible donor-A antigens: 113 (range, 47-125) versus 134 (range, 28-218) days, respectively (P = .12). Pretransplantation isohemagglutinin titers obtained before NST tended to be higher in cases with incompatible donor-A antigens, 192 (range, 64-1024), versus those with incompatible donor-B antigens, 16 (range, 8-64); however, this difference was not statistically significant (P = .22).
Clinical and laboratory findings following major ABO-incompatible NST
Data for the 14 evaluable patients undergoing major ABO-incompatible NST are shown in Table 1. Patients are grouped according to whether donor RBC chimerism occurred after day 100 (delayed donor RBC chimerism, n = 9), either with (n = 4) or without (n = 5) PRCA, or before day 100 (early donor RBC chimerism, n = 5). Values for the time after transplantation until isohemagglutinin levels decreased to 1+ or lower and the log values for pretransplantation isohemagglutinin titers were significantly different among the 3 groups (P = .017 and P = .024, respectively). There were no significant differences among the 3 groups in CD34 or CD3 content of the PBSC graft or in the time of conversion to full donor T-cell chimerism.
The 3 groups in Table 1 exhibited marked differences in the interval between the median time of conversion to full donor (loss of recipient) myeloid chimerism and the median time that isohemagglutinin levels decreased to 1+ or lower (P = .0004), as illustrated in Figure 3B. Full donor myeloid chimerism occurred 136 days before isohemagglutinins decreased in patients who developed PRCA, compared with 16 days in patients with delayed donor RBC chimerism who did not develop PRCA. In marked contrast, full donor myeloid chimerism occurred 81 days after, rather than before, isohemagglutinins decreased to 1+ or lower in patients with early donor RBC chimerism.
RBC transfusion requirements were not significantly different among the 3 groups listed in Table 1 (P = .25). Patients with PRCA required 27 (range, 21-35) RBC transfusions in the absence of other causes for transfusion support, while patients without PRCA required 12.5 (range, 2-40) RBC transfusions for disease progression, hypersplenism, GVHD, and other events. All patients with PRCA had received numerous blood transfusions prior to transplantation, as had 8 of 10 patients who did not develop PRCA.
NST patients with PRCA
Four of 14 patients (29%) developed PRCA following major ABO-incompatible NST. Patients with PRCA exhibited a rapid transition from mixed to complete donor T-cell and myeloid chimerism on days 14 (range, 14-30) and 30 (range, 14-60), respectively (Table 1). However, antidonor isohemagglutinins remained at levels 1+ or higher for 166 (range, 128-222) days after transplantation, and the onset of donor RBC chimerism was delayed until 181 (range, 134-218) days after transplantation. Reticulocyte counts in patients who developed PRCA initially recovered normally, in parallel with other patients after NST, then declined rapidly and remained depressed until the resolution of PRCA (Figure 3B). Donor RBC chimerism was detected in all cases within 1 week following the recovery of reticulocytosis (Figure4).
Effect of discontinuation of CsA on reticulocyte counts and RBC transfusion requirements.
Reticulocyte counts increased and RBC transfusion requirements resolved, accompanied by characteristic RBC serologic changes, following discontinuation of CsA in patients with PRCA after major ABO-incompatible NST. Cases are from Table 1; all cases involved group A donors and group O recipients. PRBC transfusions are shown as arrows; strength of forward type for donor RBCs and reverse type for host antidonor isohemagglutinins are shown across top of graph. ⧫, reticulocytes; ○, blood CsA levels. Reticulocyte counts recovered after CsA withdrawal, followed by the appearance of donor RBCs within 7 days. Plasma exchange procedures are indicated with double arrows. (A) UPN 113 with renal cell cancer. (B) UPN 138 with chronic myelogenous leukemia. (C) UPN 149 with paroxysmal nocturnal hemoglobinuria. Plasma exchange to lower markedly increased isohemagglutinins titers of 2048 (256 after DTT treatment) prior to CsA tapering was ineffective, producing transient isohemagglutinin decreases lasting less than 24 hours. Plasma exchange was discontinued after 2 sessions because of an idiosyncratic reaction to albumin replacement fluid. (D) UPN 178 with aplastic anemia. Platelet counts increased and platelet-transfusion independence was achieved by day 14; conversion to full donor myeloid chimerism occurred by day 30. However, reticulocyte counts remained depressed in association with persistently elevated antidonor isohemagglutinin titers for 7 months following transplant. At 6 weeks following discontinuation of CsA, a series of 4 alternate-day plasma exchanges were performed. Isohemagglutinin levels decreased by 50% after each procedure, from a titer of 32 (4 after DTT treatment) to 2, followed by rapid recovery of reticulocyte counts and appearance of donor RBCs.
Effect of discontinuation of CsA on reticulocyte counts and RBC transfusion requirements.
Reticulocyte counts increased and RBC transfusion requirements resolved, accompanied by characteristic RBC serologic changes, following discontinuation of CsA in patients with PRCA after major ABO-incompatible NST. Cases are from Table 1; all cases involved group A donors and group O recipients. PRBC transfusions are shown as arrows; strength of forward type for donor RBCs and reverse type for host antidonor isohemagglutinins are shown across top of graph. ⧫, reticulocytes; ○, blood CsA levels. Reticulocyte counts recovered after CsA withdrawal, followed by the appearance of donor RBCs within 7 days. Plasma exchange procedures are indicated with double arrows. (A) UPN 113 with renal cell cancer. (B) UPN 138 with chronic myelogenous leukemia. (C) UPN 149 with paroxysmal nocturnal hemoglobinuria. Plasma exchange to lower markedly increased isohemagglutinins titers of 2048 (256 after DTT treatment) prior to CsA tapering was ineffective, producing transient isohemagglutinin decreases lasting less than 24 hours. Plasma exchange was discontinued after 2 sessions because of an idiosyncratic reaction to albumin replacement fluid. (D) UPN 178 with aplastic anemia. Platelet counts increased and platelet-transfusion independence was achieved by day 14; conversion to full donor myeloid chimerism occurred by day 30. However, reticulocyte counts remained depressed in association with persistently elevated antidonor isohemagglutinin titers for 7 months following transplant. At 6 weeks following discontinuation of CsA, a series of 4 alternate-day plasma exchanges were performed. Isohemagglutinin levels decreased by 50% after each procedure, from a titer of 32 (4 after DTT treatment) to 2, followed by rapid recovery of reticulocyte counts and appearance of donor RBCs.
PRCA lasted 130 to 218 days and resolved only after CsA was discontinued, with no additional therapy in 3 patients and after plasma exchange in 1 patient (Figure 4D). Two plasma exchange procedures performed in another patient prior to the discontinuation of CsA were unsuccessful (UPN 149, Figure 4C). All 4 patients with PRCA failed to respond to 6- to 12-week courses of erythropoietin therapy at doses ranging from 10 000 U 3 times weekly to 20 000 U daily.
NST patients with delayed donor RBC chimerism without PRCA
Five patients exhibited a markedly delayed onset of donor RBC chimerism of 116 (range, 113-130) days, but did not develop PRCA. Conversion to full donor myeloid chimerism occurred significantly later in this group compared with those who did develop PRCA, 97 (range, 65-220) versus 30 (range, 14-60) days, respectively (P < .05). These patients initially exhibited sustained posttransplantation reticulocytosis consistent with autologous erythropoiesis (Figure 3B). At 90 days after NST, near the time of conversion to complete donor myeloid chimerism, reticulocyte counts declined significantly to 0.98% (range, 0.4% to 2.5%) from 2.96% (range, 1.5% to 4.5%) 30 days previously (P < .013). Interestingly, all patients demonstrated persistence or progression of their malignancy during the period of persistent autologous host erythropoiesis.
Additional immunohematologic observations after NST
Several patients exhibited intermittently positive direct antiglobulin tests after the onset of donor RBC chimerism. Red cell eluates in these patients were reactive against the relevant incompatible donor ABO antigen; however, there was no evidence of hemolysis. Positive red cell antibody screens prior to NST owing to anti-K (UPN 113) and anti-K, anti-C, and anti-e (UPN 149), remained reactive for 163 and 172 days after transplantation, respectively, and resolved within 5 weeks from the time that antidonor isohemagglutinins decreased to 1+ or lower.
Discussion
This study documents a high incidence of delayed donor red cell chimerism and pure red cell aplasia following low-intensity NST compared with myeloablative SCT in the setting of major ABO incompatibility. The onset of donor red cell chimerism was delayed past 100 days in 9 of 14 patients (64%) following major ABO-incompatible NST, with 4 of these patients developing pure red cell aplasia. In contrast, there were no patients with delayed donor RBC chimerism or PRCA in the 12 concurrent cases of major ABO-incompatible myeloablative SCT. The time of onset of donor red cell chimerism and the pace of decline in antidonor isohemagglutinins following myeloablative conditioning in this study were similar to those reported from prior series using dose-intensive conditioning.4,6,16,17 36-39Posttransplantation erythropoietic function was strongly affected by the pace of decline in host antidonor isohemagglutinins, which fell to clinically insignificant levels more slowly after major ABO-incompatible NST than after myeloablative SCT. NST patients with persistent antidonor isohemagglutinins who experienced an early conversion to full donor (loss of recipient) myeloid chimerism developed PRCA, while those with later conversion were protected from PRCA by a sustained bridge of autologous erythropoiesis.
Erythropoietic and serologic responses following NST were further affected by donor ABO blood group and recipient pretransplantation isohemagglutinin titers. All cases of PRCA in this study involved group A donors and group O recipients, and anti-A isohemagglutinins persisted longer than anti-B isohemagglutinins following NST, as reported previously in myeloablative SCT.16,17,38 These observations may reflect higher baseline levels of anti-A versus anti-B isohemagglutinins,38 increased cell surface density of A compared with B antigens on the RBC membrane,40 or increased complement-fixing capacity of red-cell–bound anti-A compared with anti-B.41 We also observed a strong association between the pre-NST isohemagglutinin titer and the time until posttransplantation isohemagglutinins decreased to levels causing minimal agglutination on standard serologic assays. Other studies regarding the association of PRCA with pretransplantation isohemagglutinin levels have had conflicting results, perhaps owing to the heterogeneous composition of human isohemagglutinins, variations in measurement techniques, and other factors affecting donor erythropoiesis in the posttransplantation period.1,10-13,16,19,42 43
In this study, PRCA resolved only after discontinuation of CsA. An association between CsA use and the development of PRCA after major ABO-incompatible myeloablative SCT has been previously proposed in some, but not in other, studies.12,13,16,17 CsA exerts complex actions on donor and host immune function following transplantation and has potent immunosuppressive effects on T cells, but minimal to no direct effects on B cells.44-46 The effect of adding mycophenolic acid, which blocks B-cell antibody production and inhibits both T- and B-cell DNA synthesis,47 to CsA in 3 patients in our series was difficult to evaluate. No differences in serologic or molecular end points could be distinguished among patients who did or did not receive mycophenolic acid in this small series.
A rapid decrease in antidonor isohemagglutinins was observed in 2 patients with early, severe GVHD following NST, consistent with a more pronounced graft-versus–plasma cell effect.4 There were no differences in the incidence or severity of GVHD after NST versus myeloablative SCT in this study; however, elevated antidonor isohemagglutinin levels were detected for significantly longer periods following NST, despite substantially higher T-cell contents in NST allografts. This observation highlights the critical impact that the conditioning regimen plays on host isohemagglutinin production. Reduced-intensity conditioning appeared to have a profoundly permissive effect on residual host B- and plasma-cell function, compared with dose-intensive myeloablative conditioning.
PRCA after major ABO-incompatible NST has been described in a recent case report by Veelken et al.48 In that study, full donor myeloid chimerism occurred early, within 2 weeks after transplantation, and there was no evidence of a temporary protective effect due to early autologous erythroid recovery. In contrast, the NST conditioning regimen used in our study was more immunosuppressive than myelosuppressive, permitting considerable host stem cell survival and early autologous hematopoietic recovery.23 Our data indicate that considerable temporal disparity may occur in graft-mediated effects against host hematopoietic versus host plasma cells following major ABO-incompatible NST and suggest that differences in the intensity and timing of these effects may lead to a diversity of donor/host erythropoietic functions after transplantation. Specifically, graft-mediated effects against recipient hematopoiesis in the absence of effects against recipient isohemagglutinin-producing cell populations may result in delayed donor RBC chimerism and PRCA owing to loss of recipient erythropoiesis and concomitant inhibition of donor erythropoiesis.
The efficacy of varying attempts to treat PRCA after SCT is difficult to interpret because of small patient numbers, publication bias, and occasional spontaneous resolution of PRCA.49-53Erythropoietin was ineffective in all 4 patients with PRCA in our study, highlighting the importance of the immune interactions required for eradication of isohemagglutinin production following NST. The single dramatic response to therapeutic plasma exchange we observed may have been related to a delayed graft-versus–plasma cell effect following CsA withdrawal, as isohemagglutinin levels in that patient had decreased substantially at the time of the procedure. Pretransplantation prophylactic plasma exchange to reduce isohemagglutinin levels would be unlikely to reduce the incidence of PRCA following NST, as isohemagglutinin levels often rebound within 7 to 10 days and may remain elevated for prolonged periods thereafter.2,4,54 Other reports have documented resolution of PRCA after DLI.55,56 In our study, discontinuation of CsA appeared sufficient to induce a graft-mediated immune effect against host cells involved in isohemagglutinin production, leading to resolution of PRCA. While such immune-based strategies appear effective, GHVD may also ensue.57
Strategies to further reduce transplant-related toxicity, such as lower-intensity conditioning and more prolonged administration of GVHD prophylaxis, may also be associated with prolonged host isohemagglutinin production and result in marked delays in the onset of donor erythropoiesis. In this setting, patients with ineffective pretransplantation erythropoietic function will lack the capacity to maintain effective posttransplantation autologous erythropoiesis and may be at risk for prolonged RBC transfusion requirements following NST. Alternative approaches to induce tolerance, as has been observed in dizygotic twins after in-utero exposure to ABO-incompatible erythropoietic cells, might result in stable hematopoietic chimera without adverse donor-recipient immune interactions.26 30Because ABO incompatibility remains a frequent event in SCT, future studies of these and other potential therapies are indicated to improve donor erythroid engraftment following reduced-intensity transplantation with major ABO incompatibility.
The serologic expertise of Angela Pickett and Sherry Sheldon; the contributions of Dr Elizabeth Read and Charles Carter in the design and performance of specialized cell-processing procedures; the assistance of Rhoda Enaiffe and Francesca Re in the performance of molecular chimerism assays; the many clinical contributions of Martha Marqueson, Rose Goodwin, Sheila Phang, Virginia Mayo, Adeira Green, and the nursing staff, fellows, and senior staff of the Hematology Branch, National Heart, Lung and Blood Institute; and the suggestions during review of the data and preparation of the manuscript by Dr Harvey Klein.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Charles Bolan, National Institutes of Health, Department of Transfusion Medicine, Bldg 10, Rm 1C711, 10 Center Dr, MSC 1184, Bethesda, MD, 20892-1184; e-mail: cbolan@mail.cc.nih.gov.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal