Abstract
The incidence, characteristics, risk factors for, and impact of chronic graft-vs-host disease (GVHD) were evaluated in a consecutive series of 116 evaluable HLA-identical blood stem cell transplant recipients. Minimum follow-up was 18 months. Limited chronic GVHD occurred in 6% (95% confidence interval [CI], 0%-13%), and clinical extensive chronic GVHD in 71% (95% CI, 61%-80%). The cumulative incidence was 57% (95% CI, 48%-66%). In univariate analyses, GVHD prophylaxis other than tacrolimus and methotrexate, prior grades 2 to 4 acute GVHD, use of corticosteroids on day 100, and total nucleated cell dose were significant risk factors for clinical extensive chronic GVHD. On multivariate analysis, GVHD prophylaxis with tacrolimus and methotrexate was associated with a reduced risk of chronic GVHD (hazard ratio [HR], 0.35; P = .001), whereas the risk was increased with prior acute GVHD (HR, 1.67;P = .046). When adjusted for disease status at the time of transplantation, high-risk chronic GVHD had an adverse impact on overall mortality (HR, 6.6; P < .001) and treatment failure (HR, 5.2; P < .001) at 18 months. It was concluded that there is a substantial rate of chronic GVHD after HLA-identical allogeneic blood stem cell transplantation, that clinical factors may alter the risk of chronic GVHD, and that high-risk chronic GVHD adversely affects outcome.
Introduction
Transplantation of mobilized blood stem cells from HLA-identical sibling donors has been explored extensively. In the initial reports comparing early outcome measures for allogeneic blood stem cell recipients with those for historical or concurrent control marrow recipients, the use of blood stem cells was associated with less regimen-related toxicity,1,2 earlier hematopoietic recovery,2-9 a reduction in transfusion requirements,3,4,10 fewer days of hospitalization,2,3,10 and lower initial cost11 without an increase in acute graft-vs-host disease (GVHD). Subsequent randomized trials have confirmed these benefits of blood stem cell grafts for allogeneic transplantation.12-16
In contrast, the question of whether the use of blood stem cells alters the risk of chronic GVHD in allograft recipients has been controversial; several centers have reported no impact,3,4,7,14,16,17 whereas other centers related a trend toward,2,6,13 or statistically significant,5,8,9,15,18 increase in the rate of chronic GVHD. Moreover, there is a substantial variation in the rates of chronic GVHD reported to occur after allogeneic blood stem cell transplantation.2-18 In addition, it is unclear whether relapse is altered with the use of allogeneic blood stem cells, and some researchers have suggested that the risk of relapse may depend on the occurrence of chronic GVHD in these patients.19 To address some of these inconsistencies, we evaluated the incidence, characteristics, risk factors for, and impact of chronic GVHD in a consecutive series of HLA-identical blood stem cell transplant recipients.
Patients and methods
Patients
Over a consecutive 45-month period, 168 adults received a myeloablative preparative regimen and an unmanipulated blood stem cell graft from an HLA-matched related donor. Recipients of T-cell–depleted blood stem cell transplants and those receiving minitransplant preparative regimens were not included. One hundred sixteen patients survived at least 100 days after transplantation and were evaluable for chronic GVHD (Table 1). One additional patient left the country after day 100, and follow-up data were insufficient for inclusion in this study. The medical records were reviewed retrospectively for date of onset of chronic GVHD, staging, organ involvement, and outcome. Details of blood stem cell collection and administration, peritransplant care, and GVHD prophylaxis have been described previously.20 Patients received 1 of 3 GVHD prophylaxis regimens depending on the standard at the time of transplantation or the requirement of the transplantation protocol as designated by the standard at the time the protocol was instituted.20 Patients were not assigned to a GVHD prophylaxis regimen on the basis of risk of GVHD. Written informed consent was obtained from each patient, and all protocols were approved by the Institutional Review Board of the MD Anderson Cancer Center.
Patient, donor, and graft characteristics
| No. patients | 116* |
| Median patient age (range) | 38 yrs (17-61) |
| Patient sex (male/female) | 58/58 |
| Diagnosis | |
| Leukemia/myelodysplastic syndrome | 66 |
| Lymphoma/Hodgkin disease/myeloma | 42 |
| Solid tumor | 8 |
| Disease status | |
| First remission or chronic phase | 12 |
| Second remission or accelerated phase | 31 |
| Relapse or blast crisis | 73 |
| Preparative regimen | |
| Total body irradiation–based | 30 |
| Chemotherapy alone | 86 |
| GVHD prophylaxis | |
| Cyclosporine/methylprednisolone | 32 |
| Tacrolimus/methylprednisolone | 45 |
| Tacrolimus/methotrexate | 39 |
| Prior grades 2-4 acute GVHD | 33 |
| On steroids at day 100 | 46 |
| Median donor age (range) | 39 yrs (8-64) |
| Donor sex (male/female) | 57/59 |
| Pair cytomegalovirus-seronegative | 12 |
| Total nucleated cells infused (range) | 8.7 × 108/kg (1.1-26.3) |
| CD34+ cells infused (range) | 5.7 × 106/kg (1.9-20.9) |
| CD3+ cells infused (range) | 2.3 × 108/kg (0.3-6.9) |
| CD56+cells infused (range) | 2.6 × 107/kg (1.0-8.1) |
| No. patients | 116* |
| Median patient age (range) | 38 yrs (17-61) |
| Patient sex (male/female) | 58/58 |
| Diagnosis | |
| Leukemia/myelodysplastic syndrome | 66 |
| Lymphoma/Hodgkin disease/myeloma | 42 |
| Solid tumor | 8 |
| Disease status | |
| First remission or chronic phase | 12 |
| Second remission or accelerated phase | 31 |
| Relapse or blast crisis | 73 |
| Preparative regimen | |
| Total body irradiation–based | 30 |
| Chemotherapy alone | 86 |
| GVHD prophylaxis | |
| Cyclosporine/methylprednisolone | 32 |
| Tacrolimus/methylprednisolone | 45 |
| Tacrolimus/methotrexate | 39 |
| Prior grades 2-4 acute GVHD | 33 |
| On steroids at day 100 | 46 |
| Median donor age (range) | 39 yrs (8-64) |
| Donor sex (male/female) | 57/59 |
| Pair cytomegalovirus-seronegative | 12 |
| Total nucleated cells infused (range) | 8.7 × 108/kg (1.1-26.3) |
| CD34+ cells infused (range) | 5.7 × 106/kg (1.9-20.9) |
| CD3+ cells infused (range) | 2.3 × 108/kg (0.3-6.9) |
| CD56+cells infused (range) | 2.6 × 107/kg (1.0-8.1) |
No. patients unless indicated otherwise.
Chronic GVHD grading and other study definitions
The diagnosis of GVHD was based on clinical evidence with histologic confirmation whenever possible.21 GVHD was graded as limited (localized skin or single organ involvement) or clinical extensive (generalized skin or multiple organ involvement), and platelet count was used to categorize the risk as high (platelet count less than 100 × 109/L) or low (platelet count exceeding 100 × 109/L).22 Disease status was considered early for patients in first remission or first chronic phase, intermediate for those in second remission or accelerated phase, and advanced for those in later remission, relapse, or blast crisis.
Statistical considerations
Minimum follow-up at the time of analysis was 18 months after transplantation. The actuarial rates of chronic GVHD and disease-free survival were calculated by the method of Kaplan and Meier.23 The cause-specific cumulative incidence of chronic GVHD was also calculated24 with relapse and death without relapse or chronic GVHD as competing risks. In the association of GVHD rates with continuously measured covariates, patients were initially grouped into quartiles based on covariate values (age, log cell dose). Proportional hazards regression modeling was used for univariate and multivariate analyses of chronic GVHD.25Factors found to be significant (P < .05) in the univariate analysis were included in the multivariate analysis. Factors that remained significant were retained in the final model. Factors that correlated with each other were not entered into the model simultaneously. An association was found between CD34+ cell dose group and use of methotrexate (Fisher exact test,P = .001), between CD3+ cell dose group and total nucleated cell dose group (Fisher exact test,P < .0001), and between prior acute GVHD and use of steroids at day 100 (Fisher exact test, P < .0001). Proportional hazards regression modeling with chronic GVHD entered as a time-dependent covariate was used to determine the effect of chronic GVHD on relapse, survival, and disease-free survival. A 2-sidedP < .05 was considered significant.
Results
Incidence of chronic GVHD
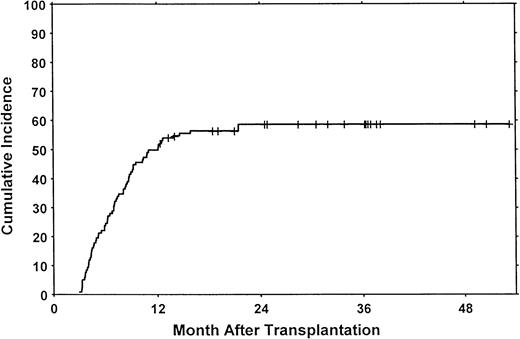
Three patients had limited chronic GVHD (actuarial rate, 6%; 95% confidence interval [CI], 0%-13%). One of these patients had only elevated liver enzymes, and 2 had only mild oral mucosal abnormalities. The actuarial rate of clinical extensive chronic GVHD at 18 months was 71% (95% CI, 61%-80%). The cumulative incidence of clinical extensive chronic GVHD at 18 months was 57% (95% CI, 48%-66%) (Figure 1). Onset was progressive in 5 patients (7%), quiescent in 20 (29%), and de novo in 43 (63%).
Clinical extensive chronic GVHD after allogeneic blood stem cell transplantation.
The cumulative risk of chronic GVHD was 57% at 18 months. Patients surviving more than 1 year without chronic GVHD are indicated by the tick marks. Competing risks were relapse without chronic GVHD (cumulative incidence, 24%) and death without relapse or chronic GVHD (cumulative incidence, 4%).
Clinical extensive chronic GVHD after allogeneic blood stem cell transplantation.
The cumulative risk of chronic GVHD was 57% at 18 months. Patients surviving more than 1 year without chronic GVHD are indicated by the tick marks. Competing risks were relapse without chronic GVHD (cumulative incidence, 24%) and death without relapse or chronic GVHD (cumulative incidence, 4%).
Risk factors for clinical extensive chronic GVHD
A number of demographic characteristics were evaluated as potential risk factors for clinical extensive chronic GVHD. When age was assessed by quartile, there was no trend for increasing risk of chronic GVHD with increasing age. When age was assessed dichotomously, there was no significant difference in chronic GVHD in patients above or below the median age of the group (Table2). Factors that were significant on univariate analysis were the GVHD prophylaxis regimen (P < .001), prior grades 2 to 4 acute GVHD (P = .013), and whether the patient was on corticosteroids at day 100 after transplantation (P = .027) (Table 2). There was no significant difference in the risk of chronic GVHD between the groups receiving cyclosporine or tacrolimus in addition to corticosteroids for GVHD prophylaxis (P = .55), so these 2 groups were combined for the multivariate analysis.
Univariate analysis of demographic risk factors for clinical extensive chronic graft-versus-host disease
| Factor . | No. patients . | Chronic GVHD HR (95% CI)* . | P . |
|---|---|---|---|
| Patient age | |||
| Younger than 38 years | 55 | 1.0 | |
| 38 years or older | 61 | 1.4 (0.8-2.2) | .2 |
| Patient sex | |||
| Male | 58 | 1.0 | |
| Female | 58 | 0.9 (0.6-1.5) | .8 |
| Diagnosis | |||
| Leukemia/myelodysplastic syndrome | 66 | 1.0 | |
| Lymphoma/Hodgkin disease/myeloma | 42 | 1.0 (0.6-1.6) | .9 |
| Solid tumor | 8 | 0.9 (0.3-3.0) | .9 |
| Disease status | |||
| First remission or chronic phase | 12 | 1.0 | |
| Second remission or accelerated phase | 31 | 1.4 (0.5-3.7) | .5 |
| Relapse or blast crisis | 73 | 1.4 (0.6-3.6) | .5 |
| Preparative regimen | |||
| Total body irradiation–based | 30 | 1.0 | |
| Chemotherapy alone | 86 | 0.97 (0.6-1.7) | .9 |
| GVHD prophylaxis | |||
| Cyclosporine/methylprednisolone | 32 | 1.0 | |
| Tacrolimus/methylprednisolone | 45 | 0.8 (0.5-1.5) | .6 |
| Tacrolimus/methotrexate | 39 | 0.3 (0.2-0.6) | < .001 |
| Grades 2-4 acute GVHD | |||
| No | 83 | 1.0 | |
| Yes | 33 | 1.9 (1.1-3.1) | .013 |
| On steroids at day 100 | |||
| No | 70 | 1.0 | |
| Yes | 46 | 1.7 (1.1-2.8) | .027 |
| Cytomegalovirus serology before transplant | |||
| Patient and donor seronegative | 12 | 1.0 | |
| Patient or donor seropositive | 104 | 0.5 (0.2-1.1) | .07 |
| Factor . | No. patients . | Chronic GVHD HR (95% CI)* . | P . |
|---|---|---|---|
| Patient age | |||
| Younger than 38 years | 55 | 1.0 | |
| 38 years or older | 61 | 1.4 (0.8-2.2) | .2 |
| Patient sex | |||
| Male | 58 | 1.0 | |
| Female | 58 | 0.9 (0.6-1.5) | .8 |
| Diagnosis | |||
| Leukemia/myelodysplastic syndrome | 66 | 1.0 | |
| Lymphoma/Hodgkin disease/myeloma | 42 | 1.0 (0.6-1.6) | .9 |
| Solid tumor | 8 | 0.9 (0.3-3.0) | .9 |
| Disease status | |||
| First remission or chronic phase | 12 | 1.0 | |
| Second remission or accelerated phase | 31 | 1.4 (0.5-3.7) | .5 |
| Relapse or blast crisis | 73 | 1.4 (0.6-3.6) | .5 |
| Preparative regimen | |||
| Total body irradiation–based | 30 | 1.0 | |
| Chemotherapy alone | 86 | 0.97 (0.6-1.7) | .9 |
| GVHD prophylaxis | |||
| Cyclosporine/methylprednisolone | 32 | 1.0 | |
| Tacrolimus/methylprednisolone | 45 | 0.8 (0.5-1.5) | .6 |
| Tacrolimus/methotrexate | 39 | 0.3 (0.2-0.6) | < .001 |
| Grades 2-4 acute GVHD | |||
| No | 83 | 1.0 | |
| Yes | 33 | 1.9 (1.1-3.1) | .013 |
| On steroids at day 100 | |||
| No | 70 | 1.0 | |
| Yes | 46 | 1.7 (1.1-2.8) | .027 |
| Cytomegalovirus serology before transplant | |||
| Patient and donor seronegative | 12 | 1.0 | |
| Patient or donor seropositive | 104 | 0.5 (0.2-1.1) | .07 |
Hazard ratio (95% CI) at 18 months in comparison with the reference group with a hazard ratio of 1.0.
No significant difference was found when chronic GVHD was assessed across quartiles of the total nucleated cell count or the cell subsets in the grafts. When assessed dichotomously, however, the rate of chronic GVHD was significantly higher in the highest quartile of total nucleated cell dose (P = .02), and there was a trend for increasing chronic GVHD in the highest quartile of CD34+cell dose (P = .08) (Table3).
Univariate analysis of graft characteristics as risk factors for clinical extensive chronic graft-versus-host disease
| Factor . | No. patients . | Chronic GVHD HR (95% CI)3-150 . | P . |
|---|---|---|---|
| Total nucleated cells | |||
| Less than 1 × 109/kg | 73 | 1.0 | .02 |
| At least 1 × 109/kg | 43 | 1.8 (1.1-2.9) | |
| CD34+ cells | |||
| Less than 8 × 106/kg | 84 | 1.0 | |
| At least 8 × 106/kg | 32 | 1.6 (0.9-2.7) | .08 |
| CD3+cells | |||
| Less than 3 × 108/kg | 84 | 1.0 | |
| At least 3 × 108/kg | 32 | 1.2 (0.7-2.0) | .4 |
| CD56+cells | |||
| Less than 4 × 107/kg | 87 | 1.0 | |
| At least 4 × 107/kg | 29 | 1.1 (0.6-1.8) | .8 |
| Factor . | No. patients . | Chronic GVHD HR (95% CI)3-150 . | P . |
|---|---|---|---|
| Total nucleated cells | |||
| Less than 1 × 109/kg | 73 | 1.0 | .02 |
| At least 1 × 109/kg | 43 | 1.8 (1.1-2.9) | |
| CD34+ cells | |||
| Less than 8 × 106/kg | 84 | 1.0 | |
| At least 8 × 106/kg | 32 | 1.6 (0.9-2.7) | .08 |
| CD3+cells | |||
| Less than 3 × 108/kg | 84 | 1.0 | |
| At least 3 × 108/kg | 32 | 1.2 (0.7-2.0) | .4 |
| CD56+cells | |||
| Less than 4 × 107/kg | 87 | 1.0 | |
| At least 4 × 107/kg | 29 | 1.1 (0.6-1.8) | .8 |
See Table 2 footnote.
On multivariate analysis, 2 factors remained in the optimal model. Methotrexate in the GVHD prophylaxis regimen was associated with a significant reduction in risk of chronic GVHD (P = .001), and a history of grades 2 to 4 acute GVHD was associated with an increased risk of chronic GVHD (P = .046) (Table4). As there was a strong association between prior acute GVHD and use of corticosteroids at day 100, these 2 factors could not be included in the same model. When use of corticosteroids was included instead of history of acute GVHD, methotrexate in the GVHD prophylaxis remained a significant risk factor (hazard ratio [HR], 0.35; 95% CI, 0.2-0.6; P < .001), and use of corticosteroids at day 100 was associated with an increased risk of chronic GVHD (HR, 1.6; 95% CI, 1.0-2.6; P = .05). Although there was also an association between a low CD34+cell dose and use of methotrexate, grouping by CD34+ cell dose did not substitute for use of methotrexate as a significant prognostic factor in the model.
Summary of regression model for extensive chronic graft-versus-host disease
| Risk factor . | HR (95% CI) . | P . |
|---|---|---|
| Methotrexate in GVHD prophylaxis | 0.35 (0.2-0.6) | .001 |
| Prior acute GVHD | 1.67 (1.0-2.8) | .046 |
| Risk factor . | HR (95% CI) . | P . |
|---|---|---|
| Methotrexate in GVHD prophylaxis | 0.35 (0.2-0.6) | .001 |
| Prior acute GVHD | 1.67 (1.0-2.8) | .046 |
We reported previously that the risk of acute GVHD after allogeneic blood stem cell transplantation was lowest in patients receiving tacrolimus and methotrexate as GVHD prophylaxis and a CD34+cell dose lower than 8 × 106/kg.20 For the 37 patients in the current series who fulfill these criteria, the actuarial rate of chronic GVHD was 51% (95% CI, 32%-68%), and the cumulative risk was 43% (95% CI, 27%-61%). Of the affected individuals, 81% had low-risk chronic GVHD.
Characteristics of chronic GVHD
Twenty-five patients had high-risk chronic GVHD, and 43 had low-risk chronic GVHD (Table 5). There were several differences between the 2 groups clinically. Patients with high-risk chronic GVHD most commonly had abnormalities of the liver, skin, gut, and mouth, whereas in patients with low-risk disease, the skin, mouth, liver, and eyes were most commonly affected (Table 5). Eosinophilia occurred more frequently in the low-risk group. In addition, for patients with high-risk chronic GVHD, the median time from transplantation to onset of chronic GVHD was significantly shorter (P = .004) and 1-year progression-free survival was significantly less (P < .001).
Clinical characteristics of patients with extensive chronic graft-versus-host disease
| . | All patients (n = 68) . | High-risk chronic GVHD (n = 25) . | Low-risk chronic GVHD (n = 43) . |
|---|---|---|---|
| Organ involvement | |||
| Rash/scleroderma | 52 (75%)* | 17 (68%) | 35 (81%) |
| Oral mucositis | 33 (49%) | 8 (32%) | 25 (58%) |
| Ocular sicca | 17 (25%) | 3 (12%) | 14 (33%) |
| Pulmonary disease | 6 (9%) | 0 (0%) | 6 (14%) |
| Liver abnormalities | 45 (66%) | 21 (84%) | 24 (56%) |
| Esophagitis | 3 (4%) | 1 (4%) | 2 (5%) |
| Nausea/vomiting | 10 (15%) | 6 (24%) | 4 (9%) |
| Diarrhea | 20 (29%) | 13 (52%) | 7 (16%) |
| Arthralgias/arthritis | 6 (9%) | 2 (8%) | 4 (9%) |
| Myositis | 2 (3%) | 0 (0%) | 2 (5%) |
| Eosinophilia | 23 (34%) | 3 (12%) | 20 (47%) |
| Effusions | 3 (4%) | 0 (0%) | 3 (7%) |
| Median day of onset after transplantation (range) | Day 209 (90-655) | Day 135 (90-383) | Day 229 (98-655) |
| 1-year progression-free survival from diagnosis of chronic GVHD (95% CI) | 47% (35%-59%) | 16% (0%-30%) | 64% (50%-79%) |
| . | All patients (n = 68) . | High-risk chronic GVHD (n = 25) . | Low-risk chronic GVHD (n = 43) . |
|---|---|---|---|
| Organ involvement | |||
| Rash/scleroderma | 52 (75%)* | 17 (68%) | 35 (81%) |
| Oral mucositis | 33 (49%) | 8 (32%) | 25 (58%) |
| Ocular sicca | 17 (25%) | 3 (12%) | 14 (33%) |
| Pulmonary disease | 6 (9%) | 0 (0%) | 6 (14%) |
| Liver abnormalities | 45 (66%) | 21 (84%) | 24 (56%) |
| Esophagitis | 3 (4%) | 1 (4%) | 2 (5%) |
| Nausea/vomiting | 10 (15%) | 6 (24%) | 4 (9%) |
| Diarrhea | 20 (29%) | 13 (52%) | 7 (16%) |
| Arthralgias/arthritis | 6 (9%) | 2 (8%) | 4 (9%) |
| Myositis | 2 (3%) | 0 (0%) | 2 (5%) |
| Eosinophilia | 23 (34%) | 3 (12%) | 20 (47%) |
| Effusions | 3 (4%) | 0 (0%) | 3 (7%) |
| Median day of onset after transplantation (range) | Day 209 (90-655) | Day 135 (90-383) | Day 229 (98-655) |
| 1-year progression-free survival from diagnosis of chronic GVHD (95% CI) | 47% (35%-59%) | 16% (0%-30%) | 64% (50%-79%) |
Data presented as no. patients (with percentage of group in column in parentheses) unless indicated otherwise.
Effect of clinical extensive chronic GVHD on relapse, survival, and progression-free survival
For all patients, survival at 18 months was 55% (95% CI, 46%-64%), and progression-free survival was 45% (95% CI, 36%-54%). The cumulative incidence of relapse was 29% (95% CI, 21%-39%). On multivariate analysis with chronic GVHD entered as a time-dependent covariate and with adjustment for disease status at the time of transplantation (early, intermediate, or advanced), development of chronic GVHD did not have a significant impact on relapse (HR, 0.6; 95% CI, 0.2-1.4; P = .2), and neither low-risk nor high-risk chronic GVHD was an independent risk factor for relapse (Table 6).
Summary of effect of chronic graft-versus-host disease on outcomes
| Outcome . | Low-risk chronic GVHD, HR (95% CI) . | P . | High-risk chronic GVHD, HR (95% CI) . | P . |
|---|---|---|---|---|
| Relapse | 0.6 (0.2-1.6) | .3 | 0.6 (0.1-2.4) | .4 |
| Overall mortality | 1.4 (0.6-3.2) | .4 | 6.6 (3.5-12.4) | < .001 |
| Treatment failure6-150 | 1.7 (0.8-3.3) | .1 | 5.2 (2.9-9.4) | < .001 |
| Outcome . | Low-risk chronic GVHD, HR (95% CI) . | P . | High-risk chronic GVHD, HR (95% CI) . | P . |
|---|---|---|---|---|
| Relapse | 0.6 (0.2-1.6) | .3 | 0.6 (0.1-2.4) | .4 |
| Overall mortality | 1.4 (0.6-3.2) | .4 | 6.6 (3.5-12.4) | < .001 |
| Treatment failure6-150 | 1.7 (0.8-3.3) | .1 | 5.2 (2.9-9.4) | < .001 |
Hazard ratio (95% CI) at 18 months is in comparison with no chronic GVHD, when chronic GVHD is entered as a time-dependent covariate and there is adjustment for disease status at the time of transplantation.
For abbreviations, see footnote to Table 2.
Treatment failure defined as relapse or death without relapse.
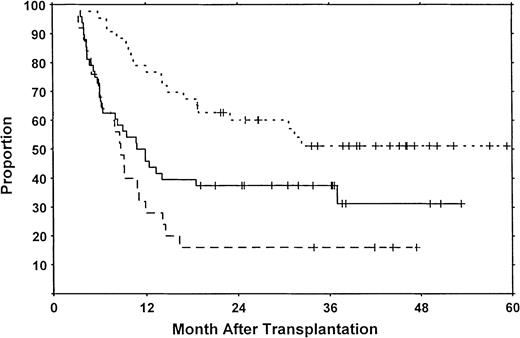
In contrast, high-risk chronic GVHD was a strong adverse factor for survival and progression-free survival when entered as a time-dependent covariate (Table 6). Moreover, when calculatations were done from the time of transplantation, patients who developed high-risk chronic GVHD had a relatively short progression-free survival (Figure2). For patients with high-risk chronic GVHD, the causes of death were GVHD-related in 85%, relapse in 10%, and other in 5%. For those with low-risk GVHD, 80% of the deaths were GVHD-related, 10% were due to relapse, and 10% were from other causes. For those who died without developing chronic GVHD, 77% of the deaths were due to relapse, and 23% to other causes. Thus, although the impact of GVHD on survival differs between the low-risk and high-risk chronic GVHD groups, GVHD was still the major cause of death within the first 18 months in both groups.
Progression-free survival from time of transplantation.
Long-term progression-free survival was 31% for patients without chronic GVHD (solid line), 51% for patients with low-risk chronic GVHD (dotted line), and 16% for patients with high-risk chronic GVHD (dashed line). All patients surviving in remission are indicated by the tick marks.
Progression-free survival from time of transplantation.
Long-term progression-free survival was 31% for patients without chronic GVHD (solid line), 51% for patients with low-risk chronic GVHD (dotted line), and 16% for patients with high-risk chronic GVHD (dashed line). All patients surviving in remission are indicated by the tick marks.
Discussion
The reported rates of chronic GVHD after allogeneic blood stem cell transplantation have varied from 38% to 95%.2-9,13-18,26-29 In our series, the actuarial rate of chronic GVHD was 77%, and almost all of our patients with chronic GVHD had clinical extensive disease. This predominance of clinical extensive over limited chronic GVHD after allogeneic blood stem cell transplantation in our study is consistent with the findings of others.5,8,13,15,27 29 Although the incidence of chronic GVHD in our patients is higher than that reported for marrow transplant recipients, the multivariate analysis identified a number of factors associated with an increased risk of chronic GVHD.
We noted that prior acute GVHD or use of corticosteroids at day 100 were prognostic factors for development of clinical extensive chronic GVHD. The late use of corticosteroids is likely to represent patients still completing treatment for acute GVHD, accounting for the association between these 2 prognostic factors. Prior acute GVHD and persistent use of corticosteroids have also been reported as risk factors for chronic GVHD after HLA-identical marrow transplantation30-35 and in one small series of allogeneic blood stem cell transplant recipients.8 Pavletic et al17 also reported cytomegalovirus (CMV) seropositivity as a risk factor for chronic GVHD in the stem cell recipients; we did not find this to be so in our series, but the number of CMV-seronegative patient-donor pairs in our study may have been too small for an accurate assessment of this risk factor.
T-cell depletion has been the only known method of GVHD prophylaxis consistently associated with a reduction in chronic GVHD after HLA-identical marrow transplantation.32 More recently, methotrexate-containing GVHD prophylaxis regimens were associated with a lower incidence of chronic GVHD after marrow transplantation,36,37 but this relationship was not confirmed by others.38 In our study, patients receiving methotrexate for GVHD prophylaxis also had a lower risk of chronic GVHD after allogeneic blood stem cell transplantation than those who received corticosteroid-based GVHD prophylaxis. However, patients in the methotrexate group had also received the lower doses of CD34+ cells, known predictors of acute GVHD, so it is unclear if use of methotrexate is an actual risk factor or a surrogate for another factor in our data set.
Several reports have suggested that the graft-vs-leukemia (GVL) effect is increased with use of blood stem cells in place of marrow for allogeneic transplantation.15,16,39 Others have noted specifically that fewer relapses occurred in patients with chronic GVHD after allogeneic blood stem cell transplantation.19,40 41In the current analysis, using chronic GVHD entered as a time-dependent covariate, chronic GVHD did not have a significant effect on the risk of relapse. However, the power of the analysis may be compromised by the small number of patients in the series, the heterogeneity of the diagnoses, and the relatively short follow-up.
The potential benefits of the GVL effect of allogeneic marrow transplantation are often blunted by the GVHD-related mortality.42-45 In fact, for the marrow transplant recipients, chronic GVHD was found to have an adverse impact on survival for patients at low risk for relapse.43 In our series, however, the adverse impact of chronic GVHD on survival was significant only for patients who developed high-risk chronic GVHD.
Chronic GVHD has been associated with excessive Th2 activity in humans.46,47 Th2 and DC2 polarization were found in healthy volunteers treated with filgrastim in vivo or in peripheral blood incubated with filgrastim in vitro,48-50 supporting the expectation that the risk of chronic GVHD would be increased with the use of filgrastim-mobilized blood stem cells for allogeneic transplantation. We here describe a population of patients with chronic GVHD, the high-risk subgroup, some of whom had typical manifestations of both acute (eg, maculopapular rash and inflammatory diarrhea) and chronic (eg, lichenoid changes of the oral mucosa) GVHD. In some cases, this has been associated with early withdrawal of GVHD prophylaxis.51 Whether the high-risk syndrome represents true chronic GVHD or a delayed form of acute GVHD is not known, as the biologic correlates have not been studied in this subgroup specifically. However, the intensity of the clinical disease in patients with high-risk chronic GVHD warrants aggressive treatment similar to that used for acute GVHD.
Although our patients had a substantial rate of clinical extensive chronic GVHD after allogeneic blood stem cell transplantation, for those using tacrolimus and methotrexate for GVHD prophylaxis and who received a conservative CD34+cell dose, the risk of chronic GVHD (51%) was similar to that reported previously for HLA-identical marrow transplant recipients using tacrolimus and methotrexate.52 The ability to optimize these prognostic variables in clinical practice suggests that the benefits of blood stem cells for allografting might be achieved without exacerbating acute or chronic GVHD, and this hypothesis should be tested in a randomized study. Whether the risk factors for chronic GVHD identified here will also apply in other clinical settings, such as with nonmyeloablative preparative regimens or CD34-selected allografts, remains to be determined.
Supported in part by The Tony Anderson Fund and Cancer Center Core grant CA 16672 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Donna Przepiorka, Baylor College of Medicine, Center for Cell and Gene Therapy, 6565 Fannin St, M964, Houston, TX 77030; e-mail: donnap@bcm.tmc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal