The prevention of apoptosis is a key function of growth factors in the regulation of erythropoiesis. This study examined the role of the constitutively active serine/threonine kinase glycogen synthase kinase-3 (GSK3), a target of the phosphoinositide-3-kinase (PI3K)/Akt pathway, in the regulation of apoptosis in primary human erythroid progenitors. GSK3 phosphorylation at its key regulatory residues S21 (α isoform) and S9 (β isoform) was high in steady-state culture, disappeared on growth factor withdrawal, and returned in response to treatment of cells with either erythropoietin or stem cell factor. Phosphorylation correlated with a PI3K-dependent reduction of 25% to 30% in measured GSK3 activity. LY294002, a specific inhibitor of PI3K, induced apoptosis in growth factor–replete erythroid cells to a degree similar to growth factor deprivation, whereas the Mek1 inhibitor U0126 had no effect, implicating PI3K and not mitogen-activated protein kinase in survival signaling. Growth factor–deprived erythroblasts, which undergo apoptosis rapidly, were protected from apoptosis by both lithium chloride, a GSK3 selective inhibitor, and inhibition of caspase activity. However, the clonogenic potential of single cells, which more accurately reflects cell survival, was maintained by lithium chloride, but not by caspase inhibition. Furthermore, lithium chloride, but not caspase inhibition, prevented the appearance of the conformational form of Bax associated with apoptosis induction. In summary, GSK3 activity is suppressed by erythropoietin and stem cell factor in human erythroid progenitor cells, and increased GSK3 activity, brought about by growth factor withdrawal, may regulate commitment to cell death through a caspase-independent pathway that results in a conformational change in Bax.
Introduction
Erythropoietin (Epo) and stem cell factor (SCF) are essential for normal erythropoiesis. Through binding and activating their respective receptors, EpoR and c-kit, an intracellular cascade of biochemical events is triggered, which, in addition to permitting proliferation and differentiation, results in the transduction of a survival signal to developing erythroid progenitors.1-6 In many cell types, the phosphoinositide-3-kinase (PI3K)/Akt (or protein kinase B) pathway is central to survival signaling in response to a variety of factors7 and this may also be the case in erythroid cells.8-10 However, other pathways, in particular the mitogen-activated protein (MAP) kinase pathway, have also been implicated in survival signal transduction.11-14
Targets of the PI3K/Akt pathway include the Forkhead transcription factors, the proapoptotic Bcl2 family member Bad, caspase 9, IκB kinase,7 and glycogen synthase kinase-3 (GSK3).15 GSK3 is a ubiquitously expressed, highly conserved, constitutively active serine/threonine kinase16and has been implicated in apoptosis regulation in primary neuronal cells.17,18 Its activity is reduced by serine (S; single-letter amino acid code) phosphorylation at S21 (α isoform) or S9 (β isoform) by Akt in insulin signal transduction15,19 and the 2 isoforms found in mammals, GSK3α and GSK3β, have a similar range of substrate specificities.16,20 GSK3 has been implicated in the regulation of a wide range of cellular processes including glycogen metabolism through glycogen synthase, translation through eIF2B, transcription through AP-1 and CREB, and cell fate specification in Drosophila and dorsoventral patterning inXenopus through β-catenin.20-22
In erythropoiesis, progenitors are critically dependent on growth factors for survival and proliferation until the intermediate normoblast stage of development, and controlling the proportion of cells that undergo apoptosis during development is one major way in which erythrocyte production is regulated.1Recently, erythroid progenitors have been shown to be dependent on PI3K for survival,8 but it is unclear in this context which of its downstream targets is critical in survival signal transduction.
We have examined the role of the PI3K/Akt target GSK3 in the regulation of apoptosis of primary human erythroid progenitors and find that both Epo and SCF suppress GSK3 activity. Furthermore, inhibition of GSK3 by lithium chloride (LiCl) prevents apoptosis induced by growth factor deprivation, maintains clonogenicity, and reduces the appearance of the conformational form of the proapoptotic Bcl2 family member Bax associated with apoptosis induction. These results implicate high GSK3 activity in apoptosis regulation in human erythroblasts and also suggest Akt is involved in its suppression.
Materials and methods
Materials
The PI3K inhibitor LY294002 and the Mek1 inhibitors U0126 and PD98059 were obtained from Biomol (Plymouth Meeting, PA). Rottlerin was obtained from Calbiochem-Novabiochem (Nottingham, United Kingdom). The pan-caspase inhibitor zVAD-FMK was from Bachem (St Helens, United Kingdom). LiCl was from Merck (Poole, United Kingdom). Recombinant human Epo was from Roche (Mannheim, Germany), recombinant human SCF was from Peprotech (London, United Kingdom), and recombinant human interleukin-3 (IL-3) was from Novartis (Basel, Switzerland). Antibodies used were as follows: antiphospho-Akt (S473), antiphospho-MAP kinase (T202/Y204), antiphospho-GSK3 α/β (S21/S9), and anti-Akt were from Cell Signaling Technology (Beverly, MA); anti-GSK3α was from Santa Cruz (Santa Cruz, CA); antiphospho-Y279/Y216 GSK3 α/β was from Biosource International (Camarillo, CA); and anti–Bax 6A7 mouse monoclonal antibody was from Pharmingen (San Diego, CA).
Erythroid primary cell culture
In accordance with local ethical guidelines, hemopoietic progenitor cells were collected either from normal donors (mobilized with granulocyte-colony stimulating factor [G-CSF]; Amgen, Thousand Oaks, CA) or from patients being treated for either lymphoma or myeloma (mobilized with G-CSF and cyclophosphamide [Pharmacia, Peapack, NJ]) on the University College London Hospitals NHS Trust bone marrow transplant program. Following immunomagnetic CD34+selection using CliniMacs or VarioMacs columns (Miltenyi Biotec, Bergisch Gladbach, Germany), which routinely resulted in more than 90% CD34+ purity, cells were cultured either directly or after a period of storage in liquid nitrogen with 10% dimethylsulfoxide (Sigma, St Louis, MO) as a cryopreservant. To generate erythroid cells, CD34+ selected cells were placed in culture in 175-cm2 tissue culture flasks (Nalge Nunc International, Rochester, NY) on day 0 at a density of 2 to 5 × 105/mL in Iscoves modified Dulbecco medium (IMDM) (Life Technologies, Paisley, United Kingdom), 20% fetal calf serum (FCS; Life Technologies), 1% penicillin and streptomycin (Sigma), 2 U/mL Epo, 20 ng/mL SCF, and 1 ng/mL IL-3 at 37°C and 5% CO2. During culture, cells were maintained at a density less than 1 × 106/mL by dilution with medium supplemented with Epo and SCF whenever necessary. By day 5 to 7, when cells were used for experiments, cell numbers had expanded approximately 20- to 30-fold and in excess of 90% of these cells were erythroid progenitors predominantly at the late burst-forming unit and colony-forming unit stages of development, as assessed by morphology, methylcellulose culture, and glycophorin A expression (data not shown).
Time-course experiments and Western blotting
To remove growth factors from cultured erythroblasts, cells on days 5 to 7 of culture were pelleted by centrifugation at 350g for 5 minutes and then washed and pelleted 3 times in phosphate-buffered saline (PBS; Life Technologies). Cells were then returned to IMDM and 10% FCS. For stimulation time courses, cells were kept without growth factors for 2 hours before stimulation with either 20 U/mL Epo or 100 ng/mL SCF, or both together. Where indicated, cells were incubated for 15 minutes with LY294002 (25 μM), U0126 (10 μM), or PD98059 (50 μM) before growth factor stimulation. At the time points shown, cells were pelleted rapidly (2000g for 30 seconds) and then resuspended in ice-cold lysis buffer. Lysates were resolved with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% acrylamide gels and transferred onto nitrocellulose membranes as described.23 Membranes were incubated with primary antibodies (diluted 1:1000 in PBS and 3% bovine serum albumin [BSA; Sigma]) overnight at 4°C, washed 4 times with PBS and 0.1% Tween, and then incubated for a further 60 minutes at room temperature with the appropriate secondary horseradish peroxidase–conjugated antimouse or antirabbit antibody (Amersham, Arlington Heights, IL) at a dilution of 1:10 000 in PBS and 3% BSA. After membranes were washed a further 4 times, protein bands were photographically visualized with enhanced chemiluminescence (ECL or ECL Plus; Amersham).
GSK3 kinase assay
The method used was modified from that of Ryves and coworkers.24 Erythroid cells were washed and incubated for 1 hour in IMDM and 1% FCS (2 experiments) or 10% FCS (3 experiments) before stimulation with growth factors. At the indicated time points, cells were pelleted and lysed in lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 5 mM EDTA, 50 mM NaF, 1% Triton X-100, 10 mM DTT, 0.5 mM Na3VO4, 5 μM Microcystin, and 1 mM Pefabloc) at 250 000 cells/12.5 μL lysis buffer. Lysate (12.5 μL) was mixed with 6.25 μL substrate mix (containing 5 mg/mL synthetic phospho-CREB substrate peptide (KRREILSRRP[pS]YR; Research Genetics, Huntsville, AL) with or without 200 mM LiCl) and the assay, performed in duplicate, was commenced with 6.25 μL ATP mix (200 mM HEPES, pH 7.5, 50 mM MgCl2, 8 mM DTT, 400 μM ATP, 4.6 kBq/μL [γ-32P]ATP). After 8 minutes at room temperature reactions were terminated by spotting on phosphocellulose disks (Life Technologies). Dry disks were washed 3 times in 0.75% phosphoric acid and once in acetone before bound radioactivity was counted with a β-scintillation counter. Specific activity due to GSK3 was determined by calculating the difference in bound quantified radioactivity between paired lithium-treated and untreated samples.
Apoptosisassay
To determine the proportion of apoptotic cells after 8 hours' incubation in various conditions, cells were pelleted at 350g for 5 minutes and then washed once in annexin V binding buffer (140 mM NaCl, 10 mM HEPES, pH 7.4, and 5 mM CaCl2) before incubation for 10 minutes on ice with 1 μL annexin V-FLUOS (Roche) in 50 μL annexin V binding buffer per sample. Specific fluorescence was then determined using an EPICS Elite flow cytometer (Beckman-Coulter, High Wycombe, United Kingdom).
Caspase 3 activity assay
Caspase 3 activity in lysates from growth factor–deprived erythroblasts was determined using a colorimetric caspase 3 assay kit (ApoAlert) according to the manufacturer's instructions (Clontech, Palo Alto, CA).
Clonogenic assays
Erythroid cells on days 4 to 6 of culture were washed and equal numbers were treated for 8 hours in IMDM, 20% FCS, and one of the following: Epo (2 U/mL) and SCF (20 ng/mL), LiCl (20 mM), zVAD-FMK (100 μM), or nil. Cells were then returned to IMDM with 20% FCS, Epo 2 U/mL, and SCF 20 ng/mL and plated out in 96-well plates at a predicted density of 0.66 cells/well. After 72 hours, wells containing 4 cells or more were scored as containing a cell that survived the 8-hour conditioning period with intact clonogenicity.
Fluorescence microscopy
Cytospin preparations were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and blocked with 20% normal goat serum (Dako, Cambridge, United Kingdom). Anti–Bax (6A7) was applied at a dilution of 1:50 in PBS and 20% normal goat serum for 1 hour at room temperature. Slides were then washed 4 times in PBS before incubation for 30 minutes with an anti–mouse fluorescein-conjugated secondary antibody (Vector Laboratories, Burlingame, CA) at a dilution of 1:250 in PBS and 20% normal goat serum. Slides were then washed 4 further times with PBS prior to application of Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories). Cells were visualized with an Olympus IX70 inverted fluorescence microscope (Olympus, Hamburg, Germany). A minimum of 200 cells were scored for Bax* positivity on each cytospin.
Results
GSK3 phosphorylation in primary human erythroblasts
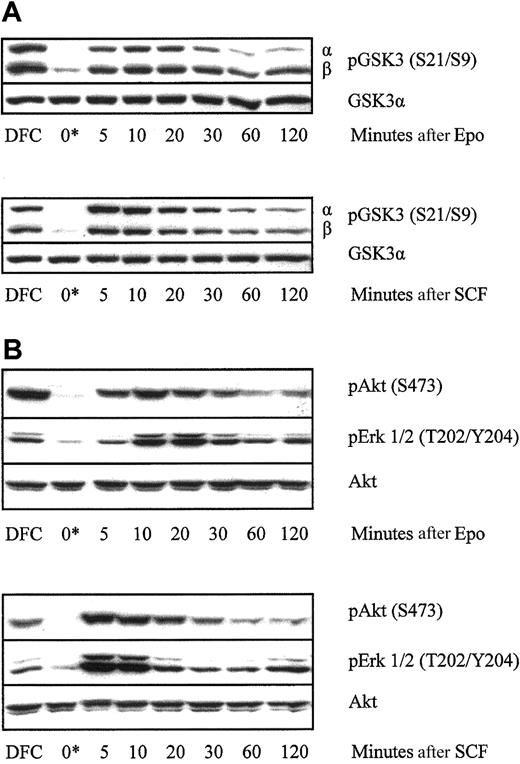
In insulin signaling, Akt phosphorylation of GSK3 at S21 (α isoform) and S9 (β isoform) leads to reduced GSK3 activity.15 Akt requires PI3K-dependent phosphorylation at S473 for optimal activity.25 The p44 MAP kinase (or extracellular signal-regulated kinase-1 [Erk1]) requires phosphorylation by Mek1 (MAP kinase or Erk kinase) on T202/Y204 for its activity.26 The pattern of phosphorylation of these 3 kinases at their key regulatory sites was examined in primary human erythroid progenitor cells. Erythroblasts lysed directly from steady-state culture contained high levels of phospho-GSK3α and GSK3β, phospho-Akt, and phospho-p42/p44 MAP kinase. On withdrawal of growth factor, phosphorylation of all 3 was rapidly and substantially reduced within half an hour and remained low until cells were re-exposed to growth factors, when it was rapidly reinduced (Figure1). Treatment of cells with Epo and SCF together did not have a noticeable additive effect over the degree of phosphorylation observed with either cytokine alone (data not shown).
Time course of phosphorylation of GSK3, Akt, and MAP kinase in primary human erythroblasts after growth factor withdrawal and re-exposure to Epo or SCF.
Primary human erythroblasts on day 6 of culture were either lysed direct from culture (DFC) or were washed and incubated in medium without growth factors for 2 hours (0*). Cells were then stimulated with either Epo 20 U/mL or SCF 100 ng/mL as indicated. Cell lysates were resolved using SDS-PAGE and probed on Western blot membranes using the indicated phosphospecific antibodies. The time course of phosphorylation of GSK3 (A) and Akt and MAP kinase (B) in a representative experiment of 4 performed is shown.
Time course of phosphorylation of GSK3, Akt, and MAP kinase in primary human erythroblasts after growth factor withdrawal and re-exposure to Epo or SCF.
Primary human erythroblasts on day 6 of culture were either lysed direct from culture (DFC) or were washed and incubated in medium without growth factors for 2 hours (0*). Cells were then stimulated with either Epo 20 U/mL or SCF 100 ng/mL as indicated. Cell lysates were resolved using SDS-PAGE and probed on Western blot membranes using the indicated phosphospecific antibodies. The time course of phosphorylation of GSK3 (A) and Akt and MAP kinase (B) in a representative experiment of 4 performed is shown.
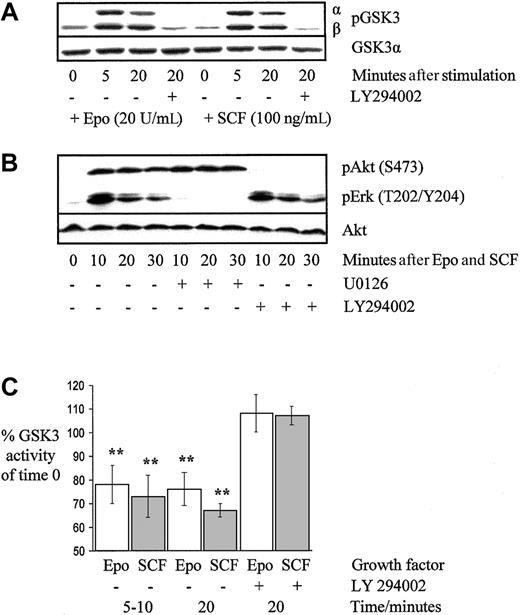
To confirm that GSK3 phosphorylation was PI3K dependent, growth factor–deprived cells were preincubated with the PI3K-specific inhibitor LY294002 (25 μM) for 15 minutes or not, before stimulation with either Epo or SCF (Figure 2A). The Mek1 inhibitor U0126 (10 μM) had no effect on GSK3 phosphorylation (data not shown). The pathway specificity of LY294002 and U0126 was confirmed by exposing cells preincubated with either one inhibitor or the other to a maximal stimulation of Epo 20 U/mL plus SCF 200 ng/mL. Figure 2B shows that LY294002 blocked Akt phosphorylation without altering MAP kinase phosphorylation and similarly U0126 blocked MAP kinase phosphorylation without altering Akt phosphorylation. Similar patterns of blockade were observed when cells were stimulated with Epo alone or SCF alone (data not shown).
GSK3 and Akt phosphorylation are PI3K dependent and phosphorylation of MAP kinase is Mek1 dependent in primary human erythroblasts.
Epo and SCF suppress GSK3 activity. Erythroblasts on day 6 of culture were deprived of growth factors for 2 hours before stimulation with Epo 20 U/mL, SCF 100 ng/mL, or both together as indicated, either with or without LY294002 (25 μM) or U0126 (10 μM) as indicated. Cell lysates were resolved using SDS-PAGE and probed on Western blot membranes using phosphospecific antibodies. The effect of PI3K blockade on GSK3 (A) and Akt phosphorylation (B) in a representative experiment of 4 performed is shown in addition to the effect of Mek1 blockade on MAP kinase phosphorylation (B). For kinase assays (C), erythroid progenitors on day 5 to 7 of culture were washed and incubated in medium without growth factors for 1 to 2 hours before stimulation with either Epo 20 U/mL or SCF 100 ng/mL. Kinase activity was determined at the times shown, either with or without preincubation with the PI3K inhibitor LY294002. The means and SEs are shown for 4 experiments (** denotes P < .05).
GSK3 and Akt phosphorylation are PI3K dependent and phosphorylation of MAP kinase is Mek1 dependent in primary human erythroblasts.
Epo and SCF suppress GSK3 activity. Erythroblasts on day 6 of culture were deprived of growth factors for 2 hours before stimulation with Epo 20 U/mL, SCF 100 ng/mL, or both together as indicated, either with or without LY294002 (25 μM) or U0126 (10 μM) as indicated. Cell lysates were resolved using SDS-PAGE and probed on Western blot membranes using phosphospecific antibodies. The effect of PI3K blockade on GSK3 (A) and Akt phosphorylation (B) in a representative experiment of 4 performed is shown in addition to the effect of Mek1 blockade on MAP kinase phosphorylation (B). For kinase assays (C), erythroid progenitors on day 5 to 7 of culture were washed and incubated in medium without growth factors for 1 to 2 hours before stimulation with either Epo 20 U/mL or SCF 100 ng/mL. Kinase activity was determined at the times shown, either with or without preincubation with the PI3K inhibitor LY294002. The means and SEs are shown for 4 experiments (** denotes P < .05).
Changes in GSK3 phosphorylation correlate with changes in kinase activity
To determine whether changes in GSK3 phosphorylation correlated with alterations in kinase activity, we used a total cell extract kinase assay method,24 which utilized a synthetic phospho-CREB peptide as a GSK3 substrate. In growth factor–deprived primary human erythroblasts, addition of Epo significantly reduced GSK3 activity by 22% ± 8% (mean ± SEM) at 5 to 10 minutes (n = 5, P = .05) and by 24% ± 7% at 20 minutes (n = 4, P = .003; Figure 2C). This reduction in activity was blocked by LY294002. SCF significantly reduced GSK3 activity by 27% ± 9% at 5 to 10 minutes (n = 5, P = .03) and by 33% ± 1% at 20 minutes (n = 4, P < .001), in a similar PI3K-dependent manner.
Use of pharmacological inhibitors implicates PI3K and not MAP kinase in survival signaling in human erythroblasts
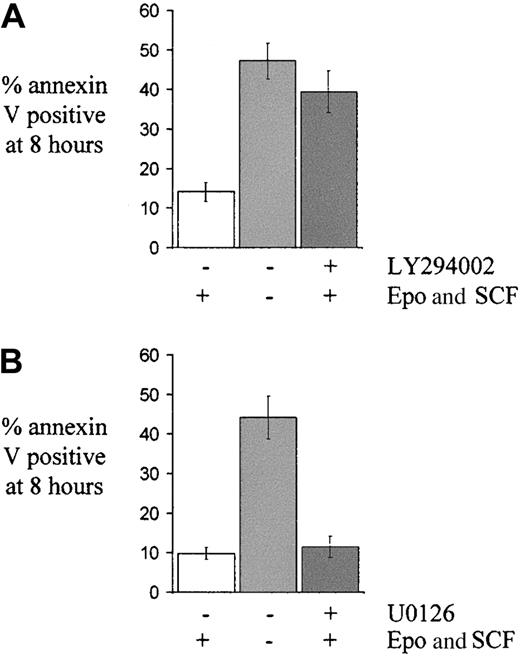
Withdrawal of growth factors from primary cultured human erythroblasts on days 5 to 7 of culture rapidly induced apoptosis. Eight hours after growth factor withdrawal, 49% ± 3% of cells were apoptotic compared with only 11% ± 1% of those supplemented with Epo and SCF (n = 13; P < .001). As described elsewhere,27 we found erythroid survival over this time course was optimal where cells were treated with both Epo and SCF. Either Epo alone or SCF alone, although reducing apoptosis significantly, was not as effective as the combination of the 2 together (data not shown). During the culture and expansion of CD34+ precursors to erythroblasts, a small proportion of cells was always found to be apoptotic, typically in the range 5% to 15%.
LY294002 was used to block PI3K and Akt activity in growth factor–replete erythroid cells and this induced apoptosis to a degree similar to that seen with growth factor withdrawal. At 8 hours, 39% ± 5% of LY294002-treated cells were apoptotic, significantly higher than the proportion seen in normal growth factor conditions (14% ± 2%; P < .001) and not substantially different from cells in growth factor–deprived conditions (47% ± %;P = .08; n = 6; Figure3A). Blockade of p42/p44 MAP kinase activation using either U0126 (Figure 3B) or PD98059 (data not shown) did not affect the proportion of apoptotic cells. Blockade of the p42/p44 MAP kinase pathway for the duration of the experiment was confirmed by finding absent p42/p44 MAP kinase phosphorylation in lysates of cells incubated with Epo, SCF, and U0126 for 8 hours (data not shown).
The effect of pharmacological blockade of either the PI3K/Akt pathway or the MAP kinase pathway on apoptosis in primary human erythroid progenitors.
Cells on day 5 to 7 of culture were washed and returned to medium either with or without growth factors (Epo 2 U/mL and SCF 20 ng/mL) or, in the case of cells given growth factors, either with (A) LY294002 (25 μM, n = 8) or (B) U0126 (10 μM, n = 5) as shown, or not. The mean percentage ± SEM of apoptotic cells at 8 hours, as determined by annexin V binding, is indicated.
The effect of pharmacological blockade of either the PI3K/Akt pathway or the MAP kinase pathway on apoptosis in primary human erythroid progenitors.
Cells on day 5 to 7 of culture were washed and returned to medium either with or without growth factors (Epo 2 U/mL and SCF 20 ng/mL) or, in the case of cells given growth factors, either with (A) LY294002 (25 μM, n = 8) or (B) U0126 (10 μM, n = 5) as shown, or not. The mean percentage ± SEM of apoptotic cells at 8 hours, as determined by annexin V binding, is indicated.
Blockade of either GSK3 activity or caspase activity prevents apoptosis of growth factor–deprived primary human erythroblasts
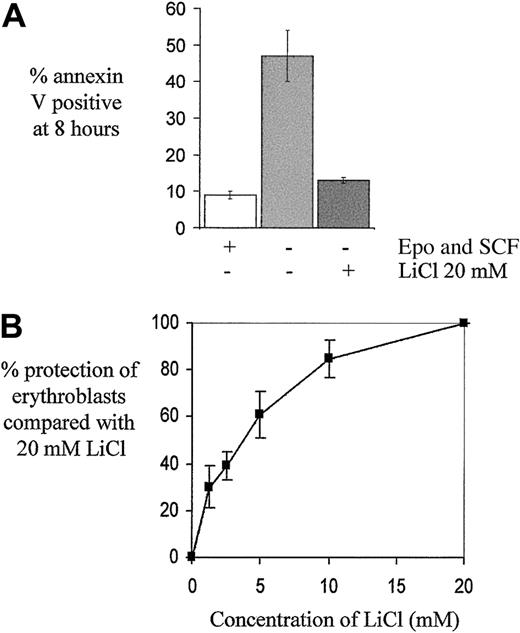
Lithium chloride, which potently inhibits GSK3 activity,28 was added at 20 mM to growth factor–deprived erythroblasts and reduced apoptosis significantly from 45% ± 4% to 20% ± 3% (n = 8, P < .001), with 10% ± 1% apoptotic cells in control growth factor–replete conditions. In 5 further experiments, LiCl was added to cells 15 minutes before washing and additionally during the PBS wash steps and this reduced the proportion of apoptotic cells further to 13% ± 1%, representing a mean reduction in apoptosis consequent on growth factor withdrawal of 88% ± 2% (Figure 4A). The addition of either 20 mM KCl or an extra 20 mM NaCl to growth factor–deprived cells had no effect on apoptosis (data not shown). In 4 further experiments, LiCl was found to inhibit apoptosis in a dose-dependent manner at concentrations near to the 50% inhibitory concentration (IC50) for GSK3 under intracellular ionic conditions (2 mM)28 (Figure 4B). Rottlerin, a compound fromMallotus philippinensis,29 also inhibits GSK3,28 and we found that 20 μM rottlerin also reduced erythroblast apoptosis, consequent on growth factor withdrawal, by 78% ± 7% (n = 3).
The effect of LiCl on growth factor–deprived human erythroblasts.
(A) Cells on day 5 to 7 of culture were washed and returned to medium either with or without growth factors (Epo 2 U/mL and SCF 20 ng/mL) or, in the case of cells without growth factors, either with or without LiCl 20 mM (n = 5). The mean percentage ± SEM of apoptotic cells at 8 hours, as determined by annexin V binding, is indicated. (B) Cells deprived of growth factors were treated with various concentrations of LiCl as indicated. The percentage protection against apoptosis (± SEM) at 8 hours compared with that obtained with 20 mM LiCl is indicated (n = 4).
The effect of LiCl on growth factor–deprived human erythroblasts.
(A) Cells on day 5 to 7 of culture were washed and returned to medium either with or without growth factors (Epo 2 U/mL and SCF 20 ng/mL) or, in the case of cells without growth factors, either with or without LiCl 20 mM (n = 5). The mean percentage ± SEM of apoptotic cells at 8 hours, as determined by annexin V binding, is indicated. (B) Cells deprived of growth factors were treated with various concentrations of LiCl as indicated. The percentage protection against apoptosis (± SEM) at 8 hours compared with that obtained with 20 mM LiCl is indicated (n = 4).
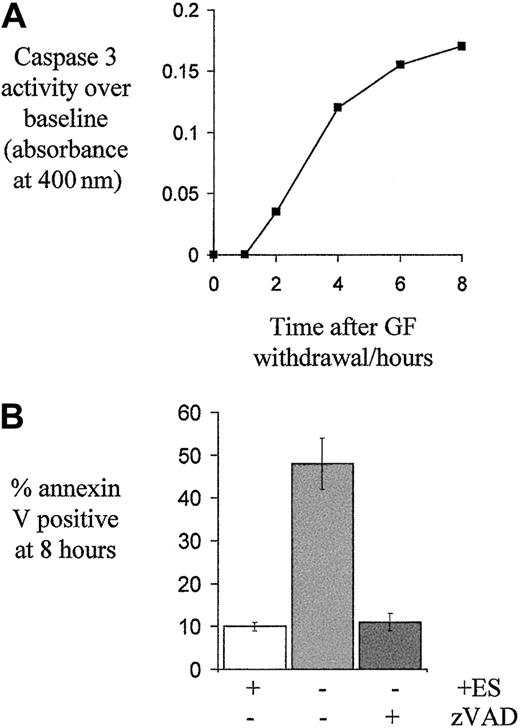
Caspase activation is instrumental in apoptosis and we found caspase 3 activity increased after growth factor withdrawal within 2 hours (Figure 5A), as shown by others.30 Blockade of caspase activity using the pan-caspase inhibitor zVAD- FMK prevented apoptosis. Only 11% ± 2% of growth factor–deprived cells treated with zVAD-FMK were apoptotic at 8 hours compared with 48% ± 6% of untreated cells (n = 6, P < .001, Figure 5B). Furthermore, examination of nuclear morphology on cytospin preparations using DAPI fluorescent nuclear staining showed that where cells had been treated with zVAD-FMK, despite 8 hours with no growth factors, nuclear morphology remained normal, unlike control cells (NIL), many of which showed nuclear changes characteristic of apoptosis (A) (Figure6B).
Caspase 3 activation in growth factor–deprived erythroblasts and the effect of zVAD-FMK on apoptosis.
(A) Caspase 3 activity in day 6 erythroblast lysates, made at the indicated times following growth factor withdrawal, was determined with a colorimetric assay. Means of 2 experiments are shown. (B) To determine the effect of caspase inhibition on apoptosis, erythroblasts on day 5 to 7 of culture were washed and then returned to medium with no growth factors, Epo 2 U/mL and SCF 20 ng/mL (ES), or no growth factors and the pan-caspase inhibitor zVAD-FMK (100 μM, n = 6). The mean percentage ± SEM of apoptotic cells after 8 hours, as determined by annexin V binding, is shown.
Caspase 3 activation in growth factor–deprived erythroblasts and the effect of zVAD-FMK on apoptosis.
(A) Caspase 3 activity in day 6 erythroblast lysates, made at the indicated times following growth factor withdrawal, was determined with a colorimetric assay. Means of 2 experiments are shown. (B) To determine the effect of caspase inhibition on apoptosis, erythroblasts on day 5 to 7 of culture were washed and then returned to medium with no growth factors, Epo 2 U/mL and SCF 20 ng/mL (ES), or no growth factors and the pan-caspase inhibitor zVAD-FMK (100 μM, n = 6). The mean percentage ± SEM of apoptotic cells after 8 hours, as determined by annexin V binding, is shown.
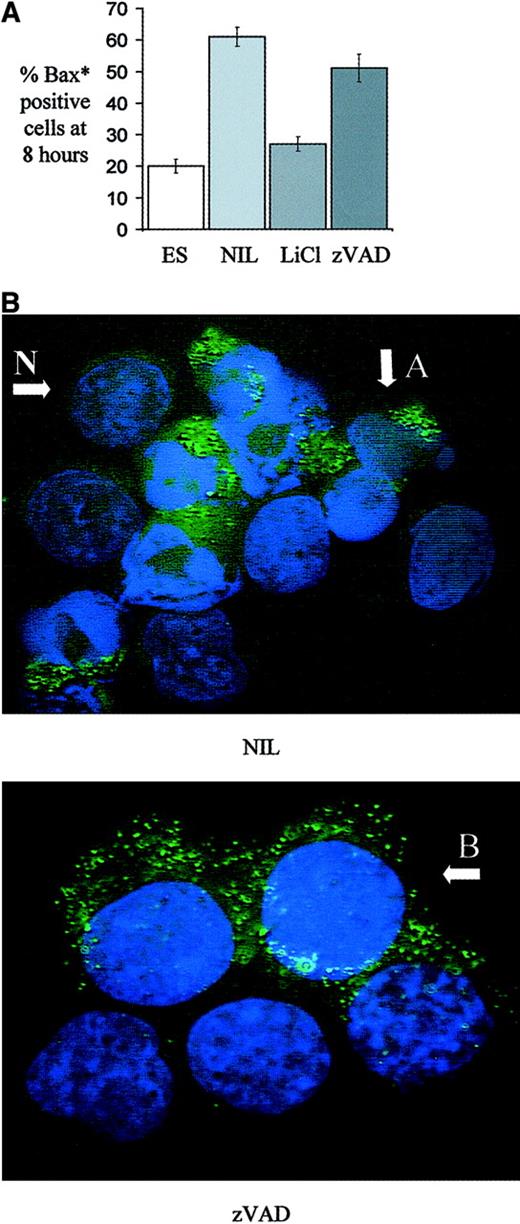
The effect of various treatments of human erythroblasts on the appearance of the conformational form of Bax associated with apoptosis.
Human erythroblasts on day 5 to 7 of culture were treated for 8 hours with medium containing either Epo 2 U/mL and SCF 20 ng/mL, no growth factors, no growth factors and LiCl (20 mM), or no growth factors and zVAD-FMK (100 μM). (A) Cells in cytospin preparations were immunostained with a conformation sensitive anti-Bax* antibody and the mean percentage ± SEM of positive cells after 8 hours is shown (n = 3). (B) Anti-Bax* immunostained cells were mounted in medium containing DAPI to visualize nuclear material. After 8 hours in growth factor–deprived conditions (NIL), apoptotic Bax*-positive cells with condensed nuclei are seen, indicated by A, as well as viable Bax*-negative cells with normal nuclear morphology, indicated by N, (× 100 oil objective). After 8 hours in the zVAD condition, although nuclear morphology remains normal in all cells, many are seen to be Bax* positive, indicated by B, (× 100 oil objective, zoom × 1.5).
The effect of various treatments of human erythroblasts on the appearance of the conformational form of Bax associated with apoptosis.
Human erythroblasts on day 5 to 7 of culture were treated for 8 hours with medium containing either Epo 2 U/mL and SCF 20 ng/mL, no growth factors, no growth factors and LiCl (20 mM), or no growth factors and zVAD-FMK (100 μM). (A) Cells in cytospin preparations were immunostained with a conformation sensitive anti-Bax* antibody and the mean percentage ± SEM of positive cells after 8 hours is shown (n = 3). (B) Anti-Bax* immunostained cells were mounted in medium containing DAPI to visualize nuclear material. After 8 hours in growth factor–deprived conditions (NIL), apoptotic Bax*-positive cells with condensed nuclei are seen, indicated by A, as well as viable Bax*-negative cells with normal nuclear morphology, indicated by N, (× 100 oil objective). After 8 hours in the zVAD condition, although nuclear morphology remains normal in all cells, many are seen to be Bax* positive, indicated by B, (× 100 oil objective, zoom × 1.5).
Clonogenicity of human erythroblasts is maintained by GSK3 inhibition but not by caspase inhibition
To determine whether the clonogenic potential, and therefore actual survival, of erythroblasts was maintained by either LiCl or zVAD-FMK, single-cell cultures were performed. Cells were washed and equal numbers then treated for 8 hours in medium with either Epo and SCF, no growth factors, no growth factors and zVAD-FMK, or no growth factors and LiCl. Cells were then transferred to 96-well plates in culture medium containing both Epo and SCF at a predicted density of 0.66 cells/well. Results showed that 45% ± 10% of growth factor–treated cells yielded a colony of 4 cells or more at 72 hours, compared with 20% ± 4% of cells with no growth factors, 42% ± 4% of cells with LiCl, and 25% ± 7% of cells with zVAD-FMK (n = 4, Figure 7). GSK3 inhibition with LiCl therefore maintained clonogenicity when compared with untreated cells (P = .006), whereas caspase inhibition did not.
The effect of LiCl or zVAD-FMK on growth factor–deprived erythroblast clonogenicity.
Equal numbers of erythroblasts on day 5 to 6 of culture were washed and treated for 8 hours in medium with no growth factors, Epo 2 U/mL and SCF 20 ng/mL (ES), or no growth factors and LiCl (20 mM) or zVAD-FMK (100 μM) as indicated. Cells were then returned to normal medium supplemented with Epo 2 U/mL and SCF 20 ng/mL in 96-well plates at a predicted density of 0.66 cells/well. The mean percentage ± SEM of wells yielding a colony of 4 cells or more 72 hours later is indicated (n = 4).
The effect of LiCl or zVAD-FMK on growth factor–deprived erythroblast clonogenicity.
Equal numbers of erythroblasts on day 5 to 6 of culture were washed and treated for 8 hours in medium with no growth factors, Epo 2 U/mL and SCF 20 ng/mL (ES), or no growth factors and LiCl (20 mM) or zVAD-FMK (100 μM) as indicated. Cells were then returned to normal medium supplemented with Epo 2 U/mL and SCF 20 ng/mL in 96-well plates at a predicted density of 0.66 cells/well. The mean percentage ± SEM of wells yielding a colony of 4 cells or more 72 hours later is indicated (n = 4).
GSK3 inhibition, but not caspase inhibition, reduces the appearance of the conformational form of Bax associated with apoptosis
During apoptosis, the proapoptotic Bcl2 family member Bax undergoes a conformational change (Bax*), which is required for its translocation from cytosol to the mitochondrial outer membrane, where it may form pores and permit cytochrome c release.31-34The conformational change is associated with the appearance of an NH2-terminal epitope that may be recognized by specific antibodies.35 Cytospins of human erythroblasts cultured for 8 hours with either Epo and SCF, no growth factors, no growth factors and LiCl, or no growth factors and zVAD-FMK were probed with a conformation sensitive anti-Bax antibody. Figure 6A shows that 20% ± 2% of cells treated with Epo and SCF were Bax* positive, compared with 61% ± 3% with no growth factors, 27% ± 2% with LiCl, and 51% ± 4% with zVAD-FMK (n = 3). Therefore, whereas LiCl treatment of growth factor–deprived cells significantly reduced Bax* positivity at 8 hours compared with untreated cells (P = .02), blockade of caspase activity did not. Figure6B shows a mixture of apoptotic Bax*-positive cells with condensed nuclear material (A) and intact Bax*-negative cells (N) in the no growth factor condition (NIL). In the zVAD condition, nuclear morphology is intact in all cells although some cells are seen to be Bax* positive (B).
Discussion
This work suggests that a pathway involving phosphoinositide-3-kinase, Akt, GSK3, and Bax contributes to the control of apoptosis induction in human erythroid progenitors. In cells mainly at the late burst-forming unit and colony-forming unit stages of development, GSK3α/β activity is regulated by phosphorylation at S21/S9, in a PI3K-dependent manner, in response to both Epo and SCF, resulting in suppressed kinase activity. Inhibition of GSK3 activity with LiCl substantially reduces apoptosis caused by growth factor withdrawal. Additionally, LiCl maintains clonogenicity and prevents the appearance of the conformational form of Bax associated with apoptosis, unlike caspase inhibition, suggesting that increased GSK3 activity has a regulatory role proximal to commitment to cell death, with caspase activation subsequent to this.
Both the PI3K/Akt pathway7 and the MAP kinase pathway11-14 have been implicated in survival signaling in primary cells. Although we found that Epo and SCF induced phosphorylation of both Akt and Erk 1/2 at their key regulatory sites in human erythroblasts, the PI3K/Akt pathway and not the MAP kinase pathway was implicated in survival signal transduction in erythroblasts because pharmacological blockade of the PI3K/Akt pathway induced apoptosis, whereas blockade of the MAP kinase pathway did not.
Of the downstream targets of Akt, we examined GSK3 for a possible role in survival signaling in erythroid cells. This is because GSK3 has been implicated in the regulation of apoptosis in Rat-1 fibroblasts, the PC12 rat pheochromocytoma cell line,36sympathetic18 and cortical neurons,17 in addition to its other well-defined biological roles. GSK3 is regulated by Akt in insulin signal transduction, reducing kinase activity by 40% in the rat L6 skeletal muscle line.15,19 Similar reductions in GSK3 activity are induced by epidermal growth factor (EGF) in A431 cells (50%)37 and nerve growth factor (NGF) in PC12 cells (30%).36 Human erythroblasts in steady-state culture show high levels of GSK3 phosphorylation at S21/S9, consistent with low GSK3 activity. This disappears with the withdrawal of growth factors and is reinduced by either Epo or SCF. As in insulin signal transduction, this effect is PI3K and not MAP kinase dependent. This suggests that the upstream kinase here is likely to be Akt, although a role for serum and glucocorticoid inducible kinase 1 (SGK1) cannot be ruled out, because this is also PI3K dependent for activity and may phosphorylate GSK3.38 The degree of inhibition of GSK3 activity induced by Epo and SCF was in the range of 25% to 30%, broadly comparable to the inhibition of GSK3 obtained by others with insulin, NGF, and EGF. It is, however, unclear how relatively small changes in GSK3 kinase activity can have important cellular effects. One possible explanation is that although whole cell GSK3 activity is assayed in a kinase assay, only a fraction of cellular GSK3 may be accessible to Akt for regulation, with the rest sequestered elsewhere in a different cellular context, with potentially different function and substrate availability.39 GSK3 requires tyrosine phosphorylation on Y279 (α isoform) or Y216 (β isoform), a residue in the activation loop of the catalytic domain, for kinase activity40 and this site is regulated in PC12 cells on NGF withdrawal and in SH-SY5Y cells treated with staurosporine.41 Using a phosphospecific antibody, we found evidence of basal tyrosine phosphorylation of both GSK3α and GSK3β but no evidence of its regulation in primary human erythroid cells (data not shown).
To determine whether GSK3 might have a role in apoptosis induction, we used LiCl to block GSK3 activity. LiCl is a potent inhibitor of GSK328 and has been shown to mimic inhibition of GSK3 in Wnt signaling in Xenopus andDictyostelium.42-44 This effect was not found with inhibitors of inositol monophosphatase,44 which is also inhibited by lithium.45 We found that 20 mM LiCl reduced the proportion of erythroblasts undergoing apoptosis consequent on growth factor withdrawal for an 8-hour period by 88%. This protective effect was found to be dose dependent over a range of lithium concentrations around and above the 2 mM IC50reported for GSK3 with lithium under ionic conditions comparable to those found intracellularly.28 Furthermore, a similar degree of protection was achieved using rottlerin, which is also a potent inhibitor of GSK3 activity.28 Together, this evidence strongly suggests that the reduction in apoptosis seen in these experiments is due to inhibition of GSK3.
Recently, a GSK3β null mouse was created, which demonstrated a role for GSK3β in suppressing apoptosis mediated by tumor necrosis factor.46 Hemopoietic stem cells from GSK3β−/− fetal livers were functionally normal and were able to reconstitute hemopoiesis fully in lethally irradiated mice. Although this might appear at odds with our findings, it is probable that GSK3α duplicates many of the functions of GSK3β, including apoptosis induction in human erythroid cells and neural tissue, because it has a similar range of substrate specificities.20Lithium carbonate is used in the therapy of bipolar affective disorder and therapy is associated with leukocytosis but not erythrocytosis.47 The cause of leukocytosis may not be related to GSK3 because the target plasma therapeutic range is 0.4 to 1.0 mM, below the IC50 for GSK3.28 Some animal studies, however, have shown that lithium may have prosurvival effects on all lineages of hemopoiesis and can induce erythrocytosis in immunodeficient mice.48
To determine a possible mechanism by which GSK3 might influence the cellular apoptotic machinery in human erythroblasts, we examined the proapoptotic Bcl2 family member Bax. Bax is normally located in the cytosol in peripheral association with the mitochondria but following a death signal translocates to the mitochondrial outer membrane31,32 consequent on a conformational change.33,49 Bax oligomers then assemble,50which have the ability to form pores and release cytochrome c.34 Concomitant with this process, an NH2-terminus epitope lying between amino acids 13 to 19 is exposed.35 This conformational change is induced by binding of Bax to the BH3-domain-only proapoptotic protein Bid, a process that may be caspase dependent51 or independent.52 Other reported inducers of conformational change in Bax include increased p38 MAP kinase activity in cortical neurons53 and blockade of pp125FAK (focal adhesion kinase) activity in mammary epithelial cells.54 We found that apoptotic human erythroblasts contained conformationally altered Bax and that its appearance was reduced by inhibiting GSK3 activity but not caspase activity. This suggests the existence of a caspase-independent, GSK3-regulated pathway that controls commitment to cell death in human erythroblasts.
Although our data suggest an important role for GSK3 in the regulation of apoptosis in erythroid cells, it is probable that other mechanisms also contribute to survival signal transduction. For example, the Forkhead transcription factor FKHRL1, a target of Akt, is phosphorylated at T32 and S253 in response to Epo and SCF in primary human erythroid cells.55 Another Akt target, the proapoptotic Bcl2 family member Bad is phosphorylated by Akt at S136, inducing dissociation from Bcl-X and binding to cytoplasmic 14-3-3 protein to promote cell survival.7 However, in this case, we did not find evidence for its regulation in response to Epo or SCF in primary human erythroid cells either by using phosphospecific antibodies or by looking for a shift in the position of Bad in SDS-PAGE–resolved lysates obtained from erythroid cells before and after growth factor stimulation (data not shown). Bcl-XL, an antiapoptotic member of the Bcl2 family of proteins, is essential for the survival through to terminal differentiation and enucleation of erythroid progenitors.56,57 Its expression increases substantially during human erythroid differentiation30 and may be induced by Epo through a Stat5 binding element in the Bcl-X promoter.58,59 However, apoptosis of in vitro–differentiated Bcl-X−/− definitive erythroid progenitors occurs very late in development, following hemoglobinization, and Epo is able to protect these cells to some degree from apoptosis, suggesting the presence of prosurvival signaling mechanisms independent of Bcl-X.57 It seems likely that although high Bcl-X expression is essential to protect hemoglobinizing erythroid precursors from apoptosis, its acute regulation does not underlie the mechanisms of apoptosis seen by us at an earlier stage of differentiation. Although in the HCD-57 cell line removal of Epo led to down-regulation of Bcl-X expression and apoptosis,60 we did not find evidence of reduction of Bcl-X levels in primary erythroid precursors deprived of growth factors for 8 hours (data not shown). Furthermore, the rapidity of onset of apoptosis in these cells, with detectable increases in caspase activity in some cells within 2 hours of growth factor withdrawal, argues against mechanisms involving transcriptional regulation and for posttranslational regulatory mechanisms.
Caspase activity has also been implicated in the regulation of erythropoiesis. Late erythroblasts express Fas ligand, which may interact with Fas expressed by earlier erythroid progenitors.61,62 This interaction activates caspases 3, 7, and 8, which are specifically targeted to the nucleus to cleave GATA-1, delaying erythroid differentiation without inducing apoptosis. This effect was reported to be dose dependently prevented by Epo acting downstream of caspase activation and reversed with inhibitors of caspase activity.62 At odds with this, limited caspase activation is suggested to be absolutely required for terminal erythroid differentiation, which may be blocked by inhibitors of caspase activity.63 We found evidence of increased caspase 3 activity as little as 2 hours after growth factor withdrawal in human erythroblasts, which correlated with rapid apoptotic cell death. Although caspase inhibition blocked apoptosis, it did not maintain clonogenicity, a finding also reported elsewhere,64suggesting that caspase activation in growth factor–deprived erythroblasts occurs subsequent to commitment to cell death and may not be amenable to regulation by growth factors.
In summary, we find that a key role of the PI3K/Akt prosurvival pathway in human erythroid progenitors is to suppress GSK3 activity. Growth factor deprivation results in increased GSK3 activity, which is associated with a conformational change in Bax and induction of cell death, irrespective of caspase blockade. Exactly how GSK3 effects these cellular changes will require further work.
Supported by the Medical Research Council (United Kingdom) with personal fellowships to T.S. and A.K. and a cooperative group grant to A.K. and D.L.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tim C. P. Somervaille, Department of Hematology, Royal Free and University College Medical School, 98, Chenies Mews, London, United Kingdom, WC1E 6HX; e-mail:t.somervaille@ucl.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal