The gray platelet syndrome (GPS) is a rare congenital bleeding disorder in which thrombocytopenia is associated with increased platelet size and decreased α-granule content. This report describes 3 new pediatric cases presenting with the classical platelet abnormalities of GPS within one family with normal parents. Examination of blood smears of the 3 patients demonstrated not only gray platelets, but also gray polymorphonuclear neutrophils (PMNs) with decreased or abnormally distributed components of secretory compartments (alkaline phosphatase, CD35, CD11b/CD18). Secondary granules were also decreased in number as assayed by immunoelectron microscopy. These data confirm that the secretory compartments in neutrophils were also deficient in this family. Megakaryocytes (MKs) were cultured from the peripheral blood CD34+ cells of the 3 patients for 14 days, in the presence of thrombopoietin and processed for immunoelectron microscopy. Although von Willebrand factor (vWF) was virtually undetectable in platelets, vWF immunolabeling was conspicuous in cultured maturing MKs, particularly within Golgi saccules, but instead of being packaged in α-granules, it was released into the demarcation membrane system. In contrast, P-selectin followed a more classical pathway. Double-labeling experiments confirmed that vWF was following an intracellular pathway distinct from the one of P-selectin. In these 3 new cases of GPS, the MKs appeared to abnormally process vWF, with secretion into the extracellular space instead of normal α-granule packaging. Furthermore, the secretory compartment of another blood cell line, the neutrophil, was also affected in this family of GPS.
Introduction
The gray platelet syndrome (GPS) is an inherited disorder of primary hemostasis with associated bleeding tendency, thrombocytopenia, and classical abnormal platelet morphology.1 The platelets appear characteristically gray after Romanovsky staining as a result of an abnormally low α-granule content. Immunoelectron microscopic analysis of gray platelets confirms that the α-granule contents appear absent.2 In contrast, the granule-limiting membranes appear present with normally expressed protein components.3 This syndrome is distinct from other inherited disorders that also affect platelet α-granules, such as α-δ storage pool deficiency and the Quebec platelet disorder. The former is combined with dense granule deficiency4 and the latter with an abnormal protease activity responsible for α-granule protein degradation.5 The α-granules are the principal storage site for hemostatic proteins such as fibrinogen, von Willebrand factor (vWF), thrombospondin, and factor V and for growth factors such as platelet-derived growth factor and transforming growth factor-β.6 In addition, plasma proteins such as albumin and immunoglobulins are endocytosed by platelets and stored in α-granules.7 The α-granule proteins are released into the open canalicular system on platelet activation and play a key role both in hemostasis and wound healing. In this report, we describe a new family presenting with GPS with a detailed description of their platelet, megakaryocytic, and polymorphonuclear neutrophil (PMN) ultrastructural abnormalities.
Patients, materials, and methods
Patients
Approval for this study was obtained from the INSERM institutional review board. Infomed consent was obtained from the patients and the parents, according to the Declaration of Helsinki.
The patients are the 3 children of a family with healthy parents. The children are 2 heterozygous twins, aged 8 years at the time of diagnosis, and their elder sister, aged 10 years. The older sister exhibited thombocytopenia (70 × 109/L) with recurrent ecchymosis and epistaxis since the age of 5 years. Up to the age of 2 years, she had many pulmonary infections. Peripheral blood smears showed that her platelets were large, agranular, and gray. In addition her PMNs also displayed a gray cytoplasmic appearance instead of their usual beige and granulous texture. On bone marrow smears, erythroblastic and granulocytic lineages were normally represented but only rare megakaryocytes (MKs) were present. The marrow specimen obtained by biopsy showed normal cellularity and confirmed that the MKs were reduced in number. A diffuse reticular fibrosis without increased collagen was noticed. Kinetic studies of autologous platelets revealed a normal life span but platelet production was apparently only one third of normal. Other investigations revealed similar biologic platelet and PMN patterns in her brother and her sister, both exhibiting thombocytopenia and prolonged bleeding times (Ivy tests: > 20 minutes).
Peripheral blood cells (platelets and PMNs) and cultured MKs from the 3 children were also studied. Ultrastructural examination of platelets demonstrated the absence of normal α-granules and prominent vacuolization. Fibrinogen as well as thrombospondin content were reduced (20% of normal).
Platelet number and morphology were normal in the parents and the grandparents of these children. The karyotypes, studied by conventional cytogenetic analysis, were normal.
Cells
Blood samples were taken from the 3 GPS children by venipuncture into plastic tubes containing ACD-C buffer (6.8 mM citric acid, 11.2 mM glucose, pH 4.2) or lithium heparin. Platelet-rich plasma (PRP) was obtained by centrifugation of PRP for 10 minutes at 180g and 22°C. The isolated platelets were obtained by centrifugation of PRP for 10 minutes at 1100g and washed 3 times with Tyrode buffer (360 mM citric acid, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 103 mM glucose, pH 7.4) containing 3.5 mg/mL bovine serum albumin (BSA; Sigma Chemical, St Louis, MO). The washed platelets were resuspended and fixed with 1% glutaraldehyde (Ladd Research, Burlington, VT) in phosphate buffer (0.1 M, pH 7.4). MKs were grown in liquid culture from circulating CD34+ blood precursors. CD34+ cells were isolated from heparin-anticoagulated blood, using a Ficoll gradient followed by a purification on magnetic beads of the Miltenyi technique. The cells were then cultured for 12 days in serum-free liquid medium containing recombinant human stem cell factor and thrombopoietin (Rhu PEG-MGDF; Amgen, Thousand Oaks, CA) at a final concentration of 10 ng/mL. Cells were fixed at day 12 of culture in 1% glutaraldehyde (Ladd Research, Burlington, VT) in phosphate buffer (0.1 mol/L, pH 7.4), and further processed for electron microscopy.8
For functional tests, PMNs were rapidly isolated at 4°C (to avoid activation) on 2% dextran T 500 (Pharmacia LKB, Uppsala, Sweden) in phosphate-buffered saline (PBS), then on a Ficoll-Isopaque gradient. After red blood cell lysis and washing with cold PBS, PMNs were adjusted to 106/mL in PBS lacking Ca++ and Mg++.9 Viability was always more than 99% in the trypan blue dye exclusion test.
Antibodies
Polyclonal rabbit antibodies were used for immunoelectron microscopy; anti-CD35 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-vWF (Dakopatts, Glostrup, Denmark), antiglycocalicin (anti-GPIb),10anti-P-selectin.11 (kind gifts from Dr Michael Berndt, Victoria, Australia) and antilactoferrin (Cappel Lab, Downington, PA) were used at 10 μg/mL. Goat antirabbit IgG coupled to 10 nm gold was purchased from British Biocell International (Cardiff, United Kingdom).
Electron microscopy
Normal PMNs, platelets, and MKs were prepared for immunoelectron microscopy as follows. After fixation, cells were washed 3 times with phosphate buffer, embedded in sucrose, and frozen in liquid nitrogen. Immunochemical reactions were performed on thin sections collected on grids according to the method of Bendayan.12 After a brief incubation of the sections with 0.1% BSA and 15% normal goat serum, they were labeled with the polyclonal rabbit antibodies diluted in Tris-buffered saline (TBS) containing 1% BSA and 4% normal goat serum for 2 hours at 22°C, washed 3 times with TBS containing 0.1% BSA, and then incubated with goat antirabbit-gold (10 nm) for 1 hour at 22°C. Double-labeling experiments were performed according to Slot and Geuze.13 The sections were counterstained with uranyl acetate and lead citrate. Samples were observed on a Philips 450 CM 10 electron microscope (Eindoven, The Netherlands).
Leukocyte alkaline phosphatase cytochemistry
Freshly prepared blood smears from the 3 patients, from their parents, and from a control subject were fixed in a solution of formol and methanol (1:9) for 1 minute, then covered with a mixture containing α-naphtyl-phosphate Na (Sigma Chemical), 0.1 g and Fast Garnet (Sigma Chemical) 0.1 g, in propanediol buffer (25 mL) and HCl N/10 (5 mL) added to distilled water (70 mL) for 25 minutes. In each sample, at least 100 PMNs were examined and the staining intensity of each of them was scored from 0 to 4 (0, no staining; 1, light beige cytoplasm; 2, deep beige cytoplasm; 3, brown staining in part of the cytoplasm; 4, homogeneously dark brown cytoplasm).
Expression of CD11b/CD18 and CD35 at the PMN surface
Heparinized blood was placed on ice and analyzed immediately. Whole blood samples were either kept on ice or incubated with PBS or N-formyl-methyonyl-leucyl-phenylalanine (fMLP; 10−6 M), at 37°C for 5 minutes. To study CD11b, CD18, and CD35 expression, samples (100 μL) from each patient were then incubated with phycoerythrin (PE)-conjugated monoclonal mouse antihuman CD11b antibody (Dakopatts), fluorescein isothiocyanate (FITC)-conjugated monoclonal mouse antihuman CD18 antibody (Becton Dickinson, Immunocytometry Systems, San Jose, CA), or FITC-conjugated monoclonal mouse antihuman CD35 antibody (Immunotech, Marseilles, France) for 30 minutes at 4°C. Red cells were lysed with FACS lysing solution. After one wash with ice-cold PBS, the cells were resuspended in 1% paraformaldehyde-PBS and kept on ice until analysis. Nonspecific antibody binding was determined on cells incubated with the same concentration of an irrelevant antibody of the same isotype.
PMN functions
Activity of reduced nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase was tested as follows. The nitroblue tetrazolium reduction assay was applied to whole blood in the presence of endotoxin (lipopolysaccharides, Escherichia coli O 55:B5, 10 μg/mL for 15 minutes) or Staphylococcus epidermidis (107 colony-forming units (CFUs)/mL for 15 minutes). After red blood cell lysis, leukocytes were cytospun and stained with fuchsin, and the percentage of PMNs containing dark blue formazan precipitates was counted microscopically.
The luminol-amplified chemiluminescence assay was performed after stimulation of isolated PMNs with either fMLP (10−6 M for 5 minutes) or phorbol-myristate acetate (PMA; 100 ng/mL for 5 minutes).
We also measured H2O2 production using a flow cytometric assay according to Bass and coworkers.14 15This technique is based on the oxidation of the nonfluorescent 2′,7′-dichlorofluorescin into highly fluorescent dichlorofluorescein (DCF), by H2O2 produced by PMA-stimulated PMNs.
Spontaneous and directed migration under agarose, toward fMLP (10−7 M) and activated serum, was measured as previously described.16
Flow cytometry analysis
Flow cytometry analysis was performed using a Becton Dickinson FACScan (Becton Dickinson Immunocytometry Systems) with a 15-mW, 488-nm argon laser. Forward and side scatters were used to identify the granulocyte population and to gate out other cells and debris. The purity of the gated cells was assessed by using FITC- or PE-conjugated CD3, CD45, CD14, and CD15 antibodies (Becton Dickinson). The green fluorescence of FITC-conjugated antibodies and DCF was recorded from 515 to 545 nm; the red fluorescence of PE-conjugated anti-CD11b was recorded from 563 to 607 nm. In all cases, unstained cells were used and the photomultiplier settings were adjusted so that the unstained cell population appeared in the lower left-hand corner of the fluorescence display. All the results were obtained with a constant photomultiplier gain. The data were analyzed using LYSIS II software (Becton Dickinson) and the mean fluorescence intensity (MFI) was used to quantify the responses. The results were compared to the normal ranges obtained from members of the laboratory staff as controls.
Results
Blood smears
Light microscopic examination of the blood smears of the 3 children was performed. Platelets displayed a characteristic gray color confirming the diagnosis of GPS. Furthermore, the PMN cytoplasm background staining was also gray with a degranulated appearance compared with normal PMNs (Figure 1A,B). The blood smears from the 2 parents were normal and platelets and PMNs displayed normal color.
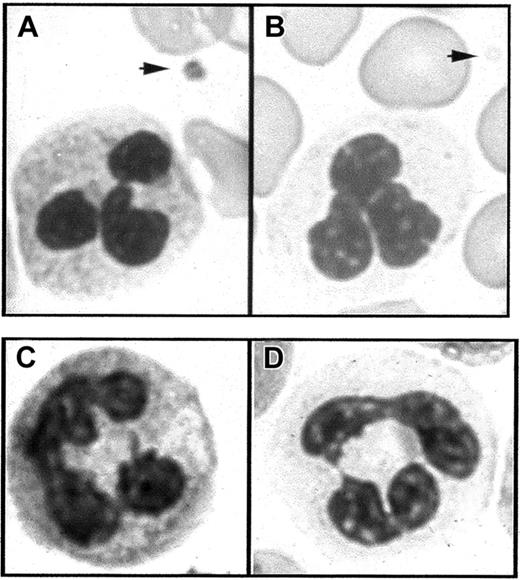
Light microscopy of blood smears.
(A) In Romanovsky-stained smears from a normal subject, the PMNs have a granular cytoplasm and a platelet displays granules in the cytoplasm (arrow). (B) In similarly stained smears from a patient with GPS, the PMN cytoplasm appears agranular and platelets are also degranulated (arrow). (C,D) Cytochemical detection of leukocyte alkaline phosphatase. PMN staining intensity is easily detected in the normal state (C) but strongly decreased in GPS compared to normal neutrophils (D).
Light microscopy of blood smears.
(A) In Romanovsky-stained smears from a normal subject, the PMNs have a granular cytoplasm and a platelet displays granules in the cytoplasm (arrow). (B) In similarly stained smears from a patient with GPS, the PMN cytoplasm appears agranular and platelets are also degranulated (arrow). (C,D) Cytochemical detection of leukocyte alkaline phosphatase. PMN staining intensity is easily detected in the normal state (C) but strongly decreased in GPS compared to normal neutrophils (D).
PMN cytochemical and ultrastructural examination
Because of the gray appearance on the smears of the PMN cytoplasm, the different neutrophil granule compartments were explored in these patients. The cytochemical detection of the leukocyte enzyme alkaline phosphatase (a specific component of the membrane of secretory vesicles) was consistently and strongly decreased compared to normal PMNs (Figure 1C,D), with a mean score of 5, 7, and 5, respectively, compared against a control mean of 62.
Myeloperoxidase (MPO)-positive primary granules were normally present, as attested by cytochemical reactions observed by electron microscopy. Immunolabeling for lactoferrin showed a strong decrease in secondary granules in the 2 patients whose PMNs could be studied by electron microscopy (Figure 2). The ratio of secondary/primary granules was diminished in these patients: control = 60%, patient Ju = 26%, patient Me = 21%. Control immunolabeling was performed by omitting the primary antibody in the reaction that led to the absence of gold particles on the structures.
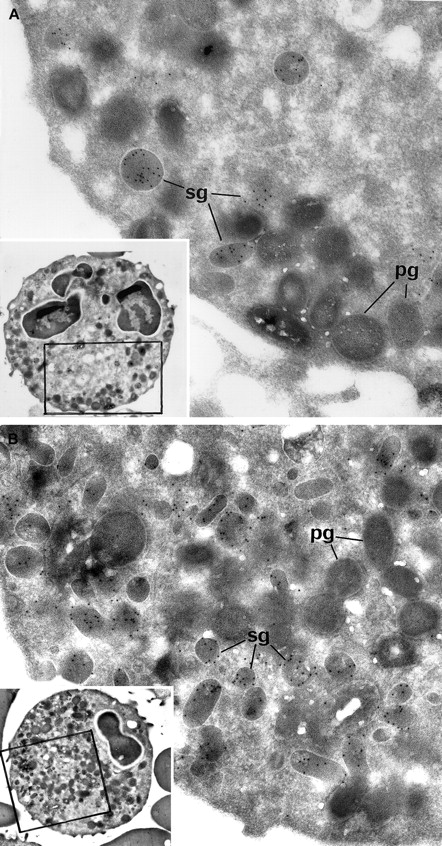
Electron microscopic view of the blood PMNs that have been cytochemically reacted for MPO and immunolabeled for lactoferrin.
(A) Patient PMNs display normal primary electron dense granules that have been opacified by the cytochemical MPO reaction and appear dark gray (pg); secondary lactoferrin-containing granules (sg) (identified by immunogold particles) are decreased in number and look larger than normal. (B) Control PMNs contain numerous lactoferrin-positive secondary granules (sg), as well as primary dark MPO+granules (pg). (Panels A and B, original magnification × 31 000; insets, × 4600.)
Electron microscopic view of the blood PMNs that have been cytochemically reacted for MPO and immunolabeled for lactoferrin.
(A) Patient PMNs display normal primary electron dense granules that have been opacified by the cytochemical MPO reaction and appear dark gray (pg); secondary lactoferrin-containing granules (sg) (identified by immunogold particles) are decreased in number and look larger than normal. (B) Control PMNs contain numerous lactoferrin-positive secondary granules (sg), as well as primary dark MPO+granules (pg). (Panels A and B, original magnification × 31 000; insets, × 4600.)
PMN flow cytometry and functional studies
Because alkaline phosphatase is a component of the membrane of secretory vesicles, which was undetectable in the 3 patients' PMNs,17 18 we investigated the distribution of the other specific components of secretory vesicles—the complement receptor CD35, which is normally restricted to the membrane of secretory vesicles; the integrins CD11b/CD18, which are expressed within both secretory vesicles and specific granules. The expression of CD35, CD11b, and CD18 (Table 1) was increased in the patients at the basal state when compared to controls. The membrane expression for those proteins was maximal on unstimulated neutrophils and did not increase on stimulation by fMLP as is the case in normal neutrophils. Interestingly, even if CD11b and CD18 expressions were highly expressed at the membrane in the PMN basal state, and barely increased after fMLP stimulation, the level of CD11b and CD18 at the membrane did not reach more than 30% of the control levels. The high basal expression of these receptors in the patients could not be explained by in vivo or in vitro PMN activation, because L-selectin expression remained in the normal range: controls = 166 ±16; patient Me = 137; patient Ju = 145.
CD35, CD11b, and CD18 expression at the surface of PMNs from the 3 patients with GPS
| . | Mean fluorescence intensity . | |||||
|---|---|---|---|---|---|---|
| PBS 4°C . | PBS 37°C . | fMLP 37°C . | ||||
| First . | Second . | First . | Second . | First . | Second . | |
| CD35 | ||||||
| Control | 15 | 27 | 17 | 41 | 106 | 141 |
| Ju | 111 | 123 | 128 | 137 | 104 | 130 |
| Ma | 89 | 97 | 97 | 99 | 93 | 117 |
| Me | 82 | 90 | 94 | 105 | 80 | 104 |
| CD11b | ||||||
| Controls | 145 ± 2 | 183 ± 12 | 2237 ± 198 | |||
| Ju | 563 | 638 | 687 | 797 | 812 | 875 |
| Ma | 607 | 705 | 806 | 824 | 732 | 862 |
| Me | 606 | 616 | 804 | 821 | 816 | 864 |
| CD18 | ||||||
| Controls | 40 | 20 | 50 | 10 | 400 | 700 |
| Ju | 116 | 144 | 131 | 129 | 150 | 181 |
| Ma | 138 | 160 | 156 | 186 | 133 | 176 |
| Me | 125 | 143 | 161 | 180 | 174 | 201 |
| . | Mean fluorescence intensity . | |||||
|---|---|---|---|---|---|---|
| PBS 4°C . | PBS 37°C . | fMLP 37°C . | ||||
| First . | Second . | First . | Second . | First . | Second . | |
| CD35 | ||||||
| Control | 15 | 27 | 17 | 41 | 106 | 141 |
| Ju | 111 | 123 | 128 | 137 | 104 | 130 |
| Ma | 89 | 97 | 97 | 99 | 93 | 117 |
| Me | 82 | 90 | 94 | 105 | 80 | 104 |
| CD11b | ||||||
| Controls | 145 ± 2 | 183 ± 12 | 2237 ± 198 | |||
| Ju | 563 | 638 | 687 | 797 | 812 | 875 |
| Ma | 607 | 705 | 806 | 824 | 732 | 862 |
| Me | 606 | 616 | 804 | 821 | 816 | 864 |
| CD18 | ||||||
| Controls | 40 | 20 | 50 | 10 | 400 | 700 |
| Ju | 116 | 144 | 131 | 129 | 150 | 181 |
| Ma | 138 | 160 | 156 | 186 | 133 | 176 |
| Me | 125 | 143 | 161 | 180 | 174 | 201 |
Whole blood was incubated with FITC-CD35, PE-CD11b, or FITC-CD18 antibodies at 4°C for 30 minutes either after maintaining the samples at 4°C or after incubation with PBS, or fMLP (10−6mol/L) at 37°C for 5 minutes. Mean fluorescence intensity was recorded as described in “Patients, materials, and methods.” Values obtained with an irrelevant antibody of the same isotype were subtracted. The CD35 and CD18 values of the day controls are in the range of other 6 healthy controls. For CD11b, the values are means ± SEM of 15 healthy controls.
Functional studies of PMN leukocytes were normal. Oxidase activity after PMA, fMLP, endotoxin, and bacterial stimulation as well as spontaneous and oriented migration were studied in the 3 children and did not differ from normal controls (data not shown).
Electron microscopy of platelets and MKs
The platelets from the 3 children displayed similar ultrastructure. Platelet size was heterogeneous, and many platelets were larger than a red blood cell or a small lymphocyte. The most striking morphologic abnormality was the lack of normal α-granules and prominent vacuolization of the cytoplasm (Figure3A). Membrane complexes, which represent abnormally distributed intracellular membrane, were also observed (Figure 3B).
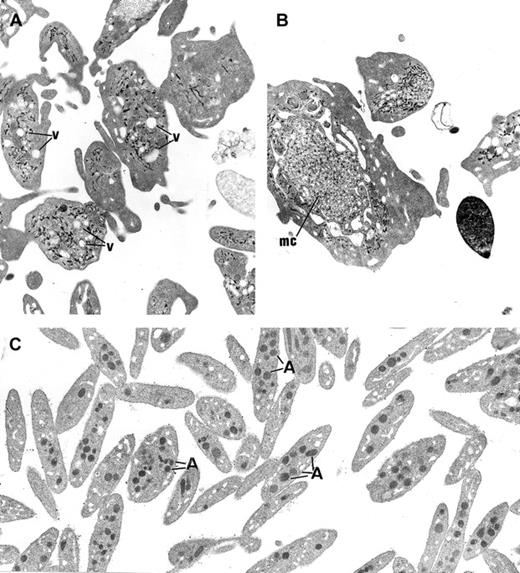
Ultrastructure of platelets.
(A) In GPS, normal α-granules are absent from the platelets and replaced by vacuolar structures (v) the size of normal α-granules. (B) Platelets of large size displaying membrane complex (mc) and devoid of normal α-granules are encountered. (C) Platelets from a normal subject are smaller and display conspicuous α-granules (A). (Panels A-C, original magnification × 10 500.)
Ultrastructure of platelets.
(A) In GPS, normal α-granules are absent from the platelets and replaced by vacuolar structures (v) the size of normal α-granules. (B) Platelets of large size displaying membrane complex (mc) and devoid of normal α-granules are encountered. (C) Platelets from a normal subject are smaller and display conspicuous α-granules (A). (Panels A-C, original magnification × 10 500.)
Intracellular trafficking of α-granule proteins was studied by immunoelectron microscopy in the 3 patients. First, gray platelets were examined for the secretory protein vWF; it was virtually absent from all putative empty α-granules (Figure4A). In contrast, in gray platelets, P-selectin was immunodetected in the membrane of empty vacuolar structures, thus identified as α-granules deprived of soluble content (Figure 4B).
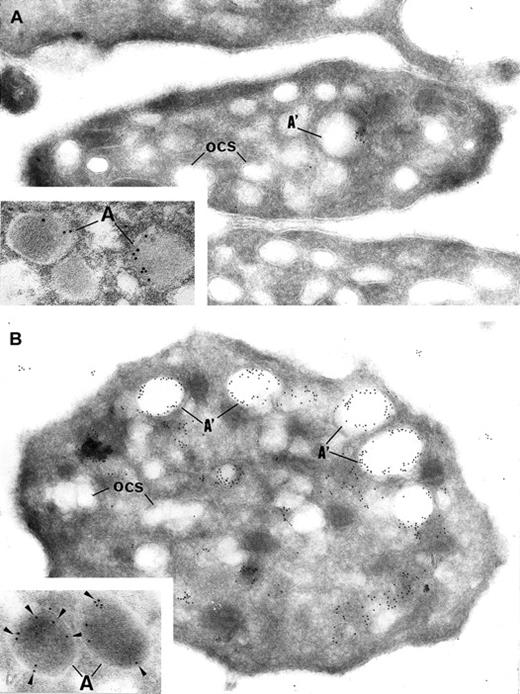
Immunoelectron microscopy.
Gray platelets immunolabeled for vWF (A) and P-selectin (B). (A) Immunolabeling for the secretory protein vWF is virtually absent from gray platelets; only trace amount of labeling is found in structures resembling empty α-granules (A′); ocs indicates open canalicular system. (B) P-selectin is immunodetected in the membrane of empty vacuolar structures, such as identified as α-granules (A′) deprived of soluble content. Insets show normal platelet α-granules immunolabeled for vWF and P-selectin. (Panels A and B, original magnification × 40 300; insets, × 65 000.)
Immunoelectron microscopy.
Gray platelets immunolabeled for vWF (A) and P-selectin (B). (A) Immunolabeling for the secretory protein vWF is virtually absent from gray platelets; only trace amount of labeling is found in structures resembling empty α-granules (A′); ocs indicates open canalicular system. (B) P-selectin is immunodetected in the membrane of empty vacuolar structures, such as identified as α-granules (A′) deprived of soluble content. Insets show normal platelet α-granules immunolabeled for vWF and P-selectin. (Panels A and B, original magnification × 40 300; insets, × 65 000.)
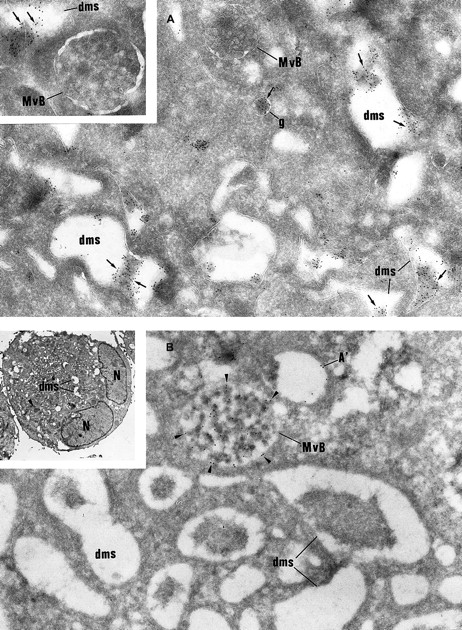
Then, intracellular trafficking of α-granule proteins was studied by immunoelectron microscopy in the 3 patients. In maturing GPS MKs, vWF immunolabeling appeared to be strongly detected within the Golgi complex, associated vesicles, and small granules (not shown), and heavy labeling was found within the lumen of the demarcation membrane system (Figure 5A). This characteristic labeling has also been observed in the cultured MKs from the 3 patients suggesting that in GPS, vWF is abundantly synthesized, not packaged or not retained in α-granules, misdirected toward the demarcation membrane system, and constitutively secreted into the extracellular medium. In maturing GPS MKs, P-selectin was detected within some vesicles arising from the Golgi complex (not shown) and nascent α-granules, which became electron lucent during maturation (Figure5B). It was also abundantly detected in the multivesicular bodies and in the membrane of occasional vacuoles (Figure 5B). Thus, and in contrast to vWF, P-selectin appeared to be normally targeted toward the membrane of vacuolar structures previously identified as empty α-granules. Double immunolabeling confirmed that P-selectin and vWF were following a distinct pattern of intracellular trafficking; P-selectin was found in the multivesicular bodies, where no GPIb was present and were thus distinct from demarcation membranes (Figure6A). vWF was detected in the lumen of demarcation membranes, identified by their GPIb content (Figure6B); multivesicular bodies were virtually deprived of vWF. Double labeling for vWF and P-selectin confirmed that the 2 proteins had followed divergent pathways because P-selectin was detected in multivesicular bodies, whereas vWF was found in the elongated channels of the demarcation membrane system (Figure 6C).
Immunolabeling of GPS MKs.
(A) vWF immunolabeling is found in small granules (g) often located near the Golgi complex and in the lumen of the demarcation membrane system (dms). Multivesicular bodies (MvB) are consistently negative (original magnification × 37 000; inset, × 46 000). (B) P-selectin is detected within multivesicular bodies (MvB) and in some vacuolar structures (A′) the size of normal α-granules. The demarcation membrane system (dms) is free of labeling (original magnification × 37 000; inset, × 2000).
Immunolabeling of GPS MKs.
(A) vWF immunolabeling is found in small granules (g) often located near the Golgi complex and in the lumen of the demarcation membrane system (dms). Multivesicular bodies (MvB) are consistently negative (original magnification × 37 000; inset, × 46 000). (B) P-selectin is detected within multivesicular bodies (MvB) and in some vacuolar structures (A′) the size of normal α-granules. The demarcation membrane system (dms) is free of labeling (original magnification × 37 000; inset, × 2000).
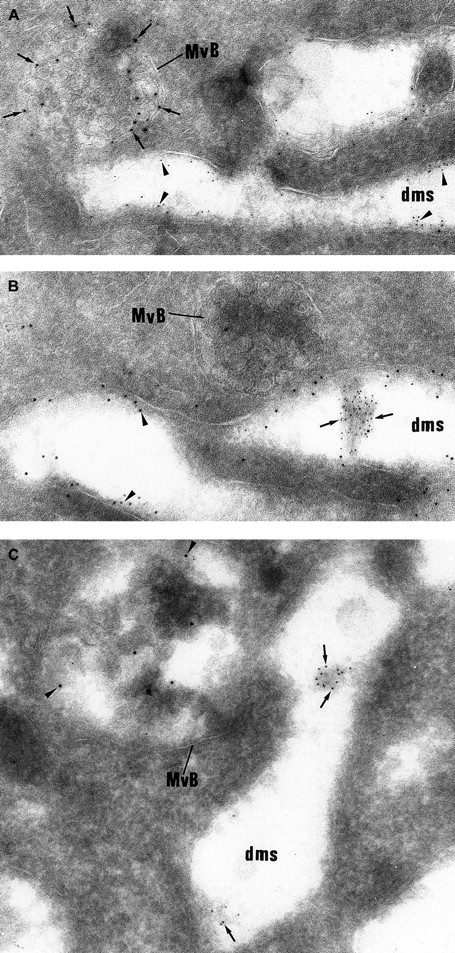
Double labeling experiments showing that vWF and P-selectin follow a distinct intracellular route.
(A) The electron lucent elongated structures display immunolabeling for GPIb (5 nm gold) and can be identified as demarcation membranes. They do not express P-selectin (10 nm gold), which is detected in multivesicular bodies (MvB). (B) vWF immunolabeling (5 nm gold) is detected in the lumen of the demarcation membrane system cisternae, which also express heavy labeling for GPIb (10 nm gold). The multivesicular bodies (MvB) are devoid of vWF labeling. (C) vWF (5-nm gold particles) is found within elongated cisternae of the demarcation membrane system, whereas P-selectin (10-nm gold particles) is detected in multivesicular bodies (MvB). (Panels A-C, original magnification × 65 100.)
Double labeling experiments showing that vWF and P-selectin follow a distinct intracellular route.
(A) The electron lucent elongated structures display immunolabeling for GPIb (5 nm gold) and can be identified as demarcation membranes. They do not express P-selectin (10 nm gold), which is detected in multivesicular bodies (MvB). (B) vWF immunolabeling (5 nm gold) is detected in the lumen of the demarcation membrane system cisternae, which also express heavy labeling for GPIb (10 nm gold). The multivesicular bodies (MvB) are devoid of vWF labeling. (C) vWF (5-nm gold particles) is found within elongated cisternae of the demarcation membrane system, whereas P-selectin (10-nm gold particles) is detected in multivesicular bodies (MvB). (Panels A-C, original magnification × 65 100.)
Control immunolabeling was performed by omitting the primary antibody in the reaction, which led to the absence of gold particles on the structures.
Discussion
This study describes a new family of 3 children with GPS. GPS is a rare congenital disorder, which can be either sporadic or inherited in an autosomal dominant fashion according to the reported observation of a large Japanese family with 24 members affected by the disease.19 The family presented here, with 2 healthy parents and the 3 affected children, suggests that the genetic transmission might also be recessive. Moreover, another family reported earlier displayed a similar inheritance pattern with healthy parents and the 2 children, a brother and a sister, with GPS.2,20,21 Nevertheless, another mode of inheritance cannot be excluded, such as germinal mosaicism, variable genic penetration, or underexpression of a gene due to an unidentified control mechanism. The α-granule proteins follow a double pattern of appearance; either they are synthesized by the MK itself or they are endocytosed from the extracellular medium.22-24As reported for normal MKs in vitro,2 vWF is synthesized by immature MKs and detected early in the Golgi area, whereas fibrinogen, which is endocytosed, is detected later within mature cells.22 In vivo studies have recently confirmed these data.25 vWF appears early also during MK maturation and is stored in a specific compartment at one pole of the α-granules.3 In gray platelets, this polar distribution of vWF within α-granules is not observed.2 It has been previously postulated that the synthesis of soluble granule proteins like vWF is normal in GPS, but these proteins are improperly packaged.2,3 21
We demonstrate here that vWF is indeed abundantly synthesized, but appears to be misdirected into the lumen of the demarcation membrane system. This protein seems to be secreted into the extracellular medium instead of being packaged (or efficiently retained) in the α-granules. Although some α-granule components are taken up from the extracellular medium and secondarily incorporated in the granules, it was firmly demonstrated that this is not the case for vWF. Indeed, normal pigs grafted with the bone marrow of a pig with severe von Willebrand disease generate platelets deprived of vWF, despite a normal level of plasma vWF.26 This rules out the hypothesis that vWF is endocytosed by MKs and could accumulate in the demarcation membranes if the system was deficient. On the other hand, the α-granule membrane component P-selectin appears to follow a normal intracellular pathway of trafficking, because it appears within multivesicular bodies and in intracytoplasmic vacuoles resembling empty α-granules and is not detected in the demarcation membranes. Multivesicular bodies have been recently shown to be a sorting compartment for α- and dense granule proteins, before their packaging within granules.27,28 Sorting of proteins within secretory granules is still poorly understood. However, it is demonstrated that many proteins aggregate in ways that facilitate association with membranes of nascent secretory granules.29 The sorting signal that directs proteins toward the aggregates is not known but could be shared by many secretory proteins and this could be missing in GPS. Protein condensation is facilitated by a slightly acidic pH and high calcium concentration in the trans-Golgi network; this is achieved by H+ and Ca++ adenosine triphosphatases.29 Whether one of these mechanisms could be impaired in GPS remains to be elucidated. In an animal model of GPS, the Wistar Furth rat, abnormal proteoglycans (serglycin) were detected in the α-granules and this might explain the α-granule content instability.30 Indeed, stored proteins may be packaged and retained within the secretory granules due to a core structural component such as serglycin; knowing that serglycin is a component of both normal platelet and neutrophil granules, it is a possible candidate that could be missing or abnormal in GPS. Therefore, this should be investigated in gray platelets. It is also noteworthy that small G-proteins are involved in intracellular vesicular transport,31 32 and their presence and function remain to be explored in GPS.
Although MKs and endothelial cells share many common processing steps of synthesis storage and regulated release of vWF within α-granules and Weibel-Palade bodies,33 the observation that vWF packaging is normal in GPS endothelial cells suggests that the anomaly in GPS is a result of a subcellular event that is specific to MKs.34 Our immunoelectron microscopic study of gray platelets and MKs shows that the disease does not affect the sorting of P-selectin into the α-granules. It was previously demonstrated by transfection experiments on the AtT20 cell line that P-selectin contains its own targeting signal that directs the protein to the limiting membrane of secretory granules and that this signal is located in the cytoplasmic tail of P-selectin.35 However, soluble proteins do not possess either membrane or cytoplasmic tails; therefore, they are probably sorted by a different mechanism, which may be abnormal in GPS. Finally, the leakage of α-granule proteins (including growth factors) into the extracellular space seems to be responsible for the development of myelofibrosis, which is a virtually constant finding in GPS.36 37
We have also extensively studied PMNs of the 3 children because of the gray staining with Romanovsky stain. Interestingly, a retrospective study of the blood smears from 2 previously published cases indicated the same gray color of PMNs. Due to the similar staining properties between platelets and PMNs, we have investigated the PMN granules. At least 3 well-defined secretory organelles have been described in PMNs: primary granules that contain MPO, secondary granules that contain lactoferrin, and secretory vesicles that contain the alkaline phosphatase and CD35 receptor in their membrane and plasma proteins (albumin) in their lumen.17,38 39
The 3 children with GPS presented an impairment of the secretory vesicle and secondary granule compartments, whereas MPO-containing primary granules were normally present. The cytochemical activity of the leukocyte alkaline phosphatase was examined in the 3 GPS children, their 2 parents, and the 2 previously reported patients.2In the 5 patients this enzymatic cytochemical activity was very low, only barely detectable, whereas it was normal in the parents. In addition, secretory vesicles provide an intracellular CD35 reservoir, from which this membrane protein may be recruited to the cell surface after fMLP stimulation.17 Our flow cytometric studies showed a maximal CD35 expression at the surface of the patient PMNs, in their resting state. This expression did not increase after fMLP stimulation. The high basal expression of CD35 without up-regulation suggests that during granulopoiesis, newly synthesized CD35 was not retained in the secretory vesicles, but was routed to the plasma membrane. This is in accordance with the findings that a promyelocytic cell line, NB4 cells, forced to differentiate into neutrophils with dimethyl sulfoxide or retinoic acid, lacks specific granules, whereas some of the cell line's endogenous protein content localizes to the plasma membrane.40,41 The integrin CD11b/CD18 is present in both secretory vesicles and specific granules. It is also up-regulated by degranulation in a hierarchical manner, playing a key role in the different steps leading to PMN infiltration of inflammatory sites. In the children with GPS, CD11b/CD18 is also highly expressed at the membrane in the basal resting state. This is in accordance with the ultrastructural observation of a decrease in specific granules.17 The high basal membrane expression of CD35, CD11b/CD18 was not due to PMN activation because L-selectin was not decreased. Indeed, this adhesion molecule is constitutively expressed at the PMN plasma membrane, and on activation, it is shed from the membrane by proteolysis.
The importance of the specific granules in neutrophil function has been shown in patients who lack specific granules.42 These patients are susceptible to repeated skin and respiratory infections and have defective neutrophil chemotaxis and adhesion. To our knowledge, the clinical consequences of secretory vesicle defect in patients has not been described. In the present cases, one of the children has suffered many respiratory infections but not the others.
This study describes a new association between thrombocytopenia and PMN abnormalities. Noteworthy, such an association is present in the May-Hegglin syndrome,43 Fechtner syndrome (a variant of Alport syndrome with leukocyte inclusions and macrothrombocytopenia),44 and Chediak-Higashi syndrome.45 The observation that the secretory compartments of 2 different lineages are affected by the same pathologic process in GPS strongly suggests a similar molecular defect in the common hematopoietic precursor.
The authors gratefully acknowledge Dr Fadila Souni for the light microscopic work on PMNs, Pr Jacques Caen for allowing the retrospective study of the PMNs of 2 GPS patients, Dr Paul Harrison for improving the manuscript, and Dr Paul-Henri Romeo for his constant support.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Elisabeth M. Cramer. INSERM U.474, MaternitéPort-Royal, 5ème étage, 123 Boulevard de Port-Royal, Paris 75014, France; e-mail: elisabeth@cochin.inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal