Abstract
Thrombin, the terminal serine protease in the coagulation cascade, is a proinflammatory molecule in vivo and induces endothelial activation in vitro. The cellular signaling mechanisms involved in this function are unknown. The role of the p38 mitogen-activated protein kinase (MAPK) signaling pathway in thrombin-induced chemokine production was studied. Phosphorylation of both p38 MAPK and its substrate, ATF-2, was observed in human umbilical vein endothelial cells (HUVECs) stimulated with thrombin, with a maximum after 5 minutes of stimulation. Using the selective p38 MAPK inhibitor SB203580, there was a significant decrease in thrombin-induced interleukin-8 (IL-8) and monocyte chemotactic protein-1 (MCP-1) protein production and messenger RNA steady-state levels. In addition, SB203580 decreased IL-8 and MCP-1 production induced by the thrombin receptor-1 agonist peptide (TRAP), suggesting functional links between the thrombin G protein–coupled receptor and the p38 MAPK pathway. Furthermore, endothelial activation in the presence of SB203580 decreased the chemotactic activity of thrombin-stimulated HUVEC supernatant on neutrophils and monocytic cells. In contrast, the p42/p44 MAPK pathway did not appear to be involved in thrombin- or TRAP-induced endothelial chemokine production, because there was no reduction in the presence of the p42/p44-specific inhibitor PD98059. These results demonstrate that the p38 rather than p42/44 MAPK signaling pathway plays an important role in thrombin-induced endothelial proinflammatory activation and suggest that inhibition of p38 MAPK may be an interesting target for anti-inflammatory strategies in vascular diseases combining thrombosis and inflammation.
Introduction
Thrombin, a well-known procoagulant molecule, activates platelet aggregation and processing of fibrinogen into fibrin.1 Three different thrombin receptors, belonging to the protease-activated receptor (PAR-1, -3, -4) family, have been identified on different cells.2-4 PAR-2, another member of this family, does not seem to be activated by thrombin but, rather, by trypsin.4 Following enzymatic cleavage of PAR-1, thrombin exhibits pleiotropic effects on cells. Notably, thrombin acts as a proliferation-inducing factor for smooth muscle cells and fibroblasts in vitro.5 In addition, thrombin appears to be a potent proinflammatory molecule in vivo and in vitro.6 Indeed, thrombin participates in the different steps of leukocyte-endothelium interactions by inducing in vitro endothelial expression of leukoendothelial adhesion molecules. Thrombin has been shown to induce P-selectin expression through a protein synthesis-independent mechanism and E-selectin as well as intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 expression through gene transcription and protein synthesis.7-10 Thus, thrombin may participate in the initial phase of rolling mediated by selectins, followed by firm leukoendothelial adhesion mediated by members of the immunoglobulin superfamily.11 Thrombin also favors leukocyte recruitment, because it is directly chemotactic for neutrophils and monocytes.12,13 In addition, thrombin induces the production of interleukin-8 (IL-8) and monocyte chemotactic protein-1 (MCP-1), 2 chemokines acting on neutrophils and monocytes.9,14,15 Interestingly, thrombin proinflammatory functions on endothelium are not mediated by the classical proinflammatory cytokines IL-1α/β and tumor necrosis factor-α (TNF-α), because they are not inhibited by specific IL-1α/β and TNF-α inhibitors or IL-1α antisense and are reproduced by a specific thrombin receptor-1 agonist peptide (TRAP).9 15
Although thrombin triggers cells following cleavage of PAR-1, downstream cellular activation signal pathways have not been clearly identified. Thrombin has been shown to induce smooth muscle cell proliferation through activation of the p42 and p44 extracellular-regulated kinase (ERK) members of the mitogen-activated protein kinase (MAPK) family.16 The MAPK family consists of 3 different subgroups of molecules, ERK-1/ERK-2, Jun-NH2-terminal kinases (JNKs), and p38, each requiring phosphorylation of tyrosyl and threonyl residues for activation.17 ERK kinases are involved in cell signaling induced by mitogens and growth factors.17 ERK kinases are activated by upstream kinases such as Raf and MAPK extracellular signal–regulated kinase kinases (MEK-1 and MEK-2) and regulate various transcription factors involved in cell proliferation and differentiation.17Alternatively, cellular stress but not mitogens appear to activate the so-called stress-activated MAPKs, JNK, and p38 MAPKs through phosphorylation of upstream MAPK kinases such as MKK4/MKK7 for JNK and MKK3/MKK6 for p38.18-20 JNK and p38 phosphorylate cellular transcription factors, including c-jun, JunD, Elk-1, and ATF-2.18-20 For example, lipopolysaccharide, IL-1, or TNF-α have been shown to induce proinflammatory cell activation such as cyclooxygenase-2 synthesis or IL-6 and chemokine production through JNK and p38 activation.21,22 Because thrombin induces p38 MAPK activation in platelets during platelet aggregation,23 24 we asked whether p38 MAPK was involved in thrombin-induced endothelial proinflammatory functions such as chemokine production.
Materials and methods
Materials
The following materials were purchased: Transwell chambers (Falcon Becton Dickinson, Grenoble, France); M199 culture medium, fetal calf serum (FCS), phosphate-buffered saline without Ca++and Mg2+ (Biowhittaker, Fontenay sous Bois, France); human α-thrombin (1000 U/mg, tested negative for contamination by plasmin, plasminogen, fibrin degradation products, human immunodeficiency virus, hepatitis virus), endothelial supplement growth factor from bovine pituitary gland, polymyxin B sulfate (Sigma Chemical, Coger, Paris, France); 51Na chromate (Cisbio International, Gif sous Yvette, France); recombinant human IL-8 and MCP-1, neutralizing goat anti–human IL-8 antibody, neutralizing mouse anti–human MCP-1 monoclonal antibody (immunoglobulin G1) (R&D Systems, Abingdon, United Kingdom); and thrombin receptor agonist peptide (TRAP-14) consisting of S-F-L-L-R-N-P-N-D-K-Y-E-P-F (single-letter amino acid abbreviations) and a control “scrambled” peptide consisting of N-E-F-S-L-P-K-P-F-R-Y-L-N-D (Neosystem Laboratories, Strasbourg, France). TRAP-14 acts as an agonist for PAR-1 and PAR-2. The selective p38 inhibitors pyridinyl imidazole SB203580 and PD169316 were from Vertex Pharmaceuticals (Cambridge, MA) and Calbiochem Merck Laboratories (Fontenay sous Bois, France), respectively. The MEK-1 inhibitor PD98059, the phosphoplus p38 MAPK antibody kit, and the p38 MAPK assay kit were purchased from New England Biolabs (Ozyme, Saint-Quentin en Yvelines, France).
Cell cultures
Human umbilical vein endothelial cells (HUVECs) were obtained and used on passage 2 or 3 as previously described.9 10Thrombin, TRAP-14, or control peptide were then added to HUVECs, and after various culture times the supernatant from each well was collected, centrifuged, and stored at −75°C prior to assay. The inhibitors SB203580, PD169316, or PD98059 were added to cultures 1 hour prior to HUVEC stimulation. All experiments described in this paper were performed in the presence of polymyxin B (7 μg/mL). THP-1 monocytic cell line was cultured in RPMI containing 10% FCS.
Endogenous p38 phosphorylation assay
The endogenous p38 phosphorylation assay was performed following the manufacturer's procedure (New England Biolabs). Briefly, HUVECs grown to confluence in 65-mm Petri dishes were activated with 8 U/mL thrombin for various times. After lysis, cell extracts were electrophoresed on a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel. Proteins were then transferred onto nitrocellulose, and membranes were probed with rabbit polyclonal anti–human p38 MAPK antibody or rabbit polyclonal anti–human phosphorylated p38 MAPK antibody. After incubation with an antirabbit secondary antibody conjugated with horseradish peroxydase, specific bands were revealed by incubation with the supersignal chemiluminescence substrate (Pierce Laboratories, Interchim, Montluçon, France) and exposed to autoradiographic films (Kodak, Biomax Light-1, Sigma Chemical).
Endogenous p38 MAPK assay
The p38 MAPK was selectively immunoprecipitated from thrombin-activated HUVEC lysates using a specific rabbit anti–human p38 MAPK polyclonal antibody (New England Biolabs) following the manufacturer's procedure. The immunoprecipitate was then incubated with ATF-2 fusion protein, p38 MAPK substrate, in the presence of ATP. ATF-2 phosphorylation on Thr-71 was measured after 12% SDS-PAGE migration followed by immunoblotting with a rabbit antiphospho–ATF-2 (Thr-71) antibody. Specific bands were revealed using chemiluminescence as described above.
RNA extraction and complementary DNA synthesis
RNA was isolated and complementary DNA synthesized following a previously reported procedure.9 Briefly, unstimulated and thrombin-activated HUVECs with or without SB203580 in 25 cm2 culture flasks were directly solubilized in RNA extraction solution (Trizol, Life Technologies, Gibco, Cergy-Pontoise, France). Total RNA was isolated and precipitated, and reverse transcription was then conducted using Moloney murine leukemia virus reverse transcriptase (Superscript RT, Life Technologies, Gibco).
Polymerase chain reaction
Polymerase chain reaction (PCR) amplification of the complementary DNA was conducted using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers as internal control as previously reported.9 Amplification consisted of 5 minutes at 94°C followed by 30 sequential cycles consisting of 1 minute at 94°C, 1 minute at 55°C, and 45 seconds at 72°C, and then a final elongation cycle of 10 minutes at 72°C in a Crocodile II thermal cycler (Appligen, Illkirch, France). Products of PCR (10 μL) were electrophoresed in a 2% agarose gel (Nusieve, Tebu, Le Perray en Yvelines, France) and then, after ethidium bromide coloration, were quantified using densitometry on a gel imager Easy Herolab (Fisher Scientific Labosi, Elancourt, France). As predicted, the amplification product (amplicon) was 247 base pairs for IL-8 and 274 base pairs for MCP-1.
Reverse transcriptase–PCR specific primers
Specific primers were 5′-TTGGCAGCCTTCCTGATT-3′ sense and 5′-AACTTCTCCACAACCCTCTG-3′ antisense for IL-825; 5′-TCCAGCATGAAAGTCTCTGC-3′ sense and 5′-TGGAATCCTGAACCCACTTC-3′ antisense for MCP-125; and 5′-CCACCCATGGCAAATTCCATGGCA-3′ sense and 5′-TCTAGACCGCAGGTCAGGTCCACC-3′ antisense for GAPDH (Genset, Paris, France).26
Cytokine assays
IL-8 and MCP-1 were measured using a specific enzyme-linked immunosorbent assay for each chemokine (Quantikine, R&D Systems).
Chemotaxis assay
Polymorphonuclear cells (PMNCs) prepared as previously reported9 or THP-1 monocytic cells were labeled with 7.4 MBq 51Cr and chemotaxis assays were performed in 3-μm or 8-μm Transwell plates, respectively, as described.9 Briefly, 106 PMNCs or THP-1 under 200 μL volume of culture medium were added to the upper chamber (and allowed to sediment for 45 minutes in the case of THP-1) before immersion of the Transwell in the bottom wells containing 700 μL culture medium alone or either recombinant IL-8, recombinant MCP-1 with or without respective neutralizing antibody, or supernatants of thrombin-activated HUVECs in the absence or presence of SB203580. The undersurface of the filter was rinsed with 2 mL ice-cold phosphate-buffered saline without Ca++ and Mg2+and containing 5 mM ethylenediaminetetraacetic acid. Both bottom well and washed filter media were collected and centrifuged. PMNC and THP-1 cell radioactivity was counted in a γ-counter (Cobra II, Packard, Rungis, France). Percentage of migration was calculated as follows: [(cpm migrated)−(total cpm added)] × 100.
Statistical analysis
Cytokine levels were expressed as the mean ± SEM of results obtained from 3 to 4 individual experiments performed in triplicate. The data were compared using the paired Studentt test.
Results
Thrombin induces endogenous p38 MAPK phosphorylation in HUVECs: a time course
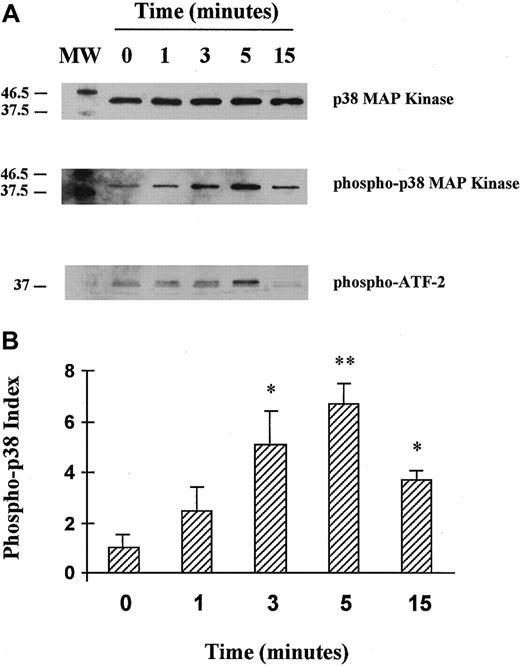
HUVECs were stimulated with 8 U/mL thrombin for 1, 3, 5, or 15 minutes, and the phosphorylated form of p38 MAPK was probed using a specific antibody. No phosphorylated form of p38 MAPK was detected in unstimulated cells, whereas HUVEC stimulation with thrombin clearly induced phosphorylation of p38 MAPK, with a maximal activation at 5 minutes of stimulation followed by a decrease after 15 minutes (Figure1A, lane 2, and Figure 1B). The p38 MAPK was then immunoprecipitated from thrombin-activated HUVEC lysates and in vitro kinase assays performed using ATF-2 as a substrate. As revealed using a specific antiphosphorylated ATF-2 antibody, thrombin induced p38 MAPK activity, with a maximum after 5 minutes of stimulation (Figure 1A, lane 3, and Figure 1B).
Activation of p38 MAPK in HUVECs stimulated by thrombin: a time course.
(A) HUVECs were unstimulated or stimulated with 8 U/mL thrombin for 1, 3, 5, or 15 minutes. HUVEC cell lysates were separated by SDS-PAGE and then transferred onto nitrocellulose membranes. Native and phosphorylated p38 MAPKs were analyzed using specific anti-p38 (lane 1) and antiphosphorylated (phospho-) p38 antibodies (lane 2). After p38 MAPK immunoprecipitation from thrombin-activated HUVEC cell lysates, the kinase activity was tested in an in vitro assay using ATF-2 as a substrate and antiphosphorylated ATF-2 fusion protein antibodies (lane 3). (B) Schematic representation of thrombin-induced p38 phosphorylation kinetics (fold activation relative to unstimulated cells is indicated, *P < .05, **P < .01, n = 3). The phospho-p38 index was calculated for each condition as follows: phospho-p38 concentration/native p38 concentration, each concentration being determined by comparing each band density using a gel analyzer.
Activation of p38 MAPK in HUVECs stimulated by thrombin: a time course.
(A) HUVECs were unstimulated or stimulated with 8 U/mL thrombin for 1, 3, 5, or 15 minutes. HUVEC cell lysates were separated by SDS-PAGE and then transferred onto nitrocellulose membranes. Native and phosphorylated p38 MAPKs were analyzed using specific anti-p38 (lane 1) and antiphosphorylated (phospho-) p38 antibodies (lane 2). After p38 MAPK immunoprecipitation from thrombin-activated HUVEC cell lysates, the kinase activity was tested in an in vitro assay using ATF-2 as a substrate and antiphosphorylated ATF-2 fusion protein antibodies (lane 3). (B) Schematic representation of thrombin-induced p38 phosphorylation kinetics (fold activation relative to unstimulated cells is indicated, *P < .05, **P < .01, n = 3). The phospho-p38 index was calculated for each condition as follows: phospho-p38 concentration/native p38 concentration, each concentration being determined by comparing each band density using a gel analyzer.
Thrombin induces endogenous p38 MAPK phosphorylation in HUVEC: a dose response
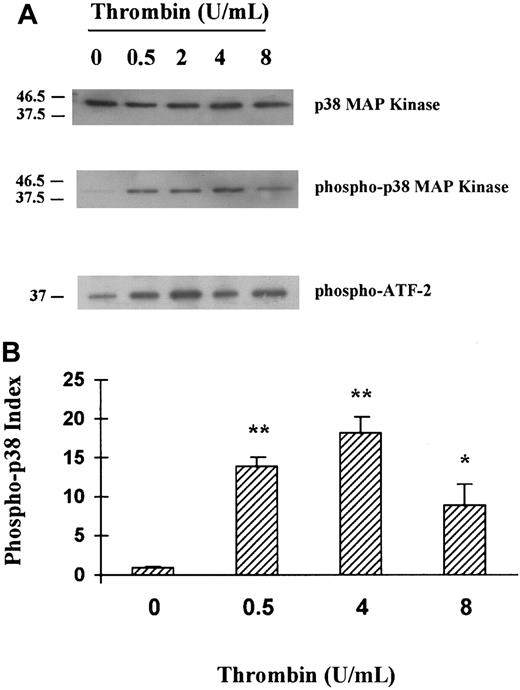
Following the above data, HUVECs were stimulated for 5 minutes in the presence of various concentrations of thrombin, and p38 MAPK phosphorylation as well as p38 MAPK in vitro activity were tested. As shown in Figure 2A (lane 2 and lane 3) and 2B, thrombin induced p38 MAPK phosphorylation and in vitro activity in a dose-dependent fashion and at concentrations as low as 0.5 U/mL.
Activation of p38 MAPK in HUVECs stimulated with thrombin: a dose response.
(A) HUVECs were stimulated with various concentrations (0.5, 2, 4, 8 U/mL) of thrombin for 5 minutes, and then using specific antibody, native p38 (lane 1) or phosphorylated p38 MAPKs (lane 2) were detected in HUVEC cell lysates, and phosphorylation of ATF-2 was tested in vitro (lane 3). (B) Schematic representation of the thrombin dose response on p38 phosphorylation (fold activation relative to unstimulated cells is indicated, **P < .01, *P < .05, n = 3). The phospho-p38 index was calculated for each condition as follows: phospho-p38 concentration/native p38 concentration, each concentration being determined by comparing each band density using a gel analyzer.
Activation of p38 MAPK in HUVECs stimulated with thrombin: a dose response.
(A) HUVECs were stimulated with various concentrations (0.5, 2, 4, 8 U/mL) of thrombin for 5 minutes, and then using specific antibody, native p38 (lane 1) or phosphorylated p38 MAPKs (lane 2) were detected in HUVEC cell lysates, and phosphorylation of ATF-2 was tested in vitro (lane 3). (B) Schematic representation of the thrombin dose response on p38 phosphorylation (fold activation relative to unstimulated cells is indicated, **P < .01, *P < .05, n = 3). The phospho-p38 index was calculated for each condition as follows: phospho-p38 concentration/native p38 concentration, each concentration being determined by comparing each band density using a gel analyzer.
The p38 inhibitor SB203580 inhibits thrombin-induced IL-8 and MCP-1 production
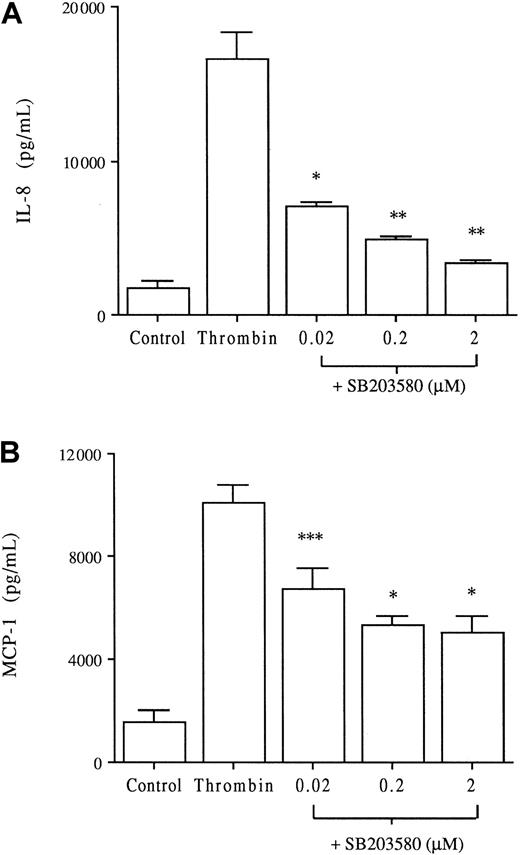
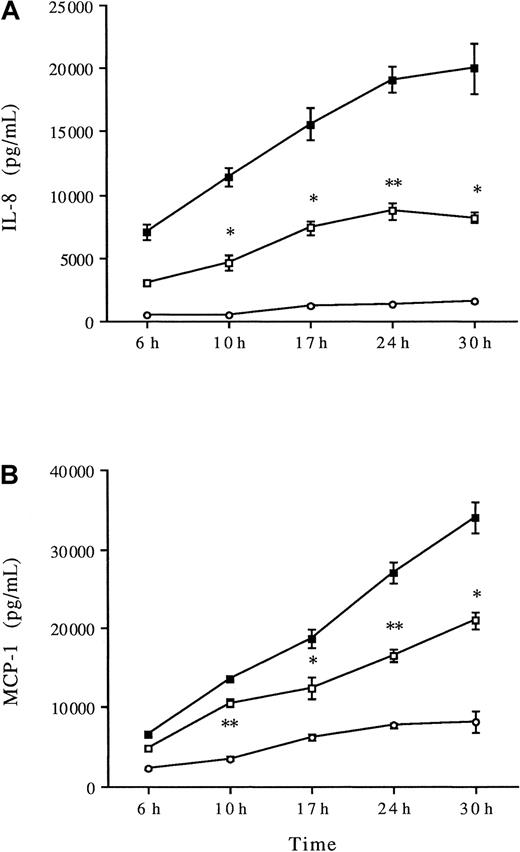
Thrombin is known to induce a dose-dependent IL-8 and MCP-1 production by HUVECs, as reported by us and others.9,15 In the following experiments, thrombin concentrations of 8 U/mL induced significant IL-8 and MCP-1 production compared with unstimulated HUVECs (P < .001, Figure 3). To elucidate the role of p38 MAPK in thrombin-induced chemokine production, the p38 inhibitor SB203580 was added at concentrations (0.02 to 2 μM) known to induce selective inhibition of p38 MAPK activity.20 Increasing concentrations of SB203580 induced a dose-dependent significant decrease of IL-8 and MCP-1 productions (Figure 3). SB203580 appears to be more effective on IL-8 than on MCP-1 production (80% inhibition vs 50%, respectively, at 2 μM SB203580). Comparable inhibition of thrombin-induced IL-8 and MCP-1 production was observed using PD169316, another selective p38 inhibitor (data not shown). HUVECs were then stimulated with thrombin in the presence or absence of 2 μM SB203580 for various periods. There was a significant decrease in IL-8 production after 10 hours of thrombin stimulation, which persisted for 30 hours (Figure4A). SB203580 also significantly decreased MCP-1 production after 10 hours of stimulation, which persisted for 30 hours (Figure 4B).
Dose-response inhibitory effect of the p38 MAPK inhibitor SB203580 on thrombin-induced IL-8 and MCP-1 production.
HUVECs cultured with or without increasing concentrations of the specific p38 MAPK inhibitor SB203580 for 1 hour were then stimulated with thrombin (8 U/mL) for 24 hours. Chemokines were measured in the supernatants IL-8 (A) and MCP-1 (B); *P < .01, **P < .001, ***P < .05 compared with thrombin alone (n = 6).
Dose-response inhibitory effect of the p38 MAPK inhibitor SB203580 on thrombin-induced IL-8 and MCP-1 production.
HUVECs cultured with or without increasing concentrations of the specific p38 MAPK inhibitor SB203580 for 1 hour were then stimulated with thrombin (8 U/mL) for 24 hours. Chemokines were measured in the supernatants IL-8 (A) and MCP-1 (B); *P < .01, **P < .001, ***P < .05 compared with thrombin alone (n = 6).
Kinetics of thrombin-induced IL-8 and MCP-1 production: effects of SB203580.
HUVECs were preincubated without (▪) or with SB203580 (2 μM) (■) for 1 hour and then stimulated with 8 U/mL thrombin for various times before chemokines were measured in the supernatants IL-8 (A) and MCP-1 (B); *P < .05, **P < .01 compared with thrombin alone (n = 3), unstimulated HUVECs (○).
Kinetics of thrombin-induced IL-8 and MCP-1 production: effects of SB203580.
HUVECs were preincubated without (▪) or with SB203580 (2 μM) (■) for 1 hour and then stimulated with 8 U/mL thrombin for various times before chemokines were measured in the supernatants IL-8 (A) and MCP-1 (B); *P < .05, **P < .01 compared with thrombin alone (n = 3), unstimulated HUVECs (○).
Extracellular signal-regulated kinases are not involved in thrombin-induced chemokine production
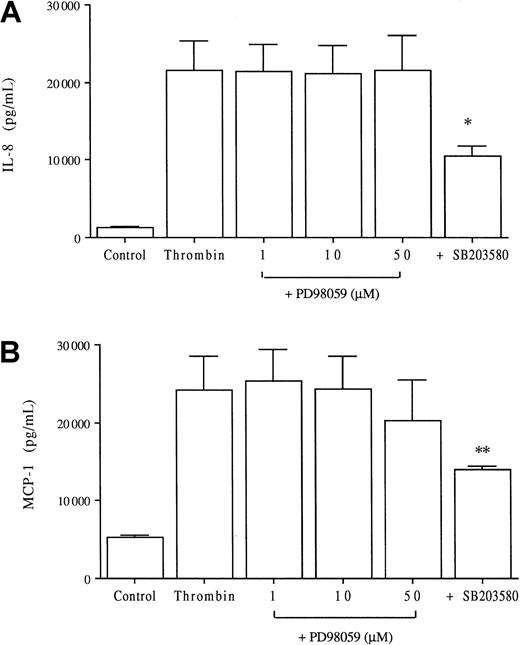
To evaluate the potential role of ERK kinases in thrombin-induced chemokine production by endothelial cells, we stimulated HUVECs in the presence of various concentrations of the specific inhibitor PD98059. This inhibitor acts upstream of ERK by selectively inhibiting MEK-1. As shown in Figure 5, PD98059 did not significantly decrease thrombin-induced IL-8 or MCP-1 production at any of the concentrations used whereas, in parallel experiments, SB203580 significantly reduced thrombin-induced IL-8 (43% inhibition) and MCP-1 (40% inhibition) production.
Lack of effect of the ERK kinase inhibitor PD98059 on thrombin-induced IL-8 and MCP-1 production.
HUVECs were cultured with or without various concentrations of the specific MEK-1 inhibitor PD98059 or 2 μM of the p38 inhibitor SB203580 and then were stimulated with 8 U/mL thrombin for 24 hours before chemokine measurements in the supernatants, IL-8 (A) and MCP-1 (B); *P < .005, **P < .01 compared with thrombin alone (n = 4).
Lack of effect of the ERK kinase inhibitor PD98059 on thrombin-induced IL-8 and MCP-1 production.
HUVECs were cultured with or without various concentrations of the specific MEK-1 inhibitor PD98059 or 2 μM of the p38 inhibitor SB203580 and then were stimulated with 8 U/mL thrombin for 24 hours before chemokine measurements in the supernatants, IL-8 (A) and MCP-1 (B); *P < .005, **P < .01 compared with thrombin alone (n = 4).
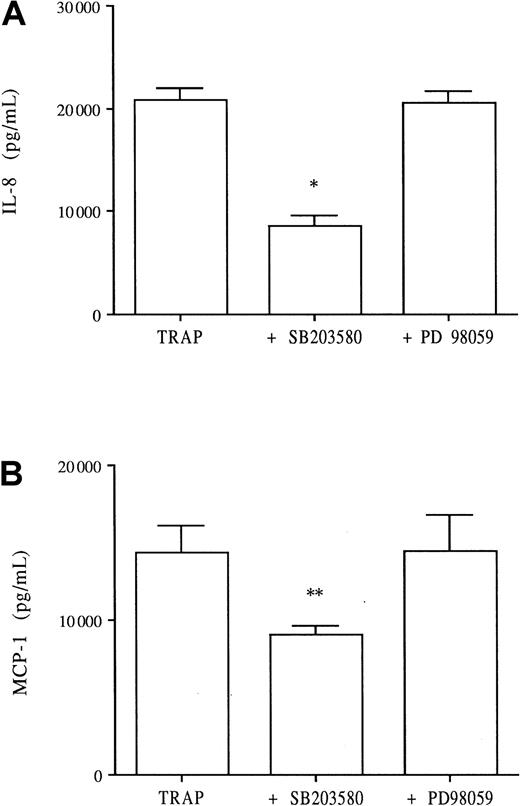
SB203580 but not PD98059 inhibits TRAP-induced IL-8 and MCP-1 productions
TRAP is a 14–amino acid agonist peptide representing the functional N-terminal region of the thrombin receptor and mimics thrombin activity. TRAP has been shown to induce both IL-8 and MCP-1 production by HUVECs in a dose-dependent fashion, whereas a scrambled peptide had no effect.9 15 TRAP at 100 μM induces HUVEC IL-8 production to levels comparable to those induced by 8 U/mL thrombin. TRAP induced significant IL-8 production by HUVECs (Figure6A), whereas the scrambled peptide did not (data not shown). When added to culture, SB203580 significantly decreased TRAP-induced IL-8 production (60% inhibition), whereas PD98059 demonstrated no inhibitory effect (Figure 6A). Similarly, TRAP induced significant MCP-1 production by HUVECs, which was significantly decreased by addition of 2 μM SB203580 (40% inhibition) but not by PD98059 (Figure 6B).
Effects of SB203580 and PD98059 on TRAP-induced IL-8 and MCP-1 endothelial production.
To determine whether the specific thrombin PAR-1 receptor was linked to the p38 or p42/44 MAPK pathway, HUVECs were stimulated with 100 μM TRAP in the presence or absence of SB203580 (2 μM) and PD98059 (10 μM). After 24 hours in culture, the supernatants were collected and assayed for chemokines, IL-8 (A) and MCP-1 (B); *P < .0001, **P < .001 compared with TRAP alone (n = 3).
Effects of SB203580 and PD98059 on TRAP-induced IL-8 and MCP-1 endothelial production.
To determine whether the specific thrombin PAR-1 receptor was linked to the p38 or p42/44 MAPK pathway, HUVECs were stimulated with 100 μM TRAP in the presence or absence of SB203580 (2 μM) and PD98059 (10 μM). After 24 hours in culture, the supernatants were collected and assayed for chemokines, IL-8 (A) and MCP-1 (B); *P < .0001, **P < .001 compared with TRAP alone (n = 3).
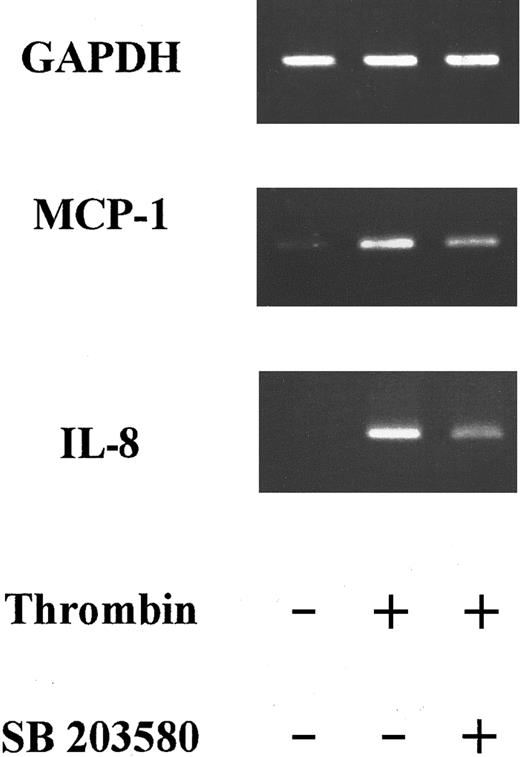
SB203580 decreased thrombin-induced IL-8 and MCP-1 mRNA steady-state levels
We and others have previously shown that thrombin stimulation increased both IL-8 and MCP-1 messenger RNA (mRNA) concentrations in HUVECs.9 15 Thrombin induced a significant increase in mRNA concentrations for both chemokines after 6 hours of stimulation. We therefore studied SB203580 effects on IL-8 and MCP-1 mRNA concentrations after 6 hours of thrombin HUVEC stimulation. Using reverse transcriptase–PCR, IL-8 mRNA was not detected in unstimulated HUVECs, but steady-state levels were elevated in thrombin-activated HUVECs after 6 hours (Figure 7). Addition of SB203580 to thrombin-activated HUVECs decreased IL-8 mRNA concentrations by almost 50% (Figure 7). MCP-1 mRNA was present in low concentrations in unstimulated HUVECs, but steady-state levels clearly increased after 6 hours of thrombin stimulation (4-fold increase; Figure 7). Addition of SB203580 induced a modest (36%) decrease in MCP-1 mRNA steady-state levels compared with thrombin-activated HUVECs.
Effects of SB203580 on thrombin-induced IL-8 and MCP-1 steady-state mRNA levels.
PCR products of RNA prepared from HUVECs stimulated with thrombin (8 U/mL) for 6 hours in the absence or presence of 2 μM SB203580 were deposited on a 2% agarose gel. SB203580 decreased IL-8 mRNA steady-state levels by almost 50% and MCP-1 mRNA by 36% (1 experiment representative of 3).
Effects of SB203580 on thrombin-induced IL-8 and MCP-1 steady-state mRNA levels.
PCR products of RNA prepared from HUVECs stimulated with thrombin (8 U/mL) for 6 hours in the absence or presence of 2 μM SB203580 were deposited on a 2% agarose gel. SB203580 decreased IL-8 mRNA steady-state levels by almost 50% and MCP-1 mRNA by 36% (1 experiment representative of 3).
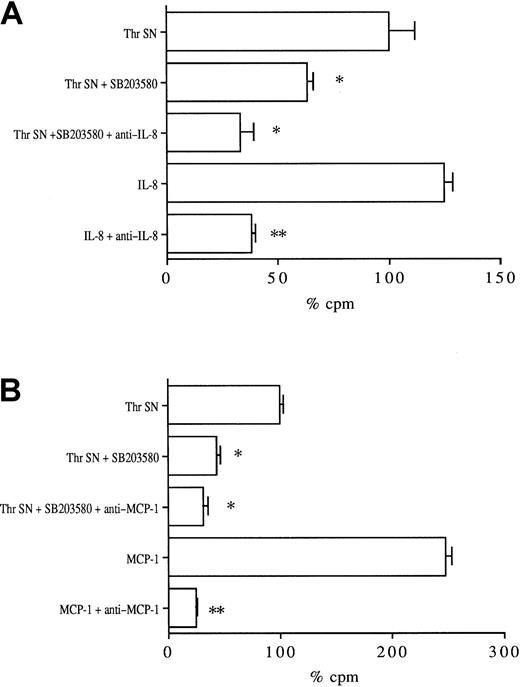
SB203580 decreases thrombin-induced leukocyte migration
Because SB203580 significantly decreased thrombin-induced IL-8 and MCP-1 productions, we asked whether SB203580 also decreased leukocyte migration in a Transwell chemotaxis assay. Thrombin-stimulated HUVEC supernatant significantly increased neutrophil migration compared with unstimulated HUVEC supernatant (150% ± 10% increase,P < .01, data not shown). As shown in Figure8A, endothelial activation in the presence of SB203580 significantly (40% ± 3%) reduced the chemotactic activity of thrombin-stimulated supernatant on neutrophils, which was decreased further (67% ± 4% inhibition) by the addition of anti–IL-8 neutralizing monoclonal antibody. As a positive control, anti–IL-8 inhibited, by 69% ± 2%, PMNC migration induced by 10 ng/mL recombinant IL-8 in this assay.
Effects of SB203580 on the chemotactic activity of thrombin-stimulated HUVEC supernatants on polymorphonuclear and THP-1 cells.
HUVECs were stimulated with 8 U/mL thrombin for 24 hours in the absence or presence of 2 μM SB203580. The supernatants were then collected, diluted, and assayed for their chemotactic activity on51Cr-labeled neutrophils (A) and monocytic THP-1 cells (B) through Transwell chamber devices. Thrombin-stimulated HUVEC supernatant chemotactic activity was considered 100% (*P < .01, **P < .005 compared with respective controls, n = 3).
Effects of SB203580 on the chemotactic activity of thrombin-stimulated HUVEC supernatants on polymorphonuclear and THP-1 cells.
HUVECs were stimulated with 8 U/mL thrombin for 24 hours in the absence or presence of 2 μM SB203580. The supernatants were then collected, diluted, and assayed for their chemotactic activity on51Cr-labeled neutrophils (A) and monocytic THP-1 cells (B) through Transwell chamber devices. Thrombin-stimulated HUVEC supernatant chemotactic activity was considered 100% (*P < .01, **P < .005 compared with respective controls, n = 3).
Thrombin-stimulated HUVEC supernatant also significantly increased THP-1 monocytic cell migration compared with unstimulated HUVEC supernatant (160% ± 8% increase, P < .01, data not shown). As shown in Figure 8B, the chemotactic activity of the supernatant obtained after stimulation of HUVECs in the presence of SB203580 was significantly reduced (55% reduction compared with thrombin supernatant) and was slightly further decreased (65%) by addition of neutralizing anti–MCP-1 monoclonal antibody. In parallel experiments, anti–MCP-1 reduced, by 75%, the chemotactic activity of 20 ng/mL recombinant MCP-1.
Discussion
Through type I and type II endothelial activation, thrombin appears to be a potent and independent proinflammatory agent in vitro and in vivo,6-10 but the intracellular mechanisms involved in this function are largely unknown. Because various proinflammatory agents, including the cytokines IL-1 and TNF-α, UV, or osmotic shock, activate the p38 MAPK pathway in cells to induce the synthesis of proteins involved in the inflammatory cascade,18-22 we asked whether the p38 MAPK pathway was involved in thrombin-induced endothelial chemokine production. In the present study, p38 MAPK was rapidly phosphorylated after thrombin activation of HUVECs and was able to phosphorylate its substrate, ATF-2. We observed that p38 MAPK participated in thrombin-induced endothelial IL-8 and MCP-1 productions, as demonstrated by the inhibitory effect of SB203580. In contrast, p42/p44 ERK kinase did not seem to be involved in thrombin-induced chemokine production. The involvement of p38 MAPK in endothelial chemokine production has been somewhat controversial because some authors using SB203580 have found no involvement of p38 MAPK in IL-1–induced HUVEC IL-8 production, whereas in the same experiments, SB203580 significantly decreased IL-1–induced IL-6 secretion.27 Using the same inhibitor however, other authors have shown that p38 MAPK is involved in osmotic shock-induced monocyte IL-8 production as well as in IL-1– or TNF-α–induced IL-8 and MCP-1 production by respiratory and umbilical endothelial cells.28-30 In agreement with data presented in our study, SB203580 at the concentrations used in these different reports is selective of p38β and p38α, 2 isoforms that have been shown to be highly present in HUVECs.31,32 Thus, we can conclude from these data that thrombin and the proinflammatory mediators IL-1 and TNF-α use the common p38 MAPK signaling pathway to activate HUVECs. In addition, selective inhibition of p38 MAPK decreased TRAP-induced IL-8 and MCP-1 productions, suggesting that the 7-transmembrane domain receptors PAR-1 or PAR-2 are linked to the p38 MAPK pathway in HUVECs. Because thrombin is not a ligand for PAR-2 and similar observations have been made in platelets during thrombin-induced aggregation,23,24 this may be a common feature of the thrombin/PAR-1 complex. Association of receptors linked to heterotrimeric G proteins with the p38 MAPK pathway has been reported in a few other cases, such as the endothelin and the muscarinic receptors and, more recently, the Kaposi sarcoma virus receptor.33-35
SB203580 decreased thrombin-induced IL-8 and MCP-1 protein as well as mRNA concentrations. From the present data, it is not possible to determine the level of action of p38 MAPK in the chemokine synthesis pathway, especially whether p38 acts at the transcriptional and/or posttranscriptional level. Several reports have shown that p38 acts at the translational level through stabilization of mRNAs containing an AU-rich sequence in their 3′ untranslated region.36Because IL-8 mRNA contains AU-rich sequences, p38 may interfere with thrombin-induced chemokine synthesis at a posttranscriptonal level.37 Alternatively, thrombin is known to activate nuclear factor κB (NF-κB) in HUVECs,38,39 and p38 may directly act at a transcriptional level through interaction with NF-κB or AP-1 activation. Using this mechanism, p38 has been reported to be involved in TNF-α–induced IL-6 mRNA synthesis in fibrosarcoma cells and, more recently, in lipopolysaccharide-induced TNF-α mRNA synthesis in neutrophils as well as in TNF-α–induced MCP-1 mRNA synthesis in HUVECs.30,40 41
In this study, the supernatants of thrombin-stimulated HUVECs in the presence of SB203580 were significantly less chemotactic for neutrophils and monocytic cells. This observation is unlikely to be the consequence of a direct effect of SB203580 on chemokine-induced leukocyte migration because in these experiments the concentrations of SB203580 contained in the supernatants were low due to dilution of the samples. Moreover, IL-8–induced neutrophil chemotaxis has been shown to be independent of p38 MAPK activation.42 Therefore, decreased leukocyte chemotaxis was likely due to a direct effect of SB203580 on thrombin-induced endothelial chemokine production.
Thrombin proinflammatory properties may be important in several inflammatory diseases characterized by diffuse intravascular or extravascular coagulation and tissue leukocyte infiltration, such as systemic vasculitis, acute and chronic allograft rejection, or rheumatoid arthritis.43,44 In rheumatoid arthritis, for example, the affected joints are characterized by the presence of proinflammatory cytokines TNF-α and IL-1 as well as IL-6, IL-8, and MCP-1 as second-wave mediators inducing chronic neutrophil and mononuclear cell infiltration.44 Extravascular coagulation is present in the joints in which tissue factor, fibrinogen, and fibronectin have been consistently detected.45Furthermore, high concentrations of thrombin have been found in rheumatoid arthritis synovial fluids, and thrombin may play a role in cartilage degradation,46 synovial cell proliferation,47 and leukocyte recruitment through adhesion molecule expression and chemokine production.8-10,14,15 SB203580 has been used in 2 murine models of collagen- or adjuvant-induced arthritis and shown to decrease the severity of local inflammation as well as bone resorption at doses of 30 to 60 mg/kg.48 The modulating effects in vitro of p38 MAPK inhibitor on thrombin-induced endothelial IL-8 and MCP-1 production and both neutrophil and monocytic cell chemotaxis reported in this study suggest that interacting with the p38 MAPK pathway downstream of the specific receptors may indeed be an interesting anti-inflammatory strategy in this kind of inflammatory disease.
We thank Dr Valettes and the Obstetrical Division of the Saint-Joseph Foundation Hospital in Marseille for providing umbilical cords and Monique Barbier for technical assistance.
Supported by National Institutes of Health grant AI15614 to C.A.D.
M.S.-S.S. has declared a financial interest in a company whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Gilles Kaplanski, Laboratoire d'Immunologie-INSERM U387, Hôpital Sainte-Marguerite, 270, blvd Sainte-Marguerite, 13009 Marseille, France; e-mail:gkaplanski@marseille.inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal