Abstract
Autologous stem cell transplantation (ASCT) improves survival in patients with previously untreated multiple myeloma (MM) and relapsed, chemotherapy-sensitive, aggressive non-Hodgkin lymphoma (NHL). Lower relapse rates seen in allogeneic stem cell transplantation have been related to early absolute lymphocyte count (ALC) recovery as a manifestation of early graft-verus-tumor effect. In ASCT, the relation between ALC recovery and clinical outcomes in MM and NHL was not previously described. This is a retrospective study of patients with MM and NHL who underwent ASCT at the Mayo Clinic between 1987 and 1999. The ALC threshold was determined at 500 cells/μL on day 15 after ASCT. The study identified 126 patients with MM and 104 patients with NHL. The median overall survival (OS) and progression-free survival (PFS) times for patients with MM were significantly longer in patients with an ALC of 500 cells/μL or more than patients with an ALC of fewer than 500 cells/μL (33 vs 12 months,P < .0001; 16 vs 8 months, P < .0003, respectively). For patients with NHL, the median OS and PFS times were significantly longer in patients with an ALC of 500 cells/μL or more versus those with fewer than 500 cells/μL (not reached vs 6 months, P < .0001; not reached vs 4 months,P < .0001, respectively). Multivariate analysis demonstrated day 15 ALC to be an independent prognostic indicator for OS and PFS rates for both groups of patients. In conclusion, ALC is correlated with clinical outcome and requires further study.
Introduction
Autologous stem cell transplantation (ASCT) improves survival in selected patients with hematologic malignancies such as multiple myeloma1 and for relapsed, chemotherapy-sensitive, aggressive non-Hodgkin lymphoma.2However, despite the survival advantage of ASCT, post-ASCT relapse rates range between 40% and 70%.3-5 The high relapse rates after ASCT have been attributed to the inability of high-dose therapy (HDT) to eradicate minimal residual disease. In contrast, the lower relapse rates observed after allogeneic bone marrow transplantation (ABMT) are considered to be related to the adoptive graft-versus-tumor immune response.6-9 Post-ABMT studies have demonstrated that early absolute lymphocyte count (ALC) recovery is associated with prolonged survival. Using an ALC cutoff value of 200 cells/μL at day 29 after ABMT in 188 patients with acute myelogenous leukemia (AML), Powles et al10 demonstrated a 3-year relapse probability rate of 42% for an ALC of fewer than 200 cells/μL versus 16% for an ALC of more than 200 cells/μL (P < .004). Pavletic et al11 analyzed ALC in 41 patients after ABMT (AML, chronic myelogenous leukemia, myelodysplastic syndrome, Hodgkin disease, chronic lymphocytic leukemia). The OS at 1-year with an ALC at day 17 after ABMT of 500 cells/μL or more was 79% versus 19% for an ALC of fewer than 500 cells/μL (P < .0024).
To assess whether early ALC recovery has prognostic significance after ASCT, we conducted a retrospective analysis of ALC at day 15 after ASCT in patients with multiple myeloma (MM) and non-Hodgkin lymphoma (NHL).
Patients, materials, and methods
Patient population
Two hundred thirty consecutive patients underwent ASCT (126 with MM, 104 with NHL) at the Mayo Clinic between February 1987 and April 1999. Data from transplant recipients were collected prospectively and entered into a computerized database. Response to therapy, relapse, and survival data are updated continuously. No patients were lost to follow-up. All patients gave written, informed consent allowing the use of their medical records for medical research. Approval for the retrospective review of these records was obtained from the Mayo Clinic Institutional Review Board and was in accordance with US federal regulations and the Declaration of Helsinki.
Prognostic factors
Information on prognostic factors for patients with MM, including age, β2-microglobulin (β2M), C-reactive protein (CRP), circulating plasma cells, lactate dehydrogenase (LDH), plasma cell–labeling index (PCLI), BM plasma cell percentage, cytogenetic analysis, absolute neutrophil count (ANC) at day 15, stem cell source (BM vs peripheral blood stem cells [PBSCs]), platelet count at day 15, number of pretransplantation chemotherapy regimens, and clinical status before transplantation were used for this study. The international age-adjusted prognostic index12 13 (age, LDH, performance status [PS], extranodal sites, and stage), in addition to the number of pretransplant treatments, stem cell source (BM vs PBSCs), ANC at day 15, platelet count at day 15, chemosensitive disease status, and complete remission (CR) status before transplantation were used for patients with NHL. The ALC was determined at day 15 after ASCT for both groups of patients.
Conditioning regimens
The conditioning regimens for patients with MM patients were as follows: 101 patients received melphalan (140 mg/m2) and total body irradiation [TBI] (12 Gy); 11 patients received melphalan (140 mg/m2), cyclophosphamide (60 mg/m2), and TBI (12 Gy); 11 patients received melphalan (200 mg/m2); 2 patients received cyclophosphamide (60 mg/m2) and TBI (12 Gy); and one patient received busulfan (16 mg/kg) and cyclophosphamide (60 mg/m2). For patients with NHL, the conditioning regimens were as follows: 7 patients received CBV (cyclophosphamide [1.5 g/m2], BCNU [300 mg/m2]), and etoposide [125 mg/m2]); 31 patients received cyclophosphamide (60 mg/m2) and TBI (12 Gy); and 66 patients received BEAC (BCNU [300 mg/m2], etoposide [100 mg/m2], ARA-C [100 mg/m2], and cyclophosphamide [35 mg/kg]). All patients underwent stem cell reinfusion after HDT. Hematologic engraftment was thought to have occurred when the ANC reached 500 cells/μL or more.
Stem cell source
The stem cell source for the ASCT included BM or PBSCs. In the MM group, 21 patients received BM stem cells, and 105 received PBSCs. In the NHL group, 49 patients received BM stem cells, 53 patients received PBSCs, and 2 patients required both. The decision to change from BM stem cell to PBSC was made when PBSCs became the standard for stem cell collection. Patients who did not obtain an adequate number of stem cells through PBSCs underwent BM harvest.
Response and survival
For patients with MM patients, CR was defined as a lack of detectable monoclonal protein in serum and urine by immunofixation. Partial remission (PR) was defined as a reduction in serum monoclonal protein and 24-hour urinary light-chain excretion by at least 50%, accompanied by a similar reduction of soft tissue plasmacytomas, if present. Disease progression was defined as a 50% increase in the serum monoclonal protein or 24-hour urinary monoclonal protein excretion over the lowest remission level. An increase in the size or number of lytic bony lesions or soft tissue plasmacytomas constituted progression. In those with CR, any detectable monoclonal protein by immunofixation constituted progression.
For patients with NHL, CR was defined as complete regression of all measurable or evaluable disease including radiologically demonstrable disease, BM involvement, or peripheral blood involvement. PR was defined as a reduction in the sum of the products of measurable lesions' longest diameters and perpendicular diameters of 50% or greater, with a 30% or greater decrease in hepatomegaly or splenomegaly (measured from the costal margin), if there was previous known liver or spleen involvement. Disease progression was defined as a 25% or more increase in the sum of the products of the longest diameter and its perpendicular diameter of measurable lesion(s) from the prestudy measurement, the appearance of new lesions, or a 2-cm increase in spleen or liver size due to lymphoma.
OS time was measured from the date of transplantation to the date of death or last follow-up. PFS was defined as time from transplantation to disease progression, relapse, or death.
Statistical analysis
OS and PFS times were analyzed using the method described by Kaplan and Meier.14 Differences between survival curves were tested for statistical significance using the 2-tailed log-rank test. The Cox proportional hazards model15 was used to assess ALC as a prognostic factor for posttransplant OS and PFS times as well as to adjust for other known prognostic factors. For analysis involving the entire cohort, the Cox model was stratified on disease type (MM vs NHL). Validity of the proportional hazards model was confirmed using the techniques of Grambsch and Therneau.16
Risk ratios reported are for risks associated with patients having high (greater than 500 cells/μL) versus low (less than 500 cells/μL) ALC values. In the MM group, prognostic factors tested included age (older than 50 years), β2M (greater than 2.7 mg/dL), CRP (greater than 0.8 mg/dL), LDH (greater than normal for age/sex), PCLI (greater than or equal to 1%), BM plasma cell percentage (greater than 40%), abnormal cytogenetics, number of pretransplant treatments (continuous variable), circulating plasma cells, ANC greater than or equal to 500 cells/μL, stem cell source (BM vs PBSCs), platelet count greater than 20 × 109/L at day 15, and clinical status before transplantation (primary refractory, plateau response, relapse off chemotherapy, and relapse on chemotherapy). For the NHL group, the prognostic factors tested included age (60 years or older), LDH (greater than normal for age/sex), stage (III/IV), extranodal sites (2 or more), performance status (ECOG, 2 or greater), number of pretransplantation treatments, stem cell source (BM vs PBSCs), platelet count more than 20 × 109/L at day 15, ANC 500 cells/μL or more, chemosensitive disease defined as CR or PR, and CR status alone before transplantation. CD34 dose, mononuclear cell counts, and total nucleated cell dose were used as continuous variables. Our data did not show any correlation between a specific cut-point and outcome for these variables.
The cutoff of an ALC of 500 cells/μL or more was based on the median of ALC for the cohort group. This choice of cut-point was supported by the data because it yielded the greatest differential in survival at 500 cells/μL based on χ2 values analyzed at different cut-points (200 to 900 cells/μL) from log-rank tests. Chi-square analysis and Fisher Exact tests were used to determine relations between nominal variables; nonparametric tests were used for continuous variables. All P values represented were 2-sided, and statistical significance was declared at P < .05.
Results
Patient characteristics
Two hundred thirty patients (126 with MM, 104 with NHL) were identified for the study; their median age was 54 years (range, 14-73 years) at the time of transplantation. Patients' characteristics are listed in Table 1 (MM) and Table2 (NHL). All patients with NHL had intermediate-grade histology. Patients received a median of 2 continuous chemotherapy regimens before ASCT (range, 1-5). None of the patients received purged or CD34-selected stem cells. Sixty-three patients in the MM group had an ALC of 500 cells/μL or more with a mean value of 1100 ± 600 cells/μL (95% CI, 938-1240 cells/μL) and a median of 870 cells/μL (range, 500-3100 cells/μL). Only 6 patients had an ALC greater than 2000 cells/μL. In the 63 patients with an ALC of fewer than 500 cells/μL, the mean value was 278 ± 117 cells/μL (95% CI, 250-307 cells/μL) and a median value of 292 cells/μL (range, 50-472 cells/μL). Three patients had an ALC of fewer than 100 cells/μL. Fifty-six patients in the NHL group had an ALC of 500 cells/μL or more, with a mean value 857 ± 219 cells/μL (95% CI, 799-917 cells/μL) and a median value of 635 cells/μL (range, 510-4440 cells/μL). Only one patient had an ALC of 4440 cells/μL. In the 48 patients with an ALC of fewer than 500 cells/μL, the mean value was 240 ± 130 cells/μL (95% CI, 203-279 cells/μL) and a median value of 230 cells/μL (range, 20-490 cells/μL). Four patients had an ALC less than 100 cells/μL. There was no association between the ALC and the number of pretransplantation chemotherapy regimens (MM, P = .76; NHL, P = .84), the type of conditioning regimens (MMP = .7852; NHL, P = .2223), or the posttransplantation ANC (MM: P = .17; NHL:P = .54).
Baseline characteristics of patients with multiple myeloma
| Characteristics . | Multiple myeloma (n = 126) . |
|---|---|
| Age (y)* | 55 (range, 33-71) |
| Sex (%) | |
| Male | 65 |
| Female | 35 |
| Disease status at transplantation (%) | |
| Plateau response | 13.5 |
| Relapsed on chemotherapy | 33.3 |
| Relapsed off chemotherapy | 38.1 |
| Primary chemorefractory | 15.1 |
| Prognostic factors for MM (%) | |
| Plasma cell labeling index 1% | 37 |
| β2-microglobulin (> 2.7 mg/L) | 59.5 |
| LDH level > normal for age/sex | 85.2 |
| Cytogenetics | |
| Normal | 64.2 |
| Abnormal | 35.8 |
| C-reactive protein level > 0.8 mg/dL | 30.3 |
| Stem cell source (%) | |
| BM | 20 |
| PBSCs | 80 |
| Platelet level > 20 × 109/L (%) | 81 |
| ALC at day 15 after ASCT (%) | |
| ≥ 500 cells/μL | 50 |
| < 500 cells/μL | 50 |
| ANC at day 15 after ASCT (%) | |
| ≥ 500 cells/μL | 95.7 |
| < 500 cells/μL | 4.3 |
| Characteristics . | Multiple myeloma (n = 126) . |
|---|---|
| Age (y)* | 55 (range, 33-71) |
| Sex (%) | |
| Male | 65 |
| Female | 35 |
| Disease status at transplantation (%) | |
| Plateau response | 13.5 |
| Relapsed on chemotherapy | 33.3 |
| Relapsed off chemotherapy | 38.1 |
| Primary chemorefractory | 15.1 |
| Prognostic factors for MM (%) | |
| Plasma cell labeling index 1% | 37 |
| β2-microglobulin (> 2.7 mg/L) | 59.5 |
| LDH level > normal for age/sex | 85.2 |
| Cytogenetics | |
| Normal | 64.2 |
| Abnormal | 35.8 |
| C-reactive protein level > 0.8 mg/dL | 30.3 |
| Stem cell source (%) | |
| BM | 20 |
| PBSCs | 80 |
| Platelet level > 20 × 109/L (%) | 81 |
| ALC at day 15 after ASCT (%) | |
| ≥ 500 cells/μL | 50 |
| < 500 cells/μL | 50 |
| ANC at day 15 after ASCT (%) | |
| ≥ 500 cells/μL | 95.7 |
| < 500 cells/μL | 4.3 |
MM indicates multiple myeloma; LDH, lactate dehydrogenase; BM, bone marrow; PBSC, peripheral blood stem cell; ALC, absolute lymphocyte count; ASCT, autologous stem cell transplantation; ANC, absolute neutrophil count.
Years expressed as the median of the group.
Baseline characteristics of patients with non-Hodgkin lymphoma
| Characteristics . | Non-Hodgkin lymphoma (n = 104) . |
|---|---|
| Age (y)* | 51 (range, 14-73) |
| Sex (%) | |
| Male | 60.6 |
| Female | 39.4 |
| Histology (%) (REAL classification) | |
| Diffuse large cell | 50 |
| Follicular large cell | 28 |
| Peripheral T cell | 8 |
| Anaplastic T cell | 6 |
| T-rich B cell | 3 |
| Angiocentric T cell | 3 |
| Angiocentric B cell | 1 |
| Natural killer cell | 1 |
| Disease status at transplantation (%) | |
| First relapse | 7.7 |
| Progression | 0.9 |
| Partial response | 68.3 |
| Complete response | 23.1 |
| Prognostic factors for NHL (%) | |
| Age ≥ 60 | 32 |
| LDH level > normal for age/sex | 57.7 |
| Performance status ≥ 2 | 7.7 |
| Extranodal sites ≥ 2 | 6.7 |
| Stage III/IV disease | 66 |
| Stem cell source (%) | |
| BM | 47.1 |
| PBSC | 50.9 |
| Both | 2 |
| Platelet level > 20 × 109/L (%) | 71.1 |
| ALC at day 15 after ASCT (%) | |
| ≥ 500 cells/μL | 53.8 |
| < 500 cells/μL | 46.2 |
| ANC at day 15 after ASCT (%) | |
| ≥ 500 cells/μL | 84 |
| < 500 cells/μL | 16 |
| Characteristics . | Non-Hodgkin lymphoma (n = 104) . |
|---|---|
| Age (y)* | 51 (range, 14-73) |
| Sex (%) | |
| Male | 60.6 |
| Female | 39.4 |
| Histology (%) (REAL classification) | |
| Diffuse large cell | 50 |
| Follicular large cell | 28 |
| Peripheral T cell | 8 |
| Anaplastic T cell | 6 |
| T-rich B cell | 3 |
| Angiocentric T cell | 3 |
| Angiocentric B cell | 1 |
| Natural killer cell | 1 |
| Disease status at transplantation (%) | |
| First relapse | 7.7 |
| Progression | 0.9 |
| Partial response | 68.3 |
| Complete response | 23.1 |
| Prognostic factors for NHL (%) | |
| Age ≥ 60 | 32 |
| LDH level > normal for age/sex | 57.7 |
| Performance status ≥ 2 | 7.7 |
| Extranodal sites ≥ 2 | 6.7 |
| Stage III/IV disease | 66 |
| Stem cell source (%) | |
| BM | 47.1 |
| PBSC | 50.9 |
| Both | 2 |
| Platelet level > 20 × 109/L (%) | 71.1 |
| ALC at day 15 after ASCT (%) | |
| ≥ 500 cells/μL | 53.8 |
| < 500 cells/μL | 46.2 |
| ANC at day 15 after ASCT (%) | |
| ≥ 500 cells/μL | 84 |
| < 500 cells/μL | 16 |
REAL indicates revised European-American lymphoma; NHL, non-Hodgkin lymphoma; for other abbreviations, see Table 1.
Years expressed as the median of the group.
CD34 cell counts (n = 57, P = .41), mononuclear cell count (n = 122, P = .86), and nucleated cell count (n = 48, P = .78) values were not significant predictors of OS or of ALC. The CD34, mononuclear cell count, and nucleated cell count were continuous variables in the analysis. Whether the ALC was above 500 cells/μL was not significantly related to the other prognostic factors tested in either of the disease groups, with the exception of a correlation between ALC and LDH (P = .03) or the clinical status in the MM group before transplantation in patients with MM (P = .01). On further investigation, relapse on and off therapy correlated with the ALC in patients with MM (P = .014 and P = .017, respectively).
Survival
By April 1999, 117 of 230 patients in the study died. Recurrent or progressive myeloma was the cause of death in 35 patients, and recurrent or progressive lymphoma was the cause in 45 patients. Twenty-five patients died of transplantation-related complications. In the MM group, 2 patients died of Staphylococcus aureussepticemia, one of aspergillus meningitis, one of cytomegalovirus pneumonitis, one of Pneumocystis carinii pneumonia, one ofEnterococcus bacteremia, one of Streptococcuspneumonia, one of Candida sepsis, one of disseminated varicella zoster, 3 of intracranial bleeding, 2 of lung failure (acute respiratory distress syndrome), 2 of renal failure, and one of liver failure. In the NHL group, 3 patients died of lung failure (acute respiratory distress syndrome), one of intracranial bleeding, 2 of AML, one of Candida sepsis, and one of pneumonia (S. aureus). In addition, in the MM group, one patient committed suicide, and one died of sudden cardiac death. In the NHL group, one died in a motor vehicle accident. There was no significant difference in the supportive care including antibiotics, growth factors, or transfusion requirements. No correlation was identified between the year of transplantation and transplantation-related mortality for both groups (MM, P < .12; NHL, P < .8). None of the patients had autologous graft-versus-host disease. The median follow-up time for all patients was 12.5 months, with a maximum of 123 months.
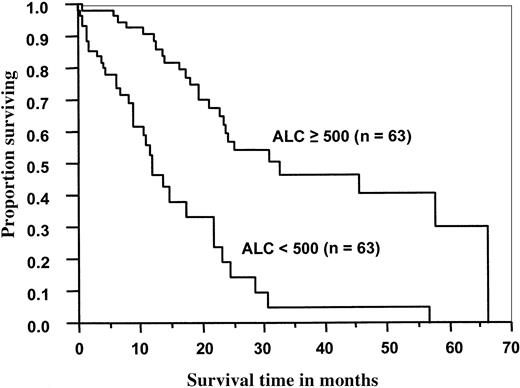
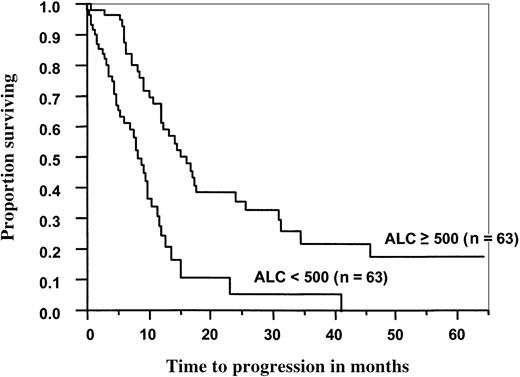
In the MM group, the median OS (Figure 1) and PFS (Figure 2) times were significantly better for patients with an ALC of 500 cells/μL or more than for those with an ALC less than 500 cells/μL (33 months vs 12 months, P < .0001; 16 months vs 8 months,P < .0001, respectively). For patients who received PBSCs, the median OS and PFS times were significantly better for patients with an ALC greater than or equal to 500 cells/μL than for those with an ALC less than 500 cells/μL (46 months vs 12 months,P < .0001; 17 months vs 8 months,P < .0001, respectively). There was no correlation with OS or PFS in the patients who received BM grafts and had higher ALC recovery rates (P < .27, and P < .86, respectively).
Overall survival of 126 patients with multiple myeloma as a function of ALC recovery at day 15 after ASCT.
Median overall survival time for patients with an ALC greater than or equal to 500 cells/μL was 33 months versus 12 months for patients with an ALC less than 500 cells/μL (P < .0001).
Overall survival of 126 patients with multiple myeloma as a function of ALC recovery at day 15 after ASCT.
Median overall survival time for patients with an ALC greater than or equal to 500 cells/μL was 33 months versus 12 months for patients with an ALC less than 500 cells/μL (P < .0001).
Progression-free survival of 126 patients with multiple myeloma after ASCT as a function of ALC recovery at day 15.
Median progression-free survival for patients with an ALC greater than or equal to 500 cells/μL was 16 months versus 8 months for patients with an ALC < 500 cells/μL (P < .0003).
Progression-free survival of 126 patients with multiple myeloma after ASCT as a function of ALC recovery at day 15.
Median progression-free survival for patients with an ALC greater than or equal to 500 cells/μL was 16 months versus 8 months for patients with an ALC < 500 cells/μL (P < .0003).
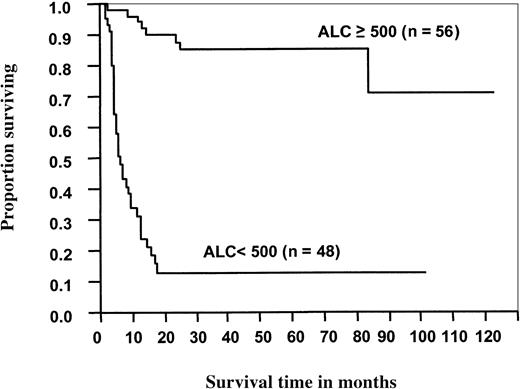
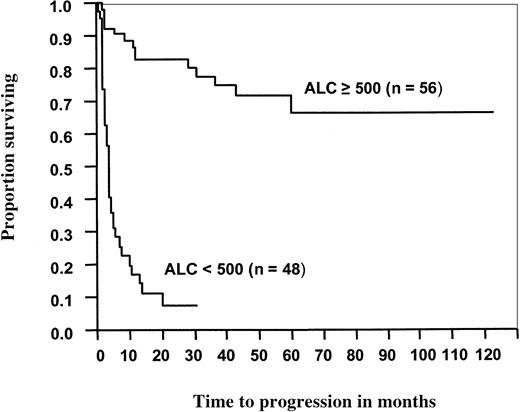
For the patients with NHL, the median OS (Figure3) and PFS (Figure4) times were significantly better for patients with an ALC greater than or equal to 500 cells/μL than for those with an ALC less than 500 cells/μL (not reached vs 6 months,P < .0001; not reached vs 4 months,P < .0001, respectively). When the ALC was greater than or equal to 500 cells/μL, median OS and PFS times were significantly better than when the ALC was less than 500 cells/μL for patients who received either PBSC grafts (not reached vs 6 months,P < .0001; and not reached vs 3.6 months,P < .0001, respectively) or BM grafts (not reached vs 5.7 months, P < .0001; and not reached vs 4 months, P < .0001, respectively).
Overall survival of 104 patients with non-Hodgkin lymphoma after ASCT as a function of ALC recovery at day 15.
Median overall survival time for patients with an ALC less than 500 cells/μL was 6 months. For patients with an ALC greater than or equal to 500 cells/μL, the median overall survival has not been reached (P < .0001).
Overall survival of 104 patients with non-Hodgkin lymphoma after ASCT as a function of ALC recovery at day 15.
Median overall survival time for patients with an ALC less than 500 cells/μL was 6 months. For patients with an ALC greater than or equal to 500 cells/μL, the median overall survival has not been reached (P < .0001).
Progression-free survival of 104 patients with non-Hodgkin lymphoma after ASCT as a function of ALC recovery at day 15.
Median progression-free survival for patients with an ALC less than 500 cells/μL was 4 months. For patients with an ALC greater than or equal to 500 cells/μL, the median progression-free survival has not been reached (P < .0001).
Progression-free survival of 104 patients with non-Hodgkin lymphoma after ASCT as a function of ALC recovery at day 15.
Median progression-free survival for patients with an ALC less than 500 cells/μL was 4 months. For patients with an ALC greater than or equal to 500 cells/μL, the median progression-free survival has not been reached (P < .0001).
Univariate analysis
Prognostic factors were tested as predictors for OS and PFS for patients with MM (Table 3) and for those with NHL (Table 4). Age, β2M, CRP, and ANC were not predictive of OS or PFS in patients with MM. ALC, stem cell source, circulating plasma cells, platelet count greater than 20 × 109/L, PCLI, LDH, number of pretransplantation chemotherapy regimens, clinical status before transplantation, and abnormal cytogenetics were significant predictors of OS and PFS in the univariate analyses. The relationship between the patients' clinical status before and after transplantation was also explored. There were no deaths in the plateau/response group, and the ALC was not prognostic for OS in this group; therefore, patients with this clinical status were omitted from the univariate analysis for OS. This variable (truncated or not) was a significant predictor of survival and PFS in these patients; the strongest prognostic component was whether a patient had a relapse while on therapy.
Univariate comparisons for patients with multiple myeloma: overall survival and progression-free survival rates
| Prognostic factors at time of transplantation . | Overall survival . | Progression-free survival . | ||||
|---|---|---|---|---|---|---|
| Relative risk . | 95% CI . | P . | Relative risk . | 95% CI . | P . | |
| Abnormal | ||||||
| cytogenetics vs normal | .39 | .23-.68 | .001 | .44 | .27-.70 | < .0007 |
| Age > 50 y vs < 50 y | 1.03 | .6-1.8 | .91 | 1.04 | .65-1.67 | .86 |
| ALC ≥ 500 cells/μL vs < 500 cells/μL | .24 | .14-.42 | < .0001 | .34 | .21-.55 | < .0001 |
| ANC ≥ 500 cells/μL vs < 500 cells/μL | .40 | .12-1.33 | .14 | .37 | .13-1.04 | .06 |
| β2M > 2.7 mg/L vs ≤ 2.7 mg/L | .58 | .33-1.01 | .054 | .7 | .44-1.11 | .13 |
| Bone marrow | ||||||
| plasma cells ≥ 40% vs < 40% | .49 | .3-.8 | .007 | .55 | .35-.87 | .01 |
| Circulating plasma cells | .39 | .23-.68 | .0007 | .46 | .28-.77 | .003 |
| CRP > 0.8 mg/dL vs ≤ 0.8 mg/dL | .57 | .31-1.04 | .067 | .65 | .38-1.1 | .11 |
| LDH level normal for age/sex vs > normal | .45 | .21-.95 | .037 | .45 | .24-.86 | .015 |
| PCLI ≥ 1% vs < 1% | .34 | .2-.6 | .0001 | .34 | .21-.55 | < .0001 |
| Platelet level > 20 × 109/L vs ≤ 20 × 109/L | .18 | .09-.36 | < .0001 | .3 | .16-.57 | .00024 |
| No. pretransplant chemotherapy regimens | 1.58 | 1.05-2.38 | .028 | 1.7 | 1.18-2.44 | .004 |
| Primary refractory | ||||||
| disease vs plateau/response | NA3-150 | 2.94 | .63-13.7 | .17 | ||
| Relapse off | ||||||
| therapy vs plateau/response | 1.163-151 | .5-2.7 | .73 | 5.99 | 1.43-25.1 | .014 |
| Relapse on | ||||||
| therapy vs plateau/response | 3.083-151 | 1.4-6.76 | .005 | 13.94 | 3.3-58.5 | .0003 |
| Stem cell source | ||||||
| PBSCs vs BM | .45 | .26-.78 | .004 | .48 | .29-.79 | .004 |
| Prognostic factors at time of transplantation . | Overall survival . | Progression-free survival . | ||||
|---|---|---|---|---|---|---|
| Relative risk . | 95% CI . | P . | Relative risk . | 95% CI . | P . | |
| Abnormal | ||||||
| cytogenetics vs normal | .39 | .23-.68 | .001 | .44 | .27-.70 | < .0007 |
| Age > 50 y vs < 50 y | 1.03 | .6-1.8 | .91 | 1.04 | .65-1.67 | .86 |
| ALC ≥ 500 cells/μL vs < 500 cells/μL | .24 | .14-.42 | < .0001 | .34 | .21-.55 | < .0001 |
| ANC ≥ 500 cells/μL vs < 500 cells/μL | .40 | .12-1.33 | .14 | .37 | .13-1.04 | .06 |
| β2M > 2.7 mg/L vs ≤ 2.7 mg/L | .58 | .33-1.01 | .054 | .7 | .44-1.11 | .13 |
| Bone marrow | ||||||
| plasma cells ≥ 40% vs < 40% | .49 | .3-.8 | .007 | .55 | .35-.87 | .01 |
| Circulating plasma cells | .39 | .23-.68 | .0007 | .46 | .28-.77 | .003 |
| CRP > 0.8 mg/dL vs ≤ 0.8 mg/dL | .57 | .31-1.04 | .067 | .65 | .38-1.1 | .11 |
| LDH level normal for age/sex vs > normal | .45 | .21-.95 | .037 | .45 | .24-.86 | .015 |
| PCLI ≥ 1% vs < 1% | .34 | .2-.6 | .0001 | .34 | .21-.55 | < .0001 |
| Platelet level > 20 × 109/L vs ≤ 20 × 109/L | .18 | .09-.36 | < .0001 | .3 | .16-.57 | .00024 |
| No. pretransplant chemotherapy regimens | 1.58 | 1.05-2.38 | .028 | 1.7 | 1.18-2.44 | .004 |
| Primary refractory | ||||||
| disease vs plateau/response | NA3-150 | 2.94 | .63-13.7 | .17 | ||
| Relapse off | ||||||
| therapy vs plateau/response | 1.163-151 | .5-2.7 | .73 | 5.99 | 1.43-25.1 | .014 |
| Relapse on | ||||||
| therapy vs plateau/response | 3.083-151 | 1.4-6.76 | .005 | 13.94 | 3.3-58.5 | .0003 |
| Stem cell source | ||||||
| PBSCs vs BM | .45 | .26-.78 | .004 | .48 | .29-.79 | .004 |
CI indicates confidence interval; β2M, β2-microglobulin; CRP, C-reactive protein; PCLI, plasma cell–labeling index; for other abbreviations, see Table 1.
Level absorbed into model since plateau/response level omitted.
Primary refractory level.
Univariate analysis for patients with non-Hodgkin lymphoma: overall survival and progression-free survival rates
| Prognostic factors at time of transplantation . | Overall survival . | Progression-free survival . | ||||
|---|---|---|---|---|---|---|
| Relative risk . | 95% CI . | P . | Relative risk . | 95% CI . | P . | |
| Age ≥ 60 vs < 60 | 1.05 | .57-1.94 | .87 | .94 | .54-1.66 | .84 |
| ALC ≥ 500 cells/μL vs < 500 cells/μL | .07 | .03-.16 | < .0001 | .09 | .04-.18 | < .0001 |
| ANC ≥ 500 cells/μL vs < 500 cells/μL | .43 | .21-.88 | .02 | .49 | .25-.95 | .034 |
| Chemosensitive disease (CR + PR) | .39 | .095-1.62 | .2 | .73 | .26-2.02 | .55 |
| CR status before transplantation | .71 | .34-1.47 | .35 | .66 | .33-1.32 | .24 |
| Extranodal sites ≥ 2 vs < 2 | 2.66 | .95-7.48 | .06 | 2.14 | .77-5.958 | .14 |
| LDH level normal for age/sex vs > normal | .59 | .33-1.06 | .077 | .81 | .47-1.39 | .44 |
| Performance status ≥ 2 vs < 2 | 2.6 | 1.09-6.16 | .03 | 2.43 | 1.03-5.71 | .04 |
| No. pretransplant chemotherapy regimens | 1.11 | .78-1.58 | .57 | .96 | .67-1.37 | .82 |
| Stage III/IV | 1.59 | .8-3.1 | .17 | 1.2 | .68-2.15 | .53 |
| Stem cell source | ||||||
| BM vs PBSCs | .85 | .46-1.57 | .61 | .84 | .48-1.47 | .55 |
| Platelet level > 20 × 109/L vs ≤ 20 × 109/L | .67 | .35-1.26 | .21 | .89 | .48-1.63 | .70 |
| Prognostic factors at time of transplantation . | Overall survival . | Progression-free survival . | ||||
|---|---|---|---|---|---|---|
| Relative risk . | 95% CI . | P . | Relative risk . | 95% CI . | P . | |
| Age ≥ 60 vs < 60 | 1.05 | .57-1.94 | .87 | .94 | .54-1.66 | .84 |
| ALC ≥ 500 cells/μL vs < 500 cells/μL | .07 | .03-.16 | < .0001 | .09 | .04-.18 | < .0001 |
| ANC ≥ 500 cells/μL vs < 500 cells/μL | .43 | .21-.88 | .02 | .49 | .25-.95 | .034 |
| Chemosensitive disease (CR + PR) | .39 | .095-1.62 | .2 | .73 | .26-2.02 | .55 |
| CR status before transplantation | .71 | .34-1.47 | .35 | .66 | .33-1.32 | .24 |
| Extranodal sites ≥ 2 vs < 2 | 2.66 | .95-7.48 | .06 | 2.14 | .77-5.958 | .14 |
| LDH level normal for age/sex vs > normal | .59 | .33-1.06 | .077 | .81 | .47-1.39 | .44 |
| Performance status ≥ 2 vs < 2 | 2.6 | 1.09-6.16 | .03 | 2.43 | 1.03-5.71 | .04 |
| No. pretransplant chemotherapy regimens | 1.11 | .78-1.58 | .57 | .96 | .67-1.37 | .82 |
| Stage III/IV | 1.59 | .8-3.1 | .17 | 1.2 | .68-2.15 | .53 |
| Stem cell source | ||||||
| BM vs PBSCs | .85 | .46-1.57 | .61 | .84 | .48-1.47 | .55 |
| Platelet level > 20 × 109/L vs ≤ 20 × 109/L | .67 | .35-1.26 | .21 | .89 | .48-1.63 | .70 |
In the NHL group, ALC, ANC, and performance status were the only significant predictors in the univariate analysis for OS and PFS. The 2 patients with BM and PBSCs were excluded because their data did not contribute any additional significant predictive ability to the model in which ALC was used.
Multivariate analysis
ALC was an independent prognostic factor for each group in multivariate analysis. For the MM group, ALC remained an effective prognostic indicator for OS (RR = 0.176; P < .0001) and PFS (RR = 0.275; P < .0006) when compared to the significant predictors identified in univariate analysis, including stem cell source, circulating plasma cells, platelet count greater than 20 × 109/L, PCLI, LDH, number of pretreatment chemotherapy regimens, clinical status before transplantation, and abnormal cytogenetics. Clinical status before transplantation was omitted from the multivariate analysis on OS. We did not want to exclude information on the 17 patients in the plateau/response group. In addition, when the truncated version of this variable was included in the model, it was not significant, whereas the ALC remained significant. This means that clinical status did not contribute any additional significant predictive ability to the model in which ALC was used. ALC was a prognostic factor for patients who received PBSC graft. In this group, ALC remained an effective prognostic indicator for OS (RR = 0.36; P < .0001) and PFS (RR = 0.46;P < .0001). There were only 21 patients in the MM group who received a BM graft. The ALC in this group was not associated with better survival (OS, P = .27; PFS,P = .86).
In multivariate analysis for patients with NHL, ALC was an independent predictor for OS (RR = 0.08; P < .0001) and PFS (RR = 0.09; P < .0001) when compared to the significant predictors identified in the univariate analysis, including ANC and performance status. It was also a prognostic factor for patients who received either PBSC or BM graft. ALC remained an effective prognostic indicator for OS (RR = 0.25;P < .0001) and PFS (RR = 0.35; P < .0001) for patients who received PBSC grafts and for OS (RR = 0.11;P < .0001) and PFS (RR = 0.08; P < .0001) for patients who received BM grafts. Table5 summarizes the significant predictors for OS and PFS in MM and NHL identified in the multivariate analyses.
Multivariate analysis for patients with multiple myeloma and non-Hodgkin lymphoma: overall survival and progression-free survival rates
| Prognostic factors . | Multiple myeloma . | Non-Hodgkin lymphoma . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR . | Overall survival, 95% CI . | P . | RR . | Progression-free survival, 95% CI . | P . | RR . | Overall survival, 95% CI . | P . | RR . | Progression-free survival, 95% CI . | P . | |
| ALC ≥ 500 cells/μL vs < 500 cells/μL | .176 | .075 -.414 | .0001 | .275 | .13 -.98 | .0006 | .08 | .04 -.17 | .0001 | .09 | .04 -.19 | .0001 |
| Circulating plasma cells | .27 | .11 -.65 | .0035 | .33 | .15 -.75 | .008 | ||||||
| PCLI ≥ 1% vs < 1% | .3 | .11 -.79 | .014 | |||||||||
| Platelet level > 20 × 109/L vs < 20 × 109/L | .18 | .06 -.5 | .001 | |||||||||
| Relapse on therapy vs plateau/response | 8.45 | 1.64 -43.58 | .011 | |||||||||
| Prognostic factors . | Multiple myeloma . | Non-Hodgkin lymphoma . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR . | Overall survival, 95% CI . | P . | RR . | Progression-free survival, 95% CI . | P . | RR . | Overall survival, 95% CI . | P . | RR . | Progression-free survival, 95% CI . | P . | |
| ALC ≥ 500 cells/μL vs < 500 cells/μL | .176 | .075 -.414 | .0001 | .275 | .13 -.98 | .0006 | .08 | .04 -.17 | .0001 | .09 | .04 -.19 | .0001 |
| Circulating plasma cells | .27 | .11 -.65 | .0035 | .33 | .15 -.75 | .008 | ||||||
| PCLI ≥ 1% vs < 1% | .3 | .11 -.79 | .014 | |||||||||
| Platelet level > 20 × 109/L vs < 20 × 109/L | .18 | .06 -.5 | .001 | |||||||||
| Relapse on therapy vs plateau/response | 8.45 | 1.64 -43.58 | .011 | |||||||||
Discussion
Our data show that ALC (greater than or equal to 500 cells/μL on day 15) after ASCT is an independent prognostic indicator compared to reported prognostic factors for OS and PFS in patients with MM17,18 and NHL.19 This is the first study describing ALC as a prognostic indicator after ASCT.
Studies have demonstrated a correlation between the number of pretransplant treatments and ANC.20,21 We found a similar correlation of slower ANC recovery with multiple pretransplant treatments in each group (MM, P = .04; NHL,P = .005). However, we did not identify a correlation between ALC and either pretransplant number of treatments, ANC, or stem cell characteristics as indicated by the mononuclear cell count, nucleated cell count, CD34 cell counts, or graft source (BM vs PBSCs). This suggests the possibility that the repopulating lymphocytes after ASCT may be derived from sources other than the transplanted stem cells, such as host lymphocytes surviving the conditioning regimens or lymphocytes present in the reinfused autograft.22
A potential explanation for the survival advantage associated with ALC recovery after ASCT in patients with MM and NHL is the possibility that early immune reconstitution may have a protective effect against residual disease progression. This is analogous to the graft-versus-tumor effect in ABMT recipients whereby the donor immune system is responsible for the eradication of residual disease in the host. Our study underscores the importance of reconstituting the immune system after ASCT, with better clinical outcomes demonstrated for patients with higher ALCs. This has led to the development of a prospective study to confirm our retrospective data. A significant limitation of our study, because of its retrospective nature, was the inability to delineate which lymphocytes subsets were involved in the ALC recovery and their relation to clinical outcome. Four sources may be involved in the ALC recovery: CD34 stem cells, reinfused graft lymphocytes, and host stem cells and host lymphocytes surviving HDT. Although our study did not find a correlation between ALC and CD34 counts, only 57 patients in the whole cohort group had a known CD34 stem cell count. Further studies are necessary to confirm the impact of CD34 counts on ALC recovery; recent studies have shown faster hematopoietic recovery with higher CD34 counts.23 It would be important to determine whether higher reinfused CD34 stem cells affect ALC recovery. As observed in allogeneic transplantation with donor lymphocyte infusion, the identification of the lymphocyte subsets in the reinfused autologous stem cell product affecting ALC recovery may provide insight into adoptive immunotherapeutic modalities to target minimal residual disease. ALC was a prognostic factor and correlated with survival in both groups of patients who received PBSCs and in the NHL group who received a BM stem cell source. Because of the small number of patients (21), no correlation was found between day 15 ALC and OS and PFS in patients with MM who received stem cells from BM harvest. Identifying methods to improve ALC recovery may be important in the quest to improve the care of patients with MM and NHL.
The reappearance of neutrophils and platelets is considered the end point of hematologic recovery after HDT and ASCT. Immunologic reconstitution is a gradual process that may not be completed until months to years after transplantation. Several studies have shown the delayed quantitative and functional recovery of T and B cells after transplantation. On the other hand, natural killer cells return to normal numbers and function as early as 1 month after transplantation, regardless of the type of stem cell transplantation (autologous24 or allogeneic25), or the treatment modality (conventional chemotherapy26 or high-dose chemotherapy). It is reasonable to propose that natural killer cells may play an important antitumor role early after ASCT controlling minimal residual disease in MM or NHL. We are exploring the role of natural killer cells in the early posttransplant period in patients with NHL.
Because this is a hypothesis-generating study, it is important to recognize that there may be many unmeasured factors that could influence ALCs and clinical outcome (such as posttransplant medications that may suppress the lymphocyte count); therefore, prospective studies are required to further analyze the impact of ALC on survival after autologous stem cell transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Svetomir N. Markovic, Department of Internal Medicine, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: markovic.svetomir@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal