Abstract
This study investigated the use of a nonablative conditioning regimen to decrease toxicity and achieve engraftment of an allogeneic blood stem cell transplant, allowing a graft-versus-malignancy effect to occur. All patients had follicular or small cell lymphocytic lymphoma after relapse from a prior response to conventional chemotherapy. Patients received a preparative regimen of fludarabine (25 mg/m2 given daily for 5 days or 30 mg/m2daily for 3 days) and intravenous cyclophosphamide (1 g/m2given daily for 2 days or 750 mg/m2 daily for 3 days). Nine patients received rituximab in addition to the chemotherapy. Tacrolimus and methotrexate were used for graft-versus-host disease (GVHD) prophylaxis. Twenty patients were studied; their median age was 51 years. Twelve were in complete remission (CR) at transplantation. All patients achieved engraftment of donor cells. The median number of days with severe neutropenia was 6. Only 2 patients required more than one platelet transfusion. The cumulative incidence of acute grade II to IV GVHD was 20%. Only one patient developed acute GVHD of greater than grade II. All patients achieved CR. None have had a relapse of disease, with a median follow-up period of 21 months. The actuarial probability of being alive and in remission at 2 years was 84% (95% confidence interval, 57%-94%). Nonablative chemotherapy with fludarabine/cyclophosphamide followed by allogeneic stem cell transplantation is a promising therapy for indolent lymphoma with minimal toxicity and myelosuppression. Further studies are warranted to compare nonablative allogeneic hematopoietic transplantation with alternative treatment strategies.
Introduction
Recurrent low-grade lymphoma is generally considered incurable using conventional treatment.1,2 One alternative, autologous stem cell transplantation (SCT), has been associated with improved disease-free survival (DFS),3 but existing studies have not demonstrated convincing improvement in survival.4-6 High-dose chemotherapy with allogeneic SCT has been investigated as treatment for low-grade lymphomas.7-9 In a 1997 study of 113 patients from the International Bone Marrow Transplant Registry,8 the 3-year probability of DFS was 49%. The recurrence rate was only 16%. This was offset, however, by a 40% incidence of treatment-related mortality. Patients in that study who were 40 years of age or older were most likely to die from treatment-related complications (relative risk of 1.85 versus 1.0).
Much of the benefit of allogeneic SCT appears to be mediated by an immune graft-versus-malignancy (GVM) effect.10,11 This suggests a strategy of administering a less toxic preparative regimen to achieve engraftment. This regimen would not completely eradicate the malignancy, but would allow development of the immune graft-versus-lymphoma effect as the primary treatment.12We piloted a study using a combination of fludarabine and cyclophosphamide given at conventional nonmyeloablative doses.13 This phase II trial was designed to assess the potential benefits of this strategy in the treatment of indolent lymphomas.
Patients, materials, and methods
Patient eligibility
We studied adult patients aged 70 years or younger who had recurrent follicular low-grade or small lymphocytic lymphoma after a prior response to conventional treatment. All had received salvage chemotherapy and had stable or responding disease. Patients were required to have a good performance status score (Zubrod ≤ 2) and no uncontrolled concomitant medical illness or active infection. All patients had an HLA-identical sibling donor willing and capable of donating filgrastim-stimulated, peripheral blood stem cells. Informed consent was obtained from all patients and donors. The treatment protocol was approved by the Institutional Review Board of the University of Texas M. D. Anderson Cancer Center.
Preparative regimen and transplantation
Donors received filgrastim, 6 μg/kg body weight, subcutaneously every 12 hours. On day 4 or 5, large-volume leukapheresis was performed to collect at least 4 × 106CD34+ cells/kg recipient body weight. The peripheral blood stem cells were unfractionated and were cryopreserved using standard techniques.
Fludarabine-based chemotherapy was used at conventional doses. This treatment without hematopoietic transplantation produces only short-term myelosuppression. The dose used was based on a previously published pilot trial in which donor cell engraftment was achieved with minimal toxicity.13 Eleven patients received fludarabine, 25 mg/m2 body surface area, administered intravenously (IV) daily on days −6, −5, −4, −3, and −2 before transplantation and cyclophosphamide, 1000 mg/m2, given IV daily on days −3 and −2 before transplantation. The schedule of fludarabine/cyclophosphamide was later changed to be given sequentially at 4-hour intervals to maximize inhibition of DNA repair and subsequent tumor kill.14 Therefore, 9 other patients received both 30 mg/m2 fludarabine and 750 mg/m2cyclophosphamide daily for 3 days on days −6, −5, and −4. Rituximab (IDEC Pharmaceuticals, San Diego, CA) was administered to these 9 patients at a dose of 375 mg/m2 administered IV on day −6 before transplantation and 1000 mg/m2 administered IV on days 1, 8, and 15 after transplantation. Allogeneic transplantation performed on day 0.
After transplantation, patients received immunosuppressive therapy with tacrolimus in combination with 5 mg/m2 methotrexate on days 1, 3, and 6. Tacrolimus was administered daily from day −2 as a continuous infusion of 0.03 mg/kg. On recovery, tacrolimus was administered orally in a twice-daily divided dose. Doses were adjusted to maintain whole blood trough blood levels at 5 to 15 mg/mL. The dose of tacrolimus was tapered by day 60 to 90 if there were signs of residual disease; otherwise it was continued for 6 months.
Filgrastim was administered in a subcutaneous injection of 5 μg/kg daily from day 0 until recovery of the granulocyte count to more than 1 × 109/L blood. Infection prophylaxis during the peritransplantation period consisted of 400 mg norfloxacin given orally twice daily, 500 mg penicillin VK given orally every 6 hours, and 200 mg fluconazole given orally every 12 hours. Patients also received 500 mg valacyclovir given orally daily and were screened biweekly for cytomegalovirus antigenemia; those who tested positive were treated with ganciclovir. All patients with neutropenic fever received broad-spectrum antibiotics. Blood product transfusions were irradiated and filtered to remove leukocytes. After recovery of the neutrophil count to more than 1.0 × 109/L blood, patients received prophylaxis against Pneumocystis cariniiinfection using trimethoprim-sulfamethoxazole given orally twice weekly or pentamidine IV every 3 weeks.
Assessment of outcome
Neutrophil count recovery was defined as the first of 3 consecutive days that the absolute neutrophil count exceeded 0.5 × 109/L blood, and platelet count recovery was defined as the day that the platelet count exceeded 20 × 109/L blood independent of platelet transfusions. Hematopoietic chimerism was evaluated on bone marrow cells by restriction fragment length polymorphisms (RFLPs) at the AY-29 or YNH24 loci15 and by cytogenetic studies in sex-mismatched cases. These assays are able to detect mixed chimerism if more than 5% recipient or donor cells are present. Toxicity was graded according to the method described by Bearman and coworkers,16 and GVHD was graded according to the consensus criteria.17
Actuarial estimates of time to GVHD, relapse, or death were calculated according to the method of Kaplan and Meier18 from the date of stem cell infusion.
Responses were scored by standard criteria in patients with lymphoma. Patients were monitored at regular intervals of 1 to 3 months with physical examinations, blood counts, bone marrow aspirate, and biopsy with flow cytometry, with RFLP analysis and with computed tomography of the chest, abdomen, and pelvis. Residual disease was analyzed by polymerase chain reaction for bcl-2 gene rearrangement at the major breakpoint and the minor cluster regions as previously described.19
Results
Patients
Between March 1997 and December 2000, 20 consecutive patients were included. Data on 3 patients were previously reported.13Patient characteristics are listed in Table1. The patients ranged in age from 31 to 68 years (median, 51 years). Eighteen patients had follicular lymphoma, and 2 patients had a small lymphocytic lymphoma. All patients had advanced recurrent disease and were previously pretreated. The number of prior chemotherapy regimens received by each patient ranged from 1 to 5 (median, 2). The median disease-free interval that patients had attained with their last form of therapy prior to salvage treatment and transplantation was 0 months (range, 0-27 months). At the time of transplantation, 12 patients were in second or greater complete remission (CR), 6 were in partial remission (PR), and 2 had progressive disease (PD). One patient had a recurrent disease after a prior autologous transplantation.
Demographic and other characteristics of the study cohort
| Patient . | Age . | Sex . | Disease histology . | Prior therapy (response) . | Salvage therapy (response) . | Stage at transplantation . |
|---|---|---|---|---|---|---|
| 1 | 54 | F | FCg1 | CHOP/COP (PR) | FND (PR) | II |
| 2 | 45 | F | FCg1 | CHOP | — | IV B |
| 3 | 46 | F | FCg1 | CHOP (CR) | CAE (PR) | II |
| FLU (CR) | ||||||
| 4 | 60 | M | FCg2 | CHOP (PR) | FND (CR) | CR |
| XRT (PR) | ||||||
| 5 | 58 | F | FCg1 | CHOP (PR) | Ifos/etoposide (CR) | CR |
| TC (PR) | ||||||
| XRT | ||||||
| IL-2 (PD) | ||||||
| ESHAP (PR) | ||||||
| 6 | 51 | M | FCg2 | FND/IFN (CR) | ESHAP (CR) | CR |
| 7 | 50 | F | FCg1 | CHOP/ESHAP/ | FND (CR) | CR |
| NOPP (CR) | ||||||
| 8 | 59 | M | FCg1 | CHOP-Bleo/XRT (CR) | COP (CR) | CR |
| XRT (CR) COP (CR) | ||||||
| 9 | 67 | F | FCg1 | CHOP/ESHAP/NOPP (CR) | FND (CR) | CR |
| IFN (CR) | ||||||
| Rituximab (PR) | ||||||
| Bryostatin (PD) | ||||||
| 10 | 50 | M | SLL | CHOP/ESHAP/NOPP (PR) | FC (CR) | CR |
| IFN (SD) | ||||||
| 11 | 56 | M | FCg2 | COP (CR) | CHOP/Rituxan (CR) | CR |
| 12 | 31 | F | FCg1 | 131I (CR) | FND (CR) | CR |
| 13 | 46 | M | FCg1 | CHOP (PR) | CHOP/Rituxan (PR) | III |
| TI (SD) | ||||||
| GCD (CR) | ||||||
| 14 | 56 | F | FCg2 | ASHAP/MBACOS/MINE (CR) | FND/R (PR) | IA |
| Lipsomal vincristine (PR) | ||||||
| 15 | 54 | F | FCg1 | CHOP (CR) | FND (PR) | IB |
| 16 | 58 | M | FCg1 | CVAD (CR) | R (CR) | CR |
| CHOP (PR) | ||||||
| 17 | 47 | F | SLL | CHOP (CR) | FND/R (CR) | CR |
| DHAP (PR) | ||||||
| 18 | 44 | M | FCg1 | FND (CR) | — | IIIA |
| IFN (CR) | ||||||
| 19 | 68 | M | FCg1 | CHOP (PR) | ESHAP (PR) | IIIA |
| 20 | 49 | F | FCg1 | COP (CR) | MINE (CR) | CR |
| CHOP (PR) | ||||||
| ESHAP | ||||||
| Rituximab (SD) |
| Patient . | Age . | Sex . | Disease histology . | Prior therapy (response) . | Salvage therapy (response) . | Stage at transplantation . |
|---|---|---|---|---|---|---|
| 1 | 54 | F | FCg1 | CHOP/COP (PR) | FND (PR) | II |
| 2 | 45 | F | FCg1 | CHOP | — | IV B |
| 3 | 46 | F | FCg1 | CHOP (CR) | CAE (PR) | II |
| FLU (CR) | ||||||
| 4 | 60 | M | FCg2 | CHOP (PR) | FND (CR) | CR |
| XRT (PR) | ||||||
| 5 | 58 | F | FCg1 | CHOP (PR) | Ifos/etoposide (CR) | CR |
| TC (PR) | ||||||
| XRT | ||||||
| IL-2 (PD) | ||||||
| ESHAP (PR) | ||||||
| 6 | 51 | M | FCg2 | FND/IFN (CR) | ESHAP (CR) | CR |
| 7 | 50 | F | FCg1 | CHOP/ESHAP/ | FND (CR) | CR |
| NOPP (CR) | ||||||
| 8 | 59 | M | FCg1 | CHOP-Bleo/XRT (CR) | COP (CR) | CR |
| XRT (CR) COP (CR) | ||||||
| 9 | 67 | F | FCg1 | CHOP/ESHAP/NOPP (CR) | FND (CR) | CR |
| IFN (CR) | ||||||
| Rituximab (PR) | ||||||
| Bryostatin (PD) | ||||||
| 10 | 50 | M | SLL | CHOP/ESHAP/NOPP (PR) | FC (CR) | CR |
| IFN (SD) | ||||||
| 11 | 56 | M | FCg2 | COP (CR) | CHOP/Rituxan (CR) | CR |
| 12 | 31 | F | FCg1 | 131I (CR) | FND (CR) | CR |
| 13 | 46 | M | FCg1 | CHOP (PR) | CHOP/Rituxan (PR) | III |
| TI (SD) | ||||||
| GCD (CR) | ||||||
| 14 | 56 | F | FCg2 | ASHAP/MBACOS/MINE (CR) | FND/R (PR) | IA |
| Lipsomal vincristine (PR) | ||||||
| 15 | 54 | F | FCg1 | CHOP (CR) | FND (PR) | IB |
| 16 | 58 | M | FCg1 | CVAD (CR) | R (CR) | CR |
| CHOP (PR) | ||||||
| 17 | 47 | F | SLL | CHOP (CR) | FND/R (CR) | CR |
| DHAP (PR) | ||||||
| 18 | 44 | M | FCg1 | FND (CR) | — | IIIA |
| IFN (CR) | ||||||
| 19 | 68 | M | FCg1 | CHOP (PR) | ESHAP (PR) | IIIA |
| 20 | 49 | F | FCg1 | COP (CR) | MINE (CR) | CR |
| CHOP (PR) | ||||||
| ESHAP | ||||||
| Rituximab (SD) |
F indicates female; M, male; FCg1, follicular center grade 1; FCg2, follicular center grade 2; SLL, small lymphocytic lymphoma; CAE, cyclophosphamide doxorubicin etoposide; CHOP-Bleo, cyclophosphamide doxorubicin vincristine prednisone bleomycin; FLU-fludarabine; COP, cyclophosphamide vincristine prednisone; FND, fludarabine mitoxantrone dexamethasone; HCVAD, fractionated cyclophosphamide vincristine doxorubicin dexamethasone; XRT, radiotherapy; TC, paclitaxel cyclophosphamide; IL-2, interleukin-2; VBD, vincristine bleomycin dexamethasone; ESHAP, etoposide Solu-Medrol cytarabine cisplatin, Ifos-ifosfamide; NOPP, mitoxantrone vincristine prednisone procarbazine; IFN, interferon; TI, paclitaxel ifosfamide; GCD, gemcitabine cisplatin Decadron; R, rituximab; MBACOS, methotrexate bleomycin doxorubicin cyclophosphamide vincristine Solu-Medrol; MINE, mesna ifosfamide novantrone etoposide; SD, stable disease.
Each patient had an HLA-identical donor. The median age of the donors was 51 years (range, 27-67 years). Positive serology for cytomegalovirus (CMV) in either donor or recipient was detected in 14 cases. There were 10 cases of sex-mismatched transplants.
Engraftment
The median number of CD34+ cells infused was 4.66 × 106/kg recipient body weight (range, 1.56-6.76 × 106). The median number of CD3+cells infused was 1.6 × 108/kg recipient body weight (range, 0.8-5.5 × 108).
All patients had prompt hematopoietic recovery. Neutrophil counts recovered to more than 0.5 × 109/L at a median of 11 days after transplantation (range, 10-16 days). There was no delay of engraftment in the 9 patients who received rituximab; their median interval to neutrophil recovery was 10 days. The median number of days with severe neutropenia with the neutrophil count less than 0.5 × 109/L blood was 6 (range, 6-14 days) and there was no difference noted whether patients received rituximab or not. Only 2 patients required platelet transfusion on more than one occasion prior to hematologic recovery. The median number of red blood cell units transfused within the same period of time was 1 (range, 0-9 units). Hematologic recovery was sustained and no patient developed graft failure.
The preparative regimen was nonmyeloablative, and mixed chimerism was expected. All patients had evidence of donor cell engraftment, as documented using a bone marrow RFLP analysis. The median percentage of donor cells at 1 month after transplantation was 80% (range, 10%-100%). Only one patient had less than 50% donor cells in his marrow at 1 month after transplantation. When tested at 3, 4, and 8 months after transplantation, this patient showed 33%, 10%, and 5% of donor cells, respectively, by chromosomal analysis. The patient developed cytopenia of suspected viral etiology 8 months after transplantation and responded to therapy with foscarnet. A subsequent RFLP analysis showed 40% donor cells in the patient's marrow.
Outcome
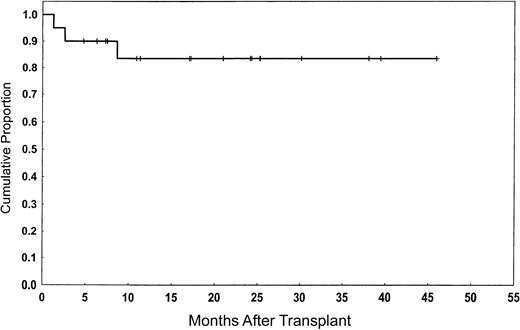
All patients achieved CR. One patient who had a PR and another who progressed at 60 days after transplantation later achieved CR concomitant with the withdrawal of tacrolimus and subsequent development of GVHD. Neither of these patients received rituximab with their preparative regimens. None of the total group have relapsed. The median follow-up period was 21 months (range, 5-46 months). Seventeen patients (85%) remain alive with a good performance status score (Zubrod ≤ 1) and in CR (Figure1). Actuarial survival in remission at 2 years was 84% (95% confidence interval [CI], 53%-84%).
Overall survival and event-free survival of the study population.
The actuarial probability of being alive and in remission at 2 years was 84%.
Overall survival and event-free survival of the study population.
The actuarial probability of being alive and in remission at 2 years was 84%.
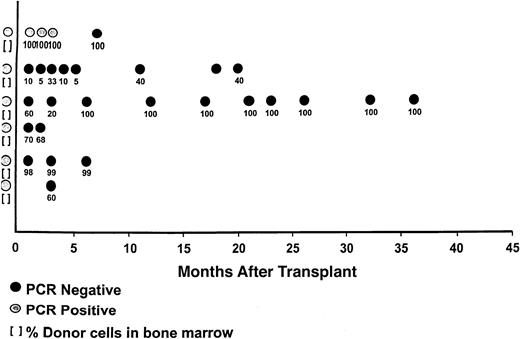
Clonal cells were detected in 6 patients who were tested before transplantation for lymphoma by marrow polymerase chain reaction (PCR) assay for bcl-2 gene rearrangement (Figure2). Patients were followed prospectively after transplantation for the presence of minimal residual disease by PCR. Results were obtained from the analysis of 29 sequential samples. Five were PCR negative. Only one patient (no.1) had a PCR-positive status. She converted to PCR negativity at 7 months after transplantation concomitant with chronic GVHD.
Molecular posttransplantation responses with corresponding chimeric results.
Six patients were PCR positive for bcl-2 at transplantation. All are currently in PCR-negative status. The one patient who was PCR positive early after transplantation converted to PCR negativity with the onset of GVHD.
Molecular posttransplantation responses with corresponding chimeric results.
Six patients were PCR positive for bcl-2 at transplantation. All are currently in PCR-negative status. The one patient who was PCR positive early after transplantation converted to PCR negativity with the onset of GVHD.
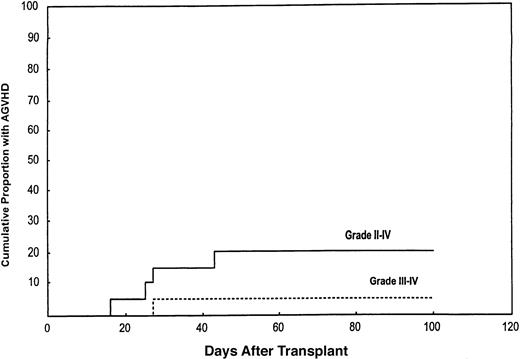
The cumulative incidence of acute grade II to IV GVHD was 20% (95% CI, 8%-45%; Figure 3). Only one patient (5%) developed grade III GVHD (95% CI, 0.7%-30%). This occurred in a patient who had central nervous system toxicity to tacrolimus, which was discontinued. She later died of complications.
Cumulative incidence of acute II to IV and III to IV GVHD in the study population.
The cumulative incidence of grade II to IV acute GVHD was 20%. The lower curve shows a cumulative incidence of 5% for grade III to IV GVHD.
Cumulative incidence of acute II to IV and III to IV GVHD in the study population.
The cumulative incidence of grade II to IV acute GVHD was 20%. The lower curve shows a cumulative incidence of 5% for grade III to IV GVHD.
The cumulative incidence of chronic GVHD was 64% (95% CI, 38%-88%). Eighteen patients were evaluable. Chronic GVHD developed in 8 patients. Five patients developed chronic GVHD, 6 to 8 months after transplantation, shortly after discontinuation of tacrolimus. It was limited in 3 patients and extensive in 2 others. All maintained a good performance (Zubrod 0) and they are currently in CR. Three additional patients developed extensive chronic GVHD related to early withdrawal of tacrolimus. One patient had exacerbation of preexisting peripheral neuropathy and her immunosuppression was discontinued at 3 months after transplantation. She responded to therapy with methylprednisolone (MP) and achieved CR in her GVHD. The 2 other patients had early withdrawal because of signs of disease progression (one case) or failure to achieve remission 60 days after transplantation. Those patients were in CR and PR, respectively, at the time of transplantation. Neither received rituximab with the preparative regimens. Both developed extensive chronic GVHD after withdrawal of immunosuppression. One responded to MP and infliximab and is currently in remission both in terms of lymphoma and GVHD. The other patient's lymphoma achieved CR but her GVHD was refractory to MP and mycophenolate mofetil. She later responded to photopheresis. She was the only patient in this report who had serious debilitation from her chronic GVHD. She developed and recovered from a Nocardia infection. However, she was later diagnosed with metastatic breast cancer, which was the cause of her death.
One other death occurred. It was related to disseminatedAspergillus infection 45 days after transplantation in a patient who received therapy with hydroxychloroquine sulfate (Plaquenil) and corticosteroid for 3 years up to her transplantation for severe arthritis.
All other nonhematologic toxicity was limited to grade 2 or less. Despite the high incidence of positive pretransplantation serology for CMV in either donor or recipient, CMV antigenemia was detected in 3 patients after transplantation and no patient developed symptomatic CMV disease. One patient had reactivation of hepatitis C infection 7 months after transplantation. He recovered with appropriate therapy and supportive care.
Discussion
Among lymphoid malignancies, low-grade lymphoma appears most susceptible to GVM effects.20 We and others have demonstrated that when high-dose chemoradiotherapy is used as preparative regimen with allogeneic transplantation to treat low-grade lymphoma, few patients relapse, but there is a 30% to 40% risk of treatment–related mortality.8 9 Treatment-related mortality is most frequent in patients older than 40 years. Low-grade lymphomas typically occur in older, often debilitated patients who are unable to tolerate ablative high-dose therapy. We hypothesized that the use of a less toxic nonablative regimen may significantly improve the outcome for patients with low-grade lymphoma.
The nonablative preparative regimen used in this study is an established therapy for indolent lymphoid malignancies. The regimen is moderately myelosuppressive and can be safely administered without transplantation.21 Rituximab was recently added to our treatment protocol with the consideration that it might be synergistic with chemotherapy and produce no overlapping toxicity.22Rituximab was used to enhance tumor control early after transplantation to allow time for the GVM effect to become established. This was based on the observation of early failures occurring in some patients who received chemotherapy alone with their preparative regimens. This has required early discontinuation of immunosuppression. Extensive and morbid GVHD subsequently developed. The dose of rituximab was based on a recent study23,24 from our institution demonstrating a dose-response relationship in patients who had chronic lymphocytic leukemia (CLL). This was also recently confirmed by Byrd and coworkers25 who demonstrated an increase in the response rate in CLL and small lymphocytic lymphoma to 45% when given thrice weekly for 4 weeks. Historically, the response rate ranged between 4% and 15% at the conventional dose and schedule of 375 mg/m2given IV weekly for 4 doses. This finding has also been indirectly supported by Magni and colleagues26 who used rituximab for in vivo purging of autologous stem cell collection prior to autotransplantation for follicular and mantle cell lymphomas. In that study, 33% of the collected stem cells were PCR negative after one course of in vivo treatment with rituximab. After the second course of treatment, 93% of cells became lymphoma-free compared to 40% in the control group who received chemotherapy alone for mobilization without rituximab. Controlled studies are needed, however, to determine the optimal dose and schedule of rituximab in the setting of nonmyeloablative allogeneic transplantation.
Results of molecular testing and cytogenetic analysis demonstrated engraftment in all patients. There was no difference in the duration of neutropenia between patients who received rituximab and those who did not. The median duration of severe neutropenia was only 6 days, nearly 1 week shorter than what is normally observed with the use of high-dose chemotherapy. This has been associated with a low incidence of serious infection. In addition, engraftment in this group of patients was durable with minimal transfusion requirements and no secondary graft failure occurred. Subsequent to the start of this study, others reported that analysis of T-cell chimerism is predictive of both engraftment and antitumor responses.27 The day 100 mortality rate was 10%, much less than the 30% to 40% risk described for younger patients with high-dose chemotherapy.9 28
Various reduced-toxicity or nonablative regimens have been proposed by other investigators. Slavin and associates29 reported results from a more intensive conditioning regimen consisting of 8 mg/kg busulfan, fludarabine, and antithymocyte globulin. This regimen, however, produced marked myelosuppression and has not been administered without hematopoietic transplantation. In addition, 25% of patients develop severe GVHD, which was the main cause of mortality. McSweeney and coworkers30 reported an alternative strategy using a low-dose total body irradiation regimen followed by the administration of cyclosporine and mycophenolate mofetil to achieve engraftment and mixed chimerism. However, 20% of patients had graft failure in that study. In addition, grade II to IV acute GVHD occurred in 39% of patients. Neither of these 2 regimens has been studied in a large series of follicular lymphomas.
Graft-versus-host disease is one of the major causes of posttransplantation morbidity and mortality, especially in elderly patients.30 The syndrome of acute GVHD results at least partially from tissue injury and cytokine release secondary to the toxicity of the preparative regimen, both of which amplify the immune graft-versus-host reaction.31 Because of the advanced age of the patients, their CMV seropositivity, and sex-mismatched donors, a relatively high incidence of acute GVHD would be expected. Grade II to IV acute GVHD occurred in 20% of the patients with only one case of grade III acute GVHD in a patient intolerant to tacrolimus. A historical control group of 44 patients receiving high-dose chemoradiotherapy and allogeneic transplantation for follicular lymphoma had a cumulative incidence of acute grade II to IV GVHD of 43% and grade III to IV GVHD of 21%.27 Both groups received comparable GVHD prophylaxis. The lesser incidence of acute GVHD in this study was probably related to the use of a less toxic preparative regimen that limited tissue injury and cytokine release. The presence of mixed T-cell chimerism may also inhibit generation of GVHD.
All treated patients achieved durable CR with a median follow-up of 21 months. Molecular remission was observed in all patients who were tested by the PCR assay after transplantation. One must remain cautious about these results considering the fact that 60% of the patients were in CR at the time of transplantation and the long natural history of indolent lymphomas. However, late relapses have been rare after allogeneic transplantation for these diseases. In the large analysis from the International Bone Marrow Registry, only one relapse occurred among 33 patients with low-grade lymphoma who were monitored more than 2 years after allogeneic transplantation.9
We conclude that the nonablative conditioning regimen of fludarabine and cyclophosphamide followed by an allogeneic SCT is a promising therapy for indolent lymphomas. Further studies with long-term follow-up are necessary and controlled trials are warranted to compare nonablative allogeneic hematopoietic transplantation with alternative treatment strategies.
Supported in part by research grants from the G & P Foundation for Cancer Research, from Berlex, and from Genentech.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Issa F. Khouri, Department of Blood and Marrow Transplantation, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Box 423, Houston, TX 77030; e-mail:ikhouri@notes.mdacc.tmc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal