Abstract
Human plasma–derived factor IX (pdFIX) concentrates are routinely used to treat patients with hemophilia B, an X-linked bleeding disorder that affects 1 in 30 000 males, but concerns remain regarding transmission of blood-borne pathogens. Therefore, the safety and efficacy of recombinant human factor IX (rFIX) were evaluated. A 20-center international trial was conducted in previously treated patients with severe or moderate (< 5 IU/dL factor IX activity) hemophilia B. Participants received rFIX for pharmacokinetic studies, treatment of or prophylaxis against hemorrhage, or surgical hemostasis, and were assessed at 3-month intervals for 2 years. Fifty-six subjects were treated. Mean incremental rFIX recovery was 0.75 IU/dL per IU/kg, 30% lower than expected for pdFIX, although the mean half-life was similar. Pharmacokinetic parameters were stable over time. Somewhat lower recoveries were seen in subjects younger than 15 years of age and in those with no detectable factor IX antigen. A total of 7362 infusions of rFIX were administered. All 1796 hemorrhages were controlled, 80.9% of which required only one rFIX infusion. Effective hemostasis was also achieved in prophylactic and surgical settings. One individual developed a low titer (1.2 Bethesda unit) transient inhibitor that spontaneously resolved. rFIX was not associated with serious adverse events, thrombogenicity, or virus transmission. rFIX is safe and effective for the treatment of hemophilia B. Despite a lower recovery compared with pdFIX, rFIX controlled hemorrhage in a wide variety of settings and may provide a safety advantage in terms of risk from blood-borne pathogens.
Introduction
Human plasma–derived factor VIII and factor IX replacement products are used routinely to treat individuals with hemophilia A (factor VIII deficiency) and hemophilia B (factor IX deficiency).1,2 Low-purity, low-specific activity factor VIII concentrates and factor IX concentrates (prothrombin complex concentrates [PCCs]) became available over 30 years ago, and their use led to dramatic improvements in duration and quality of life for persons with hemophilia. However, these products infected patients with hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV). PCCs also contained prothrombin, factor VII, and factor X and their use in hemophilia B was further complicated by thrombosis, generally attributed to the accumulation or delayed clearance of these unactivated and activated vitamin K–dependent proteins.2-5 Subsequently, high-purity plasma-derived factor IX concentrates (pdFIX) were developed. These have largely replaced PCCs and have been used successfully for those with a history of thrombosis due to PCCs.
Despite the availability of high-purity virus-inactivated plasma-derived factor VIII concentrates (pdFVIII) and pdFIX products, concerns remain about their contamination with infectious prions causing new variant Creutzfeldt-Jakob disease (vCJD).6-9Outbreaks of hepatitis A virus (HAV)10,11 and the transmission of parvovirus12,13 in persons with hemophilia, including those treated exclusively with clotting-factor concentrates considered safe against HIV and HCV,14 raise further concerns about the transmission of small nonenveloped viruses with pooled human plasma–derived clotting factor concentrates. This concern has stimulated intense efforts to develop recombinant human factor VIII (rFVIII) and factor IX (rFIX) products for use in persons with hemophilia A and B. Several rFVIII products have been available since 1992. Although the human F9 gene was first cloned nearly 2 decades ago,15 development of rFIX for clinical use was hampered by the complex posttranslational modifications required for biosynthesis of factor IX.16
These barriers have been overcome, and rFIX, a pathogen-free source of factor IX, is available to treat individuals with hemophilia B. In contrast to rFVIII products for hemophilia A, no animal or human plasma–derived protein is used during the manufacture17or formulation of rFIX, distinguishing rFIX18 as a unique replacement product for use in routine hemophilia care. Herein, we report on the safety and efficacy of rFIX in persons with severe and moderate hemophilia B for the treatment or prevention of bleeding episodes.
Patients, materials, and methods
Patients
Eligible participants had severe (factor IX activity < 1 IU/dL) or moderate (factor IX activity 1-5 IU/dL) hemophilia B and all had been previously treated with high-purity, monoclonal antibody–purified factor IX (pdFIX). Subjects with a history or evidence of a factor IX inhibitor by Bethesda inhibitor assay (BIA) or history of anaphylaxis to any factor IX–containing product were excluded. Both HIV seropositive and seronegative individuals were enrolled, but HIV+ individuals with a blood CD4 cell count of less than 400/μL were excluded. Subjects were excluded if they had serum alanine aminotransferase in excess of 5 times the upper limit of the normal range, total bilirubin more than 2 mg/dL, serum creatinine in excess of 1.25 times the upper limit of the normal range, or a platelet count of less than 140 000/μL. The presence of antibodies against HIV-1, HIV-2, HAV, HBV (surface antigen and core), and HCV was determined at baseline and throughout the study. The study was approved at each center by the local institutional review board. Informed consent was obtained from each individual (or guardian) prior to enrollment.
Study design
Clinical studies with rFIX began in February 1995. Fifty-seven individuals were enrolled at 20 sites throughout North America and Europe. One person withdrew consent prior to treatment. The final subject completed his course of treatment in April 1999. The study comprised pharmacokinetic (PK) assessment; assessment of thrombogenicity; and safety and efficacy during home treatment and management of surgical procedures. The PK assessment was repeated every 6 months, with routine clinical and laboratory evaluations at 3-month intervals. The planned study duration for each participant was 2 years.
PK assessment
A baseline PK evaluation was performed on first infusion with rFIX using a single 50 IU/kg intravenous dose. At the time of infusion, all subjects were in a nonbleeding state and without any factor IX or antifibrinolytic products during the preceding 7 days. Blood samples were drawn prior to infusion and then at 0.25, 0.5, 1, 2, 4, 8, 12, 24, 36, 48, and 72 hours. The PK parameters were derived using the observed maximum factor IX activity and the elimination half-life. Changes over time for incremental recovery and half-life were assessed using repeated measures analysis of variance. Half-life was estimated using compartmental and noncompartmental methods. Factor IX activity was measured in a central laboratory by a one-stage clotting assay using a normal plasma reference standard calibrated against a World Health Organization standard. The recovery of rFIX was calculated as the observed maximum postinfusion plasma factor IX activity (IU/mL) divided by the expected maximum plasma factor IX activity (the rFIX dose administered [IU] divided by the subject's estimated plasma volume [mL], assuming 45 mL plasma per kilogram body weight).
Thrombogenicity testing
Fibrinopeptide A and prothrombin fragment 1 + 2 (F1 + 2) were measured to assess thrombogenicity in participants during their initial PK study, at 1 hour before infusion, and 4 to 8 hours and 24 hours after infusion. A subgroup of 6 subjects consented to additional testing for D-dimer, thrombin-antithrombin complex, and F1 + 2 by separate venipuncture at 15 minutes and at 1, 2, 4, and 24 hours after infusion.
Safety and efficacy in treatment of or prophylaxis against hemorrhage
Subjects used rFIX as needed to treat hemorrhage (on demand), as prophylaxis to prevent hemorrhage, or to control surgery-related bleeding. The investigator determined the dose and frequency of rFIX administration following dosing protocols as described.1 All individuals initially used rFIX at doses similar to those used previously with pdFIX. Individualized PK testing results were used to adjust rFIX dosing when appropriate. The prescribed dosing regimen was based on body weight, vial potency, and the nature of the hemorrhage or surgery following standard guidelines.1 Each individual recorded administration details and the reason for each rFIX infusion. Participants (and the investigator if administered in the clinic) were asked to judge the bleeding response to each rFIX infusion after 24 hours, or just prior to the next dose, using a 4-point scale based on a comparison with previously used pdFIX products for similar bleeds or procedures: excellent (as much and as rapid an improvement as thebest pdFIX-related responses), good (as much and as rapid an improvement as most pdFIX-related responses), moderate (not as good as most pdFIX-related responses), or no response (no improvement). For those who were maintained on secondary prophylaxis, the rFIX dosing and frequency of administration were determined by the investigator based on the individual's prior experience with pdFIX, clinical responses to therapy with rFIX or results from PK assessments with rFIX or both. The response to secondary prophylaxis was assessed once every 3 months by the investigator using a 3-point scale: excellent (no spontaneous bleeding of any kind, no change in dosing regimen necessary), effective (no spontaneous musculoskeletal bleeding, no change in dosing regimen necessary), or inadequate (inadequate prevention of bleeding requiring a change in dosing regimen). When surgery was required, rFIX was prescribed by the investigator as indicated for the procedure following recommended guidelines.1 The investigator and surgeon assessed hemostasis, estimated blood loss, and rFIX efficacy during the perioperative period using the same 4-point scale described above.
Human rFIX
Human rFIX was manufactured in the presence of vitamin K in a characterized Chinese hamster ovary cell line free of infectious agents.19 The Chinese hamster ovary cell line was cotransfected with complementary DNAs (cDNAs) encoding human factor IX and human paired basic amino acid cleaving enzyme to ensure complete processing of profactor IX to mature biologically active γ-carboxylated factor IX.20 The cell line was grown in medium without animal or human plasma-derived protein supplements.17 A nanofiltration step was used for additional viral safety. The rFIX dosage form contains no animal or human-derived carrier proteins such as albumin.18
Results
Baseline characteristics of the 56 efficacy evaluable subjects are shown in Table 1. Fifty subjects completed the 24-month study. Five subjects withdrew from the study for personal reasons, and one withdrew because of a recurring adverse event (the urge to cough shortly after rFIX infusions), respectively. The median time on study was 741 days (mean ± SD, 710.9 ± 154.2 days). The time on study ranged between 30 days and 856 days. A total of 7362 infusions with a cumulative total of 20 923 634 IU rFIX were administered (Table 2).
Baseline characteristics of patients treated with rFIX
| Characteristic . | No. (%) . |
|---|---|
| Sex, male | 56 (100) |
| Race | |
| White | 51 (91.1) |
| African American | 2 (3.5) |
| Asian | 1 (1.8) |
| Asian-white | 1 (1.8) |
| Hispanic | 1 (1.8) |
| Age, y | |
| Median | 23 |
| Range | 4-56 |
| Hemophilia severity, %* | |
| Below 1 | 46 (82.1) |
| From 1 to 3 | 9 (16.1) |
| Higher than 3 to 5 | 1 (1.8) |
| Previous exposure days | |
| From 20 to 100 | 8 (14.0) |
| More than 100 to 250 | 22 (39.2) |
| More than 250 | 26 (46.4) |
| HIV antibody status† | |
| Negative | 49 (87.5) |
| Positive‡ | 7 (12.5) |
| Hepatitis A antibody status† | |
| Negative | 32 (57.1) |
| Positive | 24 (42.9) |
| Hepatitis B antibody status† | |
| Negative | 5 (8.9) |
| Positive | 51 (91.1) |
| Hepatitis C antibody status† | |
| Negative | 13 (23.2) |
| Positive | 43 (76.8) |
| Factor IX antigen status | |
| Negative | 33 (59) |
| Positive | 22 (39) |
| Indeterminate1-153 | 1 (2) |
| Characteristic . | No. (%) . |
|---|---|
| Sex, male | 56 (100) |
| Race | |
| White | 51 (91.1) |
| African American | 2 (3.5) |
| Asian | 1 (1.8) |
| Asian-white | 1 (1.8) |
| Hispanic | 1 (1.8) |
| Age, y | |
| Median | 23 |
| Range | 4-56 |
| Hemophilia severity, %* | |
| Below 1 | 46 (82.1) |
| From 1 to 3 | 9 (16.1) |
| Higher than 3 to 5 | 1 (1.8) |
| Previous exposure days | |
| From 20 to 100 | 8 (14.0) |
| More than 100 to 250 | 22 (39.2) |
| More than 250 | 26 (46.4) |
| HIV antibody status† | |
| Negative | 49 (87.5) |
| Positive‡ | 7 (12.5) |
| Hepatitis A antibody status† | |
| Negative | 32 (57.1) |
| Positive | 24 (42.9) |
| Hepatitis B antibody status† | |
| Negative | 5 (8.9) |
| Positive | 51 (91.1) |
| Hepatitis C antibody status† | |
| Negative | 13 (23.2) |
| Positive | 43 (76.8) |
| Factor IX antigen status | |
| Negative | 33 (59) |
| Positive | 22 (39) |
| Indeterminate1-153 | 1 (2) |
N = 56.
Percent circulating FIX activity measured by the one-stage clotting assay before administration of the initial dose of rFIX in the baseline PK evaluation. Normal is 100% or 100 IU/dL.
Antibody status was determined at study entry.
All 7 HIV+ patients were positive for HAV, HBV, and HCV in some combination.
Assay interference precludes the assessment of factor IX antigen status.
rFIX dosing and exposure days
| . | PK . | On-demand . | Prophylaxis* . | Surgery-related . | Total . |
|---|---|---|---|---|---|
| No. of patients | 56 | 55 | 47 | 20 | 56 |
| Total rFIX units (IU) administered per patient | |||||
| Cumulative | 907 264 | 8 552 140 | 9 252 060 | 2 212 170 | 20 923 634 |
| Median | 17 124 | 118 440 | 60 600 | 33 500 | 258 462 |
| Range | 2055-31 670 | 7200-810 200 | 1000-1 477 780 | 2200-423 700 | 11 778-2 141 525 |
| Dose (IU/kg) | |||||
| Median | 50.0 | 42.80 | 35.10 | 60.40 | 40.90 |
| Mean ± SD | 49.95 ± 3.53 | 46.56 ± 23.48 | 38.00 ± 17.06 | 66.25 ± 29.47 | 43.07 ± 21.32 |
| Range | 25.0-90.3 | 6.5-224.6 | 9.7-170.6 | 13.8-169.0 | 6.5-224.6 |
| No. of infusions | |||||
| Total | 263 | 2 758 | 3 909 | 432 | 7 362 |
| Median | 5.00 | 41.00 | 24.00 | 10.00 | 85.00 |
| Mean ± SD | 4.70 ± 1.04 | 50.15 ± 42.34 | 83.17 ± 130.28 | 21.60 ± 26.53 | 131.46 ± 128.69 |
| Range | 1-7 | 3-196 | 1-656 | 1-96 | 4-669 |
| No. of exposure days | |||||
| Total† | 263 | 2 633 | 3 620 | 320 | 6 823 |
| Median | 5.00 | 41.00 | 24.00 | 9.00 | 83.50 |
| Mean ± SD | 4.70 ± 1.04 | 47.87 ± 38.04 | 77.02 ± 108.28 | 16.00 ± 18.30 | 121.84 ± 103.27 |
| Range | 1-7 | 3-195 | 1-492 | 1-62 | 4-496 |
| . | PK . | On-demand . | Prophylaxis* . | Surgery-related . | Total . |
|---|---|---|---|---|---|
| No. of patients | 56 | 55 | 47 | 20 | 56 |
| Total rFIX units (IU) administered per patient | |||||
| Cumulative | 907 264 | 8 552 140 | 9 252 060 | 2 212 170 | 20 923 634 |
| Median | 17 124 | 118 440 | 60 600 | 33 500 | 258 462 |
| Range | 2055-31 670 | 7200-810 200 | 1000-1 477 780 | 2200-423 700 | 11 778-2 141 525 |
| Dose (IU/kg) | |||||
| Median | 50.0 | 42.80 | 35.10 | 60.40 | 40.90 |
| Mean ± SD | 49.95 ± 3.53 | 46.56 ± 23.48 | 38.00 ± 17.06 | 66.25 ± 29.47 | 43.07 ± 21.32 |
| Range | 25.0-90.3 | 6.5-224.6 | 9.7-170.6 | 13.8-169.0 | 6.5-224.6 |
| No. of infusions | |||||
| Total | 263 | 2 758 | 3 909 | 432 | 7 362 |
| Median | 5.00 | 41.00 | 24.00 | 10.00 | 85.00 |
| Mean ± SD | 4.70 ± 1.04 | 50.15 ± 42.34 | 83.17 ± 130.28 | 21.60 ± 26.53 | 131.46 ± 128.69 |
| Range | 1-7 | 3-196 | 1-656 | 1-96 | 4-669 |
| No. of exposure days | |||||
| Total† | 263 | 2 633 | 3 620 | 320 | 6 823 |
| Median | 5.00 | 41.00 | 24.00 | 9.00 | 83.50 |
| Mean ± SD | 4.70 ± 1.04 | 47.87 ± 38.04 | 77.02 ± 108.28 | 16.00 ± 18.30 | 121.84 ± 103.27 |
| Range | 1-7 | 3-195 | 1-492 | 1-62 | 4-496 |
Prophylaxis includes routine secondary prophylaxis 2 to 3 times each week for prevention of hemorrhage (this subgroup of patients is discussed separately in “Results”) as well as intermittent prophylactic use for the prevention of anticipated bleeding, such as bleeding that might occur following strenuous exercise.
A patient may be dosed more than once a day for different reasons.
PK assessments
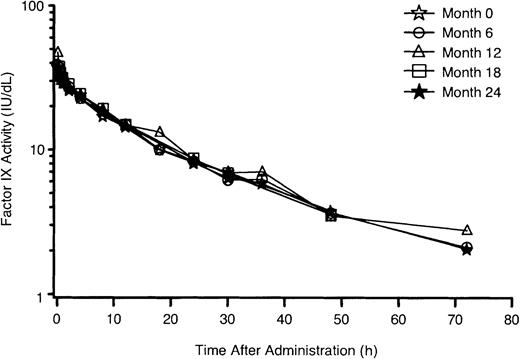
At baseline, rFIX infusion of 50 IU/kg resulted in a mean factor IX activity increase of 0.75 IU/dL per IU/kg (range, 0.34-1.38 IU/dL per IU/kg), corresponding to a recovery of 33.7% (range, 15.3%-62.2%). The mean elimination half-life was 19.3 hours (range, 11.1-36.4 hours). Repeat PK assessments every 6 months demonstrated the stability of these parameters during 2 years of exposure to rFIX (Figure 1). No significant reduction in baseline rFIX recovery or elimination half-life occurred in any previously treated patients (PTPs) over time, with the exception of one subject in whom a low-titer inhibitor developed. Subgroup analyses revealed the lowest recoveries in subjects younger than 15 years old. Lower recoveries were also seen in patients who were negative for factor IX antigen, although neither of these trends reached statistical significance (Table 3).
Mean factor IX activity versus time at months 0, 6, 12, 18, and 24.
PK parameters were estimated for 56 patients at baseline. Fifty-three patients had PK parameters estimated at 6 months, 51 patients at 12 months, and 48 patients at 18 and 24 months. The data from one patient (months 12, 18, 24) were excluded after inhibitor development.
Mean factor IX activity versus time at months 0, 6, 12, 18, and 24.
PK parameters were estimated for 56 patients at baseline. Fifty-three patients had PK parameters estimated at 6 months, 51 patients at 12 months, and 48 patients at 18 and 24 months. The data from one patient (months 12, 18, 24) were excluded after inhibitor development.
Factor IX pharmacokinetic estimates stratified by age and factor IX–antigen status3-150
| . | No. . | Incremental recovery, % . | FIX activity rise, IU/dL . | Half-life, h . |
|---|---|---|---|---|
| Age, y | ||||
| < 15 | 19 | 29.8 ± 9.9 (15.3-50.4) | 0.66 ± 0.22 (0.34-1.12) | 20.2 ± 4.0 (14.2-28.2) |
| 15-40 | 28 | 34.7 ± 9.2 (18.0-57.6) | 0.77 ± 0.20 (0.4-1.28) | 19.4 ± 5.5 (12.4-36.4) |
| > 40 | 9 | 37.7 ± 12.3 (23.3-62.2) | 0.84 ± 0.27 (0.52-1.38) | 17.2 ± 4.8 (11.1-25.0) |
| Factor IX antigen | ||||
| Positive | 22 | 36.9 ± 11.0 (17.1-62.2) | 0.82 ± 0.25 (0.38-1.38) | 18.7 ± 6.2 (11.1-36.4) |
| Negative | 33 | 31.0 ± 8.9 (15.3-50.4) | 0.69 ± 0.20 (0.34-1.12) | 19.8 ± 4.1 (12.7-28.2) |
| . | No. . | Incremental recovery, % . | FIX activity rise, IU/dL . | Half-life, h . |
|---|---|---|---|---|
| Age, y | ||||
| < 15 | 19 | 29.8 ± 9.9 (15.3-50.4) | 0.66 ± 0.22 (0.34-1.12) | 20.2 ± 4.0 (14.2-28.2) |
| 15-40 | 28 | 34.7 ± 9.2 (18.0-57.6) | 0.77 ± 0.20 (0.4-1.28) | 19.4 ± 5.5 (12.4-36.4) |
| > 40 | 9 | 37.7 ± 12.3 (23.3-62.2) | 0.84 ± 0.27 (0.52-1.38) | 17.2 ± 4.8 (11.1-25.0) |
| Factor IX antigen | ||||
| Positive | 22 | 36.9 ± 11.0 (17.1-62.2) | 0.82 ± 0.25 (0.38-1.38) | 18.7 ± 6.2 (11.1-36.4) |
| Negative | 33 | 31.0 ± 8.9 (15.3-50.4) | 0.69 ± 0.20 (0.34-1.12) | 19.8 ± 4.1 (12.7-28.2) |
Values represent the mean ± SD; range is shown in parentheses.
Assay interference precluded assessment of factor IX–antigen status in one patient.
Treatment for hemorrhage on demand
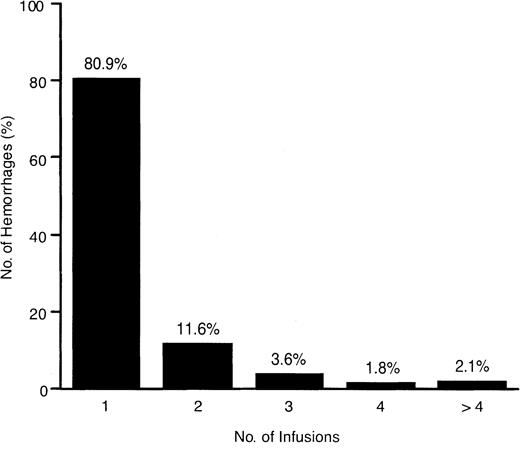
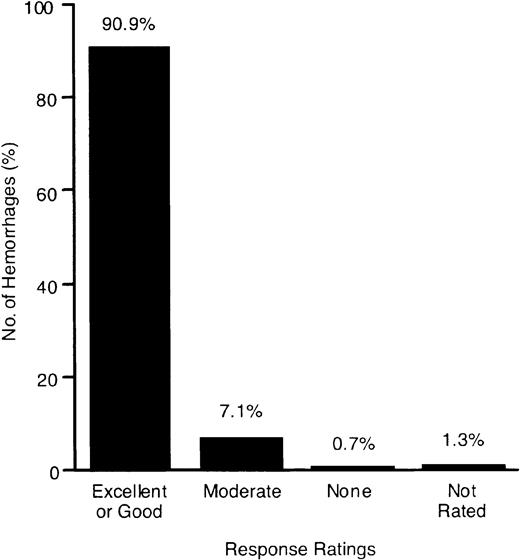
All but one PTP who was being treated with a prophylaxis regimen received rFIX for treatment of hemorrhage. A total of 2758 infusions were administered for on-demand treatment of 1796 hemorrhages (59% hemarthroses, 25% soft tissue/muscle, 16% multiple-site or other bleeding episodes). Most bleeding episodes (80.9%) were controlled with one infusion (Figure 2). The rFIX dosing used for treatment of these bleeding episodes is described in the legend for Figure 2. The majority of hemorrhages (90.9%) had an “excellent” or “good” response to rFIX treatment (Figure3). Despite a rating of “moderate” or “no response” following the initial infusion of 7.8% of hemorrhages, 47% and 15% of these bleeds still resolved without further therapy, respectively, and rFIX was effective in all bleeds.
Number of infusions required for resolution of 1796 hemorrhages.
A total of 2641 infusions were administered for on-demand treatment in 55 patients. The total number of infusions used to treat the site of any bleeding episode ranged from 1 to 123. The data exclude those from one patient after evidence of factor IX antibody was detected at month 9. Of the 2641 total infusions administered, 4% (101 of 2641) were at a dose of less than or equal to 20 IU/kg, 25% (663 of 2641) ranged from above 20 to 30 IU/kg, 18% (470 of 2641) ranged from above 30 to 40 IU/kg, 20% (537 of 2641) ranged from above 40 to 50 IU/kg, 15% (405 of 2641) ranged from above 50 to 60 IU/kg, and only 17% (461 of 2641) were used at doses above 60 IU/kg; the dose was not reported for 4 infusions. Thirty-three of the 56 PTPs used average doses for bleeding episodes and prophylaxis that were less than or equal to 50 IU/kg, whereas 23 used average doses of above 50 IU/kg. Twelve of these 23 patients used average doses between 50 and 60 IU/kg, 8 patients used average doses between 60 and 70 IU/kg, and 3 patients used higher average doses. The patient who used the far outlying average dose of 100.2 IU/kg is the patient who showed the lowest recovery in the PTP population at baseline (0.34 IU/dL per IU/kg rFIX given). The other 2 outlier patients used average doses between 70 to 80 IU/kg. One patient used self-determined doses (outside physician guidelines) and engaged in strenuous activity despite very severe hemophilic arthropathy. The other is the single PTP who developed a transient, low-titer inhibitor.
Number of infusions required for resolution of 1796 hemorrhages.
A total of 2641 infusions were administered for on-demand treatment in 55 patients. The total number of infusions used to treat the site of any bleeding episode ranged from 1 to 123. The data exclude those from one patient after evidence of factor IX antibody was detected at month 9. Of the 2641 total infusions administered, 4% (101 of 2641) were at a dose of less than or equal to 20 IU/kg, 25% (663 of 2641) ranged from above 20 to 30 IU/kg, 18% (470 of 2641) ranged from above 30 to 40 IU/kg, 20% (537 of 2641) ranged from above 40 to 50 IU/kg, 15% (405 of 2641) ranged from above 50 to 60 IU/kg, and only 17% (461 of 2641) were used at doses above 60 IU/kg; the dose was not reported for 4 infusions. Thirty-three of the 56 PTPs used average doses for bleeding episodes and prophylaxis that were less than or equal to 50 IU/kg, whereas 23 used average doses of above 50 IU/kg. Twelve of these 23 patients used average doses between 50 and 60 IU/kg, 8 patients used average doses between 60 and 70 IU/kg, and 3 patients used higher average doses. The patient who used the far outlying average dose of 100.2 IU/kg is the patient who showed the lowest recovery in the PTP population at baseline (0.34 IU/dL per IU/kg rFIX given). The other 2 outlier patients used average doses between 70 to 80 IU/kg. One patient used self-determined doses (outside physician guidelines) and engaged in strenuous activity despite very severe hemophilic arthropathy. The other is the single PTP who developed a transient, low-titer inhibitor.
Response ratings of rFIX used for treatment of 1796 hemorrhages.
For hemorrhages that required more than one treatment, only the initial response rating 24 hours following the first rFIX infusion was used in the response rating analysis. The data exclude those from one patient after evidence of factor IX antibody was detected at month 9.
Response ratings of rFIX used for treatment of 1796 hemorrhages.
For hemorrhages that required more than one treatment, only the initial response rating 24 hours following the first rFIX infusion was used in the response rating analysis. The data exclude those from one patient after evidence of factor IX antibody was detected at month 9.
Routine secondary prophylaxis for prevention of hemorrhage
In addition to those individuals who used rFIX for intermittent bleeding prophylaxis (eg, in advance of occasional physical activities that were anticipated to result in bleeding, such as strenuous exercise), 19 PTPs used rFIX for regular secondary prophylaxis regimens (average dose 40.3 IU/kg; range, 13-78 IU/kg) 2 to 3 times per week. At times, intervals between doses exceeded 72 hours. Three of the 19 PTPs did not experience breakthrough hemorrhages. The other 16 subjects had a total of 203 hemorrhages; 64 related to injury and 139 were spontaneous. Eighty-five (61%) of the 139 spontaneous hemorrhages occurred more than 72 hours after rFIX infusion, whereas only 27 (19%) of spontaneous hemorrhages were true breakthrough hemorrhages, occurring within 48 hours of rFIX infusion. This low frequency of breakthrough bleeding occurred in only 7 patients (37%) who used prophylaxis regimens. Overall, 93% of prophylaxis regimen responses were rated as “excellent” or “effective,” typical for effective secondary prophylaxis with pdFIX.
Surgical prophylaxis
Twenty-seven surgical procedures were performed in 20 PTPs, including 11 dental, 9 orthopedic (2 ankle arthrodeses, 3 total knee arthroplasties, 2 arthroscopic synovectomies, 1 hip prosthesis, 1 Achilles tendon lengthening), 2 ingrown toenail removals, 1 vasectomy, 1 lymph node biopsy, 2 cyst excisions, and 1 birthmark removal. Of all infusions administered for surgical procedures, 98% provided “excellent” or “good” hemostasis. Estimated blood loss was comparable to individuals without a coagulation disorder. One dental extraction was complicated by dislodged fibrin glue, but bleeding was controlled with rFIX.
Dosing adjustment of rFIX
Analysis of dosing patterns was evaluable for over 3 months in 54 of the 56 PTPs. Thirty-one subjects (57%) increased their dose and 23 (43%) had no change. Of the 31 PTPs who increased their dose, 11 changed because of a low recovery at baseline assessment, 15 changed because of suboptimal clinical response and low recovery, and 5 had breakthrough bleeding episodes during prophylactic treatment. Sixteen of the 31 PTPs (52%) who increased their dose were younger than 15 years of age, the cohort in which a lower recovery was observed (Table 3).
Viral transmission
There was no evidence of transmission of HAV, HBV, HCV, HIV-1, or HIV-2.
Inhibitors
A low-titer, transient inhibitor to rFIX was detected in one individual who had severe hemophilia B due to a T (nucleotide 97) to C point mutation in the F9 gene. This resulted in a Leu −24 to Pro missense mutation in the factor IX signal peptide sequence and no circulating factor IX antigen was found in the pretreatment blood sample. He had received in excess of 500 infusions of pdFIX before receiving rFIX, including a single subcutaneous dose of pdFIX (30 IU/kg) at 10 injection sites approximately 2 months before study entry. Factor IX antibodies were detected on routine enzyme-linked immunosorbent assay (ELISA) samples at month 9 after 39 rFIX exposure days, and by BIA at month 12 after 63 rFIX exposure days. PK assessment demonstrated a shortened rFIX half-life (19.1 hours at baseline to 3.6 hours), although rFIX recovery was unchanged. He continued to treat hemorrhages using rFIX every 6 to 8 hours rather than every 12 to 24 hours. By month 19, the inhibitor had disappeared by BIA and rFIX half-life had increased to 26 hours.
Thrombogenicity
There was no evidence that rFIX increased thrombin generation. One individual experienced the acute onset of a segmental renal infarction 12 days following a single dose of rFIX (3000 IU). He had received only 2 rFIX infusions in the preceding month and continued to receive rFIX for 2 days following this event. Thrombophilia investigations were negative and his symptoms resolved after 6 days. The investigator concluded that the relationship to rFIX was “unknown, but highly unlikely.” The patient continued to use rFIX, and there were no other thrombotic events in this or any other patient during the study period.
Adverse events
No serious adverse events were related to rFIX. Nine subjects experienced 55 treatment-related adverse events, including 4 subjects who showed some sign or symptom of minor allergic reaction to rFIX, although ELISA tests for antifactor IX IgE were negative. In 3 of these 4 participants, symptoms occurred once or twice and did not recur despite continued treatment with rFIX. There were no other clinically significant adverse experiences. The remaining adverse experiences were similar to those reported with pdFIX.1 3
Discussion
An important and unexpected finding of this study was that despite a mean circulating half-life similar to that of pdFIX,21the mean incremental recovery was typically 30% lower. Although minor differences in the posttranslational modification of rFIX, such as decreased complexity of N-linked glycans, decreased sulfation of Tyr155, and absent phosphorylation of Ser158 have no influence on the specific coagulant activity of rFIX,22,23 it has been speculated that these differences contribute to the observed decreased recovery compared to pdFIX.24
Studies with pdFIX have shown that subjects 15 years of age and younger have a significantly lower recovery than those who are older.25 In our study, the average incremental recovery was also lower in younger participants (< 15 years of age), although this did not reach statistical significance. Therefore, all individuals, and particularly young children, should have their recoveries evaluated to ensure proper dosing. Because the difference in recovery may lead to higher rFIX doses, when combined with the higher price of rFIX, the overall cost of hemophilia B treatment may be increased compared to the cost of treatment with pdFIX.
These results demonstrate that rFIX is safe and clinically effective in the treatment and prevention of bleeding in individuals with hemophilia B. All bleeding episodes were controlled exclusively with rFIX using standard dosing intervals, with the majority of hemorrhages responding to one rFIX infusion. Although this was not a comparative study, hemostatic efficacy of rFIX was rated against participants' previous experience with pdFIX in “on-demand” and “secondary prophylaxis” regimens, and was reported as “excellent” or “good” in 90.9% to 98% of bleeding episodes, consistent with those reported in other studies that evaluated pdFIX in individuals with hemophilia B.25-30 In addition, an analogous study testing rFVIII in PTPs with hemophilia A reported hemostatic efficacy as “excellent” or “good” in 91.2% of bleeding episodes,31 similar to our results with rFIX in PTPs with hemophilia B.
A crucial element of this study was the evaluation for potential neoantigenicity and inhibitor formation following exposure to rFIX in PTPs. There was no evidence that rFIX increased the risk of inhibitor development. Only 1 of the 56 participants showed evidence of a low-titer, transient factor IX inhibitor, which is consistent with the estimated prevalence of factor IX inhibitors (1.5%-2%) in hemophilia B.32,33 The majority of patients who develop inhibitors have gross deletions or certain nonsense mutations in the F9gene, whereas complete F9 gene deletions also confer the risk of anaphylaxis.34-37 These observations support the hypothesis that a lack of circulating factor IX protein predisposes to an immune response following factor IX replacement therapy. Although missense mutations are less commonly associated with such immune responses, this patient had a signal peptide missense mutation that prevented secretion of his factor IX protein, resulting in his factor IX antigen-negative phenotype and increased risk of inhibitor development. Alternatively, we can speculate that his prior subcutaneous administration of pdFIX contributed to the transient immune response to rFIX. PTPs with a history of inhibitor or anaphylaxis to pdFIX were excluded from this study, selecting a study population at low risk for developing immune reactions to rFIX. Because it is important to understand the true risk of immune responses to rFIX, including the development of inhibitors and minor allergic reactions, additional studies with rFIX in previously untreated hemophilia B patients are currently in progress.
As anticipated, there was no evidence of viral transmission of HIV, HAV, HBV, or HCV resulting from rFIX treatment. Continued concerns about human blood-borne pathogens, the viral safety of rFIX, and the demonstrated clinical efficacy and tolerability of rFIX reported herein lead us to conclude that rFIX offers an advantage to persons who depend on the availability of human blood for survival. Multiple steps are in place throughout the manufacturing process to ensure that rFIX is free of viral contamination. Moreover, both the production17and formulation18 of rFIX are done in the absence of animal or human plasma–derived products, thus eliminating possible contamination with viruses, vCJD, or unknown pathogens.
Recent evidence in a large animal model of vCJD demonstrates that this disease can be transmitted by transfusion with whole blood obtained during the symptom-free phase of experimental bovine spongiform encephalopathy infection.38 Furthermore, it was reported that several blood coagulation proteins, including purified factor IX, bind to PrP27-30, the protease-resistant core of the vCJD-associated prion protein (PrPSc).39 Together, these data suggest that blood may be a carrier of prions. Although it is important to emphasize that no iatrogenic cases of vCJD or other viruses have been confirmed in recipients of pdFIX, there are justified concerns about the safety of large-pool blood protein concentrates for patients such as those with hemophilia for whom continuous usage results in exposure to millions of blood donors during one's life. Given these concerns, rFIX provides a new option for the safe and effective management of patients with hemophilia B.
We are indebted to the hemophilia B study patients and their families for their participation in this study; Missy Magill, biostatistician, and other members of the basic science and clinical research teams of Genetics Institute in Andover and Cambridge, MA; the physician and nursing staffs of the numerous clinical research centers at many of the participating institutions; Dr James Meade, UNC Coagulation Laboratory, University of North Carolina, Chapel Hill, for analysis of factor IX activity and inhibitor data; Dr Steve S. Sommer, City of Hope National Medical Center, Duarte, CA, for factor IX genotype analyses; and Dr Kenneth A. Bauer, Beth Israel Deaconess Medical Center, Boston, MA, for independent interpretation of the thrombogenicity data. Lastly, we dedicate this work to the memory of Suzanne G. Courter of Genetics Institute who died unexpectedly during the final stages of preparing this manuscript. Her professional commitment to patients with hemophilia and her passion for excellence in clinical investigation are largely responsible for the execution and completion of this study.
The following institutions and investigators (alphabetical order) participated in this study as members of the Recombinant Factor IX Study Group: V. S. Blanchette (The Hospital for Sick Children, Toronto, ON, Canada); H.-H. Brackmann (Institute für Experimentelle Haematologie und Transfusionmedizin der Universität, Bonn, Germany); B. Ewenstein (Brigham and Women's Hospital, Boston, MA); P. Giangrande (Oxford Haemophilia Centre, Oxford, United Kingdom.); M. A. Heisel (Children's Health Care of Minnesota, Minneapolis, MN); W. K. Hoots (University of Texas-Houston Medical School, Houston, TX); C. M. Kessler (Georgetown University, Washington, DC); Y. Laurian (Centre de Traitement de l'Hemophilie Centre Hospitalier Universitaire Bicetre, Paris, France); J. Lusher (Children's Hospital of Michigan, Detroit, MI); C. Negrier (Hôpital Edouard Herriot Centre Regional de l'Hemophilie, Lyon, France); C. Lee and K. J. Pasi (Royal Free Hospital, London, United Kingdom); C. Philipp (Robert Wood Johnson Medical School, New Brunswick, NJ); M.-C. Poon (University of Calgary Foothills Hospital, Calgary, AB, Canada); M. V. Ragni (Hemophilia Center of Western Pennsylvania, Pittsburgh, PA); G. E. Rivard (Hôpital Ste-Justine, Montreal, QC, Canada); D. A. Roth and C. G. Rosenfield (New England Medical Center and the Floating Hospital for Children, Boston, MA); A. Shapiro (Indiana Hemophilia and Thrombosis Center, Indianapolis, IN); A. R. Thompson (Puget Sound Blood Center, Seattle, WA); J. Vermylen (University Hospital Gasthuisberg Functiemetingen Bloedingsziekten, Leuven, Belgium); and G. White (University of North Carolina, Chapel Hill, NC).
Supported by the Genetics Institute, Cambridge, MA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David A. Roth, Beth Israel Deaconess Medical Center, Center for Hemostasis and Thrombosis Research, RE-302, 41 Ave Louis Pasteur, Boston, MA 02115; e-mail: droth@caregroup.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal