Acute myeloid leukemia and adult-onset acute lymphocytic leukemia are aggressive diseases with generally poor prognoses. The primary cause of treatment failures in patients with these diseases is the emergence of multidrug resistance. Common mechanisms of resistant disease are defects in apoptotic pathways. Apoptosis is a process by which cells undergo organized self-destruction. Apoptosis is ultimately executed by caspases, a family of cysteine proteases that cleave critical intracellular proteins and execute the apoptotic program. At least 2 major pathways for caspase activation have been identified: (1) the receptor-mediated pathway which involves members of the tumor necrosis factor (TNF) family of death receptors and (2) the mitochondrial-mediated pathway involving cytochrome c–release from the mitochondria. Defects in the receptor- or mitochondrial-mediated pathway may confer prognostic information for adult patients with acute leukemia, suggesting ways that such information can be used in risk stratification. In addition, emerging knowledge about apoptosis mechanisms has laid the foundation for the eventual development of new therapies capable of overcoming blocks in apoptosis. Here we review some of the recent advances in translating information about apoptosis mechanisms into promising new strategies for improved treatment of adult leukemia.
Acute leukemia is a neoplastic disease characterized by a rapid accumulation of primitive hematopoetic cells. Acute leukemia is subclassified as myeloid or lymphoid depending on the origin of the malignant cell. Acute lymphocytic leukemia (ALL) is predominantly a disease of childhood with 75% of all cases occurring in patients younger than 15 years of age.1 In contrast, acute myeloid leukemia (AML) is more common in adults with an incidence that increases with age.2 Both AML and adult ALL are aggressive diseases with generally poor prognosis. For example, patients with AML who are younger than 55 years of age and who have no adverse prognostic features have a complete remission rate of 70% to 85% with standard induction chemotherapy, but their long-term survival is only approximately 50%. The outcome in elderly patients is worse. Patients older than 65 years of age with AML treated with standard chemotherapy have a median survival of 6 months and only 7% remain in remission at 3 years.3
The primary cause of treatment failures in adult patients with leukemia is the emergence of resistant disease. Multiple mechanisms may account for why leukemic cells become resistant to chemotherapy (Figure1). One common cause includes defects in the cell's inherent programmed cell death (apoptosis) pathways. These defects may impair the ability to achieve remission and cure with chemotherapy. In an attempt to overcome drug resistance and improve clinical outcomes, attention is turning to developing therapeutic agents that overcome defects in the apoptosis pathways. Although mostly in preclinical stages of development, these efforts offer a glimpse into the future armamentarium of agents that may eventually be available for treating leukemia.
In this review, we focus on translational approaches to apoptosis research in adult acute leukemia. More basic details of the apoptosis pathway have been reviewed elsewhere4 with some articles concentrating on individual pathway components such as the mitochondria,5,6 the TNF superfamily of receptors,7-9 Bcl-2,10,11 and caspases.12 13 Here, we will discuss the apoptosis pathways concentrating on members with important prognostic or therapeutic potential. We will then illustrate how defects in apoptosis confer prognostic information in patients with acute leukemia. By identifying patients at highest risk of resistant disease or relapse, one may be able to offer them more aggressive or alternate therapy at disease onset. Finally, we will discuss small molecules that overcome blocks in apoptosis and that will likely have a major impact in the treatment of acute leukemia.
Apoptotic pathways
Apoptosis is a process by which cells undergo organized self-destruction without eliciting an inflammatory response. Morphologically, cells undergoing apoptosis display membrane blebbing and nuclear and cytoplasmic condensation. Molecularly, classical apoptosis is caused by the activation of caspases. Caspases are a family of intracellular cysteine proteases that lie in a latent (zymogen) state in cells but become activated in response to a wide variety of cell death stimuli.
Growing evidence indicates that cell death with apoptotic features can occur without caspase activation14; the mechanisms of caspase-independent cell death still require clarification. Here, we will limit our discussion to caspase-mediated cell death, as understanding these pathways has lead to the development of agents that enhance apoptosis and may be of therapeutic value.
Caspases are organized in a cascade with upstream (initiator) caspases responsible for activating the downstream (effector) caspases. Upstream caspases contain long prodomains that interact with their specific activators. The downstream caspases contain smaller prodomains and are activated by proteolytic cleavage by upstream caspases. Once activated, the downstream caspases cleave specific protein substrates, leading to the execution of apoptosis.
At present, at least 2 major pathways of caspase activation have been revealed: (1) the receptor-mediated apoptosis pathway where the TNF family of death receptors activate upstream caspase-812,13,15 and (2) the mitochondrial-mediated apoptosis pathway where cytochrome c is released from the mitochondria and activates upstream caspase-916-19 (Figure2). Both pathways culminate in the activation of a major downstream effector caspase—caspase-3.20,21 A third minor pathway of caspase activation involving granzyme B has also been revealed. Granzyme B is a serine protease synthesized in cytotoxic T lymphocytes. Via the pore-forming protein, perforin, cytotoxic T lymphocytes inject granzyme B into target cells. Granzyme B directly cleaves and activates several caspases including procaspase-3.22-24 As such, it bypasses both the mitochondrial-mediated and receptor-mediated pathways of caspase activation. Although over 14 caspases have been identified, we will restrict our comments to caspases 3, 8, and 9 as examples of downstream effector, receptor-mediated pathway initiator and mitochondrial-mediated pathway initiator caspases, respectively.
Pathways of apoptosis.
Two major pathways of apoptosis have been discerned—the receptor- and mitochondrial-mediated pathways. Both pathways culminate in the activation of caspase-3, a major downstream effector caspase. A third minor pathway has also been identified, whereby Granzyme B directly activates caspase-3.
Pathways of apoptosis.
Two major pathways of apoptosis have been discerned—the receptor- and mitochondrial-mediated pathways. Both pathways culminate in the activation of caspase-3, a major downstream effector caspase. A third minor pathway has also been identified, whereby Granzyme B directly activates caspase-3.
Receptor-mediated pathway
The receptor-mediated pathway of apoptosis begins with the TNF family of cytokine receptors that includes Fas (CD95), DR4 (Trail-R1) and TNFRI (CD120a), and TNFRII (Figure3). These receptors differ in their ligand specificity, activating binding partners and downstream effectors. Upon ligand binding, activated TNFRI and Fas receptors recruit and bind the death effector protein Fadd/Mort-1.25,26 Bound Fadd/Mort-1 recruits procaspase-8.26 Procaspase-8 is converted to its active form and is released back into the cytosol, where it cleaves and activates the downstream effector caspase-3.20 21
Activation of apoptosis through the TNF family of death receptors.
Activated Fas and TNFR-1 launch the receptor-mediated pathway of apoptosis by recruiting the adapter protein Fadd/Mort-1. In turn, caspase-8 is activated, which cleaves procaspase-3. These receptors also induce apoptosis through an alternate pathway. Through adapter proteins TRADD and TRAF, a phosphorylation cascade is initiated, leading to the activation of JNK. Activated JNK phosphorylates substrates such as p53 and c-Jun, which leads to apoptosis through multiple mechanisms including regulating the Bcl-2 family of proteins.
Activation of apoptosis through the TNF family of death receptors.
Activated Fas and TNFR-1 launch the receptor-mediated pathway of apoptosis by recruiting the adapter protein Fadd/Mort-1. In turn, caspase-8 is activated, which cleaves procaspase-3. These receptors also induce apoptosis through an alternate pathway. Through adapter proteins TRADD and TRAF, a phosphorylation cascade is initiated, leading to the activation of JNK. Activated JNK phosphorylates substrates such as p53 and c-Jun, which leads to apoptosis through multiple mechanisms including regulating the Bcl-2 family of proteins.
This receptor family also induces apoptosis through an alternative signal transduction pathway. According to one proposed model, Fas receptors bind the adapter proteins that in turn activate the MAP3 kinase, ASK1. When activated, ASK1 launches a phosphorylation cascade that culminates in the activation of c-Jun N terminal kinase (JNK).25 Activated JNK phosphorylates substrates such as c-Jun and p53 and induces apoptosis through multiple mechanisms, including modifying and transcriptionally regulating proteins in the Bcl-2 family.27-29 Therefore, activation of Fas can induce apoptosis through multiple pathways, although some of the mechanisms still require clarification.
The TNFRI and TNFRII receptors also activate the MAPK/JNK pathway by binding the adapter proteins TRADD and TRAF that activate ASK-1.30,31 Interestingly, activation of TNFRI can also inhibit apoptosis. Activated TNFRI recruits adapter proteins such as TRADD and TRAF. These adapter proteins launch a phosphorylation cascade that frees NFκB from its inhibitor IκB. Once free, NFκB migrates to the nucleus where it mediates the transcription of survival genes31,32 including inhibitors of apoptosis protiens (IAPs).33 34
Recently, the adapter protein Bcl-10 has been identified. This molecule activates the receptor-mediated apoptosis pathway and the NFκB survival pathway. A translocation of Bcl-10, t(1;14), is found in a subset of MALT lymphomas. This translocation leads to truncation of Bcl-10 that deletes the proapoptotic region of the molecule while retaining the NFκB activating domain.35 The role of Bcl-10 in acute leukemia is less clear. The truncated Bcl-10 mutant was found in 2 of 18 leukemia and lymphoma cell lines in the absence of the t(1;14) translocation. In contrast, no pathogenic mutations in the Bcl-10 gene were seen in a survey of 94 cases of AML.36
Mitochondrial-mediated pathway
The mitochondrial-mediated pathway for caspase activation is initiated by mitochondrial damage that leads to cytochrome c–release. Cytochrome c is normally sequestered between the inner and outer membranes of the mitochondria. In response to a variety of proapoptotic stimuli, cytochrome c is released into the cytosol.16Cytochrome c then binds and activates Apaf-1. Apaf-1 activates procaspase-9, which in turn cleaves procaspase-3.17-19This pathway is dependent upon activated Bak/Bax and inhibited by Bcl-2 and its antiapoptotic family members.
Triggers of mitochondrial-mediated apoptosis
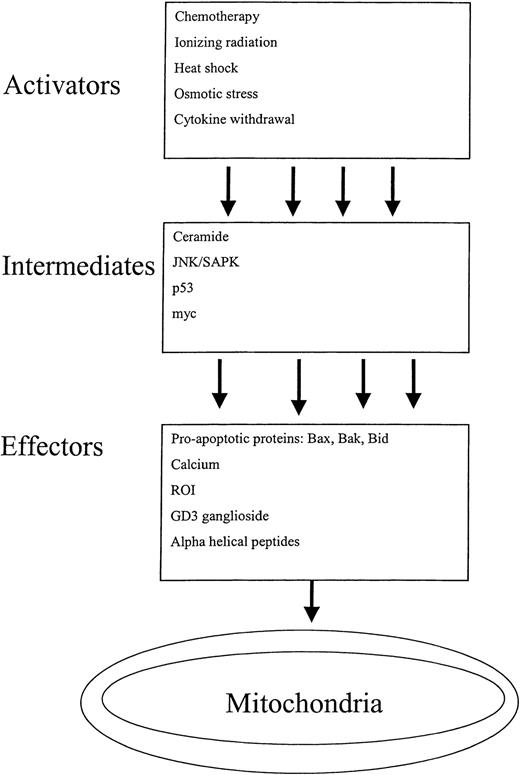
Cytochrome c–release from the mitochondria is often a starting point for discussions on caspase-mediated cell death. In reality, however, mitochondrial disruption is an end point for a multitude of diverse signals that culminate in cytochrome c–release and apoptosis. A detailed review of all agents and pathways that disrupt the mitochondria is beyond the scope of this article, but we will highlight a spectrum of triggers that activate the mitochondrial mediated pathway of apoptosis. For the purpose of this discussion, these triggers have been classified as activators, intermediates, and effectors depending on how directly they impact mitochondrial function (Figure 4).
Activators of mitochondrial-mediated apoptosis.
Many diverse triggers initiate the mitochondrial-mediated pathway of apoptosis. These triggers have been classified as activators, intermediates, and effectors, depending on how directly they impact mitochondrial function. The pathways are complex, as a single activator can unleash multiple intermediates that in turn disrupt the mitochondria though multiple effectors.
Activators of mitochondrial-mediated apoptosis.
Many diverse triggers initiate the mitochondrial-mediated pathway of apoptosis. These triggers have been classified as activators, intermediates, and effectors, depending on how directly they impact mitochondrial function. The pathways are complex, as a single activator can unleash multiple intermediates that in turn disrupt the mitochondria though multiple effectors.
Activators.
Activators refer to exogenous cellular stresses such as ionizing radiation, chemotherapy, heat shock, and cytokine withdrawal, that ultimately lead to mitochondrial disruption.5,37-39 The pathways that connect the activators to the mitochondria are complex and often poorly understood. The complexity occurs because a given activator, such as chemotherapy, can up-regulate multiple intermediates that impact the mitochondria through multiple effectors. Some of the pathways have been discerned, however. Daunorubicin can induce apoptosis by activating caspase-840 and by increasing ceramide production.41 Ara-C acts on the mitochondria-mediated pathway of caspase activation as evidenced by its ability to induce cytochrome c–release and caspase-9 activation.42 Ultraviolet (UV) radiation induces mitochondrial-mediated apoptosis through the JNK signaling pathway. Knocking out the JNK1 and JNK2 isoforms in murine fibroblasts renders these cells resistant to UV radiation–induced cytochrome c–release and apoptosis.
Intermediates.
Cell signaling pathways and oncogenes can trigger apoptosis through the disruption of the mitochondria. Here, too, the pathways connecting the intermediate triggers with the mitochondria are complex because a given trigger can disrupt the mitochondria by up-regulating multiple effectors.
The JNK cell signaling pathway is activated in response to a variety of insults, including radiation, heat shock, osmotic stress, and cytokine withdrawal.28 The JNK signaling pathway induces apoptosis in part by phosphorylating and stabilizing p53.27 Once activated, JNK also translocates to the mitochondria and phosphorylates Bcl-xL. This phosphorylation seems to inactivate Bcl-xL and potentiate apoptosis.29 Finally, JNK may mediate cytochrome c–release from the mitochondria by up-regulating the proapoptotic protein and mitochondrial-disrupter Bid.43
Ceramide is a component of the lipid signaling pathway that is synthesized de novo or through the catabolism of sphingomyelin in response to multiple cell stresses, including ionizing radiation, chemotherapeutic agents, and cytokines.44 Through conversion to GD3 ganglioside in the golgi apparatus, ceramide disrupts the mitochondrial membrane.45 In addition, ceramide induces apoptosis by activating the JNK pathway.46
In response to stimuli such as chemotherapy and radiation, p53 activates the mitochondrial-mediated pathway of apoptosis.47,48 p53 induces mitochondrial-mediated apoptosis through multiple mechanisms. It induces the transcription of the proapoptotic protein Bax.49 In addition, p53 enhances reactive oxygen species (ROS) production by transcriptionally activating genes such as PIG3.50 p53 may also disrupt the mitochondria independent of its function as a transcription factor. Studies indicate that within one hour of cell damage, p53 translocates to the mitochondria and triggers apoptosis through a mechanism that remains ill-defined, but is independent of the nucleus.51
The c-myc oncoprotein has diverse biologic properties including the induction of both proliferation and apoptosis.52 Myc deregulation can potentiate apoptosis by p53-dependent and independent mechanisms. Myc indirectly activates p53 by increasing expression of ARF.53 ARF activates p53 by biding and sequestering Mdm2, a protein that normally inhibits p53.54,55 Recently, a role for c-myc in potentiating the activation of Bax independent of an effect on Bax's translocation from the cytosol to the mitochondria has been identified.56
Rb, like p53, is a negative regulator of cell growth. Unlike p53, however, Rb overexpression inhibits apoptosis and can specifically block p53-mediated apoptosis.57
Effectors.
Direct effectors of mitochondrial disruption include both protein and nonprotein agents. Proapoptotic protein effectors of mitochondrial-mediated apoptosis include Bax, Bak, and Bid. Their effects on the mitochondria are discussed in greater detail below.
Nonprotein effectors of mitochondrial dysfunction include calcium, ROS, GD3 ganglioside, and certain chemotherapeutic agents. High levels of intracellular calcium directly damage the mitochondria.45 Mitochondrial damage is also induced by increases in ROS. Blocks in the mitochondria's electron transport chain lead to increased ROS production.58 Increased ROS damages the mitochondrial membranes and induces cytochrome c–release.52 Certain chemotherapeutic agents act directly on the mitochondria. For example, arsenic induces loss of the mitochondrial membrane potential (ΔΨM) in intact cells and a cell-free system.49 Arsenic also has indirect effects on the mitochondria by activating JNK and p53.59 60
Cytochrome c and the mitochondria
Cytochrome c–release from the mitochondria can occur with and without loss of ΔΨM and physical mitochondrial swelling. These differences relate to the site of mitochondrial damage. A description of the mechanisms of mitochondrial damage is important to understand the action of key apoptotic regulators such as Bcl-2 and Bax. Furthermore, these mechanisms serve as the basis for developing therapeutic agents that enhance apoptosis by targeting the mitochondria.
Mitochondria contain 2 membranes—the inner and outer membranes—separated by the intermembrane space (Figure5). The inner membrane surrounds the mitochondrial matrix and is the site of electron transport and adenosine triphosphate (ATP) synthesis. Under normal conditions, respiratory chain complexes pump H+ ions across the relatively impermeable inner mitochondrial membrane. Through this process, a negative electrical gradient, ΔΨM, is established across this membrane. The ΔΨM drives FO-F1 ATP synthase to produce ATP from adenosine diphosphate (ADP) and Pi with the resultant influx of H+ into the matrix. The adenine nucleotide transporter (Ant) exchanges ATP for ADP across the inner membrane. Damage to the Ant creates a conformational change leading to the formation of a permeable pore in the inner membrane. When the inner membrane becomes permeable, ΔΨM is lost, the mitochondrial matrix swells, and cytochrome c is released.61 62
Electron transport in the mitochondria.
(A) Electron transport chain. The conversion of glucose to energy in the Krebs cycle requires the reduction of NAD+ to NADH. The reoxidization of NADH occurs through the electron transport chain in the inner mitochondrial membrane. The reaction can be summarized as NADH + H+ + 1/2O2 → NAD+ + H20. (B) ATP and H+flux across the mitochondrial membranes. The reoxidization of NADH to NAD+ in the electron transport chain produces H20 and releases energy. This energy is used to drive the production of ATP in the mitochondrial matrix. The adenine nucleotide transporter (Ant) controls the flux of ADP and ATP across the inner mitochondrial membrane. The voltage dependent anion channel (VDAC) regulates the flux of ATP across the outer mitochondrial membrane. Damage to VDAC or ANT can induce cytochrome c–release and initiate the mitochondrial-mediated pathway of apoptosis. Damage to ANT is also accompanied by physical mitochondrial swelling and loss of ΔΨM.
Electron transport in the mitochondria.
(A) Electron transport chain. The conversion of glucose to energy in the Krebs cycle requires the reduction of NAD+ to NADH. The reoxidization of NADH occurs through the electron transport chain in the inner mitochondrial membrane. The reaction can be summarized as NADH + H+ + 1/2O2 → NAD+ + H20. (B) ATP and H+flux across the mitochondrial membranes. The reoxidization of NADH to NAD+ in the electron transport chain produces H20 and releases energy. This energy is used to drive the production of ATP in the mitochondrial matrix. The adenine nucleotide transporter (Ant) controls the flux of ADP and ATP across the inner mitochondrial membrane. The voltage dependent anion channel (VDAC) regulates the flux of ATP across the outer mitochondrial membrane. Damage to VDAC or ANT can induce cytochrome c–release and initiate the mitochondrial-mediated pathway of apoptosis. Damage to ANT is also accompanied by physical mitochondrial swelling and loss of ΔΨM.
The outer mitochondrial membrane contains the voltage-dependent anion channel (VDAC). The VDAC is involved in mitochondrial respiration and controls ATP efflux from the mitochondrial outer membrane.63,64 Disruptions of VDAC lead to increased permeability of the outer membrane and cytochrome c–release. In this scenario, the inner membrane remains intact, so there is no loss of ΔΨM or mitochondrial swelling.65Likewise, pore-forming proapoptotic proteins such as Bax, Bak, or activated Bid form channels in the outer membrane independent of VDAC that increase the permeabilization of the outer membrane and induce cytochrome c–release without damaging the inner membrane.6,66 67
BCL-2 family proteins
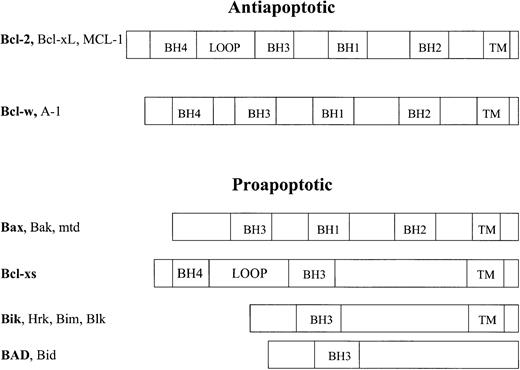
The mitochondrial-mediated pathway of apoptosis is regulated by the Bcl-2 family of antiapoptotic and proapoptotic proteins (Figure6). Bcl-2 family proteins contain shared Bcl-2 homology (BH) domains BH1, BH2, BH3, and BH4. Currently, therapeutic agents that either block or mimic these domains are being developed. The BH1 and BH2 domains are present in all of the survival factors as well as the death agonists Bax, Bak, and Mtd. These domains are likely involved in channel formation as their 3-dimensional structure resembles the pore-forming region of the diphtheria toxin.68-70 The difference in the charge and amino acid sequences between the BH1 and BH2 domains of the proapoptotic and antiapoptotic proteins may lead to different pore properties and account for the differences in function between these molecules.
Family of Bcl-2 proteins.
The proteins in the Bcl-2 family of proteins are grouped based on their proapoptotic or antiapoptotic functions and their common Bcl-2 homology (BH) domains. Some members also share common transmembrane (TM) and loop domains.
Family of Bcl-2 proteins.
The proteins in the Bcl-2 family of proteins are grouped based on their proapoptotic or antiapoptotic functions and their common Bcl-2 homology (BH) domains. Some members also share common transmembrane (TM) and loop domains.
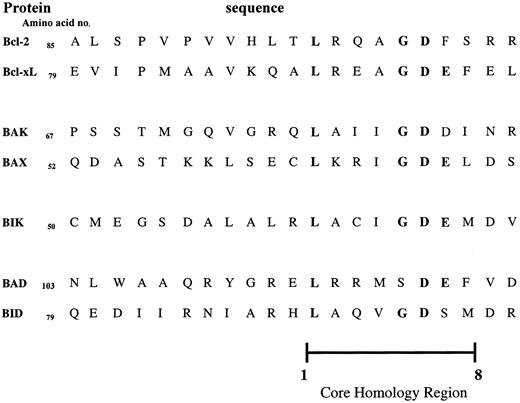
The BH3 domain is a death-promoting region common to all members of the Bcl-2 family (Figure 7). The BH3 domain contains a core sequence of 8 amino acids of which the leucine at position 1 and the aspartic acid at position 6 are critical for heterodimerization. Mutation of these residues prevents heterodimerization and abrogates the toxicity of the death agonists.71-73 In the antiapoptotic members, the BH3, BH2, and BH1 domains form a pocket. This pocket serves 2 purposes. First, it buries the BH3 domain so it cannot exert its death-promoting activity. Second, the pocket is the docking site for the BH3 domains of the death agonists.68,70 Heterodimerization with the death agonists inhibits the function of the survival factors, as discussed below.74
The BH3 domains of the Bcl-2 family of proteins.
The BH3 death domain is common to all members of the Bcl-2 family. This domain contains a core sequence of 8 amino acids (core homology region). Within this region, amino acids 1 and 6 are critical for heterodimerization between family members.
The BH3 domains of the Bcl-2 family of proteins.
The BH3 death domain is common to all members of the Bcl-2 family. This domain contains a core sequence of 8 amino acids (core homology region). Within this region, amino acids 1 and 6 are critical for heterodimerization between family members.
The BH4 domain is found primarily in the prosurvival factors and appears to be involved in protein-protein interactions with regulatory proteins outside the Bcl-2 family. This domain is involved in controlling progression of the cell cycle. It also appears to stabilize the VDAC in the outer mitochondrial membrane.75
A regulatory loop domain between the BH3 and BH4 domains is found in the antiapoptotic proteins Bcl-2, Bcl-xL, and Mcl-1. Interestingly, cleavage of Bcl-2 at the loop domain by caspase-3 releases the BH4 domain and turns Bcl-2 into a potent proapoptotic protein.76 Perhaps by cleaving the loop domain, the BH1, BH2, BH3 pocket is disrupted, leading to exposure of the death-promoting BH3 domain. The importance of this cleaved isoform in regulating the apoptosis pathways is unknown.
Bax.
The Bax family of death agonists act at the mitochondria to induce apoptosis. Three mechanisms have been identified by which Bax induces apoptosis. First, at the outer mitochondrial membrane, Bax accelerates the opening of VDAC with subsequent release of cytochrome c.65 Second, at the inner mitochondrial membrane, Bax interacts with Ant and enhances the permeability of the inner mitochondrial membrane.73 Finally, when expressed at high levels, Bax is capable of forming pores by direct oligomerization mechanisms that disrupt the permeability of the mitochondrial membranes and induce cytochrome c–release.66,67 77
Bcl-2.
The Bcl-2 class of antiapoptotic proteins are important inhibitors of the mitochondrial-mediated pathway of apoptosis and are common targets of novel therapeutic agents. These proteins inhibit apoptosis by interacting with Bax/Bak, and the mitochondria. Bcl-2 can inhibit the action of Bax/Bak by forming inactivating heterodimers.74Early reports suggested that Bcl-2 functioned as an antioxidant by preventing ROS generation.78,79 In retrospect, this function likely relates to the role of Bcl-2 at the mitochondria. Bcl-2 binds VDAC and stabilizes it, thereby preventing permeabilization of the outer mitochondrial membrane.80 These antiapoptotic molecules also homodimerize and form small ion channels in synthetic lipid vesicles.81 These channels may also exist in the mitochondrial membrane where they stabilize the membrane and prevent loss of ΔΨM cytochrome c–release. In addition to its role as an antiapoptotic protein, Bcl-2 has a separate function in controlling the cell cycle. Bcl-2 retards cells at G0, preventing them from entering into the cell cycle,82 and delays the transition from the M to G1 phase.83 The BH4 domain of the protein is responsible for part of this property, but the mechanism is poorly understood.83 84
Bcl-2 also functions at the endoplasmic reticulum (ER), where it may have distinct actions in preventing apoptosis.85 For example, in a rat-fibroblast cell line expressing an activated c-myc allele, Bcl-2 targeted to the ER is as potent an inhibitor of serum-withdrawal–induced apoptosis as Bcl-2 confined to the mitochondria.86 In addition, Bcl-2 regulates calcium flux from the ER, although the effect of this function on apoptosis is unclear.87
Mcl-1, an antiapoptotic Bcl-2 homologue, is found in the early stages of differentiation of the Mcl-1 human myeloblastic leukemia cell line and normal hematopoietic precursors.88 Like Bcl-2, Mcl-1 can form heterodimers with Bax and inhibits cell death in response to chemotherapeutic agents, UV irradiation, and growth factor withdrawal.89 In a longitudinal study of patients with relapsed acute leukemia, Mcl-1 at relapse was 2-fold higher than at presentation in 53% of patients.90 The increase in Mcl-1 may partly explain the greater degree of drug resistance seen in relapsed leukemia.
BAD, a BH3-only protein.
The BH3-only class of proteins are endogenous inhibitors of Bcl-2 and Bcl-xL and are attractive prototypes for therapeutic agents. This class of compounds inhibits Bcl-2/Bcl-xL through heterodimer formation. Among the BH3-only class of proteins, BAD was the first identified and is the best characterized. BAD was first cloned from a murine cDNA library through its ability to bind Bcl-2 in a yeast-2-hybrid assay.91 BAD heterodimerizes with Bcl-xL and Bcl-2 but not the proapoptotic members Bcl-xs, Bax, or Bak.91 To date, the only known function of BAD is to bind Bcl-2 and Bcl-xL, and block the antiapoptotic action of these proteins.71,92 BAD inhibits these antiapoptotic proteins by preventing them from binding Bax/Bak.71 92 BAD may also inhibit the ability of Bcl-2 and Bcl-xL to form channels in the mitochondrial membrane, or bind and stabilize VDAC.
The BH3 domain of BAD is necessary for its ability to bind Bcl-2 and Bcl-xL and induce apoptosis. Mutations within the BH3 domain of full-length BAD inhibit the binding of BAD to Bcl-2 and Bcl-xL and block BAD-induced apoptosis in cells overexpressing Bcl-xL.72 Furthermore, peptides corresponding to the BH3 domain of BAD competitively inhibit the binding of Bcl-xL to Bax. Mutations within these peptides permit the binding of Bcl-xL to Bax.71
Cross talk between pathways
Although the mitochondrial-mediated and receptor-mediated pathways are presented as separate entities, cross talk exists between them. For example, the 2 pathways cooperate to enhance apoptosis through Bid. Bid, a member of the BH3-only family of proapoptotic proteins, is cleaved and activated by caspase-8. When cleaved, Bid interacts with ANT as well as Bax to permeabilize the mitochondrial membrane.93 In addition, cleaved Bid forms ion channels in liposomes, suggesting that it independently can disrupt the mitochondria and induce cytochrome c–release.94,95Conversely, BAR inhibits apoptosis in both pathways. BAR was identified by screening for cDNAs capable of inhibiting Bax-induced apoptosis. BAR contains a protein-interacting domain that binds and inhibits procaspase-8 and thereby blocks the receptor-mediated pathway. BAR also inhibits the mitochondrial-mediated pathway through an unknown mechanism that involves interactions with Bcl-2 and Bax. Physically, BAR bridges the 2 pathways by forming a complex with procaspase-8 and Bcl-2, although the significance of this complex is unknown.96
Regulation of apoptotic proteins through phosphorylation
The apoptosis pathways are positively and negatively regulated through signal transduction. Teleologically, this regulation allows tight control of apoptotic signals avoiding unwanted cell death or proliferation. Therapeutically, these regulatory points are valuable targets for the development of compounds that enhance apoptosis. Bcl-2 and BAD are examples of proteins that are regulated by phosphorylation and whose regulation is being exploited for drug development.
Three known sites of phosphorylation regulate Bcl-2: ser-17, ser-70, and thr-56. Taxol induces phosphorylation on multiple residues, including ser-17, and inhibits the antiapoptotic function of Bcl-2.97,98 Phosphorylation of Bcl-2 on thr-56 in the loop domain by the cell-cycle regulator CDC2 kinase is required for activation of the role of Bcl-2 as a cell-cycle inhibitor. Mutation of thr-56 abrogates the role of Bcl-2 as a cell-cycle inhibitor, but does not alter the antiapoptotic action of the protein, indicating that the function of Bcl-2 as an inhibitor of apoptosis and a cell-cycle inhibitor can be dissociated.83,84 Some aspects of Bcl-2 phosphorylation are controversial. One report indicates that phosphorylation of ser-70 by protein kinase C (PKC) activates the antiapoptotic function of Bcl-2,99 but another study reports that taxol treatment leads to phosphorylation on the same serine and inhibits its function.100
Other sites of phosphorylation also appear important for the regulation of Bcl-2 and are currently being characterized. For example, all-trans retinoic acid (ATRA) inhibits Bcl-2 by inducing phosphorylation on a serine distinct from the sites phosphorylated by PKC and the taxol-mediated pathway.101
BAD is an example of a proapoptotic protein that is regulated by phosphorylation. Phosphorylation of ser-112, ser-136, or ser-155 inhibits the function of BAD. BAD is phosphorylated on ser-136 by Akt in the Akt/PI3K pathway102,103 and on ser-112 by PKC in the Ras/Raf/MAPK pathway.104,105 Phosphorylation at these sites promotes the binding of BAD to 14-3-3 proteins.92When bound to 14-3-3 proteins, BAD is sequestered in the cytosol and unable to interact with Bcl-2 or Bcl-xL at the outer mitochondrial membrane. The phosphorylation of ser-136 or ser-112 also induces a conformational change in BAD that increases the accessibility of ser-155 within the BH3 domain to phosphorylation by protein kinase A.106,107 Phosphorylation of ser-155 directly impairs the ability of BAD to bind Bcl-xL and Bcl-2.107 108
Pathways that dephosphorylate BAD increase its activity and promote apoptosis. Calcineurin, a calcium-dependent phosphatase, dephosphorylates BAD at both S112 and S136 and promotes BAD-induced apoptosis.109 The second messenger ceramide promotes the accumulation of dephosphorylated forms of BAD by inhibiting Akt.110
Chromosomal translocations inhibit apoptosis in acute leukemia
At a basic level, chemoresistance and treatment failures in acute leukemia represent a failure of the malignant clone to undergo apoptosis in response to chemotherapeutic agents. Resistance to apoptosis in acute leukemia is often due to aberrant gene expression. Chromosomal translocations are classic examples of aberrant gene expression in acute leukemia. The molecular basis of translocations in leukemia has been reviewed elsewhere111 and detailed discussions of the effect of chromosomal translocations on apoptosis and prognosis are beyond the scope of this article. Here, we highlight specific examples of how translocations impact apoptosis in acute leukemia.
BCR-ABL
The BCR-ABL fusion protein is created as a result of the t(9;22) translocation. This fusion protein is pathognomonic for chronic myeloid leukemia (CML) and is found in 25% of adult ALL where it confers a poor prognosis. This fusion protein dysregulates the ABL tyrosine kinase, leading to activation of several signaling pathways including Ras,112 AKT/PI3Kinase,113NFκB,114,115 and STAT.116 The BCR-ABL fusion protein inhibits apoptosis, in part, by constitutively activating STAT 5 that increases transcription of Bcl-xL.117 The tyrosine kinase inhibitor STI 571 inhibits the ABL kinase and induces apoptosis in CML and leukemia cells.118 Clinically, STI 571 has utility in the treatment of CML119 and BCR-ABL–positive ALL.120
Mixed lineage leukemia–ELL
Translocations involving the mixed lineage leukemia (MLL) gene on 11q23 are found in 4% of cases of AML and confer an intermediate prognosis.121 Over 25 fusion partners for MLL have been identified.111 Both the abnormal MLL gene and its fusion partners inhibit apoptosis, but the mechanism by which the abnormal MLL gene inhibits apoptosis is not well understood. The effects of the fusion partners are better characterized in some cases. For example, in the t(11;19) translocation, MLL is fused to the ELL/MEN gene. The dysregulated ELL gene inhibits apoptosis by binding p53 and thereby reducing its transcriptional activity.122
PML-RARα
The t(15;17) translocation produces a fusion of PML and RARα is present in most forms of AML M3.123Overexpression of the fusion transcript in U937 leukemic cells inhibits apoptosis in response to serum starvation and TNFα. The mechanism by which PML-RARα inhibits apoptosis is complex, but likely relates to inhibition of RARα-induced genes including STATs, Hox, and cell cycle regulators. For a more detailed discussion of the PML-RARα fusion transcript, the reader is referred to a recent review in this journal.123
Childhood versus adult-onset leukemia
This review focuses on adult-onset acute leukemia, but we will briefly discuss childhood acute leukemia to illustrate how differences in disease biology translate into different clinical outcomes. Childhood and adult-onset ALL are clearly different diseases. Clinically, 80% of children survive long-term after treatment of ALL, but only about 30% of adults are alive 5 years after treatment.2 Biologically, childhood and adult ALL differ as well. For example, the spectrum of chromosomal translocations varies between these 2 groups. The t(9;22) translocation, for example, is present in 25% of adult ALL and is present in only 5% of childhood ALL.124 As discussed above, chromosomal translocations such as t(9;22) inhibit apoptosis and lead to treatment failures. Studies comparing expression levels of members of the receptor- and mitochondrial-mediated pathways of apoptosis between adult and childhood ALL have not been performed. However, it would not be surprising to find higher levels of antiapoptotic proteins in adults than in children given the higher prevalence of chemoresistant and relapsed disease among adults. In support of this hypothesis, longitudinal studies reported changes in levels of apoptotic proteins in children at the time of relapse compared with diagnosis. Bcl-2 expression increased at relapse compared with diagnosis, and levels of Bax, mutant p53, Fas, and active caspase-3 declined.125-127 All of these changes could contribute to more aggressive and less responsive disease. Studies specifically comparing apoptotic members in adult and childhood ALL are warranted because they will further our understanding of the biologic differences between these 2 diseases. With a better understanding of these differences, more risk-adapted therapy can be applied.
Defects in apoptosis are important prognostically
Defects in the apoptosis pathway render leukemic blasts resistant to multiagent chemotherapy. These blocks translate clinically into reduced rates of remission, higher rates of relapse, and reduced overall survival. Studies have identified specific blocks in the receptor-mediated and mitochondrial-mediated pathways that confer prognostic information in adult patients with acute leukemia.
Receptor-mediated pathway
There is evidence to implicate defects in the receptor-mediated pathway of caspase activation with chemoresistance in leukemia. Exposure of the T-ALL cell line CEM to doxorubicin induces apoptosis and up-regulates Fas expression. A mutant CEM cell line resistant to Fas ligand fails to up-regulate Fas or undergo apoptosis in response to doxorubicin treatment.128 In contrast to this report, a Jurkat cell line resistant to Fas ligand retained sensitivity to doxorubicin-induced apoptosis.129The discrepancy between these 2 studies may reflect the numerous sites of blockage in the receptor-mediated pathway that can induce resistance to Fas ligand. A defect at the level of the Fas receptor may render a cell resistant to Fas ligand, but if doxorubicin triggers the pathway downstream of the receptor, then the cell would remain sensitive to doxorubicin. On the other hand, defects in the receptor-mediated pathway downstream of where doxorubicin acts will render cells resistant to both Fas ligand and doxorubicin. Alternatively, the discrepancy may result because chemotherapeutic agents effect multiple pathways simultaneously. As cells acquire defects in one pathway they unmask the effects of the chemotherapeutic agent on the other pathway.
Fas expression confers important prognostic information in patients with leukemia. The Fas gene encodes 2 isoforms: full-length Fas with a transmembrane domain, and a soluble form of Fas that lacks this transmembrane domain. Full-length Fas is the initiator of the receptor-mediated pathway of apoptosis. In contrast, soluble Fas is excreted into the extracellular environment where it acts as a decoy and binds Fas ligand. As such, soluble Fas is an inhibitor of apoptosis. In a study of 59 patients with adult T-cell leukemia, levels of full-length Fas expression did not influence patient survival. However, increased serum levels of soluble Fas were associated with reduced survival and remained an important predictor in multivariate analysis even after accounting for clinical risk factors.130
The prognostic importance of defects in the receptor-mediated pathway has not received much attention to date. Perhaps in the future, additional prognostic markers within this pathway will be identified. For example, caspase-8 is inhibited by the dominant-negative inhibitor I-FLICE. I-FLICE overexpression has been documented in the BCR-ABL–positive cell line K562, but its incidence and prognostic importance in patients with leukemia is unknown.131
Mitochondrial-mediated pathway
Defects in the mitochondrial-mediated pathways of apoptosis also induce multidrug resistance and confer important prognostic information. Most of the research on this pathway has focused on the Bcl-2 family of proteins.
Bcl-2.
Overexpression of Bcl-2 is an important cause of multidrug resistance. As a result, patients with AML and high levels of Bcl-2 have lower complete remission rates and a lower overall survival compared with patients with low levels of Bcl-2. For example, in a study of 82 consecutive patients with AML treated with standard induction chemotherapy, patients with high levels of Bcl-2 expression by immunofluroescent staining had a complete response rate of 29% versus an 85% complete response rate in patients with low levels. Likewise, high levels of Bcl-2 translated into decreased overall survival. The predicted survival at 2 years in patients with increased Bcl-2 was 15% versus 40% in patients with low levels of Bcl-2. Bcl-2 remained an important predictor of survival in multivariate analysis over other known prognostic markers, including age and white blood cell (WBC) count of more than 30. The influence of cytogenetics was not reported, however.132
Some studies have indicated that increased levels of Bcl-2 are not prognostic or even associated with improved survival. In patients with breast cancer, increased Bcl-2 by immunostaining correlated with improved disease-free survival,133 and in childhood ALL, increased Bcl-2 was associated with improved event-free survival.134 These results may reflect the importance of posttranslational modification of Bcl-2. For example, under certain circumstances PKC activates Bcl-2,99 so in the presence of PKC, the Bcl-2 that accumulates is functional, conferring a worse outcome. In support of this hypothesis, lower levels of PKC were associated with an improved prognosis in patients with AML.135
Bax.
In a study of 56 patients with newly diagnosed AML, increased levels of Bax expression by immunoblotting correlated with improved rates of overall survival.136 In contrast, a study of 165 patients with newly diagnosed AML135 reported that levels of Bax expression by immunoblotting did not correlate with response to induction chemotherapy or survival. However, high ratios of Bcl-2 to Bax protein conferred a poor prognosis with decreased rates of complete remission and overall survival. The prognostic discrimination from the ratio of the proteins was greater than Bcl-2 levels alone. The ratio also provided prognostic information above cytogenetics. Both studies are flawed, however, in that the patients selected were not consecutive and inclusion criteria were not predefined.
Downstream effectors.
Levels of caspase inhibitors have been correlated with survival in AML. IAPs are a family of caspase inhibitors that bind and inactivate caspases, including caspases 3 and 9. As such, they block apoptotic stimuli in both the mitochondrial-mediated and receptor-mediated pathways. In a study of 78 patients with AML, 76 had detectable levels of XIAP. Patients with the lowest levels of XIAP expression had the longest median survival.137 The importance of XIAP in the context of other clinical prognostic factors is unknown, however. Likewise, a trend to improved survival was seen in patients with low levels of the IAP inhibitor survivin.138
In another study of patients with newly diagnosed AML, increased levels of procaspase-3 in peripheral blood mononuclear cells correlated with a poor prognosis. However, patients with spontaneously increased levels of active caspase-3 at the time of diagnosis showed improved survival.139 Likewise, in 45 consecutive adult patients with ALL, increased levels of active caspase-3 correlated with an improved rate of complete remission.140 In multivariate analysis, levels of caspase-3 were no longer predictive of survival, but the small sample size may have obscured the predictive effect. The poor prognosis among patients with high levels of procaspase-3 may represent defects in caspase activation and the subsequent accumulation of uncleaved caspase-3. The better prognosis among patients with increased levels of cleaved caspase-3 may indicate competent caspase activation pathways.
In contrast to these findings, Svingen et al141 found no correlation among levels of Apaf-1, procaspases 3, 8, and 9, and response to induction chemotherapy in adult patients with AML (n = 42) or ALL (n = 18). A number of explanations may account for the lack of correlation. First, the study had a small sample size and thus had limited power to detect a difference. However, the wide variation in the levels of these apoptotic members suggests that simply increasing the number of patients would not reveal a correlation. Alternatively, this study used response to chemotherapy as its end point. The overall response rate for patients with AML after the first induction was 53%, which is lower than expected. Therefore, this group of patients may have had a particularly aggressive disease due to other molecular abnormalities that overshadowed the importance of procaspases or Apaf-1.
The apoptosis pathway as a target for novel therapeutic agents
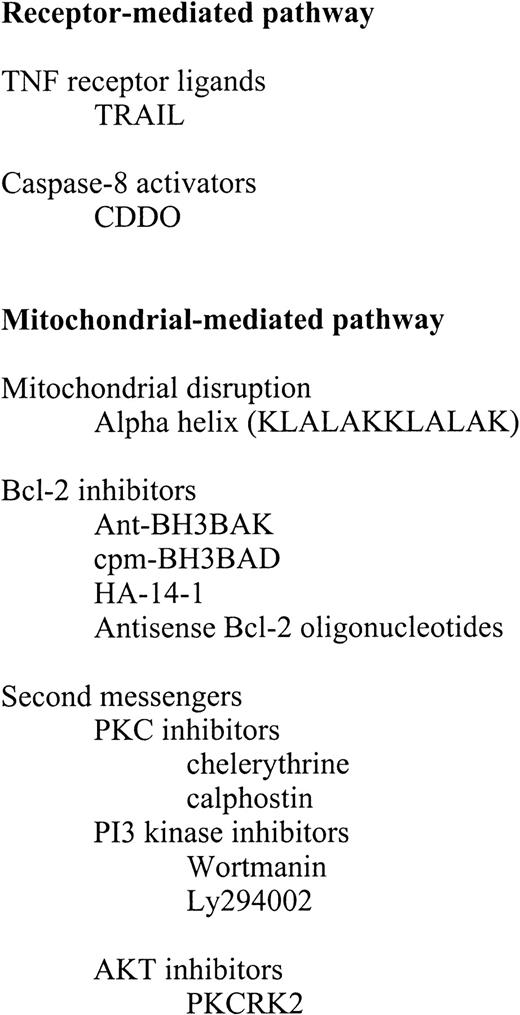
Classical chemotherapeutic agents induce cell death through a variety of mechanisms, including directly or indirectly affecting regulators of the apoptosis pathway. Rather than review the mechanism of action of chemotherapeutic agents, however, we will focus on small molecules that specifically overcome blocks in the apoptosis pathway. Attempts to develop these novel therapeutic compounds have targeted both the receptor-mediated and mitochondrial-mediated pathways of apoptosis (Figure 8).
Prototypic therapeutic agents for treatment of acute leukemia.
TNF indicates tumor necrosis factor; TRAIL; PKC, protein kinase C; and AKT.
Prototypic therapeutic agents for treatment of acute leukemia.
TNF indicates tumor necrosis factor; TRAIL; PKC, protein kinase C; and AKT.
Receptor-mediated pathway
Small molecules that target the receptor-mediated pathway can induce caspase-3 activation and overcome blocks confined to the mitochondrial-mediated pathway, such as the overexpression of Bcl-2 or Bcl-xL.
TRAIL.
Treatment with TNF can induce apoptosis through the receptor-mediated pathway, but also produces severe toxicity to normal tissue.142-144 In contrast, TRAIL (APO2L), which is similar to Fas ligand and activates the DR3 and DR4 receptors, shows selectivity for tumor cells despite the presence of the DR4 receptor on normal tissues.145 In a study by Ashkenazi et al,146 soluble TRAIL induced cell death and apoptosis in a variety of leukemic and solid tumor cell lines. In contrast, normal tissue including prostate, fibroblasts, colon, and smooth muscle cells were unaffected by exposure to TRAIL. In nude mice, intraperitoneal treatment with TRAIL induced apoptosis and shrinkage of colon cancer xenografts. No observable toxicity from TRAIL was observed in these mice or in studies of nonhuman primates. Recently, Altucci et al147 demonstrated that ATRA induces apoptosis in acute promyelocytic leukemia (APL) cells through the production and paracrine action of TRAIL. Furthermore, the addition of exogenous TRAIL to APL cells and an ATRA-resistant cell line induced apoptosis. Therefore, clinically, TRAIL may have a role in the treatment of APL perhaps in synergy with ATRA and chemotherapy. TRAIL may also be quite useful in treating patients with ATRA-resistant disease.
Caspase-8 activators.
Compounds that activate the receptor-mediated pathway at the level of caspase-8 may overcome blocks to apoptosis upstream of this level of regulation, such as at the level of the TNF-receptor family. Such compounds also can act independent of the level of expression of the TNF receptor. One such agent is 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO). CDDO is a synthetic compound that is synthesized in an 11-step reaction from oleanolic acid. This compound is a potent analogue of the naturally occurring triterpenoids which are used as herbal remedies in the treatment of inflammation and cancer. CDDO induces apoptosis in leukemic cell lines by activating procaspase-8. As the compound targets the receptor-mediated pathway, overexpression of Bcl-xL has little effect on the toxicity of this compound.148 Currently, it is unknown how CDDO leads to activation of caspase-8. In addition, the effects of CDDO on normal tissue and bone marrow remain unknown, and this information would be important before advancing this agent into clinical development.
Mitochondrial-mediated pathway
Most of the effort in developing small molecules that target defects in apoptosis has concentrated on the mitochondrial-mediated pathway and Bcl-2, in particular.
Bcl-2 inhibitors.
Bcl-2 is an attractive therapeutic target. It is overexpressed in a wide variety of malignancies including acute leukemia, and its overexpression is often associated with clinical treatment failures. Most intriguing, the temporary inhibition of Bcl-2 may not be toxic to normal cells. In support of this hypothesis, Bcl-2 knockout mice survive until birth with no histologic abnormalities in the brain, skin, intestines, or bone marrow hematopoetic precursors. Beginning one week after birth, however, these mice begin to develop renal dysfunction similar to polycystic kidney disease. By 4 weeks after birth, the mice become lymphopenic primarily due to the death of mature T cells.149 Furthermore, inhibition of Bcl-2 does not automatically induce a compensatory up-regulation of other antiapoptotic proteins. However, compounds that predominantly inhibit Bcl-xL may be less useful therapeutically due to their potential toxicity to normal tissue. In contrast to the Bcl-2 knockout mice, Bcl-xL knockout mice do not survive beyond day 13 of gestation. These mice sustain massive neuronal cell death as well as apoptosis of the hepatic haematopoietic and immature lymphocytes.150Furthermore, in the most primitive hematopoetic precursors (CD34+, lin−, CD38−, small size), Bcl-2 is expressed in only 1% to 4% of cells. In contrast, Bcl-xL is expressed in more than 95% of these cells. As these primitive cells proliferate and differentiate, Bcl-2 expression is up-regulated.151
One strategy to create Bcl-2 inhibitors has focused on developing small molecules that mimic the action of the endogenous Bcl-2–binding death agonists. An early prototype was the BH3 death domain of Bak. Holinger et al152 created a cell-permeable version of BH3Bak by fusing it to the C terminus of the 21 amino acids Antennapedia sequence that allows for entry into cells with high efficiency and low toxicity. Holinger et al demonstrated that this fusion peptide inhibited Bcl-2 and induced cell death and apoptosis in HeLa cells. Its effects on leukemic cells and its tumor specificity were not reported. One might speculate, however, that the BH3 domain on Bak might show preferential killing of malignant cells and not induce apoptosis in normal tissues. Bak induces apoptosis directly through its effects on the mitochondria, so the full-length protein likely would be toxic to normal cells. However, the role of its BH3 domain seems confined to binding and inhibiting Bcl-2, and as such, might not induce apoptosis in normal cells that do not have concurrent active death signals.
Compounds that mimic the BH3-only class of death agonists may also be useful novel therapeutic agents. BH3-only death agonists, such as BAD, inhibit the survival proteins Bcl-2 and Bcl-xL, but do not appear to have independent death-promoting activity. As such, they may show selectivity for malignant cells whose active death signals are blocked by Bcl-2/Bcl-xL overexpression. Recently, the effects of a peptide corresponding to the BH3 domain of BAD coupled to cpm, a fatty acid moiety internalization sequence, have been reported.153The decanoic fatty acid cpm (CH3[CH2]8CO) can shuttle peptides across cell membranes without untoward toxicity. Like the peptide-based internalization sequences such as ANT154,155and TAT,156 cpm represents a novel vehicle for delivering therapeutic peptides into cells.
The cpm-BH3BAD fusion peptide mimicked the action of its parent protein BAD. It bound Bcl-2 in a cell-free system and induced apoptosis in HL60 cells that overexpressed Bcl-2. In contrast, cpm-BH3BAD had minimal effect on resting and stimulated peripheral blood lymphocytes. When administered intraperitoneally into SCID mice, this peptide suppressed the growth of HL-60 tumor xenografts without inducing untoward toxicity.153 Based on the understanding of BAD, one might have predicted that this compound would have been toxic to normal tissue. As BAD binds Bcl-xL with higher affinity than Bcl-2,67 and Bcl-xL is critical for the development of hematopoetic precursors and neural cell150 one might have expected to see toxicity to normal tissues.
In a novel strategy to develop a Bcl-2 inhibitor, Wang et al157 screened a virtual library of compounds. They modeled the binding of Bak's BH3 domain to the BH1, BH2, BH3 pocket of Bcl-2. Then, using a computer simulation of binding, they screened a virtual library of 193 833 compounds for their potential ability to bind Bcl-2. From this virtual database, 28 compounds were selected for physical testing. Of these 28, one synthetic compound, HA-14-1, displayed an ability to bind Bcl-2 and induce apoptosis in HL60 cells. The selectivity of this compound has not yet been reported. Since the binding pockets of Bcl-2 and Bcl-xL differ in sequence and structure, this synthetic compound may show preferential toxicity to cells with high levels of Bcl-2 over those with increased Bcl-xL.
A different strategy to inhibit Bcl-2 involves antisense oligonucleotides. Liposomal antisense Bcl-2 oligonucleotides can induce apoptosis in HL60 cells and primary AML blasts. In addition, these oligonucleotides can sensitize the cells to treatment with Ara-C.158 Antisense oligonucleotides have been used clinically in a phase I trial. In 21 heavily pretreated patients with B-cell NHL, continuous subcutaneous infusion of an 18-mer Bcl-2 antisense oligonucleotide yielded encouraging results. There was one complete response, 2 partial responses, and 8 patients with stable disease. The only notable toxicity was thrombocytopenia that was likely secondary to the phosphorothioate backbone of the antisense molecule.159
Second messengers.
Targeting the kinases that regulate the activity of the Bcl-2 family of proteins may be another useful strategy to developing novel therapeutic agents. The therapeutic potential of kinase inhibitors has been realized in the development of the BCR-ABL tyrosine kinase inhibitor STI571. This compound induces apoptosis in leukemic cells overexpressing BCR-ABL118 and has significant clinical benefit in patients with CML119 and BCR-ABL–positive ALL.120 In addition, STI571 has activity in gastrointestinal stromal tumors expressing the c-kit receptor.160
Activation of Bcl-2 may require phosphorylation on ser-70 by PKC under some circumstances. Therefore, compounds that inhibit PKC may enhance apoptosis by blocking the activation of Bcl-2. In HL60 cells, the PKC inhibitors chelerythrine and calphostin induced apoptosis without changing levels of Bcl-2 mRNA.161 However, the phosphorylation status of Bcl-2 was not measured. Since PKC has many targets, it is unclear if these inhibitors functioned by blocking Bcl-2 phosphorylation.
Targeting the phosphorylation of BAD is another therapeutic strategy. The proapoptotic action of BAD is inhibited by phosphorylation on ser-136 through the PI3/AKT pathway. Preventing BAD phosphorylation may increase the activity of endogenous BAD and promote apoptosis. Wortmanin and Ly294002 are PI3K inhibitors and sensitize HL60 cells to undergo apoptosis after treatment with chemotherapeutic agents. Although these agents block Akt activation, they do not alter phosphorylation of BAD or the ability of BAD to heterodimerize with Bcl-2 or Bcl-xL.162 Therefore, these kinase inhibitors induce apoptosis but act through proteins other than BAD.
In another attempt to prevent BAD phosphorylation, AKT was targeted directly. The C terminal fragment from the protein kinase C–related kinase-2 (PKCRK2) was identified as an AKT inhibitor. Treatment with this protein inhibited AKT, blocked phosphorylation of BAD, and sensitized 293 cells to apoptosis.163
Direct mitochondrial toxins.
Compounds that directly disrupt mitochondrial function and induce cytochrome c–release may serve as prototypes for therapeutic agents for the treatment of leukemia. Alpha helical peptides are one such compound. Some amphipathic alpha helixes are potent naturally occurring antibiotics that disrupt the negatively charged bacterial membranes.164,165 They are not toxic to the mammalian cell outer membranes, as they are more neutrally charged and stabilized by cholesterol. When internalized into mammalian cells, alpha helixes are exposed to the negatively charged inner mitochondria that lacks cholesterol. Ellerby et al166 internalized a 14–amino acid alpha helical peptide (KLALAK)2 and demonstrated that it induced mitochondrial swelling, caspase activation, and cell death. Using an internalization sequence that hones to tumor endothelial cells, Ellerby et al demonstrated that the alpha helix could cause regression of breast cancer xenografts in nude mice without untoward toxicity to normal tissue. Potentially, alpha helical peptides could have value in the treatment of leukemia if targeted selectively.
Conclusion
Through basic investigation, the apoptosis pathway has been pieced together. Over time, further details will be elucidated, but the major players are likely in place. Now, the goal is to translate this basic work into clinical practice. Prognostic markers are being identified and prototypes for novel therapeutic agents are being developed. The challenge ahead is to further the knowledge of prognostic markers and incorporate them into current treatment protocols. On the therapeutic front, small molecules that overcome defects in apoptosis hold promise for reversing multidrug resistance. Here the challenge relates to enhancing tumor specificity, improving drug delivery, and moving the agents into clinical use.
Given the current status of these compounds, antisense Bcl-2 will likely be the first Bcl-2–targeted therapy used routinely in clinical practice. Over the next 10 years, we speculate that other small molecules will move forward into the clinical arena. A model for how these agents will be used clinically can be derived from the experience with the BCR-ABL tyrosine kinase inhibitor, STI571. STI571 was first used in patients with refractory disease, but is currently being advanced to first-line therapy. Although remissions in patients with Philadelphia chromosome–positive ALL can be obtained, relapses invariably occur, indicating that resistance to STI571 develops over time.119 120 Therefore, the ultimate place for STI571 is likely in combination with standard chemotherapy. Likewise, we speculate that small molecules that target the receptor- and mitochondrial-mediated pathways of apoptosis will initially be used as single agents or in combination with low-dose chemotherapy in patients with relapsed or refractory disease. As more experience is gained, these targeted therapies will be used up-front in the treatment of de novo disease in combination with standard induction and consolidation chemotherapy. In the future, when more small molecules that modulate the apoptosis cascade are developed, they will be used together to simultaneously target different molecular defects.
We would like to thank J. C. Reed (Burnham Institute, La Jolla, CA) and D. Barber (Princess Margaret Hospital, Toronto, Canada) for their helpful advice and discussions.
Supported by fellowships from the Canadian Institute of Health Research and the American Society of Hematology (A.D.S.).
References
Author notes
Aaron D. Schimmer, Burnham Institute, Rm 6-308, 10901 North Torrey Pines Rd, La Jolla, CA 92037; e-mail:aaron.schimmer@burnham.org.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal