Abstract
Genetically modified donor T cells with an inducible “suicide” gene have the potential to improve the safety and availability of allogeneic hematopoietic stem cell transplantation by enhancing engraftment and permitting control of graft-versus-host disease (GVHD). However, several clinical studies of gene-modified T cells have shown limited to no in vivo function of the ex vivo expanded T cells. Using the well-established dog model of allogeneic marrow transplantation, the question was asked if retrovirally transduced, donor derived, ex vivo expanded cytotoxic T lymphocytes (CTLs) that are recipient specific could enhance engraftment of dog leukocyte antigen (DLA)–haploidentical marrow following a single dose of 9.2 Gy total body irradiation and no postgrafting immunosuppression. In this setting, only 4 of 11 control recipients of DLA-haploidentical marrow without added CTLs engrafted. CTLs did not enhance engraftment of CD34+ selected peripheral blood stem cells. However, recipient-specific CTLs enhanced engraftment of DLA-haploidentical marrow in 9 of 11 evaluable recipients (P = .049). All dogs that engrafted developed multiorgan GVHD. To facilitate in vivo tracking, 8 dogs received CTLs transduced with a retroviral vector encoding green fluorescent protein (GFP) and neomycin phosphotransferase (neo). Recipients that engrafted had sharp increases in the numbers of circulating GFP+ CTLs on days +5 to +6 after transplantation. GFP+ CTLs isolated from blood were capable of recipient-specific lysis. At necropsy, up to 7.1% of CD3+ cells in tissues were GFP+ and polymerase chain reaction in situ hybridization for neoshowed infiltration of transduced CTLs in GVHD-affected organs. These results show that ex vivo expanded, transduced T cells maintained in vivo function and enhanced marrow engraftment.
Introduction
Graft-versus-host disease (GVHD) is a major complication of allogeneic hematopoietic stem cell transplantation (HSCT) and is mediated by grafted donor T cells. GVHD results in substantial morbidity and mortality, particularly in the HLA-partial mismatch setting. Attempts to prevent GVHD with T-cell depletion of marrow grafts have been associated with high rates of graft rejection and relapse of the underlying disease.1 2 Thus, the development of a strategy that would enhance donor engraftment but prevent GVHD should increase the availability of donors for patients with diseases that are treated with allogeneic HSCT.
One approach to improve the safety of HSCT is to first T-cell deplete and then add back donor T cells retrovirally transduced with a “suicide gene,” such as the herpes simplex virus thymidine kinase (HSV-tk) gene, to enhance engraftment of the T-cell–depleted donor marrow. After engraftment, selective in vivo T-cell depletion with ganciclovir could prevent the development of GVHD. To date, however, clinical trials with ex vivo expanded, HSV-tk–transduced donor T cells have not shown consistent or reliable in vivo function.3-7 Clinical progress in this strategy to control GVHD will be accelerated with a relevant large animal model.
For many years, studies of GVHD and hematopoietic engraftment in the random-bred dog model have closely predicted results of subsequent clinical HSCT studies. In the dog model, we have previously shown that T-cell depletion of donor grafts in the dog leukocyte antigen (DLA)–identical setting is associated with graft rejection.8 Both donor lymphocyte infusion and ex vivo expansion of recipient-specific donor cytotoxic T lymphocytes (CTLs) enhanced engraftment across major histocompatibility complex (MHC) barriers, but resulted in fatal GVHD.9,10 In addition, we have reported on the efficient transduction, expansion, and maintenance of alloreactivity and ganciclovir-mediated ablation of canine HSV-tk CTLs in vitro.11
We initially hypothesized that ex vivo expanded, recipient-specific CTLs could enhance engraftment of highly purified CD34+cells isolated from peripheral blood stem cells (PBSCs) mobilized by recombinant canine granulocyte colony-stimulating factor (rcG-CSF) and transplanted into DLA-haploidentical littermates. When this failed to achieve reliable engraftment, we asked if ex vivo expanded CTLs maintained in vivo function. To facilitate in vivo tracking of ex vivo expanded T cells, CTLs were transduced with a retroviral vector to express green fluorescent protein (GFP). We then studied whether GFP+ CTLs enhanced engraftment of DLA-haploidentical marrow in the setting where graft rejection was expected and, in addition, monitored the trafficking, tissue localization, and cytotoxicity of transduced CTLs.
Materials and methods
Laboratory animals
Litters of random-bred dogs were raised at the Fred Hutchinson Cancer Research Center or obtained from commercial kennels licensed by the US Department of Agriculture. The dogs weighed from 6.5 to 15.0 kg (median, 10.4 kg) and were 7 to 14 months old (median, 9 months). Dogs were observed for indications of disease for at least 60 days before entry into the study. All were immunized against leptospirosis, distemper, hepatitis, parvovirus, and papillomavirus. Research was performed according to the principles outlined in the Guide for Laboratory Animals Facilities and Care of the National Academy of Sciences, National Research Council. All dogs were housed in kennels certified by the American Association for Accreditation of Laboratory Animal Care. All dogs were examined at least twice daily. Recipient dogs were euthanized at the end of the study or after transplantation when established clinical criteria had been met for GVHD or graft rejection.
The DLA-haploidentical littermates were chosen as donor-recipient pairs on the basis of haploidentity (1 of 2 alleles identical) for highly polymorphic MHC class I and class II microsatellite markers.12 Specific DLA DRB1 allele typing was determined by direct sequencing to confirm allelic identification and assign haploidentity.13
Cytotoxic T lymphocytes
Donor-derived, recipient-specific CTLs were generated by establishing one-way bulk mixed lymphocyte cultures with cells from DLA-haploidentical littermates. PBMCs were obtained by Ficoll-Hypaque density gradient centrifugation of dog blood. Donor PBMCs were mixed with γ-irradiated (22 Gy, 137Cs source) recipient PBMCs at a 1:1 ratio. A total of 4 × 107 cells were cultured in Iscove modified Eagle medium supplemented with 10% heat-inactivated pooled normal dog serum, 2 mM l-glutamine, 1 mM nonessential amino acids, 1 mM sodium pyruvate, 50 IU/mL penicillin, 50 μg/mL streptomycin, and 0.5 μg/mL amphotericin-B (termed CTL medium) per upright T-75 flask (Corning, Ithaca, NY). On day 7, cells were restimulated (1:1) with recipient irradiated PBMCs and recombinant human interleukin (IL)-2, 100 U/mL (Chiron, Emeryville, CA). On day 9, CTLs were transferred to 6-well plates (106 cells/well) for transduction. On day 12, transduced CTLs were selected in G418 (0.6 mg/mL active drug; Roche Molecular Biochemicals, Indianapolis, IN) for 5 days in medium with 100 IU/mL IL-2. On day 17, CTLs were restimulated with allogeneic and autologous irradiated PBMCs, at a 1:20:20 ratio, respectively. Two days later, IL-2 (100 U/mL) was added. Starting on day 22, CTL medium was demi-depleted daily and replaced with fresh CTL medium without IL-2. Cells were pooled, washed, and infused into irradiated recipients on day 28. An aliquot was reserved for assessment of cytotoxity and flow cytometric analysis.
Retroviral transduction of CTLs
Virus-containing medium (VCM) was prepared from PG13 retrovirus-producing packaging cells, generating vectors with the gibbon ape leukemia virus (GALV) pseudotype.14 Two vectors based on the Moloney mouse leukemia virus were used in this study to track CTLs in vivo. LGFPSN contained the enhancedGFP gene under transcriptional control of the retroviral long terminal repeat and a downstream neomycin phosphotransferase (neo) gene under control of the simian virus-40 promoter.15 LtNGFRSN (provided by Dr D.B. Kohn, Children's Hospital, Los Angeles, CA) was similarly constructed and contained the truncated rat nerve growth factor receptor (NGFR) instead of GFP.16 The vector producer cell lines were grown in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT). VCM was collected following a 24-hour incubation at 34°C and centrifuged at 1400g for 12 minutes. The supernatant was collected and frozen at −70°C until used. Absence of replication competent retrovirus was confirmed by failure to transfer GFP expression and G418 resistance from transduced CTLs to HeLa cells. The viral titers were 8 × 105 to 2 × 106 infectious particles/mL. Thawed VCM supplemented with protamine sulfate 4 μg/mL was added to CTLs at a multiplicity of infection of 4:1, centrifuged at 1000g for 60 minutes, and incubated at 34°C overnight. CTLs were then washed and replated in CTL medium containing IL-2 (100 U/mL).
Flow cytometric analysis of CTLs
The CTLs were analyzed by flow cytometry for expression of GFP and streptavidin-phycoerythrin staining of biotinylated CD3 (CA17.6F9), CD4 (CA13.1E4), and CD8 (CA9.JD3).17 Propidium iodide (1 μg/mL) was added to exclude dead cells. CTLs transduced with LtNGFRSN were stained with the antirat NGFR monoclonal antibody (mAb) MC192 at 10 μg/mL (Chemicon, Temecula, CA) and indirect fluorescein labeling. Appropriate isotype negative control antibodies (Dako, Carpenteria, CA) were used to stain transduced cells at equivalent concentrations of primary antibody to set gates used for exclusion analysis. Heparinized peripheral blood samples from dogs receiving transduced CTLs were collected immediately before CTL infusion, at 10, 30, and 60 minutes after infusion, and then daily until necropsy. Samples were hemolyzed, washed, and analyzed for GFP expression or incubated with MC192 followed by indirect fluorescein labeling to assess NGFR expression. Cell incubations and washes were completed at 4°C, in Hanks balanced salt solution supplemented with 2% heat-inactivated horse serum (HBSS/2%HS). Analysis was performed on a FACScalibur flow cytometer (Becton Dickinson, San Jose, CA) with WinList software (Verity Software House, Topsham, ME). The absolute number of transduced cells per milliliter peripheral blood was determined by multiplication of the percent transduced cells by the total white blood cell count measured from the same venous blood sample. The limit of detection of GFP+ CTLs was approximately 1 cell/mL. GFP+CTLs were sorted from 10-mL hemolyzed blood samples of recipient dogs using a FACS Vantage cell sorter (Becton Dickinson).
Analysis of cytotoxicity
Cytotoxic activity of CTLs was measured using a standard51Cr release assay.10 Briefly, 1 × 106 concanavalin-A–stimulated dog PBMC target cells (Con-A blasts) were labeled with 50 μCi (1.8 × 106Bq) Na251CrO4 (Dupont, Boston, MA) for 1 hour at 37°C, washed 5 times, then incubated for 4 hours with effector CTL at various effector-target ratios in a total volume of 200 μL. The percentage of specific lysis was calculated using the standard formula.10
CD34+ selection of canine PBSCs
Donor dogs received rcG-CSF and recombinant canine stem cell factor (rcSCF) (gifts from Amgen, Thousand Oaks, CA) at 10 μg/kg per day in divided doses and 25 μg/kg per day in one dose, respectively, administered subcutaneously for 6 days.18 After 5 days of growth factor treatment, a shunt connecting the carotid artery with the external jugular vein was placed in the PBSC donor. The following day, leukapheresis was performed by connecting the shunt to a Cobe 2997 continuous flow centrifuge (Cobe BCT, Lakewood, CO) with a calculated processed volume of 6 to 10 L blood.18
Following Ficoll-Hypaque density gradient centrifugation of the apheresis product, cells were washed twice in HBSS/2%HS to remove excess platelets. Then, 1 × 108 cells/mL were incubated with 40 μg/mL of the biotinylated mAB 1H6, specific for canine CD34,19 for 30 minutes at 4°C. After incubation with 100μL/mL immunomagnetic streptavidin-coated microbeads (Miltenyi Biotech, Auburn, CA) for 30 minutes, cells were loaded onto a VS+ column set on a VarioMACS separation device (Miltenyi Biotech), the column was washed, and the adsorbed cells were eluted and then used for transplantation.20 Aliquots from the apheresis product, eluted cells, and flow-through fractions were analyzed by flow cytometry to assess the content of CD34+,19CD14+ (clone Tuk-4, Dako), and CD3+ cells.
Transplantation
Unmodified donor marrow was aspirated from humeri under general anesthesia and infused intravenously within 4 hours of total body irradiation (TBI).21 All recipients in this study were given a single dose of 9.2 Gy TBI (0.07 Gy/min, 60Co source).22 23 The day of infusion of donor hematopoietic stem cells was designated as day 0. No immunosuppression or exogenous cytokine/growth factor support was given after transplantation.
Care after grafting was given as previously described, which included thrice daily oral nonabsorbable antibiotics, neomycin sulfate and polymycin sulfate from day −5 until the end of the study.21 Intravenous ceftazidime (25 mg/kg per day) was begun when the white blood cell count decreased below 1 × 106/mL following TBI. Dogs received137Cs irradiated (20 Gy) red blood cell and platelet transfusions from healthy donors if indicated based on the results of daily complete blood counts (CBCs). Dogs that died underwent complete necropsy and histologic examination. The histologic criteria for GVHD have been previously described.24 25
Assessment of engraftment
Donor hematopoietic engraftment was defined by achieving 3 criteria: (1) sustained recovery of granulocyte and platelet counts in serial CBCs after the postirradiation nadir, (2) recovery of marrow cellularity, and (3) 100% donor (GAAA)n repeat polymorphism in cells from the peripheral blood and marrow. To assess donor chimerism, genomic DNA was extracted from peripheral blood or marrow at the time of necropsy, and a polymerase chain reaction (PCR)–based assay was performed with the C.2200 and C.2202 microsatellite markers, associated with MHC class I and class II genes, respectively.12 Marrow cellularity was assessed by standard pathology examination of humeral marrow aspirates along with marrow autopsy sections of rib and sternum. Graft rejection was defined as: (1) lack of sustained recovery of peripheral blood counts, (2) absence of hematopoietic marrow cellularity, and (3) absence or loss of donor hematopoietic chimerism. In all cases, dogs met all of the criteria for either engraftment or rejection.
Detection of transduced CTLs in tissue
Polymerase chain reaction in situ hybridization (ISH) for detection of neo was performed from formalin-fixed tissue sections obtained at necropsy in some of the recipient dogs as previously described.26,27 Briefly, a PCR mixture containing neo-specific primers was applied to deparaffinized and proteinase K–treated tissue sections. PCR conditions consisted of 25 cycles of denaturation (94°C, 1 minute), primer annealing (55°C, 2 minutes), and primer extension (72°C, 2 minutes). The PCR product was detected by ISH using a combination of 3neo-specific oligonucleotides labeled with digoxigenin-11-dUTP (DIG; Roche Diagnostics, Indianapolis, IN), which were in sense orientation and internal to the PCR primer binding sequences.26 The oligonucleotide probes were hybridized to tissues for 4 hours at 42°C in a humidified chamber. After thoroughly washing the slides, the hybridized probe was detected with an alkaline phosphatase–conjugated anti-DIG mAB (1500 mU/mL) and nitroblue tetrazoleum-5-bromo-4-chloro-3-indolylphosphate toluidinium substrate.28 The presence of neo DNA was indicated by a purple cell-associated precipitate and was visualized by incident light microscopy. Positive controls consisted ofneo+ CTLs immobilized in paraffin wax (not shown). Negative controls for PCR included tissue processed withoutTaq polymerase or without neo-specific primer pairs. Identical procedures completed for tissues from dogs receiving nontransduced CTLs also served as a negative control. ISH controls included an irrelevant oligonucleotide probe to an unrelated virus.29
For flow cytometric assessment of GFP+ CTLs from various tissues at necropsy, 0.5 to 1.0 cm3 fresh tissue sections were incubated for 10 minutes in ice-cold phosphate-buffered saline (PBS), washed in hemolytic buffer, and forced through an 80-μM wire mesh. Cells were isolated by Ficoll-Hypaque density gradient centrifugation and washed with cold HBSS/2%HS. Cells were stained and analyzed as described to determine the percentage of GFP+T-cell content. Up to 1 × 106 viable events were collected for each sample. Due to low numbers of viable cells from some tissues, the number of events collected was less than 10 000 in some cases.
Statistical analysis
Statistical significance of the likelihood of engraftment in the group receiving marrow only compared with recipients of marrow plus CTLs was estimated by Monte Carlo approximation based on 5000 simulations.30 Logistic regression models were also fit to accommodate potential differences in total nucleated marrow cell dose between groups.
Results
Phenotype of retrovirally transduced, recipient-specific CTLs
Donor-derived, recipient-specific CTLs were reproducibly generated and expanded in vitro as bulk cultures for infusion into lethally irradiated recipients. Characteristics of transduced CTLs infused into dogs are summarized in Table 1. CTL transduction efficiency was 3.0% to 15.2% (median, 6.9%). Following G418 selection and expansion, 84.2% to 97.7% of CTLs were GFP+. The total number of transduced CTLs yielded by the ex vivo expansion ranged from 1.1 to 3.6 × 108 (median, 2.1 × 108). The cytotoxicity of the transduced CTLs ranged from 44% to 88% specific lysis (median, 69%), and the cytotoxicity of the nontransduced CTL ranged from 55% to 84% specific lysis (median, 80%) at a 1:20 effector-target ratio. At the time of infusion, transduced CTLs were 97.1% to 99.9% CD3+ and 88.7% to 99.8% CD8+ and nontransduced CTLs were 95.5% to 99.2% CD3+ and 92.2% to 97.3% CD8+. Thus there were no apparent differences in the functional capacity of retrovirally transduced and nontransduced CTLs.
Phenotype of retrovirally transduced CTLs
| Recipient dog . | Donor dog . | Percent specific lysis . | Percent CD3+ . | Percent CD8+ . | Percent CD4+ . | Percent transduced . | Vector . |
|---|---|---|---|---|---|---|---|
| E646 | E651 | 48 | 98.2 | 91.8 | 19.4 | 14.1* | NGFR |
| E644 | E642 | 69 | 97.7 | 96.7 | 5.5 | 97.7 | GFP |
| E650 | E659 | 44 | 99.3 | 88.7 | 12.1 | 96.1 | GFP |
| E655 | E640 | 57 | 97.5 | 95.5 | 12.4 | 94.4 | GFP |
| E714 | E715 | 82 | 97.1 | 97.1 | 13.5 | 94.1 | GFP |
| E671 | E670 | 70 | 98.4 | 99.1 | 6.2 | 93.1 | GFP |
| E746 | E748 | 68 | 99.4 | 96.6 | 7.8 | 84.2 | GFP |
| E716 | E718 | 71 | 98.3 | 99.7 | 7.7 | 93.4 | GFP |
| E815 | E817 | 80 | 99.9 | 99.8 | 11.8 | 98.3 | GFP |
| E801 | E803 | 74 | 99.9 | 99.4 | 18.7 | 98.4 | GFP |
| E755 | E753 | 52 | 99.9 | 97.2 | 88.7 | 98.8 | GFP |
| Recipient dog . | Donor dog . | Percent specific lysis . | Percent CD3+ . | Percent CD8+ . | Percent CD4+ . | Percent transduced . | Vector . |
|---|---|---|---|---|---|---|---|
| E646 | E651 | 48 | 98.2 | 91.8 | 19.4 | 14.1* | NGFR |
| E644 | E642 | 69 | 97.7 | 96.7 | 5.5 | 97.7 | GFP |
| E650 | E659 | 44 | 99.3 | 88.7 | 12.1 | 96.1 | GFP |
| E655 | E640 | 57 | 97.5 | 95.5 | 12.4 | 94.4 | GFP |
| E714 | E715 | 82 | 97.1 | 97.1 | 13.5 | 94.1 | GFP |
| E671 | E670 | 70 | 98.4 | 99.1 | 6.2 | 93.1 | GFP |
| E746 | E748 | 68 | 99.4 | 96.6 | 7.8 | 84.2 | GFP |
| E716 | E718 | 71 | 98.3 | 99.7 | 7.7 | 93.4 | GFP |
| E815 | E817 | 80 | 99.9 | 99.8 | 11.8 | 98.3 | GFP |
| E801 | E803 | 74 | 99.9 | 99.4 | 18.7 | 98.4 | GFP |
| E755 | E753 | 52 | 99.9 | 97.2 | 88.7 | 98.8 | GFP |
Percent specific lysis denotes cytotoxicity by 51Cr release assay at an effector-target ratio of 20:1.
Not selected in G418.
Transplantation of CD34+ selected cells and CTLs
Previously we had reported that unmodified rcG-CSF mobilized PBSCs engrafted in all DLA-haploidentical recipients.18 We reasoned that the increased total number of CD34+ cells in mobilized PBSCs compared to marrow could provide an increased number of positively selected CD34+ cells for transplantation. In the current study, ex vivo expanded, nontransduced CTLs were added to CD34+ purified cells obtained from rcG-CSF–mobilized PBSCs to assess if engraftment could be promoted in DLA-haploidentical littermates following 9.2 Gy TBI. The CD34+ purified grafts from the DLA-haploidentical donors were highly T cell depleted, with a range of 1 to 9 × 104 (median, 4.5 × 104) CD3+/kg infused into recipients, which represented a more than 3-log depletion of T cells from the rcG-CSF plus rcSCF-mobilized PBSC apheresis product. Results from these studies are summarized in Table 2. As expected, none of the 5 recipients of CD34+ selected cells alone engrafted. However, only 1 of 5 dogs engrafted following transplantation of nontransduced CTLs plus CD34+ selected cells. The recipient that engrafted had late recovery of neutrophil counts and was euthanized on day +59 after transplantation due to severe GVHD.
Graft composition and outcome of DLA-haploidentical CD34+ selected PBSC transplants following 9.2 Gy TBI
| Recipient dog . | Donor dog . | CTL type . | Recipient wt, kg . | Donor graft cell dose . | Time after transplantation . | Findings at necropsy . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TNC/kg (×106) . | CD34+/kg (×106) . | CD3+/kg (×104) . | CTL/kg (×107) . | Day to ANC > 500/μL . | Day euthanized . | Pathology . | Hematopoietic chimerism . | ||||
| CD34+ plus ex vivo expanded CTLs | |||||||||||
| E332 | E330 | Unmodified | 14.4 | 5.0 | 4.0 | <4.0 | 4.0 | n/a | 28 | Rejection | Host |
| E506 | E507 | Unmodified | 10.5 | 1.4 | 1.0 | <1.0 | 2.9 | n/a | 8 | Rejection | Host |
| E554 | E553 | Unmodified | 13.5 | 3.3 | 2.6 | <3.0 | 1.7 | n/a | 14 | Rejection | Host |
| E639* | E607 | Unmodified | 11.7 | 4.0 | 2.2 | <1.0 | 2.0 | 39 | 59 | GVHD* | Donor |
| E538 | E536 | Unmodified | 10.0 | 2.5 | 1.7 | <3.0 | 2.8 | n/a | 21 | Rejection | Host |
| CD34+only | |||||||||||
| E294 | E295 | n/a | 9.9 | 6.0 | 4.2 | <5.0 | n/a | n/a | 21 | Rejection | nd |
| E299 | E300 | n/a | 9.7 | 5.1 | 4.0 | <5.0 | n/a | n/a | 22 | Rejection | nd |
| E511 | E514 | n/a | 10.6 | 7.9 | 3.1 | <8.0 | n/a | n/a | 19 | Rejection | nd |
| E552 | E545 | n/a | 10.3 | 9.8 | 3.0 | <9.0 | n/a | n/a | 27 | Rejection | nd |
| E581 | E577 | n/a | 14.5 | 6.6 | 3.3 | <6.0 | n/a | n/a | 18 | Rejection | nd |
| Recipient dog . | Donor dog . | CTL type . | Recipient wt, kg . | Donor graft cell dose . | Time after transplantation . | Findings at necropsy . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TNC/kg (×106) . | CD34+/kg (×106) . | CD3+/kg (×104) . | CTL/kg (×107) . | Day to ANC > 500/μL . | Day euthanized . | Pathology . | Hematopoietic chimerism . | ||||
| CD34+ plus ex vivo expanded CTLs | |||||||||||
| E332 | E330 | Unmodified | 14.4 | 5.0 | 4.0 | <4.0 | 4.0 | n/a | 28 | Rejection | Host |
| E506 | E507 | Unmodified | 10.5 | 1.4 | 1.0 | <1.0 | 2.9 | n/a | 8 | Rejection | Host |
| E554 | E553 | Unmodified | 13.5 | 3.3 | 2.6 | <3.0 | 1.7 | n/a | 14 | Rejection | Host |
| E639* | E607 | Unmodified | 11.7 | 4.0 | 2.2 | <1.0 | 2.0 | 39 | 59 | GVHD* | Donor |
| E538 | E536 | Unmodified | 10.0 | 2.5 | 1.7 | <3.0 | 2.8 | n/a | 21 | Rejection | Host |
| CD34+only | |||||||||||
| E294 | E295 | n/a | 9.9 | 6.0 | 4.2 | <5.0 | n/a | n/a | 21 | Rejection | nd |
| E299 | E300 | n/a | 9.7 | 5.1 | 4.0 | <5.0 | n/a | n/a | 22 | Rejection | nd |
| E511 | E514 | n/a | 10.6 | 7.9 | 3.1 | <8.0 | n/a | n/a | 19 | Rejection | nd |
| E552 | E545 | n/a | 10.3 | 9.8 | 3.0 | <9.0 | n/a | n/a | 27 | Rejection | nd |
| E581 | E577 | n/a | 14.5 | 6.6 | 3.3 | <6.0 | n/a | n/a | 18 | Rejection | nd |
Gy indicates unit of irradiation (1 Gray = 100 rad); TNC/kg, total nucleated cells derived from PBSCs per recipient body weight in kilograms; ANC, absolute neutrophil count; GVHD, graft-versus-host disease determined by histopathologic evaluation; nd, not determined; and n/a, not applicable.
Dog E639 developed clinical manifestations of acute GVHD on day 53 after transplantation with fever, progressive skin erythema, jaundice, anorexia, diarrhea, and weight loss. The dog was euthanized due to worsening condition. Histopathology revealed severe GVHD of the liver and gut, moderate skin GVHD.
Transduced CTLs enhance engraftment of DLA-haploidentical marrow
To determine if ex vivo expanded CTLs maintained in vivo function, 12 DLA-haploidentical recipients received transplants of donor-derived, recipient-specific CTLs and unmodified donor marrow. Results are summarized in Table 3. The initial 3 dogs in this set of experiments received CTLs that were not transduced but ex vivo expanded in conditions required for successful transduction. All 3 recipients engrafted and developed hyperacute GVHD. The subsequent 9 dogs received transduced CTLs. To track the cells in vivo, CTLs were transduced with the LtNGFRSN vector (dog E646), which resulted in cell surface expression of the truncated rat NGRF or with the LGFPSN vector (remaining 8 dogs), which resulted in cytoplasmic expression of GFP. Of the 9 dogs that received transduced CTLs plus marrow, one (dog E655) died on day +5 of radiation toxicity and was not evaluable for engraftment. Six of the 8 evaluable dogs engrafted and all 6 developed clinical and histopathologic evidence of multiorgan acute GVHD. In the concurrent control group of 11 DLA-haploidentical marrow recipients not given CTLs, 4 of 11 dogs engrafted and developed acute GVHD. The remaining 7 dogs were euthanized because of marrow aplasia. The difference in engraftment between all evaluable recipients of DLA-haploidentical marrow plus CTLs (9 of 11) and marrow recipients not given CTLs (4 of 11) was statistically significant,P = .049. Comparing engraftment in recipients of genetically modified CTLs (6 of 8) with marrow recipients not given CTLs (4 of 11) showed a suggestive but not statistically significant difference (P = .114). Logistic regression analysis that included the effect of marrow cell dose did not qualitatively change these associations.
Graft composition and outcome of DLA-haploidentical marrow transplants following 9.2 Gy TBI
| Recipient dog . | Donor dog . | CTL type . | Recipient weight, kg . | Donor graft cell dose . | Time after transplantation . | Findings at necropsy . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TNC/kg (×108) . | CD34+/kg (×106) . | CD3+/kg (×107) . | CTL/kg (×107) . | Day to ANC > 500/μL . | Day euthanized . | Pathology . | Hematopoietic chimerism . | ||||
| Marrow plus ex vivo expanded CTLs3-150 | |||||||||||
| E468 | E469 | Unmodified | 12.3 | 4.9 | nd | nd | 1.6 | 6 | 10 | GVHD | Donor |
| E466 | E467 | Unmodified | 11.7 | 4.0 | nd | nd | 2.7 | 7 | 10 | GVHD | Donor |
| E473 | E472 | Unmodified | 11.3 | 4.0 | nd | nd | 2.3 | 8 | 11 | GVHD | Donor |
| E646 | E651 | NGFR | 8.5 | 5.0 | 4.5 | 1.7 | 1.5 | 8 | 17 | GVHD | Donor |
| E644 | E642 | GFP | 7.2 | 6.6 | 6.6 | 4.5 | 4.4 | 8 | 19 | GVHD | Donor |
| E650 | E659 | GFP | 10.7 | 3.5 | 4 | 1.7 | 1.1 | 9 | 13 | GVHD | Donor |
| E714 | E715 | GFP | 8.4 | 3.5 | 4.2 | 3.1 | 1.6 | 7 | 10 | GVHD | Donor |
| E671 | E670 | GFP | 13.3 | 6.4 | 5.2 | 3.5 | 1.5 | 6 | 10 | GVHD | Donor |
| E746 | E748 | GFP | 9.2 | 6.4 | 6.5 | 4.8 | 3 | 7 | 12 | GVHD | Donor |
| E716 | E718 | GFP | 11.4 | 3.0 | 3.9 | 1.4 | 1.5 | 7 | 14 | Rejection | Mixed |
| E815 | E817 | GFP | 8.0 | 6.1 | 4 | 1.9 | 2.5 | 8 | 13 | Rejection | Host |
| Marrow only | |||||||||||
| E663 | E661 | n/a | 11.4 | 2.2 | 4.0 | 1.2 | n/a | 9 | 16 | GVHD | Donor |
| E686 | E682 | n/a | 15.0 | 2.5 | 2.8 | 2.9 | n/a | n/a | 13 | Rejection | Host |
| E696 | E693 | n/a | 12.7 | 3.1 | 4.9 | 1.5 | n/a | n/a | 13 | Rejection | Host |
| E691 | E690 | n/a | 11.0 | 2.2 | 2.6 | 1.3 | n/a | 10 | 14 | GVHD | Donor |
| E697 | E696 | n/a | 10.0 | 2.6 | 2.0 | 1.2 | n/a | n/a | 19 | Rejection | Host |
| E024 | E023 | n/a | 9.0 | 4.0 | nd | nd | n/a | n/a | 21 | Rejection | nd |
| E023 | E024 | n/a | 6.5 | 1.0 | nd | nd | n/a | 11 | 25 | GVHD | nd |
| E029 | E030 | n/a | 9.4 | 4.3 | nd | nd | n/a | 8 | 14 | GVHD | nd |
| E030 | E029 | n/a | 12.5 | 3.5 | nd | nd | n/a | n/a | 16 | Rejection | nd |
| E084 | E088 | n/a | 9.8 | 4.1 | nd | nd | n/a | n/a | 15 | Rejection | nd |
| E088 | E084 | n/a | 10.1 | 4.4 | nd | nd | n/a | n/a | 15 | Rejection | nd |
| Recipient dog . | Donor dog . | CTL type . | Recipient weight, kg . | Donor graft cell dose . | Time after transplantation . | Findings at necropsy . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TNC/kg (×108) . | CD34+/kg (×106) . | CD3+/kg (×107) . | CTL/kg (×107) . | Day to ANC > 500/μL . | Day euthanized . | Pathology . | Hematopoietic chimerism . | ||||
| Marrow plus ex vivo expanded CTLs3-150 | |||||||||||
| E468 | E469 | Unmodified | 12.3 | 4.9 | nd | nd | 1.6 | 6 | 10 | GVHD | Donor |
| E466 | E467 | Unmodified | 11.7 | 4.0 | nd | nd | 2.7 | 7 | 10 | GVHD | Donor |
| E473 | E472 | Unmodified | 11.3 | 4.0 | nd | nd | 2.3 | 8 | 11 | GVHD | Donor |
| E646 | E651 | NGFR | 8.5 | 5.0 | 4.5 | 1.7 | 1.5 | 8 | 17 | GVHD | Donor |
| E644 | E642 | GFP | 7.2 | 6.6 | 6.6 | 4.5 | 4.4 | 8 | 19 | GVHD | Donor |
| E650 | E659 | GFP | 10.7 | 3.5 | 4 | 1.7 | 1.1 | 9 | 13 | GVHD | Donor |
| E714 | E715 | GFP | 8.4 | 3.5 | 4.2 | 3.1 | 1.6 | 7 | 10 | GVHD | Donor |
| E671 | E670 | GFP | 13.3 | 6.4 | 5.2 | 3.5 | 1.5 | 6 | 10 | GVHD | Donor |
| E746 | E748 | GFP | 9.2 | 6.4 | 6.5 | 4.8 | 3 | 7 | 12 | GVHD | Donor |
| E716 | E718 | GFP | 11.4 | 3.0 | 3.9 | 1.4 | 1.5 | 7 | 14 | Rejection | Mixed |
| E815 | E817 | GFP | 8.0 | 6.1 | 4 | 1.9 | 2.5 | 8 | 13 | Rejection | Host |
| Marrow only | |||||||||||
| E663 | E661 | n/a | 11.4 | 2.2 | 4.0 | 1.2 | n/a | 9 | 16 | GVHD | Donor |
| E686 | E682 | n/a | 15.0 | 2.5 | 2.8 | 2.9 | n/a | n/a | 13 | Rejection | Host |
| E696 | E693 | n/a | 12.7 | 3.1 | 4.9 | 1.5 | n/a | n/a | 13 | Rejection | Host |
| E691 | E690 | n/a | 11.0 | 2.2 | 2.6 | 1.3 | n/a | 10 | 14 | GVHD | Donor |
| E697 | E696 | n/a | 10.0 | 2.6 | 2.0 | 1.2 | n/a | n/a | 19 | Rejection | Host |
| E024 | E023 | n/a | 9.0 | 4.0 | nd | nd | n/a | n/a | 21 | Rejection | nd |
| E023 | E024 | n/a | 6.5 | 1.0 | nd | nd | n/a | 11 | 25 | GVHD | nd |
| E029 | E030 | n/a | 9.4 | 4.3 | nd | nd | n/a | 8 | 14 | GVHD | nd |
| E030 | E029 | n/a | 12.5 | 3.5 | nd | nd | n/a | n/a | 16 | Rejection | nd |
| E084 | E088 | n/a | 9.8 | 4.1 | nd | nd | n/a | n/a | 15 | Rejection | nd |
| E088 | E084 | n/a | 10.1 | 4.4 | nd | nd | n/a | n/a | 15 | Rejection | nd |
Abbreviations are explained in the text or Table 2.
Dog E655 (not listed in table) received allogeneic CTLs and marrow but died on day +5. Necropsy showed radiation toxicity with marrow aplasia. This dog was not evaluable for engraftment and was not included in further statistical analysis.
Trafficking of transduced CTLs in vivo
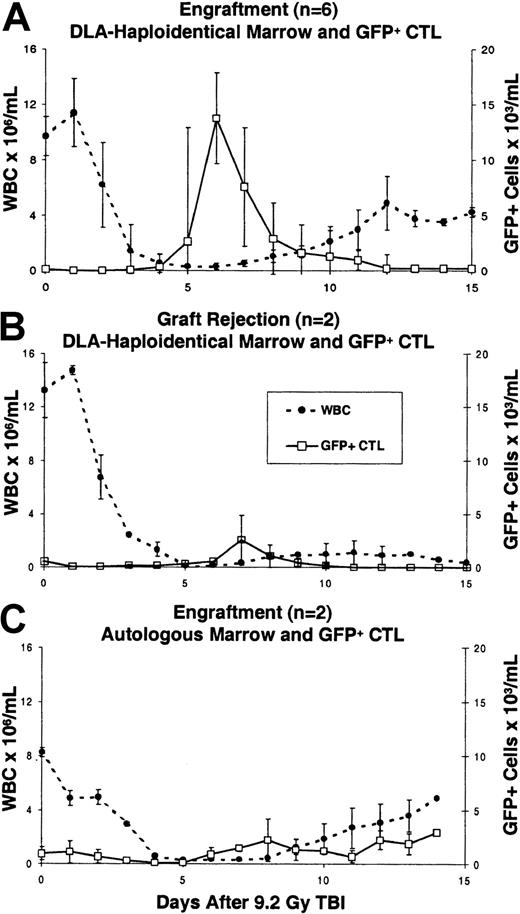
For the first 4 days after infusion of the recipient-specific CTLs into DLA-haploidentical recipients, there were low levels of circulating GFP+ cells (range, 0.005-1.05 × 103/mL) (Figure1A). When transduced CTLs were present at low levels in the peripheral blood, GFP expression in CTLs permitted more accurate tracking of transduced CTLs by flow cytometry compared to NGFR. From day 0 to +4 after transplantation the percent transduced cells in the peripheral blood was below the 0.5% to 1.0% background level of IgG1 isotype control antibody staining. Thereafter, among recipients that subsequently engrafted, there were sharp increases in the absolute numbers of transduced CTLs in the peripheral blood. The peak number of GFP+ (or NGFR+) cells in recipients on days +5 or +6 ranged from 7.8 to 66.3 × 103/mL (median 19.8 × 103/mL. The increases in circulating transduced CTLs were confirmed by semiquantitative PCR of peripheral blood DNA for neo (data not shown). Subsequently there were sharp declines in the absolute numbers of circulating transduced cells in the peripheral blood. With onset of engraftment and GVHD, GFP+ CTLs declined to 0.1 to 1.9 × 103/mL. In dogs E716 and E815 with graft rejection, GFP+ CTLs reached a peak of 4.8 and 0.5 × 103/mL, and then became undetectable by flow cytometry in the peripheral blood after days +11 and +9, respectively (Figure 1B).
Ex vivo expanded, transduced CTLs increase in the peripheral blood prior to engraftment in allogeneic recipients.
Shown are the median GFP+ (or NGFR+) cells/mL and total white blood cell count (WBC) ± SD for each group of dogs studied following 9.2 Gy TBI. Transduced cells were measured by flow cytometry of hemolyzed peripheral blood. (A) Engraftment following transplantation of allogeneic marrow and GFP+ (or NGFR+) CTLs (n = 6). There was a sharp increase in the number of transduced CTLs in the peripheral blood on days +5 to +7 after transplantation, followed by a decline in transduced CTLs on neutrophil recovery. (B) Rejection of allogeneic marrow and GFP+ CTLs. In dogs E716 and E815, GFP+ cells did not increase substantially after transplantation and subsequently were undetectable at days +11 and +9 after transplantation, respectively. Both dogs sustained an initial recovery of granulocytes to 1800 and 800/μL on days +10 and +8, respectively, followed by loss of granulocytes and GFP+ cells and emergence of donor-reactive lymphocytes. (C) Autologous marrow and GFP+CTL (n = 2). In both recipients, GFP+ cells were detected in the peripheral blood, but without a steep rise in GFP+cells prior to neutrophil recovery.
Ex vivo expanded, transduced CTLs increase in the peripheral blood prior to engraftment in allogeneic recipients.
Shown are the median GFP+ (or NGFR+) cells/mL and total white blood cell count (WBC) ± SD for each group of dogs studied following 9.2 Gy TBI. Transduced cells were measured by flow cytometry of hemolyzed peripheral blood. (A) Engraftment following transplantation of allogeneic marrow and GFP+ (or NGFR+) CTLs (n = 6). There was a sharp increase in the number of transduced CTLs in the peripheral blood on days +5 to +7 after transplantation, followed by a decline in transduced CTLs on neutrophil recovery. (B) Rejection of allogeneic marrow and GFP+ CTLs. In dogs E716 and E815, GFP+ cells did not increase substantially after transplantation and subsequently were undetectable at days +11 and +9 after transplantation, respectively. Both dogs sustained an initial recovery of granulocytes to 1800 and 800/μL on days +10 and +8, respectively, followed by loss of granulocytes and GFP+ cells and emergence of donor-reactive lymphocytes. (C) Autologous marrow and GFP+CTL (n = 2). In both recipients, GFP+ cells were detected in the peripheral blood, but without a steep rise in GFP+cells prior to neutrophil recovery.
CTLs generate allospecific cytotoxicity in vivo
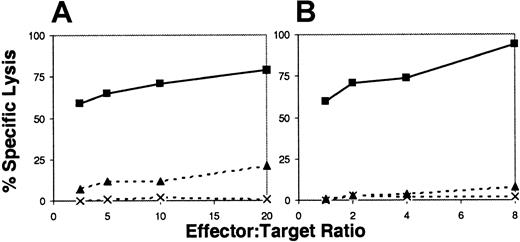
To determine if the GFP+ CTLs were capable of antirecipient activity following in vivo infusion, GFP+cells were collected from peripheral blood on day +6 after transplantation, isolated by flow cytometric cell sorting, and expanded ex vivo with irradiated donor and recipient PBMCs plus IL-2. After 7 days of in vitro expansion, there was a 30- to 110-fold increase in cells, and in all cases studied, cells were more than 99% GFP+, CD3+, and CD8+. The expanded GFP+ CTLs showed recipient-specific cytotoxicity with 72% to 94% specific lysis at an 8:1 effector-target ratio (Figure2B).
Donor CTLs generate allospecific cytotoxicity following infusion into recipients.
Representative example of 51Cr lysis results (A) immediately prior to infusion and (B) after transplantation into a DLA-haploidentical recipient. Donor CTLs lysed recipient Con-A blasts at all effector-target ratios studied (solid square with solid line), but had limited or minimal lysis of third-party (solid triangle, broken line) and autologous blasts (X, broken line). GFP+ cells were isolated by FACS cell sorting of peripheral blood on day +6 after transplantation and expanded ex vivo as described in “Material and methods.”
Donor CTLs generate allospecific cytotoxicity following infusion into recipients.
Representative example of 51Cr lysis results (A) immediately prior to infusion and (B) after transplantation into a DLA-haploidentical recipient. Donor CTLs lysed recipient Con-A blasts at all effector-target ratios studied (solid square with solid line), but had limited or minimal lysis of third-party (solid triangle, broken line) and autologous blasts (X, broken line). GFP+ cells were isolated by FACS cell sorting of peripheral blood on day +6 after transplantation and expanded ex vivo as described in “Material and methods.”
CTL distribution after allogeneic transplantation
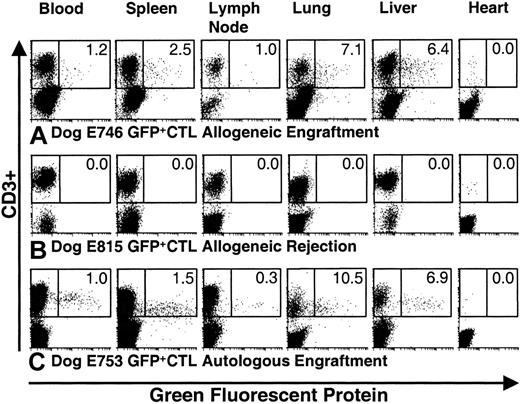
At necropsy, engrafted DLA-haploidentical recipients showed evidence of GFP+ CTLs in tissues affected with GVHD (Figures 3A and 4A,C). The highest infiltration of GFP+ CTL was observed in liver and lung, followed by spleen and lymph node (Figure 3A). In dog E746, 6.4% of CD3+cells isolated from the liver were GFP+. No GFP+ CTLs were detected in the heart (Figure 3A), thymus, and skeletal muscle (data not shown). PCR-ISH for neoconfirmed the presence of the GFP+ CTL in target tissues. In engrafted allogeneic recipients, GFP+ CTL were observed as: (1) multifocal aggregates in portal tracts, (2) aggregated in the periarteriolar lymphoid sheaths of the spleen, (3) localized in the lamina propria of the colon, and (4) scattered within the lung interstitium (Figure 4A-D, respectively). The GFP+ CTLs were distributed in tissue parenchyma in a pattern consistent with classical lymphocytic infiltrates observed with acute GVHD.25 31
GFP
+ CTLs were detected in tissues from engrafted recipients but not in recipients who had rejected donor grafts.Shown are histograms from representative experiments for detection of GFP+ and CD3+ cells obtained from blood, spleen, lymph node, lung, liver, and heart at necropsy. The boxes circumscribe CD3+ cells. The percent of CD3+cells that are GFP+ is indicated in each histogram. (A) Dog E746 engrafted and developed GVHD following 9.2 Gy TBI and transplantation of allogeneic marrow and GFP+ CTLs. GFP+ cells were detected in the blood, spleen, lymph node, lung, and liver but not in heart. In this recipient, 6.4% of CD3+ cells from the liver were GFP+. (B) Dog E815 rejected the donor graft; no GFP+ CTLs were detected in any tissue. (C) Dog E753 received autologous marrow and GFP+ CTLs following 9.2 Gy TBI. Here, 6.9% of CD3+ cells from the liver were GFP+.
GFP
+ CTLs were detected in tissues from engrafted recipients but not in recipients who had rejected donor grafts.Shown are histograms from representative experiments for detection of GFP+ and CD3+ cells obtained from blood, spleen, lymph node, lung, liver, and heart at necropsy. The boxes circumscribe CD3+ cells. The percent of CD3+cells that are GFP+ is indicated in each histogram. (A) Dog E746 engrafted and developed GVHD following 9.2 Gy TBI and transplantation of allogeneic marrow and GFP+ CTLs. GFP+ cells were detected in the blood, spleen, lymph node, lung, and liver but not in heart. In this recipient, 6.4% of CD3+ cells from the liver were GFP+. (B) Dog E815 rejected the donor graft; no GFP+ CTLs were detected in any tissue. (C) Dog E753 received autologous marrow and GFP+ CTLs following 9.2 Gy TBI. Here, 6.9% of CD3+ cells from the liver were GFP+.
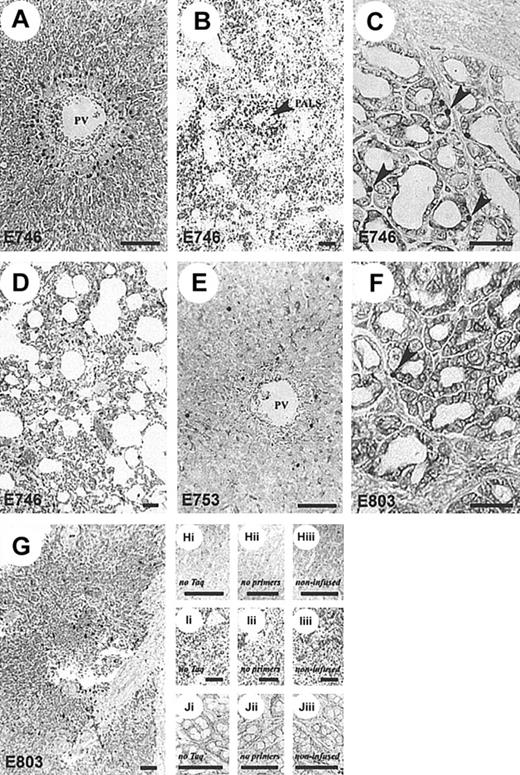
GFP+-,
neo+-transduced CTLs infiltrate target tissues. Representative histologic sections showing patterns of CTL localization in dogs receiving neo-transduced, donor-derived, recipient-specific CTLs and DLA-haploidentical marrow (A-D) compared to autologous CTLs and marrow (E-G).Neo+ CTLs localized to sites common to GVHD but only in dogs receiving allogeneic marrow. Aggregates ofneo+ cells were present in areas adjacent to the portal tracts identified by the hepatic portal vein (PV) (A) and splenic periarteriolar lymphatic sheaths (PALS, arrow) (B). Increased numbers of neo+ cells were also present within the lamina propria of the colon (arrows) (C) and scattered throughout the pulmonary interstitium (D). Autologous CTL infusions (E-G) resulted in random distribution of gene-marked T cells in liver (E), colon (F) (arrow indicates examples of neo+cells), and spleen (G). A full description of ISH-PCR assay and tissue controls (H-J), as described in “Materials and methods” and elsewhere,26 27 was used to validate these findings. Bar, 100 μm; original magnification × 100 (A, C, E, F, H, J) and × 40 (B, D, G, I).
GFP+-,
neo+-transduced CTLs infiltrate target tissues. Representative histologic sections showing patterns of CTL localization in dogs receiving neo-transduced, donor-derived, recipient-specific CTLs and DLA-haploidentical marrow (A-D) compared to autologous CTLs and marrow (E-G).Neo+ CTLs localized to sites common to GVHD but only in dogs receiving allogeneic marrow. Aggregates ofneo+ cells were present in areas adjacent to the portal tracts identified by the hepatic portal vein (PV) (A) and splenic periarteriolar lymphatic sheaths (PALS, arrow) (B). Increased numbers of neo+ cells were also present within the lamina propria of the colon (arrows) (C) and scattered throughout the pulmonary interstitium (D). Autologous CTL infusions (E-G) resulted in random distribution of gene-marked T cells in liver (E), colon (F) (arrow indicates examples of neo+cells), and spleen (G). A full description of ISH-PCR assay and tissue controls (H-J), as described in “Materials and methods” and elsewhere,26 27 was used to validate these findings. Bar, 100 μm; original magnification × 100 (A, C, E, F, H, J) and × 40 (B, D, G, I).
CTL distribution after autologous transplantation
To determine if the allogeneic origin of the CTLs and not the method of stimulation was responsible for the observed pattern of CTL trafficking and tissue distribution, the following control experiment was conducted. Autologous marrow and GFP+ CTLs were infused following 9.2 Gy TBI in 2 recipients, E753 and E803. The ex vivo expanded autologous GFP+ CTLs were allospecific to respective DLA-haploidentical littermates. Hematopoietic reconstitution occurred as expected following autologous marrow transplantation, and there was persistence of circulating GFP+ CTLs without the characteristic sharp rises of GFP+ CTLs seen in each of the DLA-haploidentical recipients (Figure 1C). To compare GFP+CTL tissue distribution with the DLA-haploidentical recipients, E753 and E803 were euthanized on day +13 and +14 after autologous transplantation, respectively. GFP+ CTLs were detected by flow cytometry in tissues at necropsy in similar tissue distribution to that observed in the engrafted DLA-haploidentical recipients (Figure3C). However, PCR-ISH for neo in liver, colon, and spleen necropsy specimens demonstrated that the autologous GFP+CTLs were diffusely scattered in tissue, at a lower cell frequency and without focal aggregates, compared to an allogeneic recipient (Figure 4E-G).
Graft rejection and eradication of donor CTLs
Two recipient dogs rejected their DLA-haploidentical donor grafts despite the infusion of recipient-specific GFP+ CTLs. Dogs E716 and E815 showed evidence of transient donor engraftment by rises in peripheral blood granulocytes (Figure 1B) to 1800/μL and 800/μL on day +10 and +8 after transplantation, respectively, and the presence of more than 98% donor chimerism by microsatellite marker analysis of peripheral blood cells on day +8. However, by day +14 and +11 there was an abrupt decline in the absolute neutrophil count to 200/μL and undetectable, respectively. On day +14 and +12, respectively, microsatellite marker analysis and flow cytometry of blood showed emergence of 100% host CD3+ T-cell receptor (TCR)-αβ+ cells. PBMCs obtained from dogs E716 and E815 on day +14 and +12, respectively, were cultured for 7 days with irradiated donor and recipient PBMCs. The 51Cr release assay showed 71% and 84% cytotoxicity specific for the donor Con-A blasts at a 20:1 effector-target ratio, respectively, but no lysis of third-party Con-A blasts. At necropsy, there was no evidence of circulating or tissue-infiltrating GFP+ cells (Figure 3B) and PCR-ISH for neo was negative. Taken together, these studies confirmed initial donor engraftment followed by graft rejection.
Discussion
In this study, donor-derived, recipient-specific CTLs, transduced with a retroviral vector that permitted tracking of cells, significantly enhanced engraftment of DLA-haploidentical marrow in the setting where graft rejection was expected. To our knowledge, these results are the first reported in a large animal model that show in vivo biologic activity of ex vivo expanded, genetically modified T cells.
Successful HSCT requires the crossing of a bidirectional immunologic barrier consisting of graft-versus-host (GVH) and host-versus-graft (HVG) reactions. Resistance to marrow grafts was increased in this MHC-mismatched setting as demonstrated in the control recipients given marrow only with 4 of 11 recipients engrafting and developing GVHD. The infused CTLs significantly enhanced DLA-haploidentical donor marrow engraftment by increasing the GVH reaction with specific suppression of the HVG reaction. The mechanism by which donor CD8+cytotoxic effector cells prevent allogeneic marrow graft rejection was shown in murine studies to involve perforin-dependent T-cytotoxic responses against MHC class I alloantigens.32 We hypothesize that in the current study the recipient-specific donor CTLs lysed or otherwise directly inhibited the function of the radiation-resistant host T and natural killer (NK) cells mediating graft rejection.33
Under the conditions examined, infused CTLs did not reliably prevent rejection of DLA-haploidentical positively selected CD34+cells despite highly specific in vitro cytotoxic activity. The failure of engraftment seen in these dogs was either due to loss of in vivo activity of CTLs with ex vivo manipulation and culture, insufficient numbers of CTLs infused, or loss of a facilitator cell population removed in the CD34+ selection process. However, ex vivo expanded CTLs enhanced DLA-haploidentical marrow engraftment. Previous experiments showed that the number of CD34+ cells infused into the current DLA-haploidentical recipients was sufficient to achieve engraftment in the autologous setting and in DLA-identical littermates following 9.2 Gy TBI.20 Based on methods used, we were unable to further increase the number of CD34+cells isolated without significantly compromising cell purity. Most likely, CD34+ selection from PBSCs resulted in the removal of a facilitating cell population that was necessary to achieve engraftment in the DLA-haploidentical setting. Candidate facilitating cells removed by CD34+ selection include CD4+cells capable of producing IL-2 to support the graft-enhancing function of the CD8+ CTLs, CD8+TCRαβ−facilitator cells,34,35 and IL-10–secreting monocytes.36 37
The use of ex vivo expanded, transduced T cells for antileukemia/lymphoma therapy has recently been described, but in vivo function has been limited to anecdotal evidence of alloreactivity and graft-versus-tumor effect.3,6 The largest reported clinical studies of genetically modified donor T cells infused into patients with disease relapse after allogeneic hematopoietic cell transplantation have been most remarkable for the lack of in vivo alloreactivity of the infused T cells.4,5 Infusion of HSV-tk–transduced, OKT-3 plus IL-2–stimulated T cells at the time of T-cell–depleted HLA-identical marrow transplantation was associated with a low incidence of GVHD and absence of an enhanced graft-versus-leukemia benefit.7 In that study, 3 of 12 patients treated developed Epstein-Barr virus lymphoproliferative disease consistent with poor immune reconstitution and loss of function of ex vivo expanded, transduced donor T cells. In murine studies, in vivo activities of ex vivo expanded cells were significantly reduced compared with freshly infused donor lymphocytes, despite the demonstration of in vitro function.38
Other investigators have reported a loss of function and a high rate of apoptosis following infusion of ex vivo expanded CD8+ T cells.39 Our data were not consistent with massive, early apoptosis of donor T cells because there was a large increase in circulating alloreactive GFP +CTLs at 5 to 6 days after transplantation followed by persistence of cells in GVHD-affected tissues. This suggests that the method of ex vivo T-cell expansion may have a significant influence on whether cells maintain in vivo function. The use of a nonspecific polyclonal mitogen such as OKT3 and supraphysiologic doses of IL-2, although resulting in massive T-cell proliferation ex vivo, may cause substantial TCR Vβ receptor skewing,40 loss of alloreactivity,41 and increased susceptibility to apoptosis following in vivo infusion.39
We observed that transduced allogeneic CTLs had a characteristic pattern of trafficking distinct from autologous cells. For the first 4 days after infusion there were low levels of allogeneic GFP+ CTLs in the peripheral blood. This likely represented the trafficking of CTLs from the peripheral circulation to the reticuloendothelial system, marrow, and lymphatic regions, presumably to target and lyse radioresistant host cells with the potential of mediating graft rejection. Among recipients of transduced allogeneic CTLs there were sharp rises in the absolute numbers of circulating CTLs on days +5 and +6 after transplantation immediately before recovery of donor-derived neutrophils. The steep increases in circulating CTLs were only observed in engrafting allogeneic recipients and likely reflected proliferative responses of the infused CTLs. Following recoveries of neutrophil counts, the absolute numbers of transduced CTLs declined to low but persistently detectable levels in the blood. This was consistent with trafficking of CTLs out of the circulation and infiltration into tissues with alloantigen presentation, the classic GVHD target organs. At the time of necropsy, there was enrichment for CTLs in organs relative to the peripheral blood.
Further evidence for specific in vivo function of the ex vivo expanded CTLs in this study was the observation that transduced CTLs infiltrated tissues with histologic evidence of GVHD and were absent from tissues unaffected by GVHD such as heart and skeletal muscle. PCR-ISH results showed aggregated transduced allogeneic CTLs at the extravascular sites of lymphocytic infiltration typical of GVHD histopathology. In contrast, CTLs infused with autologous marrow were detected by flow cytometry in the same tissues as the allogeneic CTL recipients but PCR-ISH analyses of the liver, colon, and spleen showed autologous CTLs were rare and randomly distributed. These findings are consistent with the conclusion that tissue-specific homing of the transduced CTLs had occurred and was related to the alloreactivity of the T cells despite 4 weeks of ex vivo expansion.
Alternative mechanisms to overcome graft resistance while avoiding GVHD include the use of donor cells with veto activity.42,43 In murine studies, administration of large numbers of nonhost reactive or anti–third party T cells has successfully prevented graft rejection in the MHC-mismatched transplantation setting.44-46 Similarly, a CTL clone that enhances engraftment of H2d disparate donor marrow without GVHD but requiring very large numbers of infused T-cell clones and exogenous IL-2 has been described.38 Alternatively, megadose CD34+ cells have been infused to overcome graft resistance,47 although in clinical trials substantially more intensive immunosuppression of the recipient was required and it was not always possible to achieve a cell dose more than 25 × 106 CD34+/kg for adult recipients.48 Recipient-specific alloreactive T cells used in the current study would only be clinically useful if the GVH effect can be precisely controlled with a suicide gene mechanism.
In summary, these results show that donor-derived, ex vivo expanded genetically modified CTLs maintained in vivo function and enhanced engraftment of DLA-haploidentical marrow. The CTLs did not enhance engraftment of DLA-haploidentical positively selected CD34+cells. These results establish a large animal model in which to study add-back of genetically modified T cells to enhance engraftment and control GVHD after T-cell–depleted allogeneic HSCT. In addition, this model is ideal for studies to compare different suicide gene vectors and different methods of ex vivo T-cell expansion to determine optimal use for human trials.
The authors are very grateful to the highly dedicated technicians of the shared Canine Resource Unit and the Hematology and Transplantation Biology Laboratories. Benjamin Weigler and Michelle Spector provided veterinary support. We thank Mary Beauchamp for her excellent technical assistance. We are grateful to Bonnie Larson, Helen Crawford, Lori Ausburn, and Sue Carbonneau for their outstanding secretarial support.
Supported in part by grants DK42716, HL63457, HL36444, DK56465, and CA15704 from the National Institutes of Health (NIH), Department of Health and Human Services, Bethesda, MD, and a collaborative agreement with Genetic Therapy, a Novartis company, Gaithersburg, MD. G.G. received support from NIH grant DK02753. S.J.B. was supported by NIH grants AI27757 and AI43650. M.T.L. received support from the Lady Tata Memorial Trust, London, United Kingdom. H.P.K. is a Markey Molecular Medicine Investigator. R.S. also received support from the Laura Landro Salomon Endowment Fund, and through a prize awarded by the Joseph Steiner Krebsstiftung, Bern, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
George E. Georges, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, PO Box 19024, Seattle, WA 98109; e-mail: ggeorges@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal