Abstract
Recently, a collection of surface markers was exploited to isolate viable Lin− TdT+ cells from murine bone marrow. These early pro-B cells were enriched for B-lineage lymphocyte precursor activity measured by short-term culture and had little responsiveness to myeloid growth factors. Early precursors can be propagated with remarkably high cloning frequencies in stromal cell–free, serum-free cultures, permitting this analysis of direct regulatory factors. Expression of the interleukin-7 receptor (IL-7Rα) chain marks functional precursors and IL-7 is necessary for progression beyond the CD45RA+ CD19− stage. Efficient survival and differentiation were only observed when stem cell factor and Flt-3 ligand were also present. IL-7–responsive CD19+precursors are estrogen resistant. However, B-lineage differentiation was selectively abrogated when highly purified Lin− precursors were treated with hormone in the absence of stromal cells. In addition, early stages of B lymphopoiesis were arrested by limitin, a new interferon (IFN)–like cytokine as well as IFN-α, IFN-γ, or transforming growth factor β (TGF-β), but not by epidermal growth factor (EGF). Lin− TdT+early pro-B cells are shown here to be CD27+AA4.1+/−Ki-67+ Ly-6C−Ly-6A/Sca-1Lo/−Thy-1−CD43+CD4+/−CD16/32Lo/−CD44Hi and similar in some respects to the “common lymphoid progenitors” (CLP) identified by others. Although early pro-B cells have lost myeloid differentiation potential, transplantation experiments described here reveal that at least some can generate T lymphocytes. Of particular importance is the demonstration that a pivotal early stage of lymphopoiesis is directly sensitive to negative regulation by hormones and cytokines.
Introduction
The humoral immune system is replenished throughout life with B cells produced by the bone marrow. This process is being increasingly understood in terms of selective gene expression with transcription factors playing a major role in determining how hematopoietic stem cells adopt one of 8 major fates. However, much remains to be learned about the timing of events and how they are influenced by positive and negative extracellular cues provided by the microenvironment. Multiparameter flow cytometry and cell sorting allows isolation of rare populations of bone marrow cells and it is important to order them in a differentiation sequence that culminates with the production of B lymphocytes. Expression of the enzyme terminal deoxynucleotidyl transferase (TdT) is thought to be an early, lymphocyte-specific event with functional consequences.1TdT contributes to the diversity of antibody combining sites by adding non–germline-encoded nucleotides at sites of V-DJ segment joining during immunoglobulin gene recombination.2,3 We recently developed a means to identify and sort TdT+ bone marrow cells lacking surface markers associated with any of the mature blood cell lineages (Lin−).4 5 According to tradition, they were termed “early pro-B” cells and they were highly enriched for functional B-lymphocyte lineage precursor activity. That is, early pro-B cells gave rise to CD45R+CD19− and CD19+ lymphocytes but generated few nonlymphoid cells on contact with stromal cells. These precursors comprise approximately 0.06% of the nucleated cells in marrow and may represent an important developmental compartment that must be transited by differentiating stem cells. We now report that early pro-B cells expand and differentiate under defined, stromal cell–free culture conditions. This model made it possible to examine the importance of functional interleukin-7 (IL-7) receptors (IL-7R) and other factors for their survival, proliferation, and lineage progression. Early pro-B cells were directly sensitive to factors previously described to be negative regulators of B lymphopoiesis in intact animals.
Some of the early pro-B cell characteristics are similar to ones previously used by another group to isolate “common lymphoid progenitors” (CLP).6 At least 20% of Lin− Sca-1+ IL-7Rα+ CLP could expand in culture and single cells had the potential for T, B, and natural killer (NK) cell differentiation. Stem cells with long-term repopulating potential can be distinguished from hematopoietic progenitors by the absence of CD27 together with expression of c-kit and Sca-1.7 Still other markers have been exploited to isolate categories of marrow cells with restricted potential for differentiation in nonlymphoid lineages.8 One objective of the present study was to determine features of Lin−TdT+ cells that would allow more direct comparison to CLP and other progenitor subsets. In addition to CLP, populations designated common myeloid precursors and fraction A0 pre pro-B cells were of interest.8 9 We also assessed the potential of early pro-B cells for T-lymphocyte lineage differentiation in transplantation experiments. The molecular and cellular complexity of the bone marrow was experimentally reduced with the objective of learning when differentiation fate decisions are made and how they are regulated by extracellular signals.
Materials and methods
Mice
BALB/c mice were obtained at 5 to 15 weeks of age from the Oklahoma Medical Research Foundation Laboratory Animal Resource Center or Charles Rivers Breeding Laboratories (Wilmington, ME) and used for culture experiments. C57BL/6 mice were obtained from Charles Rivers or the Jackson Laboratory (Bar Harbor, ME) and used for surface marker characterization of Lin− TdT+ cells. RAG-1−/− mice (5-10 weeks old) and 5- to 10-week-old C57BL/6 SJL mice (Ly5.1 background) were purchased from the Jackson Laboratory and used in transfer experiments.
Antibodies
Anti-CD19 monoclonal antibody (mAb) (1D3) was purified from the culture supernatant of hybridoma cells grown in our laboratory. Anti-CD45RA (14.8) mAb developed in our laboratory and the anti–Mac-1/CD11b (M1/70) mAb were used as culture supernatants of the respective hybridomas. The rat mAb to mouse IL-7Rα subunit (SB199) was established in our laboratory, purified from hybridoma supernatant and biotinylated.10Phycoerythrin (PE)-conjugated and allophycocyanin (APC)-conjugated antibodies to AA4.1 antigen were kindly provided by Dr R. R. Hardy (Fox Chase Cancer Center, Philadelphia, PA). Purified antierythroid (Ter-119) and anti–Ly6-G (Gr-1; RB6-8C5) mAbs, fluorescein isothiocyanate (FITC)–conjugated anti-CD3, (145-2C11) anti-CD8, (53-6.7) anti-CD45R/B220 (RA3/6B2), anti–Mac-1 (M1/70), and anti–Gr-1 (Ly-6G; RB6-8C5) antibodies, PE-conjugated Ter-119, anti-CD45R/B220 (RA3/6B2), anti-Flk2/Flt3 (A2F10.1), anti-CD19, (1D3) anti–Sca-1 (Ly6A/E; E13-161.7), anti-CD16/CD32 (2.4G2) and anti-Ki67 (B56) antibodies, biotinylated anti-CD27 (LG3A10) antibody and APC-conjugated anti–c-kit, anti-CD8, anti-CD45R, anti–Mac-1 antibodies were all purchased from BD Pharmingen (San Diego, CA). Purified rabbit anti-TdT polyclonal antibody and a FITC-conjugated F(ab′)2 fraction of goat antirabbit IgG antibody were purchased from Supertechs (Rockville, MD). FITC-conjugated goat antimouse IgM antibody was purchased from Zymed (San Francisco, CA).
Reagents
Recombinant mouse IL-7 was purchased from Endogen (Woburn, MA). Recombinant mouse stem cell factor (SCF), Flt-2/Flk-3 ligand (FL), and transforming growth factor-β (TGF-β) were purchased from R&D Systems (Minneapolis, MN). 1, 3, 5[10]-Estratriene-3, 17β-diol (β-estradiol) was purchased from Sigma Chemical (St Louis, MO). Epidermal growth factor (EGF) was purchased from Upstate Biotechnology (Lake Placid, NY). Mouse interferon (IFN)-α and rat IFN-γ were purchased from Life Technologies (Rockville, MD). Limitin-human immunoglobulin (IgG1) heavy-chain constant region fusion protein was prepared as previously described.11
Isolation of lineage-negative early pro-B cells
Bone marrow cells were collected from 4 to 10 mice and suspended with phosphate-buffered saline (PBS) without Ca++ or Mg++ (PBS−) and supplemented with 3% heat-inactivated fetal calf serum (staining wash). Cells were incubated with antibodies to lineage markers (Gr-1 and Mac-1 for myeloid cells, anti-CD19 and anti-CD45R for B lineage cells, and Ter-119 for erythroid cells) for 30 minutes. Then cells were washed and incubated with BioMag goat-antirat IgG-coated magnetic beads (Polysciences, Warrington, PA) for 15 minutes. Cells attached to beads were removed with a magnetic separator. Recovered cells were further incubated with new magnetic beads and subjected to a second round of magnetic separation. These enriched lineage marker–negative bone marrow cells were then stained with a cocktail of labeled antibodies to the lineage markers (FITC-conjugated anti-CD3, anti-CD8, anti–Gr-1, PE-conjugated Ter-119 and anti-CD45R) and APC-conjugated anti–c-kit antibody. In some experiments, PE-labeled anti-FL antibody and biotinylated anti–IL-7Rα antibody were also used. In this case, PE-labeled Ter-119 was eliminated and FITC-conjugated anti-CD45R was used instead of PE-conjugated antibody. Streptavidin-RED613 (Life Technologies) was used as the secondary reagent for biotinylated anti–IL-7Rα. Stained cells were subjected to sorting on a MoFlo (Cytomation, Fort Collins, CO). When reanalyzed after sorting, lineage-negative cells were generally more than 95% pure; that is, less than 5% had even slightly above background levels of staining. Subsets of these Lin−cells were usually more than 90% pure when sorted according to absence, low density, or high density of c-kit. The IL-7Rα+ cells were relatively homogenous, but the IL-7Rα− preparations included some cells with low, but above threshold staining.
Cellculture
Sorted cells were put into 24-well culture plates (Costar, Cambridge, MA) containing 1 mL X-VIVO15 medium (Biowhittaker, Walkersville, MD) containing 1% detoxified bovine serum albumin (Stem Cell Technologies, Vancouver, BC, Canada), 5 × 10−5 M 2-mercaptoethanol (2-ME), 2 mM l-glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin, and cytokines as indicated and cultured at 37°C and 5% CO2 in a humidified atmosphere. At the end of culture, cells were harvested, cell viability was determined with a trypan blue dye exclusion method, and then cells were subjected to flow cytometric analysis. For single-cell sorting experiments, cells were directly sorted into wells of 96-well U-bottom tissue culture plates (Costar) containing 100 μL of the same media. The concentrations of cytokines were IL-7, 1 ng/mL; FL,100 ng/mL; and SCF, 20 ng/mL.
Colony assay for granulocyte-macrophage progenitors
Sorted cells were put into methylcellulose medium containing murine IL-3 (mIL-3), human IL-6 (hIL-6), and murine SCF (mSCF) (MethoCult GF M3534, Stem Cell Technologies) with 200 to 1000 cells plated per dish. After 10 days of culture in a humidified CO2 incubator at 37°C, numbers of colonies were counted with an inverted microscope.
Immunofluorescence staining
For the analysis of surface antigens, cells were incubated with combinations of labeled antibodies in PBS. They were then washed and incubated with streptavidin-RED613 to detect biotinylated primary antibodies. To characterize TdT+ cells, sorted lineage-negative cells were first stained using PE-conjugated, APC-conjugated, or biotinylated antibodies in combination with streptavidin-RED613. Then cells were fixed with 1% formaldehyde in 1.25 × PBS followed by permeabilization with 70% ethanol. Then, intranuclear TdT was stained using anti-TdT polyclonal rabbit antibody and the FITC-conjugated F(ab′)2 fraction of a goat antirabbit IgG antibody. PE-conjugated anti–Ki-67 antibody was included at this step in some experiments. Stained cells were run on a FACScalibur flow cytometer (Becton Dickinson, San Diego, CA) and the data were analyzed with Flojo software (Treestar, San Carlos, CA).
In vivo transfer
Lineage marker–negative cells from C57BL/6 SJL mice were sorted to isolate the c-kitLo Flk-2/Flt-3+IL-7Rα+ fraction. One thousand to 3000 of these early pro-B cells were injected into RAG1−/− mice that had been given 400 rad irradiation. Spleens and thymuses were harvested from these mice 2 or 3 weeks after injection and stained for flow cytometric analysis.
Results
Three cytokines permit survival and differentiation of early lymphocyte precursors
Jacobsen and colleagues reported the production of CD45R/B220+ and then CD19+ lymphocytes in serum-free cultures supported only by recombinant cytokines.12 They used magnetic beads and sorting to enrich for c-kit+ Sca-1+ cells with low expression of lineage markers and their focus was on investigating roles played by SCF and Flt-3 ligand. We now report that their stromal cell–free conditions are also ideal for B lymphopoiesis in cultures initiated with the TdT+ cell-enriched Lin−c-kitLo fraction of marrow, that is, early pro-B cells. For initial experiments, bone marrow cell suspensions were rigorously depleted of lineage marker–positive (GR-1, CD11b/Mac-1, CD45RA, CD19, TER119, CD3, and CD8) cells and then separated into 3 fractions on the basis of c-kit density.4 At least one third of the cells in the c-kitHi population were myeloid precursors that proliferated in a colony-forming unit in culture (CFU-c) assay (as described in “Materials and methods”). However, small numbers of CD19+ cells were produced during a 7-day culture with SCF, FL, and IL-7 (Figure 1). Although essentially no myeloid progenitors were present in the c-kitLo subset, it produced at least 10 times more (up to 800/input cell) CD19+ B-lineage progeny in culture. Consistent with our previous study,4 small numbers of rapidly differentiating lymphocyte precursors were present in a fraction that completely lacks c-kit. As with stromal cell cocultures, the time required under defined conditions for generation of CD19+ cells from Lin− c-kitHicells was longer than when the cultures were initiated with Lin− c-kitLo/− cells (data not shown). Indeed, down-regulation of this SCF receptor coincides with loss of myeloid differentiation potential and progression through the early steps of lymphopoiesis.4 Defined culture models of this kind allow detailed investigation of the importance and signals transmitted via such receptors.
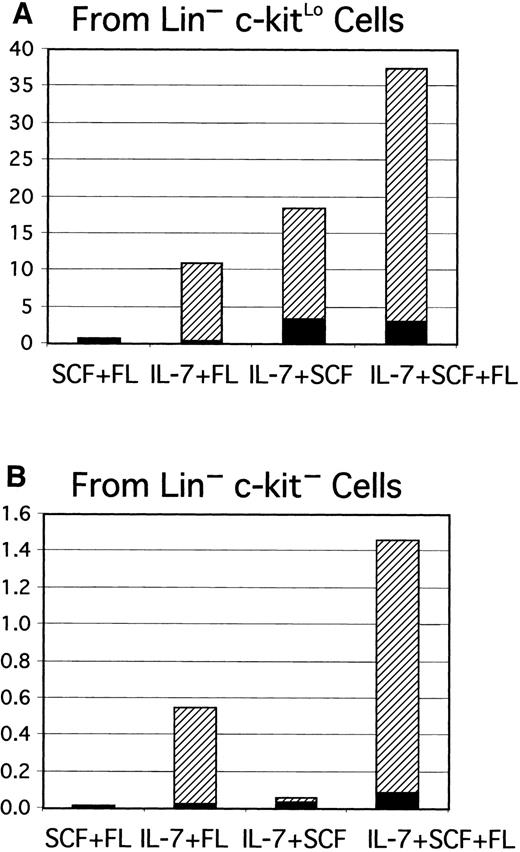
B-lineage lymphocytes arise from early precursors under defined culture conditions.
Sorted Lin− c-kitHi (7500 cells/well), Lin− c-kitLo (3000 cells/well), or Lin− c-kit− cells (10 000 cells/well) were placed in serum-free, stromal cell-free cultures. After 7 days cells were collected, counted, and analyzed by flow cytometry. Each dot represents absolute numbers of CD19+ cells per input in a single experiment. This was determined by simply dividing numbers of recovered lymphocytes by the numbers of precursors used to initiate the cultures.
B-lineage lymphocytes arise from early precursors under defined culture conditions.
Sorted Lin− c-kitHi (7500 cells/well), Lin− c-kitLo (3000 cells/well), or Lin− c-kit− cells (10 000 cells/well) were placed in serum-free, stromal cell-free cultures. After 7 days cells were collected, counted, and analyzed by flow cytometry. Each dot represents absolute numbers of CD19+ cells per input in a single experiment. This was determined by simply dividing numbers of recovered lymphocytes by the numbers of precursors used to initiate the cultures.
A requirement for IL-7 and its receptor
Gene-targeting experiments have established the importance of IL-7 for B lymphopoiesis in mice and expression of the IL-7R must represent an important differentiation milestone.13 14 Therefore, this aspect was investigated under defined culture conditions. At least some CD45R+ cells were produced from Lin−c-kitLo precursors in 1-week cultures established with any of several cytokine combinations, but CD19+ cells emerged only when IL-7 was present (Figure 2A). IL-7 was also needed for production of CD19+ cells from cultures started with Lin− c-kit− precursors (Figure 2B). Additional experiments confirmed that the combination of SCF, FL, and IL-7 was optimal and almost no cells were recovered from cultures maintained with IL-7 alone. The Lin−Flk-2/Flt-3+ c-kitLo population was then sorted into subsets on the basis of IL-7Rα expression and cultured (Figure3). The starting IL-7RαLo/− category included 9% to 37% TdT+ cells, whereas 25% to 72% of the IL-7Rα+ fraction expressed TdT. CD19+ cells were produced when cultures were initiated with IL-7RαLo/− precursors, but less efficiently than in cultures of IL-7Rα+ cells. Single Lin−Flk-2/Flt-3+ c-kitLo IL-7Rα+cells were then placed in 96 individual culture wells along with SCF, FL, and IL-7 in 2 experiments. After 11 days, growth was observed in 25 or 29 wells and 20 or 25 of them contained CD19+lymphocytes, respectively. The cloning efficiency was approximately 6-fold lower in a comparable experiment done with single Lin− Flk-2/Flt-3+ c-kitLoIL-7RαLo/− cells. When considered with the results shown above, these findings indicate that 3 functional cytokine receptors cooperate to deliver signals for survival, proliferation, or differentiation of early lymphocyte precursors, but IL-7 is particularly important for transition to the CD19+stage.
IL-7 is required for progression from the CD45R+CD19− stage to the CD45R+CD19+ stage.
Lin−c-kitLo (A) or Lin−c-kit− cells (B) were sorted to high purity and placed in serum-free, stromal cell–free cultures with the indicated combinations of cytokines. Yields of CD45R+CD19− (▪) and CD45R+CD19+ (▨) cells per input precursor were calculated as described in the legend to Figure 1.
IL-7 is required for progression from the CD45R+CD19− stage to the CD45R+CD19+ stage.
Lin−c-kitLo (A) or Lin−c-kit− cells (B) were sorted to high purity and placed in serum-free, stromal cell–free cultures with the indicated combinations of cytokines. Yields of CD45R+CD19− (▪) and CD45R+CD19+ (▨) cells per input precursor were calculated as described in the legend to Figure 1.
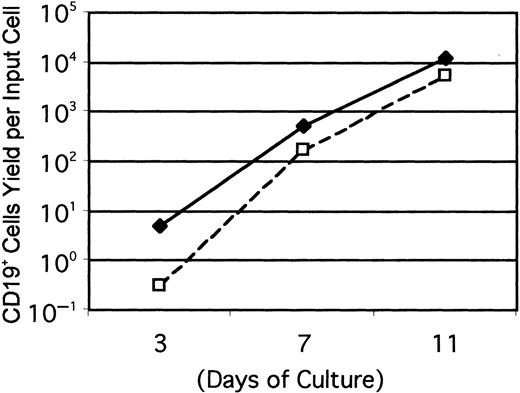
Acquisition of IL-7R corresponds with rapid B-lineage differentiation in culture.
Cultures were initiated with Lin− c-kitLoFlk-2/Flt-3+ IL-7Rα+ (♦) or Lin− c-kitLo Flk-2/Flt-3+IL-7RαLo/− (■) cells and evaluated at the indicated intervals. Numbers of CD19+ lymphocytes recovered per input precursor are shown from one of 3 independent experiments that gave very similar results.
Acquisition of IL-7R corresponds with rapid B-lineage differentiation in culture.
Cultures were initiated with Lin− c-kitLoFlk-2/Flt-3+ IL-7Rα+ (♦) or Lin− c-kitLo Flk-2/Flt-3+IL-7RαLo/− (■) cells and evaluated at the indicated intervals. Numbers of CD19+ lymphocytes recovered per input precursor are shown from one of 3 independent experiments that gave very similar results.
Early lymphocyte precursors are directly influenced by negative regulators
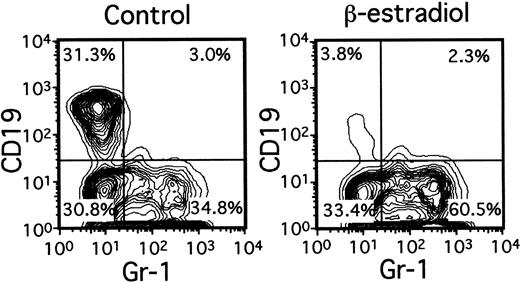
A variety of evidence suggests that lymphopoiesis in the thymus and bone marrow is limited by sex steroids, but the nature of the target cells remains elusive.15 Lin−c-kitHi cells with the potential for lymphoid or myeloid differentiation were placed in serum-free, stromal cell–free cultures containing only SCF, FL, and IL-7 (Figure4). Although total numbers of Gr-1+ myeloid progeny were slightly increased by inclusion of β-estradiol in the medium (1.1- to 2.5-fold), production of CD19+ lymphocytes was almost completely prevented. Titration experiments indicated that 10−8 M concentrations of the hormone were sufficient for maximum suppression (not shown). The next putative stage of differentiation also appeared to be directly sensitive to estrogen. That is, the ability of Lin−c-kitLo or Lin− c-kit− marrow cells to generate CD19+ lymphocytes in 1-week cultures was suppressed 78% ± 11% (Table 1) or 92% ± 3%, respectively, by β-estradiol. Interestingly, absolute numbers of CD45R+ CD19− cells were actually increased in estrogen- containing cultures as compared to control cultures (Table 1). The same accumulation of CD45R+CD19− cells (168.2% ± 34.8% of controls) occurred in estrogen-treated cultures initiated with Lin−c-kitHi precursors. We previously found that estrogen had no influence on the IL-7–driven clonal proliferation of lymphoid progenitors in semisolid agar cultures.16 Indeed, the same hormone preparations found here to be suppressive for early stages of lymphopoiesis were ineffective in CFU–IL-7 assays (not shown). Although late events in B lymphopoiesis can be influenced by estrogen only via effects on stromal cells, early precursors may be direct hormone targets.16
Early lymphocyte precursors are directly and selectively inhibited by estrogen in culture.
Lin− c-kitHi BM cells were placed in serum-free, stromal cell-free cultures with or without β-estradiol (10−8M). Gr-1+ myeloid and CD19+lymphoid cells determined by flow cytometry are shown after 7 days of culture.
Early lymphocyte precursors are directly and selectively inhibited by estrogen in culture.
Lin− c-kitHi BM cells were placed in serum-free, stromal cell-free cultures with or without β-estradiol (10−8M). Gr-1+ myeloid and CD19+lymphoid cells determined by flow cytometry are shown after 7 days of culture.
Early lymphocyte precursors are direct targets for negative regulation by hormones and cytokines
| Factor (concentration) . | CD45R+, CD19−cells . | CD45R+, CD19+ cells . |
|---|---|---|
| β-Estradiol (10−8 M)* | 239.8 ± 208.7 | 22.0 ± 11.0 |
| Limitin (200 ng/mL)† | 5.6, 4.7 | 1.2, 0.1 |
| Interferon-α (10 U/mL)† | 14.9, 3.3 | 5.9, 4.2 |
| Interferon-γ (10 U/mL)† | 19.5, 17.8 | <0.1, 0.3 |
| TGF-β (1 ng/mL)† | 8.3, 9.0 | 11.0, 9.1 |
| EGF (50 ng/mL)† | 94.1, 62.9 | 82.2, 87.2 |
| Factor (concentration) . | CD45R+, CD19−cells . | CD45R+, CD19+ cells . |
|---|---|---|
| β-Estradiol (10−8 M)* | 239.8 ± 208.7 | 22.0 ± 11.0 |
| Limitin (200 ng/mL)† | 5.6, 4.7 | 1.2, 0.1 |
| Interferon-α (10 U/mL)† | 14.9, 3.3 | 5.9, 4.2 |
| Interferon-γ (10 U/mL)† | 19.5, 17.8 | <0.1, 0.3 |
| TGF-β (1 ng/mL)† | 8.3, 9.0 | 11.0, 9.1 |
| EGF (50 ng/mL)† | 94.1, 62.9 | 82.2, 87.2 |
TGF-β indicates transforming growth factor β; EGF, epidermal growth factor.
Highly purified Lin− c-kitLo cells were held for 1 week in serum-free, stromal cell-free cultures. Yields of CD45R+CD19+ and CD45R+CD19− cells per input precursor were calculated and then normalized as percentages of control values. These data represent means ± SD from 5 independent experiments.
Results from each of two experiments are separated by commas.
B lymphopoiesis was preferentially suppressed when limitin, a new IFN-like cytokine, was injected into mice.11 This factor dramatically reduced the yield of CD19+ cells in 1-week stromal cell-free cultures and, in contrast to estrogen, also reduced numbers of CD45R+ CD19− cells (Table 1). This was true when the cultures were initiated with either Lin−c-kitHi marrow cells or the more B-lineage precursor-rich Lin− c-kit− fraction (not shown). Gene-targeted mice and binding assays were used to determine that limitin uses the type I IFN receptor11 and IFN-α is also known to diminish B lymphopoiesis in vivo.17 Therefore, we performed a comparison between limitin and conventional IFNs in defined cultures (Table 1). IFN-α, IFN-γ, and limitin were equivalent in suppressing the production of CD45R+ CD19+cells from early precursors. In contrast to estrogen, the IFN-like cytokines also prevented the generation of CD45R+CD19− cells. Therefore, the B-lymphocyte lineage is also a direct target for negative regulation by these cytokines.
Transforming growth factor-β represents another previously studied inhibitor, but one with the potential for limiting myeloid as well as lymphoid differentiation.18 We now show that both the expansion and lymphoid lineage differentiation of Lin−precursors are sensitive to TGF-β, using a neutralizing antibody to confirm specificity (Table 1 and data not shown). Although some evidence indicates that EGF negatively influences hematopoietic cells,19 we saw no evidence for this in our stromal cell–free cultures (Table 1).
Comparison of early pro-B cells with other committed precursors on the basis of surface marker expression
Lin− TdT+ cells were uniformly positive for CD27, an antigen recently found to be absent from long-term reconstituting stem cells7 and when a PE-conjugated antibody was used, more than half of the Lin−TdT+ cells also displayed the AA4.1 antigen20(Figure 5). Large and medium-sized Lin− TdT+ cells could be distinguished on the basis of forward light scatter, but we found no differences between them in terms of these surface markers. Both medium and large Lin− TdT+ cells stained for the Ki-67 proliferation-associated antigen (Figure 5 and data not shown).21 Ly-6C is a protein of unknown function on some bone marrow cells and we previously found that CD45R+Ly-6C+ cells had little potential for differentiation to CD19+ lymphocytes in culture.5 We now report that less than 8% of Lin− TdT+ cells were positive for the Ly-6C antigen.
Additional characteristics of Lin−TdT+ bone marrow cells.
Sorted Lin− bone marrow cells were stained for surface antigens followed by intracellular TdT staining and analysis by 4 parameter flow cytometry (described in “Materials and methods”). The Lin− TdT+ cells in each panel are shown as black dots, whereas all remaining cells are shown as yellow dots. Percentages of the total population of Lin−TdT+ cells in each quadrant are also given. Cells were also stained for the Ki-67 proliferation antigen (bottom panel). Expression of Ki-67 in Lin− TdT+ cells (bold) was compared with that in Gr-1Lo bone marrow cells (dotted) and in IgM+ B cells (plain) along with the isotype control (tinted).
Additional characteristics of Lin−TdT+ bone marrow cells.
Sorted Lin− bone marrow cells were stained for surface antigens followed by intracellular TdT staining and analysis by 4 parameter flow cytometry (described in “Materials and methods”). The Lin− TdT+ cells in each panel are shown as black dots, whereas all remaining cells are shown as yellow dots. Percentages of the total population of Lin−TdT+ cells in each quadrant are also given. Cells were also stained for the Ki-67 proliferation antigen (bottom panel). Expression of Ki-67 in Lin− TdT+ cells (bold) was compared with that in Gr-1Lo bone marrow cells (dotted) and in IgM+ B cells (plain) along with the isotype control (tinted).
In C57BL/6 mice, 42% to 73% of Lin− TdT+cells were positive for Ly-6A/Sca-1, a marker extensively used to isolate stem cells and early lymphocyte precursors.22-24However, we found that early pro-B cells were not homogeneous with respect to Sca-1 density. There was a tendency for the c-kitHi cells to have higher amounts of Sca-1 than did the c-kitLo/− TdT+ cells (Figure 5). We found no expression of Thy1.2 on Lin− TdT+ cells, but they were uniformly positive for CD43 (not shown).
Heterogeneity was also found with respect to CD4 or the CD16/CD32 Fcγ receptors identified with the 2.4G2 mAb (Figure 5). Therefore, neither of these 2 markers would allow clean discrimination of Lin− TdT+ cells. CD44 is another marker that has been used to characterize early precursors in the thymus and we found that all Lin− TdT+ cells in bone marrow had a uniformly high density (not shown). The Lin−c-kitLo TdT+ fraction of bone marrow cells is highly enriched for B lymphocyte precursors and the flow cytometry data permit a more direct comparison with lymphocyte precursor populations described by others. We conclude that most of these early pro-B cells are CD27+ AA4.1+/−Ki-67+Ly-6C−Ly-6A/Sca-1Lo/−Thy-1−CD43+CD4+/−CD16/32Lo/− and CD44Hi. This extends our previous characterization of them as a Lin− c-kitLo Flk-2/Flt-3+IL-7Rα+/ −TdT+ fraction of murine bone marrow.
Marrow fractions enriched for early B-lineage precursors include T-lymphocyte precursors
The emphasis of our studies has been on identifying functional precursors for B-lymphocyte lineage cells within bone marrow and the term “early pro-B cells” seemed appropriate for Lin−c-kitLo Flk-2/Flt-3+ IL-7Rα+cells.5 However, some of these surface characteristics were also used by others to isolate “common lymphoid progenitors”6 and it was important to learn more about the differentiation potential of early pro-B cells. Immunodeficient Rag-1−/− mice were injected with Lin−c-kitLo Flk-2/Flt-3+ IL-7Rα+cells (Table 2). This subset contained cells with the potential to reconstitute T- as well as B-cell lineages. Thus, a population of Lin− c-kitLoFlk-2/Flt-3+ IL-7Rα+ cells that efficiently gives rise to CD19+ lymphocytes also includes T-lineage precursors.
Early pro-B cells can reconstitute T as well as B lymphoid lineages in vivo
| . | CD45.1+, IgM+ B cells (×103)* recovered . | CD45.1+CD4+ or CD8+ T cells (×103)†recovered . | ||
|---|---|---|---|---|
| Day 14 . | Day 21 . | Day 14 . | Day 21 . | |
| Experiment 1 | 850, 340 | 850, 450 | 17 000, 1500 | <100, 15 000 |
| Experiment 2 | 120, <100 | 140, 160 | 6100, <100 | 13 000, 40 000 |
| . | CD45.1+, IgM+ B cells (×103)* recovered . | CD45.1+CD4+ or CD8+ T cells (×103)†recovered . | ||
|---|---|---|---|---|
| Day 14 . | Day 21 . | Day 14 . | Day 21 . | |
| Experiment 1 | 850, 340 | 850, 450 | 17 000, 1500 | <100, 15 000 |
| Experiment 2 | 120, <100 | 140, 160 | 6100, <100 | 13 000, 40 000 |
There were fewer than 105 CD45.1+ cells per organ in uninjected control mice (data not shown).
B-cell reconstitution was determined by the presence of CD45.1+ IgM+ cells in the spleen.
T-cell reconstitution was determined by the presence of CD45.1+ CD4+ cells or CD45+CD8+ cells in the thymus.
Discussion
The focus of this study was on a population of bone marrow cells that lacks lineage-associated surface markers but expresses intracellular TdT. Our previous analysis indicated that they represent important intermediates between multipotential stem cells and functional B-cell precursors. We show here that a fraction enriched for Lin− TdT+ cells efficiently survive in well-defined culture conditions and differentiate under the positive influence of 3 cytokines. They are also sensitive to previously described negative regulators, suggesting they may represent an important control point for B lymphopoiesis. We have learned that the population includes cells with the potential for T-lymphocyte lineage differentiation and shares other properties with common lymphoid progenitors described by another laboratory.
The serum-free, stromal cell–free culture conditions developed by Jacobsen and colleagues12 proved ideal for our analysis of early pro-B cells. Remarkably, the same required cytokines were those whose receptors were used as sort criteria. That is, the cells we placed in culture bore mAbs to c-kit, IL-7Rα, and FLk-2/Flt-3. Despite this receptor ligation, at least one in 4 had the potential to respond to the corresponding factors in serum-free culture. Addition of these cytokines in various combinations and with cells isolated with different sorting strategies was informative. For example, SCF was an effective stimulus even when cells were sorted as negative for the corresponding receptor (Figure 2). We previously found that a subset of c-kit− cells transiently reacquired this receptor in culture.4 Also, the minimum receptor density required for cytokine responsiveness might be below that detectable by flow cytometry. Gene-targeting experiments indicate that SCF, IL-7, and FL contribute to efficient lymphocyte production in mice.25Ligation of c-kit by SCF is not essential for B lymphopoiesis,25,26 and we observed small numbers of CD19+ cells without addition of SCF to our cultures. Similarly, CD19+ lymphocytes were produced in reduced numbers when FL was omitted from the medium. However, these 2 factors synergize to maintain a normal-sized population of pro-B cells in vivo25 and were needed for optimal production of B-lineage lymphocytes from early pro-B cells. It is now important to discriminate roles played by each and determine how they interact with other positive signals delivered by stromal cells. An additional goal must be to determine why human lymphocyte precursors have more complex and not completely overlapping requirements in culture.27 28
Our findings complement and extend previous studies regarding roles played by IL-7. Jacobsen and colleagues concluded that the cytokine is important for commitment of multipotential progenitors to lymphocyte development.12 We showed that cells bearing the IL-7R differentiated slightly better than those lacking this molecule, and particularly when assessed at short culture intervals (Figure 3). This suggests that IL-7RαLo/− progenitors require time to become responsive to the cytokine and the 2 fractions were indistinguishable with respect to IL-7Rα density when examined by flow cytometry after 1 week of culture (not shown). However, the presence of more TdT+ cells in the IL-7Rα+subset than in the IL-7RαLo/− fraction suggest the frequency of B-cell progenitors may not have been equivalent in the 2 starting populations. These findings would be consistent with a sequence of differentiation events where expression of TdT precedes display of the IL-7Rα by cells with the potential for acquisition of CD45R and CD19. Other studies indicate that IL-7 may not only support survival of precursors, but stimulate the immunoglobulin gene rearrangement process.29 Cells did not progress beyond the CD45R+ stage to yield CD19+ lymphocytes in our cultures unless IL-7 was included. Targeting of genes for IL-7, the IL-7Rα chain, or the γc chain component of the IL-7R, as well as the intracellular mediator JAK3 kinase all have severe consequences for B lymphocyte lineage differentiation in the mouse.13,14,30,31 However, IL-7 is unlikely to be the only factor involved in this process for several reasons. The lymphocyte deficiency is more severe in IL-7Rα−/− mice than in IL-7Rγc−/− or IL-7−/− mice, and it has been frequently suggested that thymic stromal lymphopoietin (TSLP) plays a partially overlapping role.13,14,32,33 It is noteworthy that there is little progression to the sIgM+B-cell stage in our cultures supported by SCF, FL, and IL-7. There is some indication from culture studies that TSLP augments the final stages in B-lymphocyte formation.33
It has become increasingly apparent that negative, as well as positive stimuli, control rates of blood cell production and 3 of the inhibitory agents investigated here are effective in vivo. Castration and androgen receptor abnormalities increase B-cell production, whereas it is selectively suppressed during pregnancy and after treatment with sex steroids.34-37 These observations suggest that steady-state lymphocyte production is under hormonal control, but much remains to be learned about relevant target cells and molecular mechanisms. We previously found that IL-7–responsive pro-B cells are insensitive to estrogen, but that stromal cell cocultures initiated with earlier precursors were suppressed.16 The findings indicated that stromal cells might be induced to produce a soluble negative regulator of B lymphopoiesis. This possibility is still open but we show with stromal cell–free cultures and highly purified precursors that early pro-B cells are also potential hormone targets. Efforts to identify estrogen-regulated genes and consequences of hormone therapy should include an analysis of this rare cell population.
We recently discovered limitin, an IFN-like cytokine that preferentially suppresses B lymphopoiesis.11 This activity has also been described for type I IFN17 and limitin can use the same receptor.11 We demonstrate here that early pro-B cells are directly and similarly suppressed by limitin, type I IFN, and type II IFN. Stromal cells, macrophages, and T lymphocytes are all potential sources of these factors within bone marrow.38-40 However, B-cell precursor populations appear to be normal in type I IFN receptor-mutated mice41 (and data not shown). In contrast to sex steroids, these agents may only influence lymphopoiesis under conditions of trauma, inflammation, or disease.
Transforming growth factor-β is another known inhibitor of B lymphopoiesis and early pro-B cells were directly responsive to it. This extends earlier observations that IL-7–responsive pro-B cells and a pre-B lymphoma are sensitive to TGF-β.42,43 However, it is not a cytokine that is preferentially active on the B-lymphocyte lineage.18,44 In addition to the potentially direct effect on hematopoietic precursors, TGF-β may negatively influence stromal cell IL-7 production.45
Epidermal growth factor was of interest for several reasons. This is an estrogen-inducible cytokine and one that can elicit estrogen receptor–mediated signaling.46,47 Therefore, involvement of EGF in or mimicking of sex steroid–elicited responses seemed possible. We show here that early pro-B cells are unaffected by EGF. However, EGF may influence components of the bone marrow microenvironment because it promotes growth of bone marrow–derived stromal cells48 as well as fetal thymic architecture49 in culture.
This panel of negative regulators included 2 that selectively inhibit lymphopoiesis, but not myelopoiesis and this direct comparison now makes it possible to appreciate differences in their actions.11,16 50 Although limitin blocked formation of CD45R+ CD19− cells in culture, these presumptive intermediates actually accumulated in cultures containing estrogen (Table 1). We will report elsewhere that very early hematopoietic precursors in intact mice are estrogen sensitive, but it is possible this response is mediated indirectly via stromal cells (K.L.M., manuscript in preparation). Conventional IFNs and TGF-β are known inhibitors of nonlymphoid hematopoietic cells as well as lymphocyte precursors.18,44,51As discussed above, estrogen is the only one of these agents likely to be important as a lineage-specific negative regulator under normal steady-state conditions.
One important objective of this study was to learn more about the surface marker characteristics of Lin− TdT+early pro-B cells. The Ly-6A (Sca-1) antigen is useful for identifying stem cells from some strains of mice22-24 and common lymphoid progenitors were isolated with this as a sort criterion.6 We found nearly complete overlap between the “common lymphoid progenitor” and early pro-B cells on the basis of Sca-1 expression (Figure 5). However, there was a range of densities and a tendency for c-kitHi cells to express more than those that were c-kitLo/−. This is consistent with previous findings that stem cells express higher levels of Sca-1 than CLP.6 Sca-1 and c-kit may be progressively down-regulated in parallel as lymphocyte precursors differentiate.4 As expected, early pro-B cells differ from long-term repopulating stem cells by uniform expression of CD27.7 The presence of the Ki-67 antigen indicates that the Lin− TdT+cells are cycling, as previously concluded for CLP on the basis of Hoechst dye staining.6,52 CLPs were defined by Kondo and colleagues on the basis of IL-7Rα expression.6 Although most of the Lin− TdT+ cells in murine bone marrow express this receptor component,5 questions remain about the timing and requirement for its acquisition. We now show that display of the IL-7R signals readiness to differentiate and that the cytokine is necessary for transition to the CD19+ stage under defined culture conditions.
Several subsets of nonlymphoid progenitors have recently been found among the Lin− Sca-1− fraction of bone marrow and resolved on the basis of FcγR expression.8Lin− TdT+ cells were not homogeneous for this marker and it does not seem promising for their enrichment (Figure 5). However, most did not have the high FcγR levels characteristic of many granulocyte-macrophage restricted progenitors. We previously found that functional B-cell precursors lack CD34, another distinction from most myeloid progenitors.5 8
Differences in batches of labeled mAbs and mouse strains as well as other technical reasons complicate comparisons between laboratories concerning extremely rare subsets of bone marrow cells. The functional B-cell precursors we refer to as early pro-B cells only partially resemble cells designated “fraction A0” by Hardy and colleagues.9 Some, but not all, early pro-B cells display the C1qRp receptor recognized by the AA4.1 antibody and many, but not all, expressed the CD4 antigen used by Hardy to define fraction A0.9 CD4 has long been used to identify the earliest thymic immigrants and cells with T-lineage potential within bone marrow.53 That is consistent with our finding that early pro-B cell populations include T-lineage precursors. On the other hand, others found little or no B-lymphocyte lineage potential associated with CD4+ bone marrow cells.54,55Furthermore, we found variable, low level expression of CD4 on CD45R+ CD19−Ly-6C+ marrow cells that also failed to differentiate in culture.5 We also found that Lin−TdT+ cells were negative for Thy-1.2. This is consistent with a recent report that a Lin− Sca-1+ c-kit+Thy-1− population is enriched with respect to B-lymphocyte lineage potential, whereas a Lin− Sca-1+c-kit+ Thy-1Lo population includes myeloid progenitors.56 In summary, we previously determined that functional B-cell precursors were enriched among the Lin−c-kitLo Flk-2/Flt-3+IL-7Rα+/−TdT+ fraction of murine bone marrow. The present findings show that the Lin−TdT+ cells are CD27+AA4.1+/−Ly-6C−Ly-6A/Sca-1Lo/−Thy-1−CD43+CD4+/−CD16/32Lo/− and CD44Hi.
We demonstrated that functional T-lymphocyte lineage progenitors were present in the Lin− c-kitLoIL-7Rα+ Flk-2/Flt-3+ fraction of bone marrow (Table 5). This result is entirely consistent with the similarities we found in surface markers expressed by early pro-B cells and the common lymphoid progenitors described by others.6 However, caution must be exercised with respect to terminology and interpretation of differentiation pathways. Kondo and colleagues showed that at least some individual cells with a given phenotype have B and T lymphocytes, but not myeloid differentiation potential.6 It remains to be seen if cells with that unique capability represent a major and obligatory intermediate step between stem cells and lymphocyte precursors. Our previous studies and data presented here are informative about the characteristics of early B-lineage precursors. The efficiency and time required for differentiation of early pro-B cells suggests this is a major pathway to the pre-B cell stage and although myeloid differentiation options appear to have been lost, at least some cells in the population retain T-lineage potential. Some type of single cell analysis, perhaps along the lines used by Katsura and colleagues,57 would be required to learn what fraction of early pro-B cells should be designated common lymphoid progenitors. The dependence of these cells on 3 cytokines and their direct sensitivity to inhibitors suggests they may represent an important control point for lymphocyte formation.
Note added in proof: Thurmond and colleagues recently described transplantation experiments with estrogen receptor α knockout mice and conclude that hematopoietic cells can be direct hormone targets (Endocrinology. 2000;141:2309).
The authors thank Dr Lisa Borghesi for critical reading of the manuscript, as well as Ms Viji Dandapani and Mr Jim Henthorn for cell sorting. The assistance of Ms Shelli Wasson in manuscript preparation is also appreciated.
Supported by grant AI 20069 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paul W. Kincade, Immunobiology and Cancer Program, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal