Abstract
Mutations of receptor tyrosine kinases are implicated in the constitutive activation and development of human malignancy. An internal tandem duplication (ITD) of the juxtamembrane (JM) domain-coding sequence of the FLT3 gene (FLT3/ITD) is found in 20% of patients with acute myeloid leukemia (AML) and is strongly associated with leukocytosis and a poor prognosis. On the other hand, mutations of the c-KIT gene, which have been found in mast cell leukemia and AML, are clustered in 2 distinct regions, the JM domain and D816 within the activation loop. This study was designed to analyze the mutation of D835 of FLT3, which corresponds to D816 of c-KIT, in a large series of human hematologic malignancies. Several kinds of missense mutations were found in 30 of the 429 (7.0%) AML cases, 1 of the 29 (3.4%) myelodysplastic syndrome (MDS) cases, and 1 of the 36 (2.8%) acute lymphocytic leukemia patients. The D835Y mutation was most frequently found (22 of the 32 D835 mutations), followed by the D835V (5), and D835H (1), D835E (1), and D835N (1) mutations. Of note is that D835 mutations occurred independently of FLT3/ITD. An analysis in the 201 patients newly diagnosed with AML (excluding M3) revealed that, in contrast to the FLT3/ITD mutation (n = 46), D835 mutations (n = 8) were not significantly related to the leukocytosis, but tended to worsen disease-free survival. All D835-mutant FLT3 were constitutively tyrosine-phosphorylated and transformed 32D cells, suggesting these mutations were constitutively active. These results demonstrate that the FLT3 gene is the target most frequently mutated to become constitutively active in AML.
Introduction
Class III receptor tyrosine kinases (RTKs), consisting of FLT3, KIT, FMS, and PDGF receptor, share structural characteristics such as 5 immunoglobulin-like domains in the extracellular regions and a juxtamembrane (JM) domain, 2 kinase domains (TK1 and TK2) separated by a kinase insert (KI) domain, and a C-terminal domain in intracellular regions.1 Ligand binding to the extracellular domain of RTK leads to receptor dimerization, stabilizing a conformation of the catalytic domain with the activation loop (A-loop) in an open conformation. This conformation of the active site accommodates adenosine triphosphate (ATP) and substrate binding, enabling transphosphorylation of the A-loop, stabilizing the catalytic domain in an active conformation.2 Receptor dimerization and the subsequent phosphorylation of tyrosine residues accompanies RTK activation, followed by induction of multiple intracellular signaling pathways leading to cell proliferation and activation. Amplification, overexpression, or somatic mutation of RTK results in increased receptor signaling, causing tumorigenesis. Several mutations of RTKs are implicated in the constitutive activation and development of human malignancy.3
Recently, an internal tandem duplication (ITD) of the JM domain-coding sequence of the FLT3 gene (FLT3/ITD) was found.4 This is present in about 20% of patients with adult acute myeloid leukemia (AML) and in about 3% of those with myelodysplastic syndrome (MDS); it is strongly associated with the leukocytosis and poor prognosis in AML patients.5-12Although the duplicated sequence varied in both position and length, it was always in-frame and limited to the JM domain, resulting in an elongated product. Regardless of the type of ITD, FLT3/ITD mutants are constitutively dimerized and autophosphorylated on tyrosine residues, causing the activation of STAT5 and mitogen-activated protein (MAP) kinase.13,14 In addition, FLT3/ITD-transfected murine interleukin (IL) 3-dependent cell lines, such as Ba/F3, FDC-P1, and 32D, are able to proliferate without IL-3 and form a blastoma when inoculated into syngeneic mice.15
In-frame deletion and missense mutations in the c-KIT JM domain have been identified in mastocytomas and gastrointestinal stromal tumors and shown to cause constitutive activation of the receptor.16 Furthermore, an activating mutation of D816 (single-letter amino acid codes) within the A-loop of c-KIT has been found in AML cases as well as human mast cell leukemia cell line HMC-1.17,18 This mutation is thought to cause constitutive activation by triggering the A-loop into an active conformation. Since this Asp codon is highly conserved in RTKs, and substitutions to Tyr or Val in murine c-FMS and murine FLT3 result in constitutive activation of the receptors,19 20 the Asp within the A-loop is likely to be a key regulatory residue of the RTKs.
In this study, we first analyzed the mutation of D835 within the A-loop of FLT3 in a large series of human hematologic malignancies. Next, we analyzed the clinical characteristics of the AML cases with D835 mutation in comparison with ITD mutation. Finally, we analyzed the biologic significance of D835 mutation by introducing full-length mutant FLT3 complementary DNA (cDNA) into Cos7 cells and the murine IL-3–dependent cell line 32D.
Patients, materials, and methods
Patients and samples
The study population included 429 AML, 29 MDS, 36 acute lymphocytic leukemia (ALL), 14 adult T-cell leukemia (ATL), 17 malignant lymphoma (ML), 10 chronic lymphocytic leukemia (CLL), 40 multiple myeloma (MM), 11 chronic myeloid leukemia in blast crisis (CML-BC), and 3 essential thrombocytosis (ET) patients. The diagnosis of hematologic malignancy was based on morphology, histopathology, the expression of leukocyte differentiation antigens, and the French-American-British (FAB) classification (Table1). For the normal control, peripheral blood mononuclear cells from 30 healthy volunteers working at a company were used. We obtained informed consent from all patients and volunteers to use their samples in this study.
Analysis of the FLT3 gene mutations in hematologic malignancies
| No. of analysis . | D835 mutation . | ITD . | |||
|---|---|---|---|---|---|
| (N) . | (%) . | Mutation type and number . | (N) . | (%) . | |
| AML | |||||
| M0 4 | 1 | (25) | Y: 1 | 0 | (0) |
| M1 63 | 2 | (3.1) | Y: 2 | 16 | (25.4) |
| M2 99 | 3 | (3.0) | Y: 3 | 10 | (10.1) |
| M3 141 | 11 | (7.8) | Y: 7, V: 2, E: 1, Y&E*: 1 | 23 | (16.3) |
| M4 70 | 4 | (5.7) | Y: 4 | 19 | (27.1) |
| M5 40 | 9 | (22.5) | Y: 5, V: 3, H: 1 | 10 | (25.0) |
| M6 6 | 0 | (0) | 1 | (16.7) | |
| M7 6 | 0 | (0) | 2 | (33.3) | |
| Total 429 | 30 | (7.0) | Y: 22, V: 5, E: 1, H: 1, Y&E: 1 | 81 | (18.9) |
| MDS | |||||
| RAEB 6 | 0 | (0) | 0 | (0) | |
| RAEB in T 13 | 1 | (7.7) | I836L + D†: 1 | 1 | (7.7) |
| CMMoL 10 | 0 | (0) | 0 | (0) | |
| Total 29 | 1 | (3.4) | I836L + D: 1 | 1 | (3.4) |
| CML-BC 11 | 0 | (0) | 0 | (0) | |
| ALL 36 | 1 | (2.8) | N: 1 | 0 | (0) |
| ATL 14 | 0 | (0) | 0 | (0) | |
| ML 17 | 0 | (0) | 0 | (0) | |
| CLL 10 | 0 | (0) | 0 | (0) | |
| MM 40 | 0 | (0) | 0 | (0) | |
| ET 3 | 0 | (0) | 0 | (0) | |
| Normal 30 | 0 | (0) | 0 | (0) | |
| No. of analysis . | D835 mutation . | ITD . | |||
|---|---|---|---|---|---|
| (N) . | (%) . | Mutation type and number . | (N) . | (%) . | |
| AML | |||||
| M0 4 | 1 | (25) | Y: 1 | 0 | (0) |
| M1 63 | 2 | (3.1) | Y: 2 | 16 | (25.4) |
| M2 99 | 3 | (3.0) | Y: 3 | 10 | (10.1) |
| M3 141 | 11 | (7.8) | Y: 7, V: 2, E: 1, Y&E*: 1 | 23 | (16.3) |
| M4 70 | 4 | (5.7) | Y: 4 | 19 | (27.1) |
| M5 40 | 9 | (22.5) | Y: 5, V: 3, H: 1 | 10 | (25.0) |
| M6 6 | 0 | (0) | 1 | (16.7) | |
| M7 6 | 0 | (0) | 2 | (33.3) | |
| Total 429 | 30 | (7.0) | Y: 22, V: 5, E: 1, H: 1, Y&E: 1 | 81 | (18.9) |
| MDS | |||||
| RAEB 6 | 0 | (0) | 0 | (0) | |
| RAEB in T 13 | 1 | (7.7) | I836L + D†: 1 | 1 | (7.7) |
| CMMoL 10 | 0 | (0) | 0 | (0) | |
| Total 29 | 1 | (3.4) | I836L + D: 1 | 1 | (3.4) |
| CML-BC 11 | 0 | (0) | 0 | (0) | |
| ALL 36 | 1 | (2.8) | N: 1 | 0 | (0) |
| ATL 14 | 0 | (0) | 0 | (0) | |
| ML 17 | 0 | (0) | 0 | (0) | |
| CLL 10 | 0 | (0) | 0 | (0) | |
| MM 40 | 0 | (0) | 0 | (0) | |
| ET 3 | 0 | (0) | 0 | (0) | |
| Normal 30 | 0 | (0) | 0 | (0) | |
Both D835 and ITD mutations are essentially restricted to AML. The D835Y mutation was most frequently found (22 of the 32 D835 mutations), followed by the D835V (5 of 32), D835H (1 of 32), D835E (1 of 32), and D835N (1 of 32) mutation.
AML indicates acute myeloid leukemia; MDS, myelodysplastic syndrome; RAEB, refractory anemia with excess of blast; RAEB in T, refractory anemia with excess of blast in transformation; CMMoL, chronic myelomonocytic leukemia; CML-BC, chronic myeloid leukemia in blast crisis; ALL, acute lymphocytic leukemia; ATL, adult T-cell leukemia; ML, malignant lymphoma; CLL, chronic lymphocytic leukemia; MM, multiple myeloma; ET, essential thrombocytosis.
D835Y and D835E mutations were found in one AML (M3) patient; cloning analysis showed that these mutations occurred in different alleles.
This mutation consisted of the insertion of three nucleotides (TTG) between D835 and I836, and AT to GA substitutions at the first and second nucleotides of I836, resulting in insertion of Leu and an Ile to Asp amino acid change (I836L + D).
Reagents and cells
Recombinant murine IL-3 was generous gift of Kirin Brewery (Tokyo, Japan). Recombinant human FLT3 ligand (FL) was purchased from R&D Systems (Minneapolis, MN). All endonucleases were purchased from New England Biolabs (Beverly, MA). All reagents for polymerase chain reaction (PCR) were purchased from Applied Biosystems (Foster City, CA). Murine IL-3–dependent myeloid progenitor cell line, 32D, was obtained from the RIKEN cell bank (Tsukuba, Japan) and maintained in RPMI 1640 medium (Gibco BRL, Gaithersburg, MD) supplemented with 10% fetal calf serum (FCS; Gibco BRL) and 1 ng/mL murine IL-3. The Cos7 cells were maintained in Dulbecco modified essential medium (DMEM; Gibco BRL) supplemented with 10% FCS.
Screening of the ITD and D835 mutation of theFLT3 gene
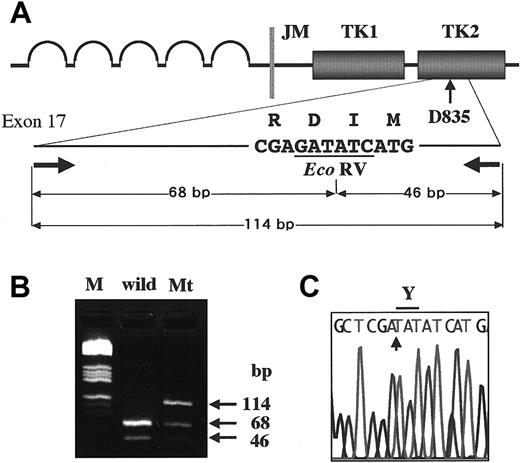
High-molecular-weight DNA was extracted from the samples by the standard method. FLT3/ITD was examined by amplification of the JM domain from exon 11 to 12, followed by electrophoresis on an agarose gel as previously reported.5 To detect mutations at D835, we used the restriction fragment length polymorphism-mediated PCR assay, because D835 and I836 codons were encoded by the nucleotide GATATC, which forms the Eco RV restriction site. We amplified exon 17 of the FLT3 gene by genomic PCR using the primers 17F, 5′-CCGCCAGGAACGTGCTTG-3′, and 17R, 5′-GCAGCCTCACATTGCCCC-3′, as previously reported.5Amplified products were digested with Eco RV, and subjected to electrophoresis on an agarose gel (Figure1). If the amplified products showed the undigested band, it was cut out from the gel, purified with a QIAquick gel extraction kit (Qiagen, Chatsworth, CA), and directly sequenced on a DNA sequencer (310; Applied Biosystems) using a BigDye terminator cycle sequencing kit (Applied Biosystems). In some samples that could not be sequenced directly, Eco RV-undigested fragments were cloned into pT7Blue T-vector (Novagen, Madison, WI) and sequenced.
Detection of D835 mutations in the
FLT3 gene. To detect D835 mutations, we amplified exon 17 by PCR, and then digested it with the EcoRV endonuclease (A). The amplified products of wild type were digested to 2 bands (68 bp and 46 bp) by the EcoRV. When amplified products contained D835 mutations, undigested bands (114 bp) were visualized on agarose gel electrophoresis. M indicates the molecular weight marker (HaeIII digested pBR332 plasmid DNA) (B). The undigested bands were directly sequenced (C). In this sample, the first nucleotide G of D835 was substituted with T, resulting in an Asp to Tyr amino acid change (D835Y).
Detection of D835 mutations in the
FLT3 gene. To detect D835 mutations, we amplified exon 17 by PCR, and then digested it with the EcoRV endonuclease (A). The amplified products of wild type were digested to 2 bands (68 bp and 46 bp) by the EcoRV. When amplified products contained D835 mutations, undigested bands (114 bp) were visualized on agarose gel electrophoresis. M indicates the molecular weight marker (HaeIII digested pBR332 plasmid DNA) (B). The undigested bands were directly sequenced (C). In this sample, the first nucleotide G of D835 was substituted with T, resulting in an Asp to Tyr amino acid change (D835Y).
Construction of the FLT3 mutant
Full-length human FLT3 cDNA, kindly provided by Dr Oliver Rosnet (INSERM, France), was recloned into pMKIT-Neo mammalian expression vector, kindly provided by Dr Toshio Kitamura (University of Tokyo, Japan). Mutations of D835, found in clinical samples, were introduced into human wild FLT3 cDNA using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. All constructs were confirmed by sequencing.
Phosphorylation analysis of mutant FLT3
The Cos7 cells were transfected with wild-type and mutant FLT3 constructs using LipofectAMINE (Gibco BRL) according to the manufacturer's instructions. After 48 hours of culture, cells were serum starved for 24 to 48 hours before incubation with or without human FL at 50 ng/mL for 10 minutes, and then harvested in lysis buffer as previously described.13 Lysates were immunoprecipitated with rabbit antihuman FLT3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and protein G Sepharose (Amersham Pharmacia Biotech, Uppsala, Sweden). The precipitated samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted onto Immobilon polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA). Immunoblotting was performed with antiphosphotyrosine antibody (4G10; Upstate Biotechnology, Lake Placid, NY). The membranes were incubated with the stripping buffer, then reprobed with anti-FLT3 antibody. Signals were developed by using the enhanced chemoluminescence (ECL) system (Amersham Pharmacia Biotech).
Generation of the mutant FLT3-expressing 32D cell lines
Wild-type and ITD mutant FLT3-expressing 32D cell lines were reported previously.14 15 The 32D cells were transfected with full-length D835 mutant FLT3 cDNAs cloned in the pMKIT-Neo vector by using TransFast (Promega, Madison, WI), then selected by G418 (Gibco BRL). Expression of FLT3 products was confirmed by flow cytometer (FACSCalibur; Becton Dickinson, San Jose, CA) using an antihuman FLT3 monoclonal antibody (SF1.340; Immunotech, Marseille, France) and Western blotting. Each 32D cell line, which expressed D835 mutant FLT3 stably, was washed 3 times with the RPMI 1640 medium containing 10% FCS, then cultured without IL-3.
For cell proliferation assay, 1 × 105 cells were seeded in 24-well culture dishes with or without murine IL-3, and then viable cells were counted daily by trypan blue dye-exclusion assay.
Statistical analysis
The relationships of clinical characteristics among FLT3 D835 and ITD mutations were analyzed in 201 patients with AML, excluding those with type M3, who were treated with the same therapeutic protocols according to the Japan Adult Leukemia Study Group (JALSG). Differences in median variables in age, peripheral white blood cell (WBC) counts, platelet counts, and serum lactic dehydrogenase (LDH) concentration were analyzed with the Mann-Whitney U test. Analysis of frequencies was performed using the Fisher exact test for 2 × 2 tables or the Pearson χ2 test for larger tables. Survival probabilities were estimated by the Kaplan-Meyer method, and differences in the survival distributions were evaluated by the log-rank test. The prognostic significance of the clinical variables was assessed using the Cox proportional hazards model. These statistic analyses were performed with StatView-J 5.0 (Abacus Concepts, Berkeley, CA). For all analyses, the P values were 2-tailed, and aP value of less than .05 was considered statistically significant.
Results
D835 mutation is found in AML, MDS, and ALL
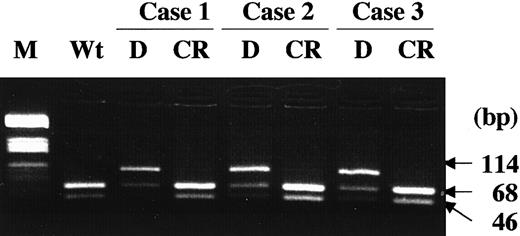
We examined the D835 and ITD mutations of the FLT3 gene in a total of 589 patients with hematologic malignancies (Table 1). We found several kinds of missense mutations of D835 in 30 of the 429 (7.0%) AML, 1 of the 29 (3.4%) MDS, and 1 of the 36 (2.8%) ALL patients. Among AML patients, D835 mutations were found in 7.0% (30 of 429), an incidence significantly lower than that of ITD mutation (81 of 429, 18.9%, P < .001, Fisher exact test). According to the FAB classification, D835 mutation was frequently found in the M5 type (P = .015, Pearson χ2 test); 1 of 4 (25%) of M0, 2 of 63 (3.1%) of M1, 3 of 99 (3.0%) of M2, 11 of 141 (7.8%) of M3, 4 of 70 (5.7%) of M4, 9 of 40 (22.5%) of M5, 0 of 6 of M6, and 0 of 6 of M7 cases. In 3 AML patients, whose leukemia cells had the D835 mutations at the initial diagnosis, the mutations were lost at the complete remission (CR; Figure 2). Furthermore, no mutation was found in peripheral blood mononuclear cells from 30 healthy volunteers.
D835 mutations were lost at CR.
In 3 AML patients, whose leukemia cells had the D835 mutations at the initial diagnosis, the mutations were lost at the CR. M indicates molecular weight marker (HaeIII digested pBR332 plasmid DNA); Wt, wild-type FLT3; D, diagnosis; CR, complete remission.
D835 mutations were lost at CR.
In 3 AML patients, whose leukemia cells had the D835 mutations at the initial diagnosis, the mutations were lost at the CR. M indicates molecular weight marker (HaeIII digested pBR332 plasmid DNA); Wt, wild-type FLT3; D, diagnosis; CR, complete remission.
Sequence analysis showed that there were several kinds of D835 mutations, though all were missense. The first nucleotide G of D835 was most frequently substituted with T (22 of the 32 D835 mutations), resulting in an Asp to Tyr amino acid change (D835Y). The T substitution for the second nucleotide A of D835, resulting in an Asp to Val change (D835V), was found in 5 patients. Furthermore, D835H, D835E, and D835N mutations were each found in one patient. Of interest is that D835Y and D835E mutations were found in one AML (M3) patient, whereas cloning analysis showed that these mutations occurred in different alleles. In one MDS (RAEB in T) patient, a different mutation was found at I836. This mutation consisted of the insertion of 3 nucleotides (TTG) between D835 and I836, and AT to GA substitutions at the first and second nucleotides of I836, resulting in insertion of Leu and an Ile to Asp amino acid change (I836L+D).
Of note is that both D835 and ITD mutations were found in only one AML (M3) patient. To clarify whether these mutations occurred on the same allele, we amplified the region from JM through TK2 domains by reverse transcriptase-mediated PCR. After Eco RV digestion, the amplified products were separated through a polyacrylamide gel. The result showed that the product with ITD was completely digested byEco RV but not the product without ITD, suggesting that these mutations occurred on different alleles (data not shown).
Clinical characteristics and prognosis of AML patients with or without FLT3 gene mutations
Among the AML patients, 201 individuals excluding those with M3 who were treated with the AML87, AML89, and AML92 protocol of the JALSG21-23 were evaluated for their clinical characteristics and initial therapy response (Table2 and Figure3). Of these patients, 46 (22.9%) had only an ITD mutation (ITD), 8 (4%) had only a D835 mutation (D835-Mt), and 147 (73.1%) had neither (Wt, wild type). The presence of ITD or D835 mutations was related neither to age, sex, or the occurrence of hepatosplenomegaly or extramedullary involvement, nor to the CR rates for initial induction therapy. WBC counts were significantly higher in the ITD group than the Wt group (P < .0001), whereas those in the D835-Mt and the Wt groups were the same. Serum LDH levels were significantly higher in the ITD group than the Wt group (P = .009), whereas those in the D835-Mt and the Wt groups were the same. The ITD mutation was infrequent in the M2 FAB type (P = .0025) and in the leukemia with t(8; 21) (P = .047); there was no significant difference in the incidence of D835 mutation among FAB types and cytogenetic findings.
Clinical characteristics of 201 acute myeloid leukemia (excluding M3) patients
| . | Total (N = 201) . | ITD (N = 46) . | D835-Mt (N = 8) . | Wt (N = 147) . |
|---|---|---|---|---|
| Age y | 49 | 56 | 53.5 | 48 |
| (15-85) | (15-77) | (17-70) | (15-85) | |
| WBC count (×109/L) | 24.7 | 54.6* | 25.7 | 20.8 |
| (0.9-632) | (2.1-632) | (5-139.5) | (0.9-33.7) | |
| FAB | ||||
| M0 | 3 | 0 | 0 | 3 |
| M1 | 48 | 14 | 2 | 32 |
| M2 | 83 | 8† | 2 | 73 |
| M4 | 47 | 17 | 2 | 28 |
| M5 | 15 | 5 | 2 | 8 |
| M6 | 4 | 1 | 0 | 3 |
| M7 | 1 | 1 | 0 | 0 |
| Cytogenetics | ||||
| t(8;21) | 28 | 2‡ | 1 | 25 |
| inv16 | 6 | 2 | 0 | 4 |
| t(9;22) | 3 | 0 | 0 | 3 |
| del(5) or del(7) | 11 | 2 | 0 | 9 |
| Others | 41 | 5 | 2 | 34 |
| Normal | 82 | 23 | 4 | 55 |
| ND | 30 | 11 | 1 | 18 |
| Outcome | ||||
| CR | 147 | 32 | 6 | 109 |
| Failure | 51 | 13 | 2 | 36 |
| Unevaluable | 3 | 1 | 0 | 2 |
| . | Total (N = 201) . | ITD (N = 46) . | D835-Mt (N = 8) . | Wt (N = 147) . |
|---|---|---|---|---|
| Age y | 49 | 56 | 53.5 | 48 |
| (15-85) | (15-77) | (17-70) | (15-85) | |
| WBC count (×109/L) | 24.7 | 54.6* | 25.7 | 20.8 |
| (0.9-632) | (2.1-632) | (5-139.5) | (0.9-33.7) | |
| FAB | ||||
| M0 | 3 | 0 | 0 | 3 |
| M1 | 48 | 14 | 2 | 32 |
| M2 | 83 | 8† | 2 | 73 |
| M4 | 47 | 17 | 2 | 28 |
| M5 | 15 | 5 | 2 | 8 |
| M6 | 4 | 1 | 0 | 3 |
| M7 | 1 | 1 | 0 | 0 |
| Cytogenetics | ||||
| t(8;21) | 28 | 2‡ | 1 | 25 |
| inv16 | 6 | 2 | 0 | 4 |
| t(9;22) | 3 | 0 | 0 | 3 |
| del(5) or del(7) | 11 | 2 | 0 | 9 |
| Others | 41 | 5 | 2 | 34 |
| Normal | 82 | 23 | 4 | 55 |
| ND | 30 | 11 | 1 | 18 |
| Outcome | ||||
| CR | 147 | 32 | 6 | 109 |
| Failure | 51 | 13 | 2 | 36 |
| Unevaluable | 3 | 1 | 0 | 2 |
Mean (minimum to maximum) values are indicated for age and WBC count. Number of cases is shown in FAB, cytogenetics, and outcome.
ITD indicates internal tandem duplication; WBC, white blood cell; FAB, French-American-British; ND, not determined; CR, complete remission.
P < .0001.
P = .0025.
P = .047.
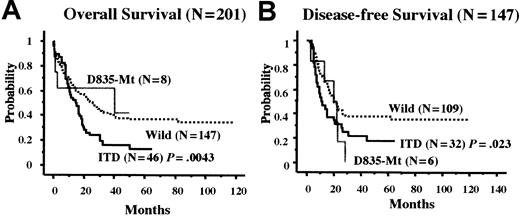
Kaplan-Meier curves according to the D835 and ITD mutations.
(A) Overall survival of 201 patients with AML excluding M3. (B) Disease-free survival of 145 patients who achieved CR. Statistical difference was evaluated by the log-rank test.
Kaplan-Meier curves according to the D835 and ITD mutations.
(A) Overall survival of 201 patients with AML excluding M3. (B) Disease-free survival of 145 patients who achieved CR. Statistical difference was evaluated by the log-rank test.
At a median follow-up time of 50 (3-118) months, 68 of 201 patients (33.8%) were alive. The predicted overall survival (OS) rates at 50 months were 13.1%, 41.7%, and 37.1% in the ITD, D835-Mt, and Wt groups, respectively (Figure 3A). The ITD group had a worse prognosis than the Wt group (P = .0043), whereas the D835-Mt group did not. Disease-free survival (DFS) was further analyzed in 147 patients who achieved CR. The predicted DFS rates at 50 months were 17.5% and 37.8% in the ITD and Wt groups, respectively. The ITD group had a worse DFS than the Wt group (P = .023). In all 6 patients with the D835-Mt, leukemia relapsed within 28 months. However, the difference was not significant (Figure 3B).
D835 mutation is an activating mutation
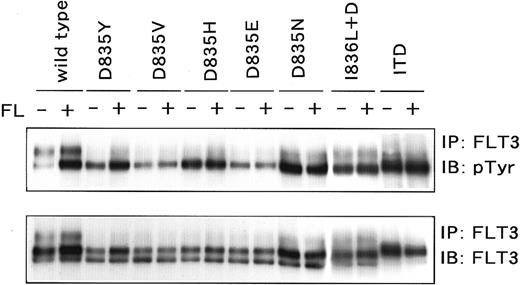
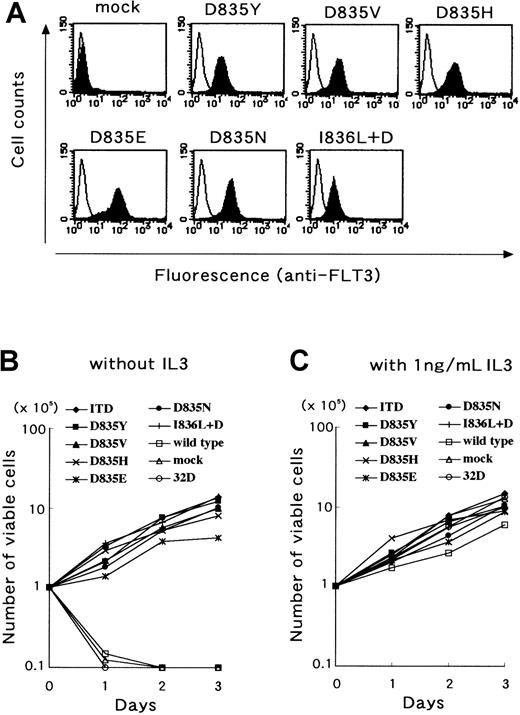
We next examined whether the D835 mutations found in this study resulted in constitutive activation of the FLT3 receptor by introduction of the D835 mutant FLT3 cDNAs into Cos7 and 32D cells. The wild-type FLT3 cDNA was introduced as a negative control and the ITD mutant was introduced as a positive control. When transfected into Cos7 cells, all D835 mutants were FL-independently tyrosine phosphorylated as well as the ITD mutant (Figure4). In addition, we established all D835 mutant-expressing 32D cell lines. Stable expression of mutant FLT3 was confirmed by flow cytometer and Western blotting. After cloning procedures, the clones with the highest expression on their surface were chosen (Figure 5A) and used following analyses. Wild-type FLT3-expressing 32D cells could not proliferate without IL-3 as well as parental and mock-transfected 32D cells. However, all D835 mutant-expressing 32D cells proliferated without either IL-3 or FL at the same level as the ITD mutant-expressing cells (Figure 5B), and the proliferation rates were the same as those with IL-3 (Figure 5C). These results confirmed that the D835 mutations were gain-of-function mutations.
Phosphorylation status of the D835 mutants.
Cos7 cells were transfected with D835 mutant cDNA. Mutants were immunoprecipitated and probed with antiphosphotyrosine antibody. All mutants were constitutively tyrosine phosphorylated without FL stimulation. Because of the higher amounts of the loading samples, the phosphorylated band of wild-type FLT3 looks relatively high.
Phosphorylation status of the D835 mutants.
Cos7 cells were transfected with D835 mutant cDNA. Mutants were immunoprecipitated and probed with antiphosphotyrosine antibody. All mutants were constitutively tyrosine phosphorylated without FL stimulation. Because of the higher amounts of the loading samples, the phosphorylated band of wild-type FLT3 looks relatively high.
D835 mutant FLT3 transformed 32D cells.
The 32D cells were transfected with D835 mutant cDNA. Stably mutant FLT3-expressing cells were cloned and the surface expression was analyzed by flow cytometer. Expression levels were the same among transfectants (A). Stably mutant FLT3-expressing cells were cultured without FL and IL-3. Mutant FLT3-expressing cells showed autonomous proliferation, as well as FLT3/ITD-expressing cells (B). Furthermore, the proliferation rates of mutant FLT3-expressing cells were the same as those with IL-3 (C).
D835 mutant FLT3 transformed 32D cells.
The 32D cells were transfected with D835 mutant cDNA. Stably mutant FLT3-expressing cells were cloned and the surface expression was analyzed by flow cytometer. Expression levels were the same among transfectants (A). Stably mutant FLT3-expressing cells were cultured without FL and IL-3. Mutant FLT3-expressing cells showed autonomous proliferation, as well as FLT3/ITD-expressing cells (B). Furthermore, the proliferation rates of mutant FLT3-expressing cells were the same as those with IL-3 (C).
Discussion
In this study, we analyzed D835 mutations of the FLT3gene in a large series of hematologic malignancies, because the Asp within the A-loop of RTKs might be a key residue for the receptor activation. Although we found 32 D835 mutations in a total of 589 patients with hematologic malignancy, they were essentially found in AML or MDS patients in accordance with the ITD mutation.6,7 Interestingly, we found D835 mutation in one ALL patient. It was reported that ITD mutation was found in 2 ALL patients whose leukemia cells expressed myeloid antigens.9However, leukemia cells of our ALL patient had a B-cell precursor phenotype, but did not express myeloid antigens, suggesting that D835 mutation might be involved in lymphoid lineage cells.
To exclude the possibility of polymorphism, we also analyzed normal individuals and the patients both at initial diagnosis and at CR. In all normal individuals, no D835 mutation was found. Furthermore, in the patients with D835 mutation at initial diagnosis, the mutation was lost at CR. These results confirmed that D835 mutations of FLT3 are somatic mutations associated with leukemia.
The incidence of D835 mutations was significantly lower than that of ITD mutations; both mutations were mainly found in AML. D816 mutations of c-KIT, which are equivalent to D835 of FLT3, were found in many patients with mastocytosis as well as AML.16 Although most of the D816 mutations of c-KIT were an Asp to Val substitution (D816V), the major D835 mutation of FLT3 was an Asp to Tyr substitution (D835Y). Furthermore, although 3 kinds of D816 c-KIT mutations (D816V, D816Y, and D816F) have been found in patients with mastocytosis and AML, D835H, D835E, and D835N mutations of FLT3 were found in addition to D835Y and D835V mutations. However, the mutants found in this study showed constitutive activation of the receptor in accordance with mutant c-KITs, in which the Asp residue was mutated to a series of other amino acids.24
In one patient with AML (M3), 2 kinds of mutations (D835Y and D835E) were found. Cloning analysis demonstrated that these 2 mutations did not occur on the same allele. Likewise, D835 and ITD mutations were found in one patient with AML (M3), but further analyses showed that these mutations occurred on different alleles. These results suggest that continuing mutations of the FLT3 gene seem to occur in leukemia cells. However, this raises the question of whether all kinds of mutations have the same potential functions in leukemia cells. Because it remains unclear whether all mutations have the same kinase activity, and are associated with the same signal-transduction pathway, we could not entirely rule out the possibility that different kinds of mutations are additively or synergistically associated with the progression of leukemia.
Clinical characteristics were analyzed in 201 patients with newly diagnosed AML excluding the M3 cases. In contrast to ITD mutations, D835 mutations did not significantly affect any clinical variables or prognosis. However, these results do not indicate whether D835 mutations have an adverse effect on leukemia cells because such a mutation was found in only 8 patients (4%). Indeed, all 6 patients in the D835-Mt group who achieved CR relapsed within 28 months, whereas there was no significant difference from the Wt group. If D835 mutations are not considered, ITD mutations do not become a poor prognostic factor for DFS. However, if D835 mutations are considered, the ITD group had a significantly lower DFS than the Wt group (P = .023). We previously reported that age 60 years or older and cytogenetics data were the strongest unfavorable factors for DFS in a multivariate analysis in these patients.8 We, therefore, analyzed the effect of D835-Mt on DFS in the 101 patients who were under 60 years old and did not have the karyotypic abnormalities associated with poor prognosis, specifically t(9; 22), 11q23 alterations, del(5) or del(7). Among them, 19 patients had an ITD mutation, 5 had a D835 mutation, and 77 had neither. Although the D835-Mt group was too small for statistical analysis, it showed a tendency for a worse prognosis (P = .09).
It has been reported that N-RAS gene mutations were found in 10% to 20% of patients with AML and associated with several clinical variables.25,26 Previously, we also reported that N-RAS gene mutations were found in 28 of the same 201 AML patients and associated with leukocytosis.8 However, N-RAS gene mutations were found in only 3 patients with FLT3/ITD and not in patients with D835 mutations, suggesting that N-RAS and FLT3 gene mutations occur independently. Because it has been demonstrated that the MAP kinase pathway is activated by either FLT3 or RAS,10 27 the leukemia clone, in which MAP kinase is activated, seems not to acquire the other mutation. To exclude the effect of N-RAS gene mutations, we reanalyzed the relationship between D835 mutations and clinical variables in 173 patients without N-RAS mutations. However, we found no significant differences in the patients with D835 mutations. To clarify the clinical significance of the FLT3 gene mutations, a larger scale analysis is required.
Although D835 mutants were constitutively activated and caused the autonomous proliferation of 32D cells like ITD mutants, it remains to be clarified whether or not the level of kinase activity and signal-transduction pathway are the same between them. In addition, it has been demonstrated that the level of kinase activity differed with the amino acid substituted for the Asp residue (D814 in c-KIT and D802 in c-FMS) within the A-loop of murine c-KIT and c-FMS. In murine c-KIT, all mutants except D814C had an increased amount of tyrosine phosphorylation without ligand stimulation, and D814Y, D814V, D814L, D814I, and D814W mutants, especially, revealed markedly elevated kinase activity.24 In murine c-FMS, D802Q, D802R, and D802G were inactivating mutations, whereas all other mutants were active.19 According to these results, all types of c-KIT or c-FMS mutations, which corresponded to FLT3 D835 mutations found in this study, caused constitutive activation of the receptor, suggesting that D835 mutations have adverse effects on leukemia cells. This is further supported by the report that Tyr and Val substitutions for Asp, which occupy most of the mutations found in this study (27 of 32, 84%), had the strongest kinase activity in c-KIT.24However, it should be examined whether all D835 mutations found in this study have the same adverse effects on leukemia cells.
In conclusion, this study demonstrated novel activating mutations of FLT3 in patients with AML, MDS, and ALL. Because one third of AML patients have a mutation in the FLT3 gene, the aberrant signal transduction pathways from the mutant FLT3 would serve as an important molecular target for treatment.
This study was performed in cooperation with the Leukemia Study Group of the Ministry of Health and Welfare and JALSG. We would like to thank the participating physicians for sending patients' samples: Drs Tohru Kobayashi, Kosei Matsuei, Shin Matsuda, Yasushi Nakayama, Kiyoshi Kitano, Naomichi Arima, Hikaru Kobayashi, Masatomo Takahashi, Hiroshi Morishita, Isao Maekawa, Hiroshi Furuya, Nobuhiko Kimura, Michiko Ogawa, Shigeru Hoshino, Takahiro Okamoto, Junichi Tamura, Shigeki Ohtake, and Shunichi Kumakura. We also thank Dr Kazuhito Yamamoto for technical advice and Ms Yoko Kudo and Ms Yoko Tagawa for secretarial and technical assistance.
Supported by Grants-in-Aid from the Japanese Ministry of Health and Welfare; the Ministry of Education, Science and Culture; Kowa Life Science Foundation; Mochida Memorial Foundation for Medical and Pharmaceutical Research; Tokai Science Academy; Foundation for Promotion of Cancer Research in Japan; and Takeda Science Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hitoshi Kiyoi, Department of Infectious Diseases, Nagoya University School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya 466-8560, Japan; e-mail: kiyoi@med.nagoya-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal