Abstract
The active form of vitamin D3, 1,25(OH)2D3, inhibits proliferation and induces differentiation of a variety of malignant cells. A new class of vitamin D3 analogs, having 2 identical side chains attached to carbon-20, was synthesized and the anticancer effects evaluated. Four analogs were evaluated for their ability to inhibit growth of myeloid leukemia (NB4, HL-60), breast (MCF-7), and prostate (LNCaP) cancer cells. All 4 analogs inhibited growth in a dose-dependent manner. Most effective was 21-(3-methyl-3-hydroxy-butyl)-19-nor D3(Gemini-19-nor), which has 2 side chains and removal of the C-19. Gemini-19-nor was approximately 40 625-, 70-, 23-, and 380-fold more potent than 1,25(OH)2D3 in inhibiting 50% clonal growth (ED50) of NB4, HL-60, MCF-7, and LNCaP cells, respectively. Gemini-19-nor (10−8 M) strongly induced expression of CD11b and CD14 on HL-60 cells (90%); in contrast, 1,25(OH)2D3 (10−8 M) stimulated only 50% expression. Annexin V assay showed that Gemini-19-nor and 1,25(OH)2D3 induced apoptosis in a dose-dependent fashion. Gemini-19-nor (10−8 M, 4 days) caused apoptosis in approximately 20% of cells, whereas 1,25(OH)2D3 at the same concentration did not induce apoptosis. Gemini-19-nor increased in HL-60 both the proportion of cells in the G1/G0 phase and expression level of p27kip1. Moreover, Gemini-19-nor stimulated expression of the potential tumor suppressor, PTEN. Furthermore, other inducers of differentiation, all-trans-retinoic acid and 12-O-tetradecanoylphorbol 13-acetate, increased PTEN expression in HL-60. In summary, Gemini-19-nor strongly inhibited clonal proliferation in various types of cancer cells, especially NB4 cells, suggesting that further studies to explore its anticancer potential are warranted. In addition, PTEN expression appears to parallel terminal differentiation of myeloid cells.
Introduction
The present chemotherapy of cancer uses agents that are usually toxic to normal cells. On the other hand, induction of cellular differentiation may supplement the use of cytotoxic drugs in several forms of neoplasia, like the successful use of all-trans-retinoic acid (ATRA) in the treatment of acute promyelocytic leukemia. The physiologically active form of vitamin D3, 1,25(OH)2D3, is a member of the secosteroid hormone family, which controls calcium homeostasis and bone metabolism. 1,25(OH)2D3 can induce differentiation and inhibit the growth of a number of malignant cell types, including myeloid leukemia, breast, prostate, colon, skin, and brain. Several studies suggested that growth inhibition by 1,25(OH)2D3 may be attributed to inhibition of the G1 to S transition in the cell cycle, which probably is due at least in part to stimulation of expression of the cyclin-dependent kinase inhibitors (CDKIs), p21waf1 and p27kip1 as well as induction of programmed cell death.1-4 In a clinical study, oral administration of 1,25(OH)2D3 to preleukemic patients was only partially effective5; calcemic side effects prevented the administration of the dosage of the compound needed to achieve the concentration of 1,25(OH)2D3 in vivo, which was known to be necessary from our in vitro studies.5,6Therefore, synthesis of vitamin D3 analogs with potent antiproliferative and differentiation activity against cancer cells with decreased risk of hypercalcemia has received considerable attention.7-14
Recently, PTEN/MMAC1/TEP1, a tyrosine phosphatase, was identified and mapped to chromosome 10q23.3.15-17PTEN gene mutations have been observed in a variety of human cancers including breast, prostate, brain, lymphoma, and leukemia.15,18-23Germline deletion of PTEN in the mouse resulted in early embryonic lethality, and heterozygous mice developed malignant neoplasms.24 25 These findings strongly suggested that PTEN is a candidate tumor suppressor.
In this study, a class of newly synthesized vitamin D3analogs having 2 identical side chains attached to carbon-20 was analyzed. We focused particularly on the most active analog, which has a deletion of C-19, 21-(3-methyl-3-hydroxy-butyl)-19-nor D3(Gemini-19-nor). This new vitamin D3 analog was more potent than 1,25(OH)2D3 in mediating growth inhibition, differentiation, apoptosis, G1/G0arrest of the cell cycle, and expression of p27kip1. Furthermore, we observed that this compound induced the expression of PTEN in myeloid leukemic cells as the cells underwent differentiation.
Materials and methods
Cells and compounds
The myeloid leukemia (HL-60), breast cancer (MCF-7), and prostate cancer (LNCaP) cell lines were obtained from American Type Culture Collection (Rockville, MD). The NB4 promyelocytic leukemia cell line was provided by Dr Lanotte (INSERM, Hospital Saint-Louis, Paris, France). HL-60, NB4, and LNCaP were cultured in RPMI 1640 with 10% fetal calf serum (FCS). MCF-7 cells were maintained in Dulbecco modified Eagle media with 10% FCS. All 4 cell lines were maintained in a 37°C incubator containing 5% CO2. After informed consent, mononuclear cells from normal bone marrow were collected by separation on Ficoll-Hypaque (Pharmacia, Piscataway, NJ) gradients at a density of 1.077, and washed in Iscove modified Dulbecco medium (IMDM) containing 10% FCS.
All vitamin D3 analogs were synthesized by Milan R. Uskokovic (Hoffmann-La Roche, Nutley, NJ). The analogs are shown in Figure 1. The vitamin D3compounds were dissolved in absolute ethanol at 10−3 M as stock solution, which were stored at −20°C and protected from light. 12-O-Tetradecanoylphorbol 13-acetate (TPA) and ATRA were purchased from Sigma (St Louis, MO).
Soft agar colony assay
Cells were cultured in a 2-layer soft agar system for either 7 days (HL-60 and NB4) or 10 days (MCF-7 and LNCaP) as described previously.26 Normal bone marrow cells were cultured for 14 days in methylcellulose medium M3234 (Stem Cell Technology, Vancouver, BC, Canada) containing 20 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF). MCF-7 and LNCaP cells were trypsinized, washed, counted, and plated into 24-well, flat-bottom plates with a total of 1 × 103 cells/well in a volume of 400 μL/well. The feeder layer was prepared with agar that had been equilibrated at 42°C. Prior to this step, vitamin D3compounds were pipetted into the wells. After incubation, the colonies were counted. All experiments were done at least 3 times using triplicate plates per experimental point.
Analysis of differentiation
Expression of cell surface antigens was determined by flow cytometry. HL-60 cells were cultured with either 1,25(OH)2D3 or Gemini-19-nor (10−8and 10−7 M) for 4 days. After twice washing with phosphate-buffered saline (PBS), cells were incubated for 30 minutes with fluorescein isothiocyanate (FITC)-conjugated murine antihuman CD11b or antihuman CD14 antibody (DAKO, Carpinteria, CA). Murine IgG1 antibody (DAKO) was used as negative control. Cells were analyzed by a FACScan (Becton Dickinson, Mountain View, CA). HL-60 cells were assessed for their ability to produce superoxide as measured by reduction of nitroblue tetrazolium (NBT), by morphology as detected on cytospin preparations stained with Diff-Quick Stain Set (Baxter Healthcare, Miami, FL). All experiments were independently done at least 3 times. All data were statistically analyzed by Studentt test.
Cell cycle analysis
Cell cycle analysis was performed on HL-60 cells incubated for 4 days with either 1,25(OH)2D3 or Gemini-19-nor at either 10−8 or 10−7 M. The cells were fixed in chilled methanol overnight before staining with 50 μg/mL propidium iodide (PI), 1 mg/mL RNase, and 0.1% NP40. Analysis was performed immediately after staining using a FACScan (Becton Dickinson) and CELLFit program (Becton Dickinson). All experiments were independently performed at least 3 times. All data were statistically analyzed by Student t test.
Apoptosis analysis
To study induction of apoptosis by vitamin D3analogs, annexin V assay (Annexin V-FITC Apoptosis Detection Kit; Pharmingen, San Diego, CA) was performed according to the manufacturer's instructions. Briefly, cells were harvested after exposure with either 1,25(OH)2D3 or Gemini-19-nor (10−8 and 10−7 M), washed twice with PBS, incubated with FITC-conjugated annexin V and PI for 15 minutes, and measured by FACScan (Becton Dickinson). All experiments were independently done at least 3 times. All data were statistically analyzed by Student t test.
Western blot analysis
Cells were washed twice in PBS, suspended in lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS], 0.5% sodium deoxycholate, 1% NP40, 100 μg/mL phenylmethylsulfonyl fluoride, 2 μg/mL aprotinin, 1μg/mL pepstatin, and 10 μg/mL leupeptin), and placed on ice for 30 minutes. After centrifugation at 15 000g for 20 minutes at 4°C, the supernatant was collected. Protein concentrations were quantitated using the Bio-Rad assay (Bio-Rad Laboratories, Hercules, CA). Whole lysates (40 μg) were resolved by 4% to 15% SDS-polyacrylamide gel, transferred to an immobilon polyvinylidene difuride membrane (Amersham, Arlington Heights, IL) and probed with anti-p27kip1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), anti-PTEN antibody (Santa Cruz Biotechnology), and anti-GAPDH antibody (Research Diagnostics, Flanders, NJ). The blots were developed using the enhanced chemoluminescence (ECL) kit (Amersham). Band intensity was measured using a densitometer and fold increase in expression as compared to control, untreated cells was calculated.
Results
Effect of vitamin D3 analogs on clonal proliferation
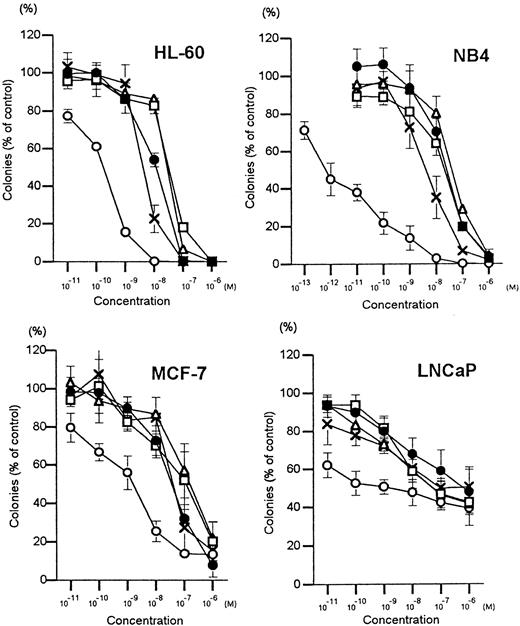
The myeloid leukemia (HL-60 and NB4), breast cancer (MCF-7), and prostate cancer (LNCaP) cells were cloned in soft agar in the presence of various concentrations of vitamin D3 analogs. All 4 Gemini vitamin D3 analogs and 1,25(OH)2D3 inhibited clonal growth of all 4 cell lines in a dose-dependent manner (Figure2). The effective dose that inhibited 50% colony formation (ED50) was determined (Table1). Gemini-19-nor was approximately 70-, 40 625-, 23-, and 380-fold more potent than 1,25(OH)2D3 in mediating clonal growth inhibition of HL-60, NB4, MCF-7, and LNCaP cells, respectively. Gemini 1-F-25-OH was 3- to 6-fold stronger than 1,25(OH)2D3 in suppressing clonal growth of the myeloid leukemia cell lines (HL-60 and NB4), but it had the same potency as 1,25(OH)2D3 against MCF-7 and LNCaP cells. Furthermore, the potency of Gemini 5,6-trans and Gemini 3-epi were nearly equivalent to 1,25(OH)2D3 for all 4 of the cell lines. Because Gemini-19-nor was found to be the most potent compound, all additional experiments focused on this analog.
Dose-response effects of vitamin D3compounds on clonal proliferation of cell lines.
Results are expressed as a mean (± SD) percentage of control plates containing no vitamin D3 analogs. = , 1,25(OH)2D3; c, Gemini-5,6-trans; Δ, Gemini-3-epi; O, Gemini-19-nor; X, Gemini-1-F-25-OH. Each point represents a mean of 3 independent experiments with triplicate dishes.
Dose-response effects of vitamin D3compounds on clonal proliferation of cell lines.
Results are expressed as a mean (± SD) percentage of control plates containing no vitamin D3 analogs. = , 1,25(OH)2D3; c, Gemini-5,6-trans; Δ, Gemini-3-epi; O, Gemini-19-nor; X, Gemini-1-F-25-OH. Each point represents a mean of 3 independent experiments with triplicate dishes.
Inhibition of clonal proliferation of cancer cell lines by vitamin D3 analogs
| Cell lines . | Inhibition of clonal proliferation, ED50 (M) . | ||||
|---|---|---|---|---|---|
| 1,25(OH)2D3 . | Gemini-19-nor . | Gemini-3-epi . | Gemini-5,6-trans . | Gemini-1-F-25-OH . | |
| HL-60 | 1.2 × 10−8 | 1.7 × 10−10 | 2.8 × 10−8 | 1.2 × 10−8 | 4.3 × 10−9 |
| NB4 | 2.6 × 10−8 | 6.4 × 10−13 | 3.9 × 10−8 | 2.1 × 10−8 | 4.0 × 10−9 |
| MCF-7 | 3.7 × 10−8 | 1.6 × 10−9 | 1.6 × 10−7 | 1.2 × 10−7 | 4.1 × 10−8 |
| LNCaP | × 10−7 | 1.7 × 10−9 | 5.0 × 10−8 | 7.0 × 10−8 | 1.0 × 10−7 |
| Cell lines . | Inhibition of clonal proliferation, ED50 (M) . | ||||
|---|---|---|---|---|---|
| 1,25(OH)2D3 . | Gemini-19-nor . | Gemini-3-epi . | Gemini-5,6-trans . | Gemini-1-F-25-OH . | |
| HL-60 | 1.2 × 10−8 | 1.7 × 10−10 | 2.8 × 10−8 | 1.2 × 10−8 | 4.3 × 10−9 |
| NB4 | 2.6 × 10−8 | 6.4 × 10−13 | 3.9 × 10−8 | 2.1 × 10−8 | 4.0 × 10−9 |
| MCF-7 | 3.7 × 10−8 | 1.6 × 10−9 | 1.6 × 10−7 | 1.2 × 10−7 | 4.1 × 10−8 |
| LNCaP | × 10−7 | 1.7 × 10−9 | 5.0 × 10−8 | 7.0 × 10−8 | 1.0 × 10−7 |
Results derived from Figure 2; the concentration of vitamin D3 compound that induced 50% clonal inhibition (ED50). HL-60, human myeloblastic leukemia cells; NB4, human acute promyelocytic cells; MCF-7, estrogen-receptor positive breast cancer cells; LNCaP, androgen receptor-positive prostate cancer cells.
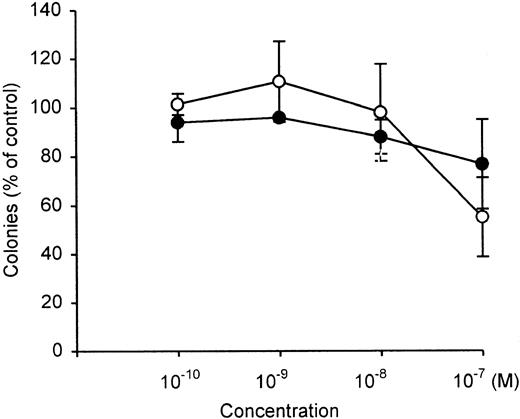
Clonal growth of normal bone marrow-committed myeloid stem cells (colony-forming units-granulocyte/macrophage [CFU-GM]) were not inhibited by either 1,25(OH)2D3 or Gemini-19-nor over a concentration range of 10−10 M to 10−8 M (Figure 3). At 10−7 M, both compounds inhibited by 20% to 40% the clonal growth of normal bone marrow CFU-GM.
Dose-response effects of vitamin D3compounds on clonal proliferation of normal bone marrow-committed myeloid stem cells (CFU-GM).
Results are expressed as a mean (± SD) percentage of control plates containing no vitamin D3 analogs. = , 1,25(OH)2D3; O, Gemini-19-nor. Each point represents a mean of 3 independent experiments with triplicate dishes.
Dose-response effects of vitamin D3compounds on clonal proliferation of normal bone marrow-committed myeloid stem cells (CFU-GM).
Results are expressed as a mean (± SD) percentage of control plates containing no vitamin D3 analogs. = , 1,25(OH)2D3; O, Gemini-19-nor. Each point represents a mean of 3 independent experiments with triplicate dishes.
Effect of Gemini-19-nor on differentiation of leukemia cell lines
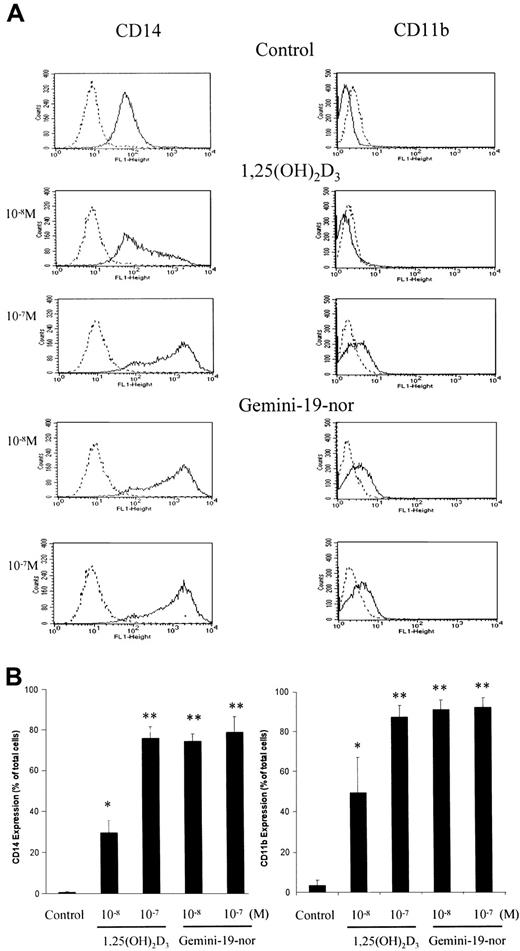
The induction of expression of the cell surface antigens CD11b and CD14 occurs on HL-60 cells as they undergo differentiation. The ability of Gemini-19-nor and 1,25(OH)2D3 to induce CD11b and CD14 was analyzed using flow cytometry (Figure4). A 4-day exposure of HL-60 cells to either 1,25(OH)2D3 (10−7 M) or Gemini-19-nor (10−7 M) resulted in both producing nearly 90% and 80% CD11b+ and CD14+ cells, respectively. At 10−8 M, Gemini-19-nor and 1,25(OH)2D3 induced 90% and 50% CD11b+ cells, and 75% and 30% CD14+ cells, respectively.
Induction of cell surface antigens on HL-60 cells by vitamin D3 compounds.
HL-60 cells were treated for 4 days with different concentrations (10−8 or 10−7 M) of either 1,25(OH)2D3 or Gemini-19-nor and then analyzed for expression of either CD11b or CD14 using flow cytometry. (A) Dashed line indicates negative control antibody; solid line, CD11b or CD14. (B) Column indicates mean (± SD) of 3 independent experiments. *P < .05; **P < .01 as determined by Student t test difference compared with the control group. Histograms show representative results from one experiment.
Induction of cell surface antigens on HL-60 cells by vitamin D3 compounds.
HL-60 cells were treated for 4 days with different concentrations (10−8 or 10−7 M) of either 1,25(OH)2D3 or Gemini-19-nor and then analyzed for expression of either CD11b or CD14 using flow cytometry. (A) Dashed line indicates negative control antibody; solid line, CD11b or CD14. (B) Column indicates mean (± SD) of 3 independent experiments. *P < .05; **P < .01 as determined by Student t test difference compared with the control group. Histograms show representative results from one experiment.
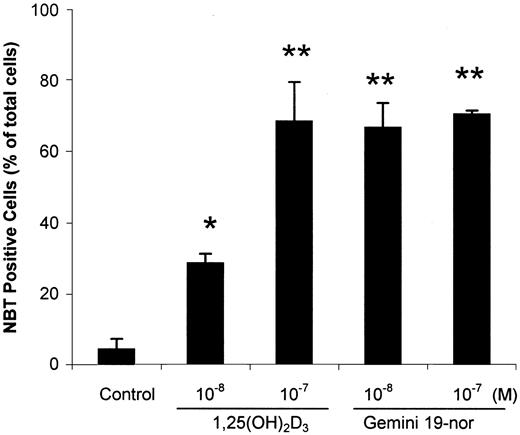
The capacity of HL-60 cells to produce superoxide as measured by the reduction of NBT was another marker of differentiation that was used. Gemini-19-nor was more potent than 1,25(OH)2D3with either Gemini-19-nor or 1,25(OH)2D3(10−8 M, 4 days) inducing 70% and 30% NBT+cells, respectively (Figure 5).
Effect of vitamin D3 compounds on reduction of NBT by HL-60 cells.
HL-60 cells were treated for 4 days with different concentrations (10−8 or 10−7 M) of either 1,25(OH)2D3 or Gemini-19-nor and then analyzed for reduction of NBT. Column indicates mean (± SD) of 3 independent experiments. *P < .05; **P < .01 as determined by Student t test difference compared with the control group.
Effect of vitamin D3 compounds on reduction of NBT by HL-60 cells.
HL-60 cells were treated for 4 days with different concentrations (10−8 or 10−7 M) of either 1,25(OH)2D3 or Gemini-19-nor and then analyzed for reduction of NBT. Column indicates mean (± SD) of 3 independent experiments. *P < .05; **P < .01 as determined by Student t test difference compared with the control group.
Effect of Gemini-19-nor on induction of apoptosis
Several vitamin D3 analogs and 1,25(OH)2D3 have been shown to cause apoptosis of several type of cancer cells.27 28 Gemini-19-nor and 1,25(OH)2D3 (10−8 M, 4 days) induced 20% and 6% of HL-60 cells, respectively, to undergo apoptosis (Figure 6). At a higher concentration (10−7 M, 4 days), both compounds produced apoptosis of about 20% of HL-60 cells (Figure 6).
Induction of apoptosis by vitamin D3compounds.
HL-60 cells were treated for up to 4 days with different concentrations (10−8 or 10−7 M) of either 1,25(OH)2D3 or Gemini-19-nor and then analyzed by flow cytometry for cells that were annexin V-FITC+ and PI−, which represent those in the early stages of apoptosis. (A) The upper right quadrant of each histogram shows necrotic cells that were PI+ and annexin V+. The lower right quadrant shows apoptotic cells that were not necrotic (PI−) and annexin V+. Histograms show representative data from one experiment. (B) Percentage of apoptotic cells after 4 days. Column indicates mean (± SD) of 3 independent experiments. *P < .05 as determined by Studentt test difference compared with the control group.
Induction of apoptosis by vitamin D3compounds.
HL-60 cells were treated for up to 4 days with different concentrations (10−8 or 10−7 M) of either 1,25(OH)2D3 or Gemini-19-nor and then analyzed by flow cytometry for cells that were annexin V-FITC+ and PI−, which represent those in the early stages of apoptosis. (A) The upper right quadrant of each histogram shows necrotic cells that were PI+ and annexin V+. The lower right quadrant shows apoptotic cells that were not necrotic (PI−) and annexin V+. Histograms show representative data from one experiment. (B) Percentage of apoptotic cells after 4 days. Column indicates mean (± SD) of 3 independent experiments. *P < .05 as determined by Studentt test difference compared with the control group.
Analysis of the cell cycle and expression of p27kip1
The effect of Gemini-19-nor and 1,25(OH)2D3 on the cell cycle of the HL-60 cells was determined. A significant accumulation (P < .05) of cells in the G1/G0and G2/M phases of the cell cycle occurred, with a concomitant decrease in the proportion of those in S phase after 4 days of culture with either 1,25(OH)2D3(10−7 M) or Gemini-19-nor (10−8 and 10−7 M) (Figure 7).
Cell cycle modulation by vitamin D3compounds.
HL-60 cells were cultured for 4 days with either 1,25(OH)2D3 (10−7 M) or Gemini-19-nor (10−7 M), fixed, and stained with PI, and the cell cycle status was analyzed using flow cytometry. Column indicates mean (± SD) of 3 independent experiments. *P < .05 as determined by Student t test difference compared with the control group.
Cell cycle modulation by vitamin D3compounds.
HL-60 cells were cultured for 4 days with either 1,25(OH)2D3 (10−7 M) or Gemini-19-nor (10−7 M), fixed, and stained with PI, and the cell cycle status was analyzed using flow cytometry. Column indicates mean (± SD) of 3 independent experiments. *P < .05 as determined by Student t test difference compared with the control group.
The cyclin-dependent kinase inhibitor, p27kip1, may act as a key regulator of G1/G0 accumulation induced by vitamin D3. Both 1,25(OH)2D3 and Gemini-19-nor induced expression of p27kip1 in a dose-dependent manner at 4 days of exposure of HL-60 cells as determined by Western blot analysis (Figure 8A). Consistent with the cell cycle results, Gemini-19-nor strongly induced expression of p27kip1 at a lower concentration (10−9 M) than 1,25(OH)2D3 (10−7 M). A time course study of HL-60 cells indicated that Gemini-19-nor (10−7 M) induced p27kip1 protein expression by approximately 7-fold at day 2 and 14-fold by day 4 of culture (Figure8B).
Induction of expression of p27kip1 and PTEN by vitamin D3 compounds.
(A) Dose-dependent study of p27kip1 and PTEN expression in HL-60 cells analyzed by Western blot. Cells were either untreated (control) or cultured with 10−9 to 10−7 M of either 1,25(OH)2D3 or Gemini-19-nor for 4 days. GAPDH was used as a loading control. (B) Time course study of p27kip1 and PTEN expression in HL-60 cells studied by Western blot. Cells were either untreated (Control) or cultured with Gemini-19-nor (10−7 M) for 0.5 to 4 days. GAPDH was used as a loading control. The densities of the bands were measured using densitometery. (C) Induction of PTEN expression by TPA and ATRA in HL-60 cells. Cells were either untreated (control) or cultured with either TPA (10−9 M) or ATRA (10−7 M) for 4 days. GAPDH was analyzed as a loading control.
Induction of expression of p27kip1 and PTEN by vitamin D3 compounds.
(A) Dose-dependent study of p27kip1 and PTEN expression in HL-60 cells analyzed by Western blot. Cells were either untreated (control) or cultured with 10−9 to 10−7 M of either 1,25(OH)2D3 or Gemini-19-nor for 4 days. GAPDH was used as a loading control. (B) Time course study of p27kip1 and PTEN expression in HL-60 cells studied by Western blot. Cells were either untreated (Control) or cultured with Gemini-19-nor (10−7 M) for 0.5 to 4 days. GAPDH was used as a loading control. The densities of the bands were measured using densitometery. (C) Induction of PTEN expression by TPA and ATRA in HL-60 cells. Cells were either untreated (control) or cultured with either TPA (10−9 M) or ATRA (10−7 M) for 4 days. GAPDH was analyzed as a loading control.
Induction of expression of PTEN
Recent studies showed that the tumor suppressor PTEN was mutated or otherwise dysregulated in several types of human tumors. It is involved in the normal regulation of cell growth, the cell cycle, and apoptosis.29,30 We evaluated the effect of vitamin D3 compounds on protein expression of PTEN in HL-60 cells that have no mutation, deletion, or methylation of this gene.22 The nontreated, control HL-60 cells had a very low level of expression of PTEN as determined by Western blot analysis (Figures 8A,B). Expression markedly increased by 16-fold in HL-60 cells at 4 days of culture with 10−9 M Gemini-19-nor; under the same culture conditions, the 1,25(OH)2D3(10−9 M) did not induce detectable levels of PTEN (Figure8A). At 10−7 M, Gemini-19-nor and 1,25(OH)2D3 increased the expression of PTEN by about 32-fold and 14-fold, respectively. A time course study showed that Gemini-19-nor enhanced expression of PTEN by 6-fold at 0.5 days and about 36-fold at 3 days of exposure (Figure 8B).
We also examined if other inducers of differentiation of HL-60 cells could up-regulate expression of PTEN. The phorbol diester, TPA, induces macrophage-like differentiation31 and ATRA induces granulocyte-like differentiation of HL-60 cells. Four days of culture with either TPA (10−9 M) or ATRA (10−7 M) induced PTEN expression in HL-60 cells (Figure 8C).
Discussion
A recent study revealed that a vitamin D3 compound that has 2 side chains on C-20 (Gemini) was more active than 1,25(OH)2D3 in its ability to inhibit clonal growth of malignant cells.32 Therefore, we synthesized additional novel Gemini compounds and examined their biologic effects on cancer cells. In this study, we evaluated 4 compounds from the newly synthesized family of Gemini. The Gemini-19-nor, which has 2 side chains on C-20 and the removal of the C-19, was the most potent inhibitor of clonal proliferation of myeloid leukemia, breast, and prostate cancer cells. It was more active than 1,25(OH)2D3 in these 3 types of cancers. In particular, the analog showed marked activity with the NB4 acute promyelocyte leukemia cells and was 40 625-fold more potent than 1,25(OH)2D3. Therefore, we focused on the activity of this analog compared with 1,25(OH)2D3.
Previously, we reported that analogs of 1,25(OH)2D3 that had removal of their C-19 moiety (19-nor 1,25D3 analogs) were active against prostate, breast, and myeloid leukemia cells.12,13,33,34The 19-nor analog with the code name LH (1,25[OH]2-16-ene-23-yne-26,27-F6-19-nor-D3) was most potent against cancer cells of breast (ED50: MCF-7, 8 × 10−10 M)12 and prostate (ED50: LNCaP, 1 × 10−9 M),13and the 1,25S(OH)2-16,23-diene-26-F3-19-nor-D3was most potent against myeloid leukemia cells (ED50: HL-60, 4 × 10−11 M; NB4, 5 × 10−11M).34 When compared with these 19-nor analogs, Gemini-19-nor was about 100-fold more potent than 1,25S(OH)2-16,23-diene-26-F3-19-nor-D3when studied with NB4 cells, whereas it had either comparable or weaker activity than LH and 1,25S(OH)2-16,23-diene-26-F3-19-nor-D3with HL-60, MCF-7, and LNCaP cells. Therefore, even though study conditions were not completely identical, these data suggest that in some types of leukemia, Gemini-19-nor may be a more potent inhibitor of proliferation than 1,25S(OH)2-16,23-diene-26-F3-19-nor-D3, which was the most active vitamin D3 analog against myeloid leukemia cells in previous studies. In contrast to the effect on tumor cell lines, Gemini-19-nor (10−10 M to 10−8 M) did not inhibit the clonal growth of normal human bone marrow committed myeloid stem cells (CFU-GM).
Exposure of HL-60 myeloid leukemia cells to either 1,25(OH)2D3 or Gemini-19-nor induced the expression of the cell surface markers, CD11b and CD14. Gemini-19-nor (10−8 M) induced expression of CD11b and CD14 in about 90% and 75% of cells, whereas the same concentration of 1,25(OH)2D3 induced expression on only 50% and 30% of cells, respectively. Similarly, Gemini-19-nor (10−8 M) induced 70% of HL-60 cells to become NBT+, compared to only 30% for the same concentration of 1,25(OH)2D3. Therefore, these results suggested that Gemini-19-nor was more potent than 1,25(OH)2D3 as an inducer of myeloid differentiation.
Gemini-19-nor also mediated apoptosis. Previously, we reported that vitamin D3 analogs in concert with a RXR ligand induced apoptosis of myeloid leukemia cells and caused levels of expression of Bcl-2 to decrease suggesting an association between the 2 events.3,4,35 However, other experiments found that a vitamin D3 analog could induce apoptosis of an HL-60 variant without a reduction of cellular levels of Bcl-2.4Another group reported that vitamin D3 compounds induced apoptosis via a novel caspase- and p53-independent pathway, and apoptosis was inhibited by forced expression of Bcl-2.36These findings suggested that vitamin D3 might use several pathways to induce apoptosis.
Previous studies showed that vitamin D3 analogs caused accumulation at the G1/G0 phase of the cell cycle, and this block may be mediated by p21waf1 and p27kip1 CDKIs.2,26,37 Gemini-19-nor D3 also produced G1- to S-phase block of the cell cycle and induced p27kip1 expression. These results support the hypothesis that CDKIs mediated at least in part the antiproliferative affects of the vitamin D3 compounds by induction of a G1/G0 accumulation. A block in the G2/M checkpoint has also been previously observed in HL-60 cells treated with 1,25(OH)2D3,38 and Gemini-19-nor was able to more potently induce a G2/M block compared to 1,25(OH)2D3. This effect has been attributed to a decrease in levels of p34(cdc),39a protein kinase which associates with B-type cyclins and controls transition through G2/M. Therefore Gemini-19-nor may be able to decrease levels of this protein to a greater extent than 1,25(OH)2D3.
Recent studies indicated that the candidate tumor suppressor, PTEN, could block the phosphatidylinositol 3′-kinase (PI3K)/Akt signaling pathway, resulting in cell death or inhibition of growth or both.29,30 Activated Akt mediates cell survival by inhibition of mitochondrial release of cytochrome c, inactivation of Forkhead transcription factors (FKHR), and phosphorylation of BAD and caspase-9.40 The overexpression of exogenous PTEN induced apoptosis of malignant cells.41-45 Moreover, a genetic link between the Fas proapoptotic pathway and PTEN was suggested, because Fas-mediated apoptosis was impaired in the germline heterozygous PTEN+/− murine model.46 In addition, PTEN induced G1 cell cycle arrest and this was associated with an increased expression of p27kip1.45 47-53 In the present study, Western blotting analysis demonstrated that PTEN expression was up-regulated by vitamin D3 compounds in HL-60 cells, and it paralleled the induction of apoptosis, expression of p27kip1 and G1 cell cycle arrest. These findings suggest that PTEN might enhance apoptosis and G1 cell cycle arrest in transformed cells exposed to a vitamin D3 analog, and it might help explain the multiple pathways of apoptosis mediated by vitamin D3 compounds.
Several studies indicated that PTEN induced cell differentiation in glioma cells.54 55 Therefore, we analyzed inducers of myeloid differentiation other than vitamin D3. We choose TPA, a stimulator of the protein kinase C pathway that induces macrophage-like differentiation, and ATRA, which binds the nuclear hormone receptor, retinoic acid receptor and induces granulocytic differentiation. As shown in Figure 6C, TPA and ATRA significantly induced PTEN expression. These observations suggest that PTEN expression is associated with monocytic and granulocytic differentiation. We do not know if this marked increase in PTEN expression is the cause or the effect of terminal differentiation of HL-60 cells. Further studies are required to define the role PTEN plays in this process of myeloid differentiation.
Taken together, the new vitamin D3 analog Gemini-19-nor D3 strongly inhibited growth of transformed cells, and produced myeloid differentiation, apoptosis, and G1/G0 arrest associated with elevated levels of p27kip1. Moreover, the vitamin D3analog induced expression of PTEN. These observations suggest that the anticancer effects of vitamin D3 might be regulated in part via PTEN. This analog may provide an adjuvant therapy for myeloid leukemia, especially acute promyelocytic leukemia, and may be effective in other types of cancers.
Supported by US Defense and National Institutes of Health grants as well as the Lymphoma Foundation, Parker Hughes Trust, Horn Foundation, and the C. and H. Koeffler Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
H. Phillip Koeffler, Division of Hematology/Oncology, Cedars-Sinai Medical Center, UCLA School of Medicine, 8700 Beverly Blvd, B-208, Los Angeles, CA 90048.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal