Abstract
Major mechanisms underlying poor immune responses to autologous tumor-associated antigens are overwhelming tumor kinetics and the absence of effective T-cell costimulation by antigen-presenting cells. To address these issues, leukemia and lymphoma mice were treated with the combination of chemotherapy and systemic immunotherapy with recombinant soluble murine B7–immunoglobulin G (IgG) molecules. In this report, 3 murine models were used, a radiation-induced SJL acute myeloid leukemia, a transplantable spontaneous SJL lymphoma, and the C57BL/6 EL-4 thymic lymphoma. Various treatment modalities were evaluated: single treatments with either B7-IgG or chemotherapy as well as combination therapies. The results demonstrate the following: (1) in all tumor models, the combination of chemotherapy and soluble B7-IgGs is more potent than either therapy alone, leading to cure of tumor-bearing animals; (2) the therapeutic responses are T-cell–dependent, because combined therapy is not efficacious in severe combined immunodeficient mice; (3) the rejection of tumor cells leads to the development of tumor-specific immunity, because cured mice are immune to the rejected tumor but not to a different syngeneic tumor; and (4) 51Cr release assays show that rejection of tumor cells leads to the development of very potent tumor-specific cytotoxic T-lymphocyte activity. On the basis of these results, it is proposed that chemotherapy-mediated tumor reduction, together with consequent augmented tumor-antigen presentation to activated T cells, are primary mechanisms leading to curative responses. The safety profile of the B7-IgG fusion proteins and their synergy with chemotherapy strongly suggest that the combination regimen is a promising strategy in cancer treatment.
Introduction
Major recent discoveries that have drastically modified the nature of T-cell–directed immunotherapy in cancer are the cloning of several tumor-associated antigens (TAAs) that elicit autologous cytotoxic T-cell responses1,2 and the discovery of new molecules and pathways involved in T-cell activation and costimulation.3,4 This knowledge has guided the design of numerous preclinical and clinical studies that, in turn, have generated remarkable insight into the mechanisms controlling host responses to cancer cells. Thus, it is now accepted that few spontaneous tumors are immunogenic, but most, if not all, are antigenic.5,6However, what ultimately determines the outcome of an endogenous antigen encounter is the context in which that particular antigen is presented to T cells.7,8 In the absence of danger signals that accompany tissue destruction and inflammation (typically observed during viral infection), the outcome is immune ignorance.9 10 At the level of antigen-presenting cell (APC)–T-cell interactions, the local danger signals translate primarily into the up-regulation of T-cell costimulatory signals provided by APCs at the time of antigen presentation.
Costimulation is defined as a signal necessary for optimal T-cell activation and survival delivered to T cells along with the T-cell receptor (TCR) signal, provided by APCs, ie, activated B cells, macrophages, and dendritic cells.11,12 Over the years, the CD28/B7 T-cell costimulatory pathway has emerged as the key regulator of T-cell responses. The signal involves the interaction of the T-cell surface antigen CD28 with the members of the B7 family molecules B7.1 (CD80) and B7.2 (CD86) expressed on APCs.13,14 Following a TCR-mediated signal, ligation of CD28 results in up-regulation of the interleukin 2 (IL-2)–receptor, increased IL-2 messenger RNA transcription, T-cell proliferation, cytokine secretion, and up-regulation of several T-cell-activation–related molecules.15 In contrast to CD28-mediated costimulation, the CD28 homologue cytotoxic T-lymphocye antigen-4 (CTLA-4, CD152) delivers a down-regulatory signal.16,17 In this context, CTLA-4–mediated immune regulation appears to be critical for the maintenance of immunological homeostasis.18
The discovery of the B7-CD28/CTLA-4 pathway led to the speculation that lack of T-cell costimulation could be an important mechanism conferring low immunogenicity, even to tumors expressing major histocompatibility complex (MHC) molecules, which are able to present tumor antigens to T cells.19,20 The hypothesis that introduction of B7 genes into tumors might result in effective tumor immunogenicity has been demonstrated in several tumor models. The pioneering work of Chen et al21 and Townsend and Allison22 showed that genetic modification of melanoma cells to express B7.1 led to reduced tumorigenicity of the modified cells and to the development of tumor-specific immunity. Subsequent studies demonstrated the efficacy of B7.1-based tumor vaccines in myeloid leukemia models.23,24 A striking observation that emerged from these studies was the lack of efficacy against bulk disease; in this context, B7.1-based tumor vaccines were efficacious only when given in early stages of the disease. It has been proposed that this is simply due to drastically increased replication rates of tumor cells that may preclude the opportunity for developing an adequate immune response.25 26
In an attempt to overcome the practical problems currently related to genetic modification of patients' tumor cells, we have developed soluble B7–immunoglobulin G (IgG) (B7.1-IgG and B7.2-IgG) fusion proteins and have recently shown their efficacy as single therapy in several murine solid-tumor models.27 In this report, we tested the efficacy of soluble B7-IgG in murine systemic leukemia and lymphoma models. Our results show that in poorly immunogenic and aggressive systemic tumor models, single therapy with either B7-IgG fusion proteins or cytoreductive chemotherapy has variable efficacy, but neither therapy alone is able to cure tumor-bearing animals. However, when both systemic immunotherapy and chemotherapy are combined, leukemia and lymphoma tumors are rejected and treated mice are cured.
Materials and methods
Mice
Female SJL/J mice and female C57BL/6 severe combined immunodeficient (SCID) mice, 6 to 8 weeks old, were purchased from Jackson Laboratories (Bar Harbor, ME). Female C57BL/6 mice, 6 to 8 weeks old, were purchased from Harlan Sprague-Dawley (Indianapolis, IN). The animals were kept at the animal facility of Genetics Institute (Cambridge, MA) according to the Institute's guidelines.
Tumor models
We used 3 murine hematological tumor models in this study: a radiation-induced SJL/J acute myeloid leukemia (AML) model,24 a transplantable spontaneous SJL lymphoma (Ly) that has developed at Genetics Institute's animal facility and has been passaged in vivo several times, and the C57BL/6 EL-4 thymic lymphoma. In all experiments, frozen spleen (AML) or lymph node (Ly) mononuclear cells (isolated from moribund leukemic or lymphoma mice) were used. The EL-4 cell line was purchased from American Type Culture Collection (Rockville, MD) and was maintained in vitro at 37°C in RPMI 1640 medium containing 10% fetal calf serum, 2% glutamine, and 1% penicillin-streptomycin. For the establishment of tumors, 105 AML or Ly cells were injected intravenously (IV) in the tail vein of SJL mice, and 106 EL-4 cells were injected subcutaneously (SC) in the flank of C57BL/6 mice. Tumor-bearing animals either died within 20 to 35 days after tumor inoculation (AML/Ly) or were killed when tumors reached a size of approximately 400 to 600 mm2 (EL-4).
Murine recombinant B7-IgG fusion proteins
The development and purification of soluble B7.1- and B7.2-IgG fusion proteins have been previously described.27 Briefly, complementary DNA encoding the signal and the extracellular domains of murine B7.1 or B7.2 were joined to the genomic DNA encoding the hinge–CH2-CH3 domains derived from a murine IgG2a antibody. B7-IgG molecules are dimeric and can bind to CD28 and/or CTLA-4 on T cells and to Fc receptors (FcRs) on macrophages and APCs. In some experiments, either mutated B7.2-IgG fusion protein (the IgG2a region is mutated to ablate binding to Fcγ receptor I [FcγRI]and complement C1b) or control isotype murine IgG2a was used.
Chemotherapy protocols
Leukemia protocol was as follows: cytarabine (Cytosar-U, Pharmacia & Upjohn, Kalamazoo, MI), 2 consecutive intraperitoneal (IP) injections of 200 mg/kg, 6 hours apart, on days 7, 14, and 21; doxorubicin hydrochloride (Adriamycin RDF, Pharmacia & Upjohn), 1 IP injection of 6 mg/kg on days 7 and 14. Lymphoma protocol was as follows: cyclophosphamide monohydrate (Sigma, St Louis, MO), 1 IP injection of 100 mg/kg on days 7 and 21; doxorubicin hydrochloride, 1 IP injection of 6 mg/kg on days 7 and 21. The choice of the compounds was based on their use in clinical leukemia and lymphoma chemotherapy protocols.
Combination therapy
Mice were injected with live tumor cells on day 0 and subsequently treated with chemotherapy and one injection of 100 μg B7.2-IgG (or 50 μg of each B7.2- and B7.1-IgG) on days 7, 14, 21, and 28. B7-IgG was diluted in 200 μL of sterile saline and administered by subcutaneous injections at the described doses and schedules. Tumor size in EL-4 and survival of mice in AML and Ly were monitored twice weekly. Except for survival experiments, mice were killed when tumors reached a size of approximately 400 to 600 mm2.
Proliferation assays
Spleens were harvested from nontreated leukemic (AML) mice and from mice that had received 1 (day 7) or 2 (days 7 and 14) courses of chemotherapy (AML plus chemotherapy). Single-cell suspensions were prepared as previously described. Cells were cultured at 2 × 105 cells per well in flat-bottomed 96-well plates coated with a suboptimal dose (100 ng/mL) of anti-CD3 monoclonal antibody (mAb) 145-2C11(Pharmingen, San Diego, CA) and increasing amounts of plate-bound B7.2-IgG. Response to costimulation with anti-CD3 plus anti-CD28 (1 μg/mL) was used as positive control. Proliferation of responder cells was measured after 72 hours by the incorporation of 3H thymidine (1 μCi per well) for the last 18 hours of incubation. In tumor cell proliferation assays, 106 tumor cells per milliliter (2 × 105cells per well in U-bottomed 96-well plates) were cultured with 10 μg/mL of soluble B7.1- or B7.2-IgG or control IgG, and their proliferation was measured 72 hours after culture initiation.
Murine IL-2 enzyme-linked immunosorbent assay
Levels of murine IL-2 in culture supernatants were determined by a sandwich enzyme-linked immunosorbent assay that used specific antimurine mAbs for capture and detection (Endogen, Woburn, MA). The sensitivity of the assay is 5 pg/mL.
Immunostaining and flow cytometry analysis
Splenocytes were isolated from AML and AML-plus-chemotherapy mice and were stained as previously described.24 The following mAbs (Pharmingen) were used for flow cytometry studies: Gr-1+ (RB6-8C5), CD3e (145-2C11), CD4 (L3T4), CD8a (53-67).
51Cr release cytotoxic T-lymphocyte assays
Spleens were collected from mice 11 weeks after EL-4 tumor inoculation/rejection, and single-cell suspensions were prepared. Splenocytes (5 × 106) were cocultured with irradiated (7335 cGy) EL-4 cells (1 × 105) in 2 mL complete RPMI per well of a 24-well tissue-culture plate (Costar, Cambridge, MA). At 6 days later, splenocytes were harvested and used as effector cells in cytotoxic T-lymphocyte (CTL) assays. EL-4 cells or control AML cells (2 × 106) were labeled with 200 μCi 51Cr (New England Nuclear, Boston, MA) for 90 minutes, washed twice, and used as targets (5000 per well) in the CTL assays. The standard 4-hour CTL assays were set up with various effector-to-target (E/T) ratios as previously described.
Statistical analysis
Most individual experiments consisted of 10 mice per treatment group. The data analyzed represent the results of 2 or 3 individual experiments. The statistical survival analysis was performed by means of the standard Mantel-Cox log-rank test. Cytokine values and proliferation results are the mean ± SD. The statistical significance between various groups was analyzed by Studentt test.
Results
Effects of systemic chemotherapy
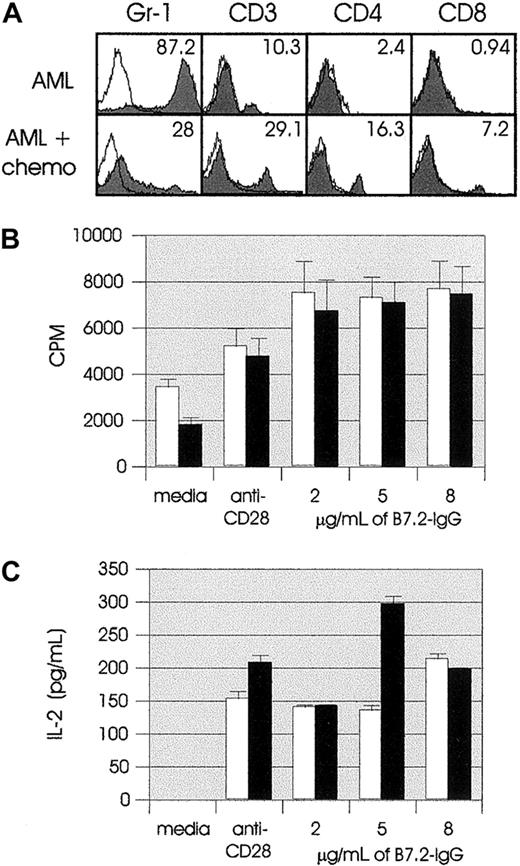
In an attempt to mimic clinical situations as closely as possible, we used chemotherapy regimens consisting of drugs that are major components of leukemia and lymphoma clinical protocols. Because most cytoreductive drugs target dividing cells, it is conceivable that any proliferating cells of the immune system would also be affected. Therefore, we first determined what the in vivo effects of chemotherapy were on both tumor cells and T cells. We treated leukemic mice on days 7 and 14 with Ara-C plus doxorubicin (AML plus chemotherapy). On day 14 or 21 (1 week after the first or second dose of chemotherapy), AML-plus-chemotherapy mice and control AML mice were killed, and their splenocytes were used for in vitro studies. Immunostaining and flow cytometric analysis on day 14 showed that after one course of chemotherapy, AML-plus-chemotherapy spleen had decreased numbers of Gr-1+ cells (marker for the leukemic cells)24and increased numbers of T cells as compared with AML spleen (data not shown). The effect of chemotherapy was more prominent in day-21 flow cytometric analysis. As shown in Figure1A, there was a dramatic decrease in the percentage of Gr-1+ cells in AML-plus-chemotherapy spleen as compared with AML (28% vs 87.2%), whereas the percentage of CD3, CD4, and CD8 T cells was significantly increased in AML-plus-chemotherapy spleen (29.1% vs 10.3%, 16.3% vs 2.4%, and 7.2% vs 0.94%, respectively).
Effects of systemic chemotherapy.
Two groups of SJL mice (10 mice per group) were injected IV (tail vein) with live 105 AML cells on day 0. One group (AML, ■) received no treatment; the second group (AML plus chemotherapy, ▪) was treated with chemotherapy (chemo) as described in “Materials and methods.” (A) Flow cytometric analysis of AML and AML-plus-chemotherapy splenocytes on day 21 (1 week after the second dose of chemotherapy). In all panels, solid histograms represent the expression of the indicated markers by splenocytes. (B) AML and AML-plus-chemotherapy splenocytes were cultured at 2 ×105cells per well in U-bottomed 96-well plates coated with suboptimal dose (100 ng/mL) of anti-CD3 mAb 145-2C11 and the indicated amounts of plate-bound B7.2-IgG. Response to costimulation with anti-CD3 plus anti-CD28 (1 μg/mL) was used as positive control. Proliferation of responder cells was measured after 72 hours by the incorporation of3H thymidine (1 μCi per well) for the last 18 hours of incubation. Results are representative of 2 independent experiments and are shown as the mean ± SD of 8 cultures. (C) AML and AML-plus-chemotherapy splenocytes were cultured as described in panel B. IL-2 concentrations were determined in 24-hour tissue-culture supernatants. Results are representative of 2 independent experiments and are shown as the mean ± SD of 8 cultures.
Effects of systemic chemotherapy.
Two groups of SJL mice (10 mice per group) were injected IV (tail vein) with live 105 AML cells on day 0. One group (AML, ■) received no treatment; the second group (AML plus chemotherapy, ▪) was treated with chemotherapy (chemo) as described in “Materials and methods.” (A) Flow cytometric analysis of AML and AML-plus-chemotherapy splenocytes on day 21 (1 week after the second dose of chemotherapy). In all panels, solid histograms represent the expression of the indicated markers by splenocytes. (B) AML and AML-plus-chemotherapy splenocytes were cultured at 2 ×105cells per well in U-bottomed 96-well plates coated with suboptimal dose (100 ng/mL) of anti-CD3 mAb 145-2C11 and the indicated amounts of plate-bound B7.2-IgG. Response to costimulation with anti-CD3 plus anti-CD28 (1 μg/mL) was used as positive control. Proliferation of responder cells was measured after 72 hours by the incorporation of3H thymidine (1 μCi per well) for the last 18 hours of incubation. Results are representative of 2 independent experiments and are shown as the mean ± SD of 8 cultures. (C) AML and AML-plus-chemotherapy splenocytes were cultured as described in panel B. IL-2 concentrations were determined in 24-hour tissue-culture supernatants. Results are representative of 2 independent experiments and are shown as the mean ± SD of 8 cultures.
We next determined the proliferative and cytokine response of AML-plus-chemotherapy splenocytes to in vitro B7.2-IgG costimulation. On day 14, no differences were observed between AML-plus-chemotherapy and AML splenocytes (Figure 1B), and both appeared to proliferate more vigorously with B7-IgG costimulation than with costimulation by anti-CD28 mAb. Furthermore, as shown in Figure 1C, IL-2 secretion in response to B7-IgG costimulation was comparable to stimulation through CD28. (The IL-2 levels in response to 5 μg/mL AML-plus-chemotherapy culture presented in Figure 1C were not observed in any of our other, similar in vitro costimulation experiments. These data are included because they are part of the series of other parameter measurements in the same experimental system). Overall, no major differences were observed between AML-plus-chemotherapy and AML splenocytes, indicating that this chemotherapy regimen did not cause detectable immunosuppression of the leukemic mice with regard to thymidine uptake and cytokine secretion. The day-21 proliferation and IL-2 level assays were performed only on AML-plus-chemotherapy splenocytes (as shown in Figure 1A, day-21 AML spleens were heavily infiltrated with Gr-1+ cells), and the results were comparable to those from day-14 assays (data not shown). Collectively, these results demonstrate that treatment of murine AML with doxorubicin and Ara-C significantly reduces the leukemic burden, without having a detrimental effect on in vitro T-cell proliferation and IL-2 secretion in response to B7.2-IgG costimulation.
In vitro effects of B7.2-IgG on tumor cells
Soluble B7.2-IgG fusion protein can potentially bind on CD28/CTLA-4 and FcγRs on cells. Because EL-4 cells express CD28 and all 3 tumor cell types express FcγRs, we investigated what the in vitro direct effects of soluble B7.2-IgG were on tumor cells. Tumor cells were cultured as described in “Materials and methods,” and their immunophenotype (fluorescence-activated cell sorting analysis), viability (trypan-blue exclusion), and proliferative response (3H thymidine uptake) were examined. In all types of experiments, no significant differences were observed among the media, IgG, or B7-IgG cultures (data not shown), indicating that there is no direct effect of soluble B7.1- or B7.2-IgG on tumor cells with regard to their immunophenotype, viability, and proliferative profile.
Combination therapy leads to cure of leukemia and lymphoma mice
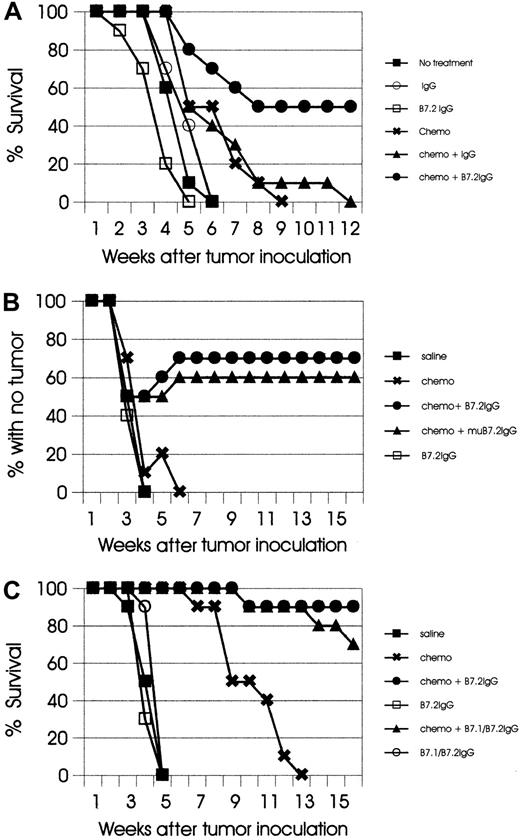
Single and combined treatment modalities (with B7.2-IgG, B7.1/B7.2-IgG, mutated form of B7.2-IgG, or control IgG) were evaluated in each tumor model, and no statistically significant differences were observed with the use of different sets of circumstances. Each experiment was repeated 2 to 3 times with similar results, and data from one representative experiment per model will be presented in this report. In the experiment shown in Figure2A, single B7.2-IgG or control IgG treatment in the AML model had no effect. In most experiments, AML mice treated with single chemotherapy had 1 to 2 weeks' prolonged survival, as compared with untreated animals (P < .05), but eventually all mice developed lethal leukemia. However, when both treatments were combined, 50% of the mice were cured (Figure2A).
Combination therapy leads to cure of leukemia and lymphoma mice.
(A) SJL mice (10 mice per group) were injected IV (tail vein) with live 105 AML cells on day 0 and subsequently treated with the indicated types of treatment, as described in “Materials and methods.” Mice treated with the combination of chemotherapy plus B7.2-IgG had 50% cure; all other groups developed lethal leukemia. The results are representative of 3 independent experiments. (B) C57BL/6 mice (10 mice per group) were injected SC in the flank with live 106 EL-4 cells and subsequently treated as described in “Materials and methods.” Mice with no treatment, chemotherapy alone, and B7.2-IgG alone, were killed when tumors reached a size of approximately 400 to 600 mm2. In the chemotherapy-plus-B7.2-IgG group, 70% of the mice remained tumor free, and in the chemotherapy-plus-mutated B7.2-IgG group, 60% of the mice remained tumor free. The results are representative of 2 independent experiments. (C) SJL mice (10 mice per group) were injected IV with live 105 SJL Ly cells on day 0 and subsequently treated with the indicated types of treatment, as described in “Materials and methods.” Mice treated with chemotherapy alone had significantly prolonged survival (P < .01), but eventually developed lethal tumors. In the chemotherapy-plus-B7.2–IgG group, 90% of the mice remained tumor free, and in the chemotherapy-plus-B7.1/B7.2–IgG group, 70% of the mice remained tumor free. The results are representative of 2 independent experiments.
Combination therapy leads to cure of leukemia and lymphoma mice.
(A) SJL mice (10 mice per group) were injected IV (tail vein) with live 105 AML cells on day 0 and subsequently treated with the indicated types of treatment, as described in “Materials and methods.” Mice treated with the combination of chemotherapy plus B7.2-IgG had 50% cure; all other groups developed lethal leukemia. The results are representative of 3 independent experiments. (B) C57BL/6 mice (10 mice per group) were injected SC in the flank with live 106 EL-4 cells and subsequently treated as described in “Materials and methods.” Mice with no treatment, chemotherapy alone, and B7.2-IgG alone, were killed when tumors reached a size of approximately 400 to 600 mm2. In the chemotherapy-plus-B7.2-IgG group, 70% of the mice remained tumor free, and in the chemotherapy-plus-mutated B7.2-IgG group, 60% of the mice remained tumor free. The results are representative of 2 independent experiments. (C) SJL mice (10 mice per group) were injected IV with live 105 SJL Ly cells on day 0 and subsequently treated with the indicated types of treatment, as described in “Materials and methods.” Mice treated with chemotherapy alone had significantly prolonged survival (P < .01), but eventually developed lethal tumors. In the chemotherapy-plus-B7.2–IgG group, 90% of the mice remained tumor free, and in the chemotherapy-plus-B7.1/B7.2–IgG group, 70% of the mice remained tumor free. The results are representative of 2 independent experiments.
In the EL-4 model, single therapy with either B7.2-IgG or chemotherapy alone had minimal or no effect. Treatment with B7.2-IgG or the mutated form of B7.2-IgG (which does not bind high-affinity FcγRs and complement C1q)27 plus chemotherapy led to cure of 70% and 60%, respectively (Figure 2B). In this model, a bimodal pattern of tumor growth was observed in 10% to 20% of treated mice; ie, the mice developed palpable tumor masses that then regressed during the following 2 weeks, indicating a therapeutic response to the combination regimen. In the AML and EL-4 models, treatment with both B7.1- and B7.2-IgG fusion proteins and chemotherapy showed comparable efficacy to treatment with B7.2-IgG plus chemotherapy (data not shown).
In the SJL Ly model, single therapy with fusion proteins showed no therapeutic effect (Figure 2C). Chemotherapy alone significantly prolonged the survival of treated mice (P < .01), but no cures were observed. In the same experiment, B7.2-IgG plus chemotherapy led to 90% cure, and the combination of B7-IgGs plus chemotherapy to 70% cure.
Collectively, these results demonstrate that, in all models tested in this study, the combination of B7-IgG fusion proteins with chemotherapy leads to cure of leukemia- and lymphoma-bearing mice.
Therapeutic responses to combination therapy are T-cell dependent
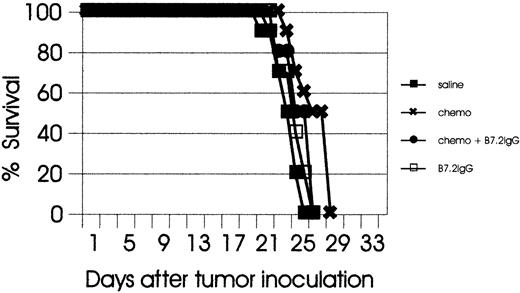
It has been previously shown in tumor models that B7-IgG–mediated curative responses are CD8+T-cell–dependent.27 To examine the role of T cells in the therapeutic responses to combination therapy, we evaluated single therapies (B7-IgG or chemotherapy) and combination therapy in T- and B-cell–deficient (SCID) mice. C57BL/6 SCID mice bearing EL-4 tumors were treated with B7.2-IgG, chemotherapy alone, or the combination of both. The tumors grew more rapidly in SCID mice than in normal C57BL/6 mice, and by day 28 after tumor inoculation, all mice had succumbed to lethal tumors. As shown in Figure 3, combination therapy had no therapeutic effect in SCID mice, clearly indicating the indispensable role of T cells for the efficacy of the combination regimen. Combination experiments in C57BL/6 SCID mice were also pursued in another systemic leukemia model (C1498 myeloid leukemia model), and similar results were observed (K.D.-J., unpublished data, March 2000).
Therapeutic responses to combination therapy are T-cell–dependent.
C57BL/6 SCID mice (10 mice per group) were injected SC in the flank with live 106 EL-4 cells and subsequently treated with the indicated types of treatment. All treatment groups, including combination therapy, succumbed to lethal tumors.
Therapeutic responses to combination therapy are T-cell–dependent.
C57BL/6 SCID mice (10 mice per group) were injected SC in the flank with live 106 EL-4 cells and subsequently treated with the indicated types of treatment. All treatment groups, including combination therapy, succumbed to lethal tumors.
Combination therapy leads to long-lasting tumor-specific immunity
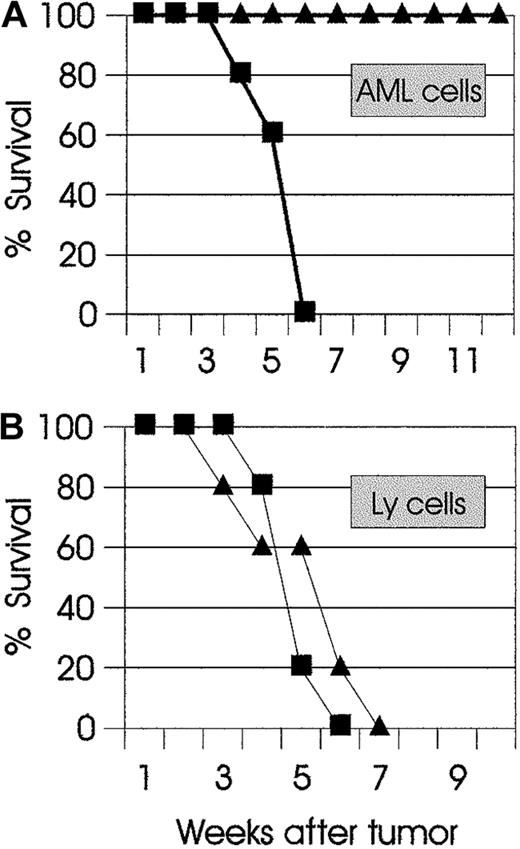
One of the main goals of combining cytoreductive chemotherapy with immunotherapy is to activate the adaptive immune system and thus trigger the development of effector and memory cytotoxic T cells. Whereas effector T cells in this setting may have a beneficial role in eliminating residual tumor cells that have escaped chemotherapy, long-term disease-free survival can be achieved only when potential tumor relapses are under the critical control of memory cytotoxic T cells. Therefore, we sought to determine if combination therapy was able to support the development of antitumor memory T cells. AML mice that had been cured with combination therapy were challenged 4 months later with live AML cells. As shown in Figure4A, all mice were immune to challenge and rejected the leukemic cells. At 2 months after the AML challenge and rejection, the same mice were inoculated with syngeneic lymphoma (Ly) cells. As shown in Figure 4B, the challenged mice developed lethal lymphoma at the same time as naive mice that had been inoculated with Ly cells. These findings demonstrate that the mechanisms mediating curative responses in combination therapy also lead to the development of long-lived, tumor-specific memory cells.
Combination therapy leads to long-lasting tumor-specific immunity.
(A) SJL AML mice (▴) that had been cured with combination therapy were challenged 4 months later with live 105 AML cells. Naive SJL mice (▪) were used as control. All SJL AML mice were resistant to the challenge and rejected the AML cells. (B) At 2 months later, the same mice were injected with a different syngeneic tumor (SJL Ly cells). Control naive (▪) and challenged mice (▴) developed lethal lymphoma. The results are representative of 2 independent experiments.
Combination therapy leads to long-lasting tumor-specific immunity.
(A) SJL AML mice (▴) that had been cured with combination therapy were challenged 4 months later with live 105 AML cells. Naive SJL mice (▪) were used as control. All SJL AML mice were resistant to the challenge and rejected the AML cells. (B) At 2 months later, the same mice were injected with a different syngeneic tumor (SJL Ly cells). Control naive (▪) and challenged mice (▴) developed lethal lymphoma. The results are representative of 2 independent experiments.
Combination therapy leads to long-lasting tumor-specific CTL activity
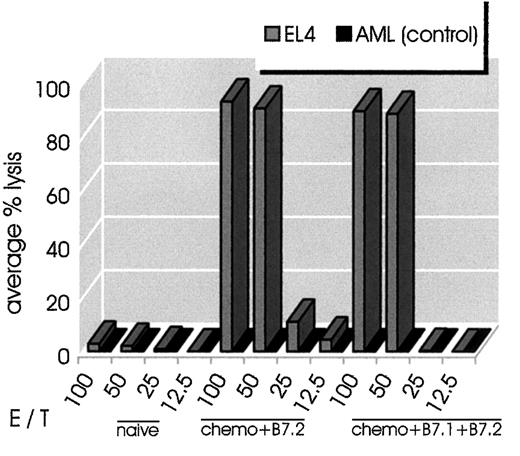
To further characterize the antitumor memory responses of cured mice, we performed in vitro CTL assays. Spleens were harvested from mice that had been cured of EL-4 lymphoma (with B7.2-IgG plus chemotherapy or the combination of B7-IgG plus chemotherapy), and splenocytes were assayed for in vitro CTL activity. As shown in Figure5, both groups of cured mice generated very potent cytolytic responses upon stimulation with EL-4 cells. The response was EL-4–specific (H-2b) because the same cells did not lyse alloantigen-presenting AML (H-2s) cells.
Combination therapy leads to long-lasting tumor-specific CTL activity.
Splenocytes from mice that had been cured from EL-4 lymphoma (chemotherapy plus B7.2-IgG or chemotherapy plus B7.1- plus B7.2-IgG) were stimulated in vitro with irradiated EL-4 cells as described in “Materials and methods.” At 6 days later, cells were harvested and used as effector cells in the indicated E/T ratios. Target cells (H-2b EL-4 or control H-2s AML cells) were incubated with 51Cr for 90 minutes. The standard 4-hour CTL assays were set up in a total volume of 0.2 mL per well in 96-well microtiter plate. All conditions were set up in quadruplicate. As control in the experiment, normal C57BL/6 splenocytes were tested for CTL activity on EL-4 and AML cells. Splenocytes from combination-treated mice had strong CTL activity against EL-4 cells but not AML cells.
Combination therapy leads to long-lasting tumor-specific CTL activity.
Splenocytes from mice that had been cured from EL-4 lymphoma (chemotherapy plus B7.2-IgG or chemotherapy plus B7.1- plus B7.2-IgG) were stimulated in vitro with irradiated EL-4 cells as described in “Materials and methods.” At 6 days later, cells were harvested and used as effector cells in the indicated E/T ratios. Target cells (H-2b EL-4 or control H-2s AML cells) were incubated with 51Cr for 90 minutes. The standard 4-hour CTL assays were set up in a total volume of 0.2 mL per well in 96-well microtiter plate. All conditions were set up in quadruplicate. As control in the experiment, normal C57BL/6 splenocytes were tested for CTL activity on EL-4 and AML cells. Splenocytes from combination-treated mice had strong CTL activity against EL-4 cells but not AML cells.
Discussion
In this report, we demonstrate that the addition of soluble B7.2-IgG to conventional leukemia and lymphoma chemotherapy regimens has remarkable synergistic effects in murine leukemia and lymphoma models, leading to curative T-cell–dependent antitumor responses. The establishment of long-lived tumor-specific memory T cells was confirmed with in vivo challenge experiments and in vitro CTL assays.
Various mechanisms may contribute to the synergy observed in combination therapy, as opposed to the limited efficacy of single therapies in our studies. Chemotherapy alone, as shown in the AML model, significantly reduces the leukemic burden, but cannot eradicate minimal residual disease. Apparently, this is the reason chemotherapy-treated animals finally succumb to lethal leukemia, a situation resembling the relatively short duration of remission observed in AML patients with partial remission to induction chemotherapy.28,29 In addition to tumor-burden reduction, cytoreductive chemotherapy generates a plethora of TAAs, which, as is the case with all non–self-antigens, are eventually expressed in the context of MHC molecules on APCs and can potentially initiate antigen-specific T-cell activation. However, chemotherapy overall fails to generate clinically overt antitumor memory responses, primarily for 2 reasons: first, it temporarily reduces the T-cell pool by targeting the relatively low numbers of proliferating T cells, and secondly, the sudden availability of abundant tumor antigens is probably not accompanied by signals required for the maturation process of immature dendritic cells (DCs), the most potent APCs and initiators of immunity.30,31 In the presence of a maturation signal, DCs express higher levels of costimulatory and MHC molecules and can then activate resting T cells.32 In our studies, neither tumor reduction and increased TAA presentation (chemotherapy) nor T-cell activation (B7-IgG) alone can cure leukemia and lymphoma mice. The efficacy of the combination regimen suggests that B7-IgG soluble molecules are probably strengthening APC–T-cell interactions during antigen presentation, which in combination with chemotherapy-mediated tumor reduction can lead to curative immune responses.
How does soluble B7-IgG fusion protein work? Because of the nature of the fusion molecule, there is little doubt that in vivo it will bind to both CD28/CTLA-4– and FcγR-expressing cells. We have shown that B7.2-IgG indeed binds in vitro to CD3+, Mac-1+, and B220+ murine splenocytes.33 The binding of B7.2-IgG on T cells can potentially involve both ligands, CD28 and CTLA-4. Although at this point we have no experimental evidence what role, if any, CTLA-4 plays in this system, we have reproducible results showing that murine naive T cells proliferate and secrete cytokines (IL-2 and interferon [IFN]–γ) in response to in vitro costimulation with soluble B7-IgG.33 One possible explanation why CD28 binding, rather than CTLA-4–mediated suppression of T cells, might be the primary pathway leading to activation is that CD28 is constitutively expressed on most T cells, whereas no detectable levels of CTLA-4 molecules are expressed on nonactivated T cells.34 35 All together, the in vitro and in vivo results strongly suggest that B7.2-IgG binding on T cells mediates an activation signal, and that most probably CD28 is the dominant responding molecule. Future studies with the use of CD28 knockout (KO) mice will provide more information on the mechanistic function of B7.2-IgG in this matter.
The indispensable role of T cells in combination therapy was confirmed in studies with SCID mice, in which all experimental groups, with or without treatment, rapidly developed lethal tumors. The relatively complex nature of combination therapy has made us hesitant to use additional in vivo compounds for CD4+ and CD8+ T-cell depletion and determination of each subset's role in combination therapy. We plan, however, on specifically addressing the role of CD4+ and CD8+ cells in future work by (1) in vivo selective depletion of CD4/CD8 cells at the time of rechallenge, (2) adaptive transfer experiments, and (3) T-cell depletion of long-term survivors and determination of whether there is tumor regrowth at that time.
In addition to CD28/CTLA-4, B7.2-IgG also binds in vitro (and potentially in vivo) to FcγR-expressing cells. Experiments in vitro have shown that soluble B7.2-IgG binding in SCID macrophages induces up-regulation of several activation-related molecules, including B7.1, CD40, MHC class I, and adhesion molecules.33 It is conceivable that similar in vivo effects of B7.2-IgG binding on APCs (which are loaded with an excess of tumor antigens) would potentially have significant contribution to the curative responses of the combination regimen. In our studies, the in vivo use of the mutated form of B7-IgG had comparable efficacy to the nonmutated form, indicating that abrogation of high-affinity (CD64) FcγRI binding is dispensable in the combination therapy. It has been shown that murine low-affinity FcγRIII (CD16)–mediated endocytosis and antigen presentation are dependent on the immunoreceptor tyrosine-based activatory motif (ITAM) of the associated gamma-chain.36-38 Since murine low-affinity FcγRII (CD32) contains only immunoreceptor tyrosine-based inhibitory motif (ITIMs) signaling motifs and therefore mediates inhibitory signals,39 we believe that any FcγR-binding–related activatory responses occur through FcγRIII (CD16)–mediated signals. We aim to specifically define the role of FcγR–B7.2-IgG in vivo interactions with the use of various FcγR KO mice.
A critical issue in the combination regimen is the appropriate timing for the immunoadjuvant. It is well known that chemotherapy induces peripheral leukopenia whose severity and duration depend on the type and dose of chemotherapeutics.40 Whereas high-dose, myeloablative regimens are accompanied by prolonged peripheral leukopenia, conventional chemotherapy is related primarily to neutropenia and, to a lesser extent, to lymphopenia. Several studies in humans have shown that among lymphocyte subsets, B cells are the cells most affected by chemotherapy, followed by CD4+ T cells, with CD8+ T cells remaining relatively well preserved.41-44 Interestingly, the remaining clonogenic T lymphocytes derived from acute leukemia patients with therapy-induced leukopenia have shown a broad cytokine response to in vitro activation.45 We have previously shown in the SJL AML model that absolute peripheral lymphocyte numbers return to normal within a week after chemotherapy with cytarabine and doxorubicin.26 On the basis of these findings, we reasoned that the interval between injections of B7-IgG should be 1 week or longer. Because 2 of the B7-IgG injections in the lymphoma models and 3 in the AML model were given on the same day with chemotherapy, it is conceivable that a percentage of T cells responding to B7/CD28 interactions by proliferation would be affected by chemotherapy. However, the favorable clinical outcome of the combination regimen in our studies suggests that a significant number of B7-IgG–activated T cells can nevertheless resist chemotherapy and eventually become long-lived antitumor memory cells.
A major potential advantage for the use of B7.2-IgG as an adjuvant to chemotherapy is its expected high safety profile. The protein has not shown any in vivo toxicity, even when injections of 500 μg were given to mice (unpublished results, May 1999). Studies in the MethA tumor model (which has reproducibly shown therapeutic responses to single B7.2-IgG treatment) have shown that the efficacy of the fusion protein is not ablated in IFN-γ27or IL-12p35 KO mice (unpublished results, October 1999). These observations dissociate the immune mechanisms mediated by IFN-γ and IL-12 and the efficacy of B7-IgG in therapeutic tumor models, and suggest that cytokines with less toxicity than IL-12 and IFN-γ may characterize the microenvironment, where tumor presentation occurs, or, alternatively, that B7-IgG induces cytokines only in specialized microenvironments.
The combination of immunotherapy with chemotherapy is an emerging form of cancer treatment. With the addition of an immune-boosting agent that, in principle, forces “provoked” immunity, conventional cancer therapy could conceivably be made more effective without increasing its toxicity.46 This may be manifested in greater durability of response rather than absolute clinical response rate. It is anticipated that individual immunomodulatory compounds will not synergize with all cytotoxic drugs, owing to differential immunosuppressive effects, and at present it is a great challenge to identify successful combinations in preclinical tumor models. The chemotherapy regimens used in this report apply beyond leukemia and lymphoma, since the combination of anthracyclines and cyclophosphamide is broadly used in the clinic and is one of the most widely used regimens in breast cancer treatment.47 The safety profile of combining B7.2-IgG with chemotherapy in preclinical tumor models, together with the potent therapeutic effect, has directed our efforts toward the development of strategies for clinical evaluation.
We thank Lori Block and Terri Haire for technical help and Drs Stan Wolf and Frank Borriello for critical review of the manuscript.
All authors were employed by Genetics Institute at the time that this study was conducted.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
K. Dunussi-Joannopoulos, Genetics Institute, 1 Burtt Rd, Andover, MA 01810.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal